Abstract
Objective:
The purpose of the review is to portray the theoretical concept on neurological disorders from research data.
Background:
The freak changes in chemical response of nerve impulse causes neurological disorders. The research evidence of the effort done in the older history suggests that the biological drug targets and their effective feature with responsive drugs could be valuable in promoting the future development of health statistics structure for improved treatment for curing the nervous disorders.
Methods:
In this review, we summarized the most iterative theoretical concept of structure based drug design approaches in various neurological disorders to unfathomable understanding of reported information for future drug design and development.
Results:
On the premise of reported information we analyzed the model of theoretical drug designing process for understanding the mechanism and pathology of the neurological diseases which covers the development of potentially effective inhibitors against the biological drug targets. Finally, it also suggests the management and implementation of the current treatment in improving the human health system behaviors.
Conclusion:
With the survey of reported information we concluded the development strategies of diagnosis and treatment against neurological diseases which leads to supportive progress in the drug discovery.
Keywords: Neurological disorders, computational aided drug design, structure based drug design, targets, drug delivery
1. INTRODUCTION
Neurological disorders are simply defined as engaged impairment or interruptions in nervous system. It coordinates with body’s all movement and functions [1]. The nervous system is considered as our body's command center and it is obvious that the brain comprises neurons, axons and synapses. This states that the brain is a complex structure which has the ability to store and process information through the myriad of sensory inputs. The neural axons connect the cortical and sub cortical processing center which denotes the network to be convoluted [2-4]. The brain is composed of specialized regions which interact to perform a complex task [5].
The function of neuron, transmission of signal and cell to cell communication are the processes intervened by the protein and its alteration which brings about a dysfunctional physiological state [6]. Function of the nervous system is devastated by the Neurological disorder which results in abnormal Central nervous system (CNS) and paraneoplastic neurological syndromes (PNS) growth, physical as well as mental stress, changes in behavior and thoughts [7, 8]. Dysfunctions of brain range from high disability and fatal neuromuscular condition ALS (amyotrophic lateral sclerosis) as well as mild and treatable depression.
The nervous system is affected mainly due to the Oxidative stress. During respiration, the aerobic organisms are vulnerable to oxidative stress since the oxygen is related to free molecules like hydroxyl (OH·), superoxide (O2–·) and hydrogen peroxide, produced by mitochondria. The reactive oxygen species produced is thought to be around 2% of total oxygen dependable during respiration however varies with different parameters [9, 10]. Due to lack of reactive oxygen species, oxidative metabolism leads to oxidative stress which causes neurological disorders. Neurological disorders affect mostly to the patients and their families, which often take out the qualities of being human. The chemical components present in the brain which motivates and focus are named as Neurotransmitters. The complex interaction between them helps to shift mood and changes our mind [11]. The neurons and the supportive glial cells belong to the transmitting signals which are built in the complex network of cellular components. The analysis of the transcriptome depicts that 69% of all human proteins (n=20344) are expressed and 1134 of these genes show an elevated expression in the brain in comparison to other tissue types. An analysis of these genes with an elevated expression in the brain shows diverse patterns of the expression in various neurons, glial cells and neuropil. The mesh-work of axons, dendrites, synapses and extracellular matrix was embedded in the central nervous system cells [12-14].
Neuroproteome is the term that describes the collection of proteins in the nervous system [15]. At present, the analysis of proteins were performed the affinity-based technologies and mass spectrometry methods. The functional mediators in many diseases including neurological disorders are protein and this neurological disorder associates protein deregulation. The susceptibility of developing disease is associated with genotype, while in others no such linkage has been observed. Regardless of the underlying cause, proteins play a major role in disease pathogenesis and generally in the focus of disease related research. The analyzed proteins are suitably found to be a drug target based on its expression level in normal and diseased condition [16, 17].
Once the drug target is identified as suitable for curing the disease, mostly structure based approaches are often used for identification of lead compounds. Structure based drug design is the growing, iterative and powerful approach includes the structural evaluation of target and drug discovery process. It has the ability to reduce the time as well as cost in developing ideas of new effects and potential drug lead molecules. This review intends to describe the present scenario in understanding the neuroproteome. The involvement of the neuroproteome in some most common neurological disorders is also studied. The technology used for the study of proteins and the disputes in relation to the structure based approaches were recapitulated [18-20].
1.1. Neurological Diseases and its Mechanism
The subsequent subdivision describes the disease characteristics, understanding of protein involvement and investigation efforts within some of the most common disorders affecting the nervous system.
1.1.1. Alzheimer’s Disease
The neurodegenerative disease that is progressive, impairs the memory and cognitive judgment is often accompanied by mood swings, disorientation and eventually delirium is Alzheimer’s disease. The most common cause of the neurodegenerative disorder rest with memory loss, decline in language skills, poor statement, confusion and other cognitive impairment that becomes the major threat in the ageing population [21-23]. Fyn kinase, GSK-3-β kinase and Cyclin dependent kinase-5 (CDK5) are the set of signaling pathways which behaves as the important donor to the synaptic dysfunction in Alzheimer’s disease [23]. Multiple features such as amyloid-β and τ protein aggregation, excessive metal ions, oxidative stress and reduced acetylcholine level play an important role in the pathogenesis of Alzheimer’s disease. Amyloid-β oligomer produces toxicity, as well as amyloid-β misfolding describes the importance of oligomerisation. Tau proteins bind to the microtubules in axons and stabilize them under normal conditions which exert pivotal roles. The pathological conditions of tau proteins result in hyper phosphorylation. The proteolytic cleavage of the amyloid precursor protein (APP) produces the Amyloid β which is a toxic peptide [24-26].
Emerging techniques were revealed by researchers and still they are passionate to discover new techniques to treat Alzheimer's. The drugs available currently help to treat the symptoms of Alzheimer but does not help in the treatment of the underlying disease or delay its progression [27]. An innovative drug of Alzheimer would treat the underlying disease either through stopping or delaying the cell damage which leads to the worsening of symptoms. The neuro- transmitters were regulated with the help of few drugs and the brain chemicals transmit the message between neurons subsequently. This transmitter helps to maintain thinking, memory, communication skills and helps to deal with the certain behavioral problems [28]. On the other hand, these drugs do not show the perfect effect on the underlying disease process and are effective for some, but not for all people. They may be helpful only for a limited time. Numerous promising drugs were in development and testing. Several researchers focus towards screening of leads in the specificity of AD, causing over expressed proteins as drug target. The proteins, namely APP, APOE, BACE, and tau play important roles in the pathology of AD. Therefore, efforts are being made to develop new inhibitors for BACE, PS-1 and γ-secretase for treatment of AD [29-31].
Recently, research scientists from Duke University have declared that new processes have been developed which contributes to the development of the disease is observed in the study of Alzheimer’s in mice. They state that the immune cells which protect the brain normally begin to consume arginine, a vital nutrient [32, 33]. The success of this study continues with the suitable drug candidate which blocks the mechanism and they would be able to prevent the ‘plaques’ formation in brain. These are the characteristic of Alzheimer’s disease and also increase memory loss in the mice. The hallmarks of Alzheimer’s disease are the neuronal, synaptic loss, extra cellular amyloid deposits and degenerate filament due to the intracellular deposition (neurofibrillary tangles). The major hallmarks are the cognitive impairment and memory loss. In AD, brain-damaged neurons, neuritisarehighly insoluble, Aβ42 peptide deposits and neurofibrillary tangles provides stimuli for inflammation. Accumulation of Aβ peptide is caused by the gene mutations like PS2 and APP human mutation [34, 35].
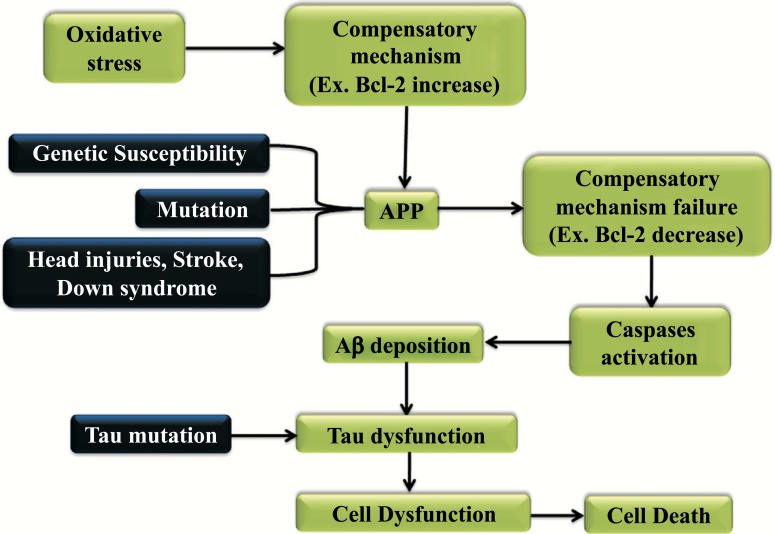
The mechanism of the Alzheimer’s disease is represented in Fig. (1). When the oxidative stress reflects the imbalance between the production of free radicals the detoxification through neutralization causes the compensatory mechanism to increase. Environmental and genetic factors when comes to affect the protein APP this leads to a compensatory mechanism to be failed and in turn activates the Caspase and the Aβ deposition. Later, when the Tau mutation occurs this leads to tau dysfunction that indirectly leads to cell dysfunction and cell death [36, 37].
Fig. (1).
Mechanism and effect of Alzheimer’s disease.
1.1.2. Niemann-Pick Type C Disease
The rare genetically inherited Niemann-Pick type C disease is an autosomal-recessive as well as neurovisceral disorder sourced by genetic mutation, abnormal storage intracellularly of un-esterified glycosphingolipids and cholesterol in the endosomal or lysosomal compartments [38]. Niemann-Pick type C disease (NPC) belongs to lipid storage disease available for infants, children, or adults. Neonates can be prone to getting ascites and liver disease due to the infiltration of the liver or respiratory defects from penetration of the lungs. Liver or pulmonary diseases in infants were described as hypotonia and they have a delay in the development [39]. The common management of the disease occurs in the mid-to-late childhood with the insidious onset of ataxia, dementia and vertical supranuclear gaze palsy (VSGP). Seizures and Dystonia are common. Finally, Dysphagia and Dysarthria become disabling and makes the oral feeding impossible. Usually, death occurs in the late second or third decade from aspiration pneumonia and adults are more prone to get affected with dementia or psychiatric symptoms [40].
Niemann-Pick Type C (NPC) is a different disease than Type A or B (ASMD). Metabolizing cholesterol as well as other lipids within the cell is difficult for the patients affected with the Niemann pick type C disease. In the liver and spleen cholesterol has been accumulated in excessive amount whereas in brain other lipid accumulation persists. ASM activity shows secondary reduction caused due to the NPC and this leads to the consideration of the three types of the same form of the disease. Neurological symptoms begin between the ages of 4 and 25.40 cm most cases [41]. In general, the later neurological symptoms begin, the slower the progression of the disease. The biochemical testing method is used for the diagnosis of NPC and reveals the impaired cholesterol esterification and the staining of positive Filipin in cultured fibroblasts. In NPC disease, the lack of function in NPC1/NPC2 or lysosomal acid lipase results in cellular cholesterol trafficking [42]. The individuals with NPC have NPC1 and the mutations were occurred. NPC2 were diagnosed in less number of individuals and also the mutations occur. In NPC1 and NPC2 molecular genetic testing was performed and the mutations caused by the disease were detected in nearly 94% of individuals. The Mutation occur in NPC gene causes to type C1 & C2, where protein produced from NPC gene are not able to involve in the movement of cholesterol and lipids in cells and the accumulation of lipids causes the cell death [43].
Primary irrational indicators may be of hepatic, pulmonary, neurological or psychiatric in nature. Cerebellar ataxia, cataplexy, progressive dementia, movement disorders, epileptic seizures are all associated with the NPC disease [44]. Miglustat is the first disease-specific approved drug for the treatment of neurological manifestations to stabilize or slow the irreversible neurological damage. There are very limited data of effective treatment with Miglustat to prevent neurological symptoms for asymptomatic patients [45]. But, there is a need for raising disease awareness and improving early detection, for optimal disease management. Hydro- xypropyl-beta-cyclodextrin has been granted by the FDA into orphan drug and labeled 2-hydroxypropyl-β-cyclodextrin (HPβCD) can be used for the effective treatment for Niemann-Pick type C disease [46-49].
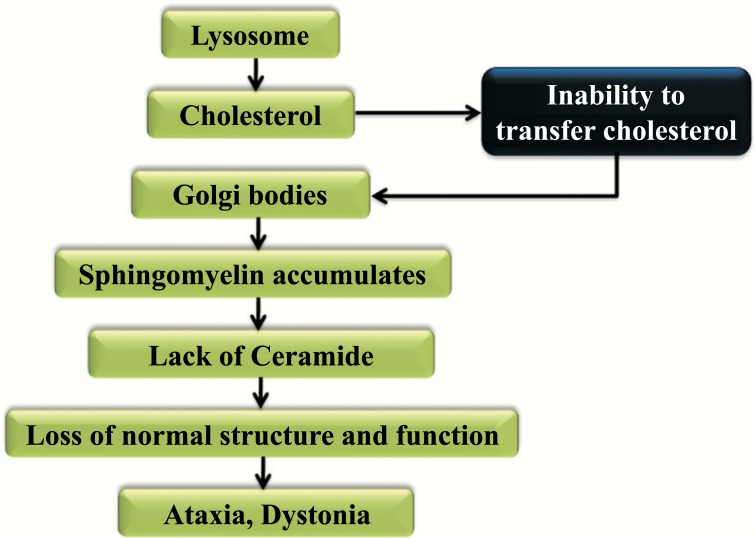
Cholesterol is esterified as well as hydrolyzed by LAL for the production of fatty acids with the free cholesterol. This free cholesterol is then transmitted to NPC2. When NPC2 or NPC1 is absent, cyclodextrin replace their function in promoting cholesterol efflux. For better understanding, the mechanism is symbolized in Fig. (2). Free cholesterol is transported from glia to neurons through the ApoE complex and the permeation of the blood-brain barrier is not performed by plasma. The accumulation of the unesterified cholesterol and gangliosides in Lysosomes were happening because of the trafficking from the endosomal system by the free cholesterol. Lysosomes break down the food and inability to transfer cholesterol to the Golgi bodies, which followed by the accumulation of Sphingomyelin results in the lack of ceramide. It leads to the loss of normal function with the protein leading structure for the development of ataxia and dystonia [50-54].
Fig. (2).
Mechanism and effect of Niemann Pick type C disease.
1.1.3. Parkinson’s Disease
The progressive movement disorder and chronic disease is the Parkinson's disease (PD) which means that the symptoms continue and worsen over time. It is the second most chronic and movement disorder of the nervous system worldwide. This disease involves the death and malfunction of the vital nerve cells in the brain named neurons, [55, 56]. The region substantia nigra in the brain consists of neurons, which are primarily affected by Parkinson’s. The symptoms that caused by these diseases is the tremors, muscle rigidity, bradykinesia and stooping [57]. The dying neurons produce a chemical substance termed as dopamine, which sends messages to the part of the brain and controls the coordination along with movement during the progression of PD. This makes the people who have uncontrollable movement [58]. Stages of Parkinson’s disease include various stages, and in the beginning state - the infected person shows the very mild symptoms that generally do not interfere with daily activities. Here mostly, the one side of the body shows Tremor and other movement symptoms. Later, the second stage shows tremor, rigidity and other movement symptoms affect both sides of the body. Third stage shows mid stage of progression of the disease with loss of balance and slowness of movements are hallmarks of this phase. Fourth stage shows much severity and the patience can't stand alone without any assistance. Fifth stage is the most advanced and debilitating stage of Parkinson’s disease and the patient experience hallucinations and delusions. α-synuclein and LRRK2 are the major genes known to produce dominantly inherited PD [59-62].
The role of synuclein and the molecular pathology is understood with the available resources and later LRRK2 are seen and the regular function and the compulsive role of the genes in PD are studied. PTEN induced kinase 1 (PINK1) dysfunction is also one of the important leads to the loss of dopaminergic neuron loss in PD [63]. Recently, researchers have announced two lead compounds, namely GW5074 and indirubin-3’-monooxime, blocks the action of the LRRK2 enzyme and prevents the death of mouse neurons. The PD and the mutation in LRRK2 gene were linked together, which was discovered by the researchers with great interest to works on drugs for the alteration of the LRRK2 enzyme. These studies suggest that in animal models of PD the neurons may be protected by the LRRK2 blocking. Based on the compounds that inhibits LRRK2 new therapies can be suggested which can be developed for the people with PD who constitutes the LRRK2 mutation [64, 65]. The mutations in the gene which codes for the parkin protein have been linked to the Parkinson’s disease and this uses the tag damaged mitochondria as waste by cells. When the tagged mitochondria was damaged and degraded by Lysosomes, which is the cell’s trash disposal system. The mutations that were known in parkin prevent the tagging of mitochondria and results in the accumulation of unhealthy mitochondria [66-68].
Few other researchers are search towards the identification of suitable drug target and also some medicinal drugs and pharmacological therapies that may have facilitated to relieve from PD. In regards to the pathogenic process the lack of understanding may be the reason for the non-availability of effective treatment in which the treatment could prevent the onset and the progression of the disease. However, investigative and drug development efforts should be diversified to fully address the multi-factoriality of the disease. Dopaminergic neurons play an important role in the Parkinson’s disease. The death of the dopaminergic neurons results in the Parkinson’s disease.
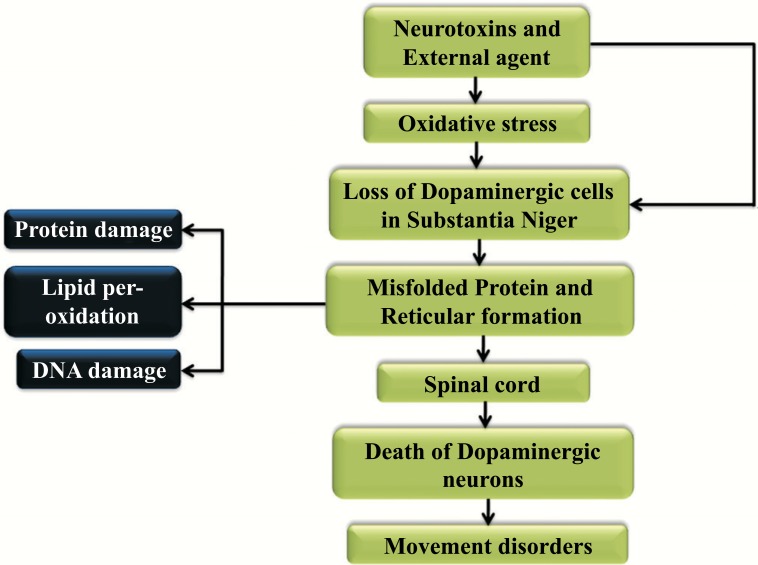
Misfolding of the proteins and dysfunction of the ubiquitin-proteasome pathway is pivotal in Parkinson disease pathogenesis. Substantia nigra degenerates in Parkinson’s disease (PD) when the nigrostriatal pathway is disrupted that reduces the striatal dopamine and it produces PD symptoms which is very much depicted in the (Fig. 3) [69]. In mammals as well as the humans the movement of the neuronal circuits in spinal cord helps in the locomotion [70]. The defective utilization of the afferent input which is in combination with the secondary compensatory process in the central motor disease shows the involvement of typical activity namely the spasticity [69, 70]. The representation of the mechanism and effect of the Parkinson’s disease is shown in Fig. (4). Neurotoxins and external agents lead to the oxidative stress, which indirectly results in the dopaminergic cell loss in Substantia Niger and where the misfolded protein and reticular formation is developed. This protein misfolding leads to the protein, DNA damage and the Lipid peroxidation. This happens over the connection in the spinal cord. As a result, there exists the death of dopaminergic neurons and this tends to exhibit the disease [71-75].
Fig. (3).
Mechanism and effect of Parkinson’s disease.
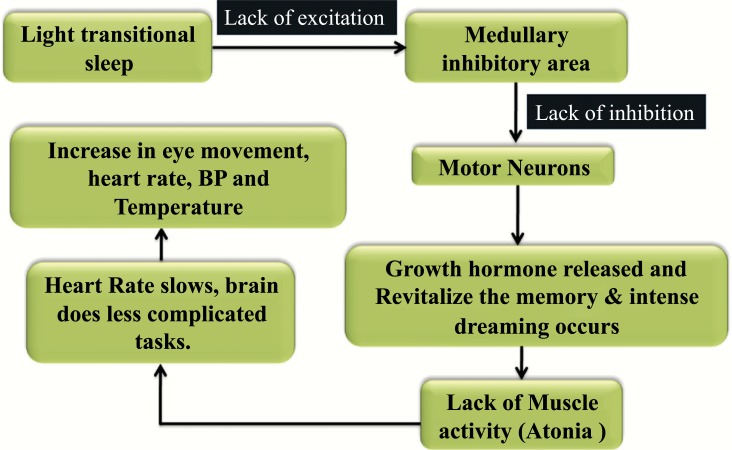
Fig. (4).
Mechanism and effect of Rapid Eye Movement sleep behavior disorder.
1.1.4. Rapid-eye-movement (REM) Sleep Behaviour Disorder (RBD)
The disorder which depends on the sleep behavior is the rapid-eye movement (REM) and this results in the abnormal behavior in the sleep phase with the rapid eye movement and the disorder is stated as the REM sleep behavior disorder (RBD) [76]. This disorder is characterized by certain dream enacting behaviors like punching, shouting and falling out of bed and these characteristics are related to unpleasant dreams and the loss of normal REM-sleep muscle atonia. It is also a Parasomnia. The transition between the different states was named as the rapid eye movement (REM) sleep and wakefulness and this belongs to the phase of sleep. The different states were associated with the dreaming and the non-rapid eye movements (N-REM) sleep. Numerous characteristics were defined during each state and the understanding of the disorder of REM behavior plays an important aspect all through REM sleep [77, 78]. In brain, the electrical activity was recorded by the electroencephalogram that looks similar to the electrical activity occurring during awake. In REM sleep behavior the neurons were more active like when they are during waking and is characterized by temporary muscle paralysis [79, 80]. Associated with the other neurodegenerative diseases, namely the Parkinson’s dementia with Lewy bodies and multiple system atrophy which becomes idiopathic. Patients suffering with RBD have no waking motor or cognitive complaints [81].
REM sleep behavior disorder is probably at increased risk for neurodegenerative disease. Underlying RBD is one of the most fascinating scientific nosiness in medicine and neurology. Our scientific community is awaking people to educate on the requirement of treatment and diagnosis of RBD [82]. Researchers have recently identified the robust connection to neurodegenerative disease, particularly Parkinson's disease. The rapport between RBD and Parkinson disease is complex; whereas the people with Parkinson’s disease will not always develop RBD [83]. The treatment of rapid eye movement sleep behavior disorder (RBD) can be exciting in some patients with fundamental neurodegenerative conditions. The drug compound Clonazepam has proven to be a highly successful treatment for RBD and the precise process of mechanism of clonazepam in RBD is not very clear but they may reflect in some of its serotonergic properties. In addition to clonazepam, melatonin, nlafaxine, mirtazapine, levodopa, carbamazepine, clonidine, and L-tryptophan are some of the drugs in specific to cure this RBD. Unfortunately, the functional mechanism of these drugs is still unclear and so identifying the suitable drug targets will be more beneficiary for the RBD [84]. The skeletal muscle atonia which is normal is lost when the rapid eye movement affects the patient with the protruding motor activity and the dreaming. Medullar magnocellular reticular formation, created the concrete pathway of the spinal motor neuron inhibition. The midbrain and forebrain structure is connected to the parts substantia nigra, forebrain, thalamus, frontal cortex, hypo- thalamus. This occurs in disproportionally greater frequency in dementia and Parkinson’s disease (PD) with Lewy bodies [85].
Abnormalities of electro-encephalographic activity, cerebral blood flow & cognitive, perceptual and autonomic functions, motor neurons inhibition, lack and revitalization of the memory is associated with the lack of muscle activity. The abnormal and violent motor manifestations are the characteristics of the Rapid eye movement (REM) disorder [86]. The Motor neurons in the medullary inhibitory area lack the excitation and the inhibition of the transitional sleep. These results in the release of Growth hormone, revitalize the memory, develops atonia during dreaming, the heart rate slows down and the brain does only the easy tasks. Later, due to this action there increase the eye movement, temperature, BP and heart rate. The light transitional sleep happening in the medullary inhibitory area is a victim of the lack of excitation and inhibition of the motor neurons [87-89].
1.1.5. Amyotrophic Lateral Sclerosis (ALS)
The major adult-onset of the neurodegenerative disorders is the greatest aggressive Amyotrophic Lateral Sclerosis (ALS) that affects motor neural cells of the brain and the spinal cord that cause muscle weakness and impacts physical function. The movements of the muscle were controlled by the nerve cells, but when it is affected by ALS these nerve cells die. In this regard, the muscles progressively weaken and at last will be wasted [90, 91]. The probable reasons of the ALS were identified by the researchers and these comprise gene mutation, chemical imbalance, disorganized immune response and protein mishandling. Patients affecting with ALS tend to live only maximum for three to five years after the sign of the first symptoms. In ALS particular mutations in the gene were observed and they propose the changes in the processing of the RNA molecules that gives the function of the gene regulation as well as the activity helps in the ALS-related motor neuron degeneration. Various mutations also reported which shows the incriminate defects in the protein recycling [92]. There are so many other prospective defects in the structure as well as the shape of the motor neurons and the increased susceptibility to environmental toxins. In general, it is progressively clear that a numerous type of cellular defects leads to motor neuron degeneration in ALS. Even though, the ALS pathogenesis is available and the understanding of it was done extensively, but unfortunately no effective therapy have developed to cure the ALS [92-95]. Currently, there is one FDA approved drug, Riluzole uses for treatment of patients with ALS whereas it has also been found to slow the rate of development of the disease in some individuals. But, Scientists have made number of strategies and approaches to improve the value of life for people with ALS [96-98].
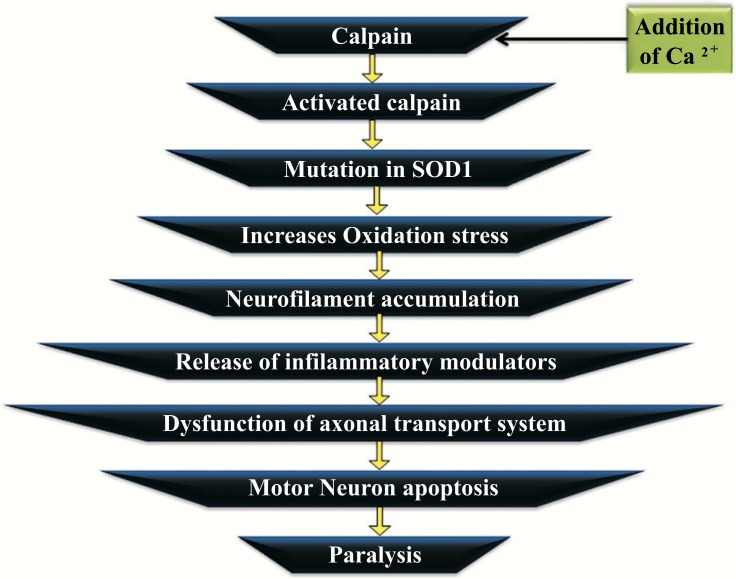
The normal physiologic neuronal function is convinced with the help of the calcium sensitive protease and the calpain satisfy the requirement which is a universal component. Alteration in calcium homeostatis lead to determined pathway activation of calpain in a number of neurodegenerative diseases. The cleavage of the numerous neuronal substrates is the result of the clinical activation of calpain and this cleavage affects the neuronal structure through negative aspects and leads to the inhibition of the required neuronal survival mechanism. The calcium abnormality results in the calpain stimulation in ALS. The disease, ALS is the disorder of protein aggregation. The copper/zinc superoxide dismutase (SOD1) is mutated and this aggregates in the Amyotrophic lateral sclerosis [99, 100]. Calpain gets activated by the addition of Ca2+ and leads to the mutation in the protein SOD1. This increases oxidation stress and as a result leads to neurofilament accumulation. The release of inflammatory modulators promotes the dysfunction of the axonal transport system and there develop the motor neuron apoptosis. The degeneration of motor neuron characterizes the Amyotrophic lateral sclerosis that structurally resembles apoptosis. Fig. (5) represents the mechanism and effect of the Amyotrophic lateral sclerosis. The neuronal death is progressed into 3 sequential stages, namely chromatolysis, somatodendritic attrition and apoptosis. The increase of calcium level is associated with neuro- degenerative disease. Calpain catalyses the conventional proteolysis. The calpain activation occurs in the early phase of ALS which will be a cellular defence mechanism [101, 102].
Fig. (5).
Mechanism and effect of Amyotrophic Lateral sclerosis.
1.1.6. Epilepsy
The ancient Greek word which describes the disorder of the central nervous system is the Epilepsy. In this disease the activity of the nerve cell in the brain develops disruption sensations, loss of consciousness and cause seizures or periods of unusual behavior [103, 104]. When the brain directs the wrong signals through the nerve cells or neurons automatically seizures will happen. This can lead to the emotions and the strange sensations or make people behave strangely. Worldwide millions of people are experienced by Epilepsy, a chronic disorder characterized by paroxysmal brain dysfunction resulting from abnormal neuronal activity. People affected with epilepsy were characterized as destructive, potentially fierce, underdeveloped, weak sluggish, antisocial and physically unattractive. The fundamental characteristic feature of epilepsy is persistent and senseless attacks [105, 106].
Based on the development of the epileptic seizures, they are broadly classified based on the onset whether it is partial and those which are generalized. The partial epileptic seizure arises through one hemisphere of the brain and those that are generalized seizures begin in the involvement of both hemispheres [107]. Currently, to cure this disaster is a lack of well-known treatments. But, different methods are developed for the design of new more selective and effective drugs against epilepsy. Research performed by the scientist shows that the primary cause of the epilepsy in all children, adults and the elderly people and they affects the brain and as a result brain trauma, stroke and tumors [108]. Current research focuses on the development of new model systems that is used frequently for the screening of potential treatments for the epilepsy. The cause of epilepsy is observed with the help of the identification of the genes or genetic information which influences the cause and this may allow the doctors to prevent the disorders as well as to predict what kind of treatment would be more beneficial to individuals with specific types of epilepsy. The research, based on how the neurotransmitter interact with the brain cells for the control of nerve firing as well as the contribution of the seizures by the non-neuronal cells is performed by scientist [109].
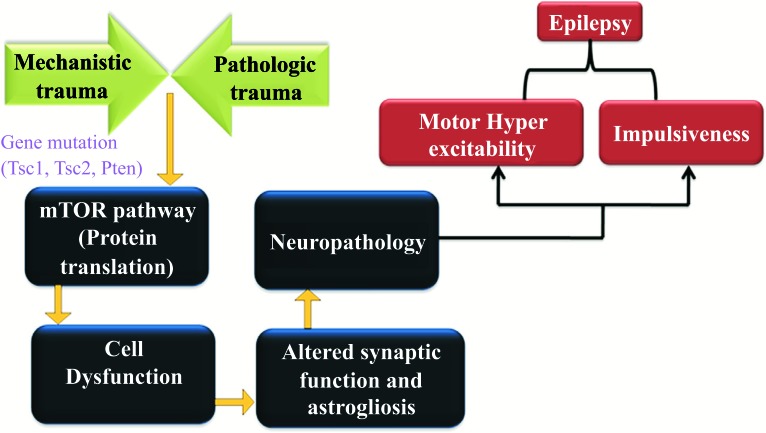
The national institutes of health funds for the research which could be more potent for the flexible brain implant, which can be used to treat the seizures. Researchers are developing a new instrument which helps the assistance of the brain and also trying to improve the MRI as well as the other brain scans which may assist in diagnosing the epilepsy and identify the source or focus of the seizures in the brain. Epileptic seizures develop from excessively synchronous and the discharge of a group of neurons. Neuronal excitability is due to the persistent increase of the epileptic syndrome, which is the major feature of epilepsy. The mechanism and effect of the disease, Epilepsy are represented in the Fig. (6). There are diverse causative factors such as oxygen, trauma, deprivation, infection, metabolic rearrangements and tumors associated with the cellular discharges. The most important part of the epilepsy is the disorder of neuronal migration in the mTOR pathway [110, 111]. The abrasion of the pathology and the mechanism of the genes affect the mTOR pathway, especially the protein translation. It promotes the cell dysfunction and the alteration of the synaptic function, leading defects to the complete neuropathology in terms of hyper excitability and impulsiveness [104].
Fig. (6).
Mechanism and effect of Epilepsy.
1.2. Factors Associated with Neurological Disorder
1.2.1. Role of Cholesterol in CNS associated with Neurological Disorders
Cholesterol is a 27-carbon sterol compounds that show amphipathic properties and plays both physiological and structural functions in the human body. The human brain contains largest amount of cholesterol than any other organ which present the close relation between cholesterol and nervous system [112]. This component is the indispensable which plays an important role in the plasma membrane of all the eukaryotic cells and serves as a precursor for the biosynthesis of steroid hormones, vitamin D and bile acids [113]. It is accentuated in both the central and peripheral nervous systems of mammals. But the foremost role of cholesterol is highlighted in the central nervous system (CNS) which is composed of myelin sheaths as well as an important constituent of synaptic vesicle membrane in which an appropriate membrane structure is essential for the proliferation of salutatory impulses laterally on the axon as well as in the synaptic connectivity among neurons [114]. Past evidence has accumulated about the genetic changes in cholesterol metabolism leads to a wide continuum of neurological problems, linked to several neurological disorders, including Huntington’s disease (HD), Alzheimer’s disease (AD), Parkinson’s disease (PD) and Niemann-Pick type C disease as well as stroke and brain trauma [115, 116].
The consequence of sterol in the mechanism of the atherosclerosis and other cardiovascular diseases is also well recognized, whereas correlations occur amongst brain disorders and cholesterol and these are immobile and poorly categorized. It is profuse in synaptosomal membranes that influence stability, synapse formation and neurotransmitter that helps in the release of the pre-synaptic level and the cholesterol molecule focuses generally in the inner leaflet of the lipid bilayer which gives the principal structural role. The constituent of lipid rafts which anchors or regulates the numerous neurotransmitter receptor activity (e.g. GABA receptors and AMPA-type glutamate receptors) as a function of the post synaptic terminals and other post-synaptic elements on the membrane. Micro-tubular conveyance of synaptic vesicles in the subsequent fusion, cytosol, and release via SNARE protein interaction depends on the high cholesterol levels [117, 118]. Cholesterol requirement is very high in the CNS which is responsible for membrane curvature. The neuronal transmission of the impulse at the cellular level is aided with the help of myelin sheaths and the specializations of the membrane which is derived from the oligodendrocytes and in which the axon of the several neighboring neurons were wrapped with the axon. Due to enrichment of cholesterol, the reduced permeability is available in the myelin sheaths to the ion which allows the propagation axon with the electrical impulse rather than the oligodendrocytes membranes. Further studies on the cholesterol metabolism in the nervous system can discover to pave the way for novel inhibitor to treat neurological disorders [119-121].
1.2.2. Role of Environmental Factors in Neurological Disorders
The human nervous system is the controller of the behaviors with different level of complexity. Many neurological disorders are not well understood and have less genetic information of their functional proteins and genes. The majority constitutes are environment factor which directly influences the nervous system during the diseases progress [122]. Research has been shown many of toxins, pesticides or other exposures/agents related to environmental features that play a significant role in the progress of neurological disorders [123]. For an example, MPTP (1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine) which is a toxic product and the active state (MPP+) affects the catecholamine neurons and leads to death, it also used to craft the animal models of Parkinson’s disease (PD). In case of Alzheimer’s disease, most of the metals such as aluminum, zinc or copper have been recognized as more risky environmental factors. Some pesticides and mercury have been identified as exposure to lead ALS. More research on the role of environmental factors in neurological disorders may lead to be latent risk factors [124, 125].
1.2.3. Role of Stress in Neurological Disorder
Stress plays a very significant role in the commencement of various ailments like immunological disorders, cardio- vascular diseases pathophysiological consequences of aging. In Neurological diseases, stress triggers certain mechanism and sometimes destroys some mechanism of pathophysiological importance which is highly associated -with neurological disorder [126]. Numerous movement disorders are of neurological origin andare pretentious of stress and trauma. There is a high degree of connection between neuro- degeneration and stress. The stress on the nervous system due to the pathophysiological effects and the function of hippocampus are closely related [127, 128]. The contribution of stress in the degeneration of neurons, especially in the hippocampus formation is strong. Stress is an important factor that causes deficits in memory performance and this effect in turn is important for pathophysiological processes connected with the Neurological disorders. The structure that plays a major part in the loading of several actions in the long term remembrance loss is the stress-sensitive hippocampus [129]. The stages of anxiety, fright and provocation induces the learning deficits and memory loss that leads to the deterioration and damage of neurons in the brain. The effect of stress on the hypothalamic pituitary adrenal axis represents the pathophysiological component in neurodegenerative diseases. The genetic, internal, external and environmental factor alters the vulnerability to the neurodegenerative disorders as well as the inflammatory diseases. The stress associated with anxiety and depression has been discussed which demonstrates it as a mechanism related to anxiety and chronic stress. Stress is the important characteristic that mediates, promote and even cause mental disorders like depression [130]. The role in the susceptibility, progress and outcome of neurodegenerative diseases/mental disorders are influenced by stress [126].
1.2.4. Accidental Damages in Neurological Disorders
The occurrence of auto reactive B cells and antibodies in body were detected when there is an existence of neurological disorders. The contributory role of these components was well established in neurological disorders [131]. Axonal damage has been recently recognized as the key predictor in various central nervous system disorders that includes head trauma, metabolic encephalopathy’s, multiple sclerosis and spinal cord trauma [132]. The important factors that influence the body condition to be prone to the neurodegenerative disease is the oxidative stress. This is associated in both normal aging and countless neurodegenerative diseases. The neuronal loss is the result of the oxidative stress and is initiated with the antioxidant molecule decline of Glutathione (GSH). This GSH has several roles with regard to the nervous system, namely the possible neurotransmitter, free radical scavenger and redox modulator of ionotropic receptor activity. When the exhaustion of this GSH happens there develops oxidative stress which in turn increases the level of toxic molecules [133, 134]. The role of oxidative stress is significant in various neuro- degenerative diseases like Alzheimer’s, Amyotrophic Lateral sclerosis and Lo Gehrig’s disorder [135].
1.3. Computational Drug Design
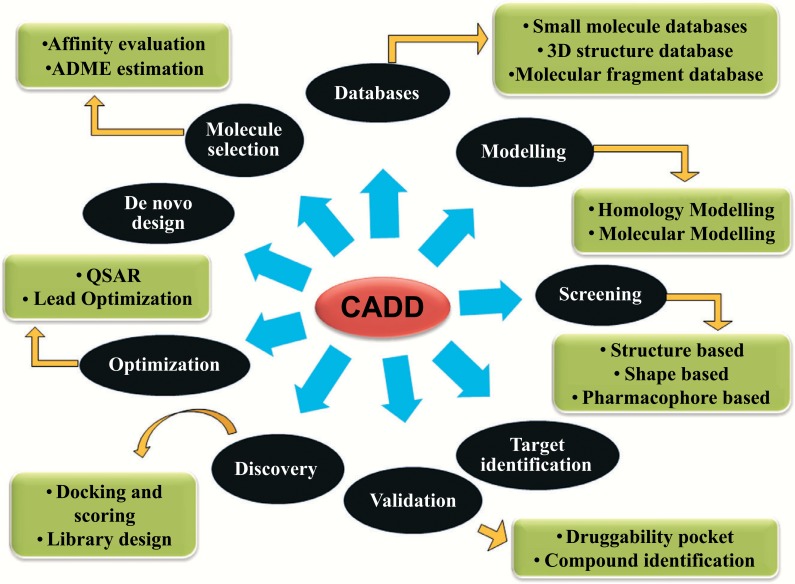
In the biomedical arena, a Modern strategy of computer-aided drug design (CADD) is an emerging way to facilitate the discovery, design and optimization of the new effects and potential therapeutic agents. Computer-aided drug design (CADD), represents the role of computers and the applications of tools with the help of computers in the drug design process [136]. Drug design process is a vast area in which it is specific to target a drug and this process commence when a chemist identifies the drug candidate. which displays an attractive biological profile and will get completed when both the chemical synthesis and the activity profile of the novel chemical entity are optimized [137]. The drug is an organic small molecule which inhibits or activates the purpose of a bimolecular such as a protein which in turn results in a remedial assistance to the patient. Universally, the drug design involves in the development of small molecules which is complementary in contour and charge to the biomolecular target to which they binds and therefore will bind to it [138]. Drug design relies on computer modeling techniques and the topology of the target and the compound frequently as well as not necessary. This is referred to as the computer-aided drug design. Computational approaches are playing the eminence and important role in drug discovery which reduce the time as well as cost for developing new drug against various biological diseases [139]. The most precious part of the drug design is the prediction of small molecule which binds to the target macromolecules. De novo drug design is a continuous process where the three dimensional organization of the receptor is used to develop a novel molecules. This also makes the involvement of the determination of structure with the lead target complexes and the modification of the lead with the help of different tools [140, 141]. This technique was used for the development of new chemical classes of compounds which are similar to the sub-compounds to the target. Pharmaceutical drug developments with the computer design technique intrinsically are in need of the complex drug design [142]. The overview of the various components of the Computer Aided Drug Designing (CADD) is represented in Fig. (7).
Fig. (7).
Overview of Computer Aided Drug Designing.
The predictions of the binding affinity with the help of the modelling techniques are successful. There are numerous properties, namely the bioavailability, lack of side effects and metabolic half life, etc., which is optimized when the ligand become safe and efficacious drug [143, 144]. The characteristics are generally difficult for the optimization of the rational drug design techniques. In drug discovery there are numerous approaches where-as especially the traditional approaches rely on the step wise synthesis and the screening program for the large compounds for the optimization of the activity profiles [145]. Until and unless we know the disease and the receptor responsible for the disease, it is not possible for us to design the drug. In rational drug design the initial step necessary for the process of the technique is the identification of the molecular target which critics about the disease or the infectious pathogen. The necessary requirement of the discovery of the drug is the determination of the molecular structure of the target, in which the word rational shows better meaning and sense [146, 147]. The foremost types of the drug design are of two types. The first type is referred to as the ligand-based drug design followed by the structure – based drug design. Structure-based (SBDD) and ligand-based (LBDD) drug design are extremely important and active areas of research in both the academic and commercial realms [148].
1.3.1. Role of Computer Aided Drug Design
In today’s fast growing world, with the marvelous ongoing growth in the field of pharmaceutical drugs, most of the drugs have discovered to cure for an enormous variety of diseases. To diminish the time & cost, computational approaches emerged in 1960’s by Hansch and Fujita, used to analyze the drugs & the mechanistic pathways of the disease, also applied to predict the activities of the compounds, the technique known as Computer aided drug design (CADD) [149, 150]. The emerging tool to rationalize drug discovery, development and optimization are the CADD. It is being utilized to facilitate the target identification, validation, optimization of the ADMET (absorption, distribution, metabolism, excretion and Toxicity) profile of a drug and safe drugs. Large number of selected compounds which can be either the natural products or the synthesized compounds is tested through the biological assays or screens, which takes long time also high price tag. But, CADD has the ability to produce and verify the biological activity of huge quantity of compounds within a tinyphase of time than biological assays. With the help of CADD is possible to explain the molecular basis of the therapeutic activity [139, 151].
CADD has played imperative role in the development of therapeutic agents over past decades. Optimization of the structure for the pharmacological tests in the pipeline of drug discovery is carried out with the help of Computer aided drug designing [152]. Computational assessment of the binding affinity of inhibitors before synthesis is the major component of CADD paradigms. The approach of CADD is applied based on the availability of the experimentally determined 3D structure of the target molecule. Computer aided molecular design has a very significant influence in drug designing. The approach of influencing can either be structure based or the ligand based drug design. Increased computational possibility and the development of sophisticated modelling algorithms are available for accurate predictions of the inhibitors as well as the targets [153-156]. The neural network is the novel approach that shows the ability in the various modelling processes. Various neural networks are studied with the help of the pathway of Blood brain barrier permeability [157]. The quantum chemical descriptor is one of the important components of the Quantitative structure activity relationship that describes the activity and susceptibility of the compound quantitatively [158]. The central approach of the structure based computer aided drug design helps in the development of the compounds which binds tightly to the biological targets where there will be a large reduction in the free energy, improved ADMET properties and the targets which have reduced off-target effects. If the structure based drug designing is being a thriving application, then these scheme will result in the best lead compound that can be further authorized in the in vitro and in vivo studies [152, 159].
1.3.2. Structure-based Design
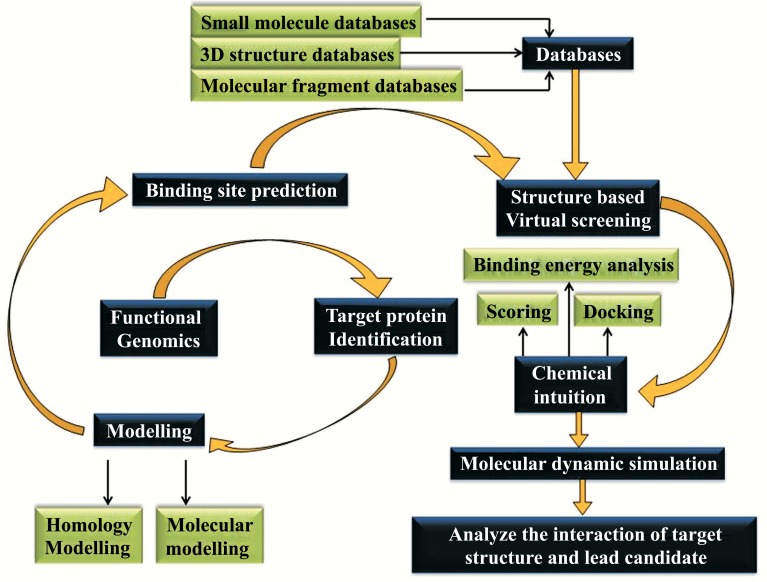
The three dimensional structure of the biological target is the main source for the structure-based drug design (or direct drug design) requires the three dimensional structure of the biological target which is obtained through methods such as x-ray crystallography or spectroscopy [160]. There is also a possibility for the designing of the ligands through the screening protocol as well as the screening against the homology model which contains the highest degree of confidence. The main idea of the structure based drug design is to use the 3D protein structure which predicts the ligands to bind to the target [161]. The structure of the biological target and the candidate drugs helps in the prediction of the high affinity when they bind and the selectivity to the target can also be designed with the help of interactive graphics as well as the insight of the medicinal chemist [162]. Alternatively a wide range of computerized computational approaches may be suggested for the development of new drug candidates. In past decades the structure-based drug design becomes a fast growing and highly facilitated field. It is also a robust and useful process includes the selection of biological objective, the evolution of protein structure of target, lead identification, lead development and optimization of drug candidate [163]. The ability to analyze the 3D structure of the biological target also helps in the development of the assessment of the structure based drug design. The methods which are highly in need through the methods such as x-ray crystallography and NMR spectroscopy, which is the main concept of structure based drug design. Structure based drug discovery has become a very potent and imperative tool, helps the genomics and proteomics that lead to the breakthrough of the great number of candidate drug targets. To overcome the problems arising during the development of effective drug in the pharmaceutical area, SBDD approaches (Protein Modelling, Docking, library design, fragment based design, molecular dynamics, etc.) are taken a step forward to design potential drug based on the structural information of the target and the properties of the ligand [164-166].
Modern computational structure based drug design has established a novel platform in designing the potential inhibitor against the neurological disorders [167]. Various targets of neurological disease have been studied thoroughly. GABA-AT protein and Htt protein from HD has been studied with the help of the SBDD approaches. GABA-AT protein is a neurotransmitter which is a significant source of the regulation of muscles in accordance with the help of neurons. In order to overcome this regulation problem GABA-AT protein helps to get a solution [168-171]. Fig. (8) represents the flowchart of Structure-Based Drug design approach.
Fig. (8).
Flow chart of Structure Based Drug Designing.
1.3.3. Implementation of Structure Based Drug Design in Neurological Disorder
The interplay of many dysregulated physiological processes characterise the complex multifactorial disorders that in turn relate to neurodegenerative diseases. The Parkinson’s disease (PD) involves the multiple perturbed cellular function that includes mitochondrial dysfunction and autophagic dysregulation that is preferentially sensitive to dopamine neurons. A network with the protein P62, GABARAP, GBRL1 and GBRL2 formed with the union on high preference of accessibility. These proteins are predicted to be important for the cross talk between the mitochondrial dysfunction, autophagy and MPP+ neurotoxicity in the pathological state. Thus, the protein-protein interaction network analysis helps in the identification of key mediators of neurotoxicity [172]. Glycogen synthase kinase 3β (GSK-3β) which belongs to the serine/threonine protein kinase has been materialized as a key target in drug discovery and these biological target is implicated through 4d in multiple cellular processes and associated with various diseases. This GSK-3β inhibitors proved as a useful remedial compounds in treating the condition associated with the prominent level of enzyme action such as a neurological disorder and type2 diabetes. The proprietary compound libraries as well the functional activity of the selected compounds were obtained in the virtual screening study that helps in the analyzing of the human GSK-3β. The novel hit compounds were identified with the help of the in silico screening approaches. Molecular docking studies assist in the way for the finding of novel chemical scaffold for GSK-3β inhibition and this provides the base for a rational structure based design [173].
Neurodegenration in neurons appears to happen in different levels. The path of the process is from the molecular level to systemic level. Computer aided drug design helps on behalf of the formation of the process of drug discovery and the scientific advance in the region of molecular structure characterization. Contribution of the approach towards the progress of the latest drugs against the neurodegenerative diseases is high elevated. Structure based virtual screening comprises the docking protocol that follows binding of the alkaloids to the apocynaceae family commencing through an in house data bank to decide on the particular structures based on the potential inhibitory activity cohesive with the human AChE. This approach facilitates for the alkaloids used in the advanced studies of the treatment of neurological disorder [174, 175].
Schizophrenia as well as the Parkinson’s disease were the problems of the neurological and the psychiatric conditions in which the treatment of the consecutive disease to be discussed whereas the human dopamine D4 receptor on the GPCR target is involved in the treatment. With reference to the study of homology modelling of the target, the rational structures of the receptor with the help of the template were developed. The model we gained from the homology modelling was further used for the molecular dynamics simulation and the stability has been checked. The mechanistic and the structural features of the receptor like the preserved disulfide bride and ionic lock were measured in modelling experiments. The model of the human D4 receptor was appeared to be the promising path through the future for the study of designing of the drug for the described drug target [176].
mGluR5 is the significant target correlated to more than a few neurological diseases. Development of ligands is focused on the orthosteric binding pocket which happens due to the deficient of selective ligands. The binding pocket of the transmembrane in the allosteric region has been in the requirement has been developed. The molecular docking of the mGluR crystal structure and model helps to satisfy the analyses of the non as well as the group selective compounds of orthosteric ligands. The binding mode analyses of the non and group selective orthosteric ligands is based on the molecular docking in mGluR crystal structure and models [177].
Human carbonic anhydrase VII is involved in several neurological diseases which is expressed in the brain and is validated as the attractive target for the treatment of epilepsy and neurological pain. In order to identify the new chemical entities as carbonic anhydrase inhibitors the structure based approach is used. Library of compounds was screened against the pharmacophore model obtained based on the carbonic anhydrase inhibitors and the most interesting hits were docked into the crystal structure and thus the studies have been carried out. This helps in the identification of the best hit compound binds to the receptor [178, 179].
In several neurological conditions, the Serotonin receptor subtype 1A(5-HT(1A)R) is implicated whereas the potent 5-HT(1A)R agonists where the therapeutic potential for the treatment of anxiety, depression, Parkinson’s and schizophrenia disease. The structure of the receptor is not available and hence to support the scientific research homology modelling of 5-HT(1A)R is followed through the support of the latest released high resolution structure similar to the query sequence [180].
1.3.4. Binding Site Analysis
The binding site of the protein is the important aspect in the structure based drug design in which the understanding of the structure and function of the binding site is the corner stone. Understanding of the development of the drug design requires the knowledge of both the location and the physical properties of the binding site. The binding pocket of the protein were analyzed in which the key interaction sites were derived and it prepares the necessary data for the Ligand fragment link [181]. The three dimensional structure of the protein and the pre-docked ligand in the PDB format, and the atomic properties were the basic input for the development in the process of drug design. The initial and effortless models of ligand binding are the lock and key archetype where it observes the ligand-receptor binding as well as the rigid shape-matching process. The mainstream of the association of ligand binding constitutes the side-chain and loop arrangement. Minor side chains are addressed by the soft potentials where non-polar Vander Waals clashes is involved [182, 183]. Recent developments provided the insights that the modelling of protein by the receptor arrangements which will be in process prior to the virtual screening [184]. Active site of the protein which means the binding site where the inhibitors bind were analyzed and identified through Ligsite. It is the platform for the prediction of the active site in which the concept of the surface solvent event and also the surface residue’s conservation degree [185].
1.4. Rational Drug Discovery
In comparison to the traditional methods of drug discovery, the trial and error testing of the chemical substances on the cultured cells or the animals are the important source of the drug discovery especially the rational drug discovery. The matching of the apparent effects to the treatments is performed by the rational drug discovery and the hypothesis modulate the specific biological target and thus the rational drug discovery begins where the biological target have the therapeutic use [186]. For the biomolecule to be chosen as a drug target, some essential piece of information is required. The initial evidence is that the modulation of the target which will have the therapeutic value [187]. The knowledge of the rational drug discovery emerges from the disease linkage studies and this shows an association of the mutations in the biological target as well as the certain disease states. The next information required for the development of the drug discovery in rational drug design is that the target is druggable. It means that the target is capable of binding to a small molecule whereas its activity can be modulated by the small molecule [188].
When a suitable drug target is identified, there may be a chance that this target can be available normally as the cloned target and expressed. For the establishment of the screening assay, the expressed target is used comprehensively. The three dimensional structure of the target may be determined by the in silico approach. The small molecules that bind to the target were searched with the help of the screening library for the potential drug compounds. The approach of the screening helps in the assessment and the obtaining of the compound that can be potent. Virtual screening assay (‘a wet screen’) can also play the part of the drug development. The available of the drug target paves the way for the use of the virtual screening techniques for the development of the lead candidate [189-191]. Preferably, the candidate drug compounds poses to be the “drug-like” in which they possess the properties that are predicted for the lead to show the oral bio-availability, metabolic stability, minimal toxic effects and chemical ideally the candidate drug compounds should be “drug-like”, that is they should possess properties that are predicted to lead to oral bioavailability, plenty of chemical properties, metabolic stability, and minimal toxic effects. Numerous methods are accessible for the estimation of drug likeness such Lipinski's Rule of Five and Lipophilic efficiency. The lipophilic efficiency deals with the range of scoring methods [192, 193]. Enormous methods of the prediction of the drug metabolism are proposed in the scientific literature and the latest example is the SPORCalc. Based on the complexity of the process of drug design, there are two terms of interest which are stated as serendipity and the bounded rationality [194].
1.4.1. Common Targets of Neurological Disorders
Delivering drugs to the neurological targets has been a major challenge. Much evidence explained that the blood-brain barrier (BBB) is a highly selective permeability barrier within the brain, blocks most of the all small and large molecules delivering to the targets. Most of the neuro- degenerative disorders such as Alzheimer’s disease, Parkinson’s disease, Prion diseases, Huntington’s disease, Front temporal dementia and Motor Neuron disease are familiar in the way of sharing a noticeable feature namely the aggregation and the deposition of abnormal protein [195, 196]. Some of common targets of neurological disorders are mentioned in table given below (Table 1).
Table 1.
Common Targets of Neurological disorders.
| Disease | Toxic Protein | Genes Related with the Protein | Refs. |
|---|---|---|---|
| Alzheimer's disease | Aβ | APP - Pathogenic mutation due to toxic improvement | [26, 197] |
| Presenilin - 1, Presenilin - 2 - Pathogenic mutation due to the loss of function | |||
| Parkinson's disease | tau | α- synuclein - toxic improvement | [198] |
| α- synuclein | |||
| Parkin - Loss of toxic function | |||
| UCHL1 - loss of toxic function | |||
| Prion disease | PrpSc | PRNP - Toxic improvement | [199] |
| Polyglutamine disease | Polyglutamine containing protein | Different genes with CAG repeat | [200] |
| Tauopathy | tau | tau - Improvement of toxicity | [201] |
| Familial amyotrophic lateral sclerosis | SOD1 | SOD1 - Improvement of toxicity | [201] |
Alzheimer’s disease is characterized by the presence of two lesions, first is the plaque, an extracellular lesion made largely of the β-amyloid (AB) peptide and the second is the tangle, an intracellular lesion made up of the cytoskeletal protein tau. In case of Parkinson disease, large numbers of genes are associated; some of them are noticeable like PARK1, PARK2 and the recently identified gene ubiquitin COOH-terminal hydrolase (PARK5). The important hallmark of the Parkinson disease is the deposition within dopaminergic neurons of Lewy bodies and cytoplasmic inclusions composed of synuclein gain. Cholecystokinin (CCK) & Gastrinrelated peptides include the family of peptide hormones and neuropeptides, plays various physiological actions on the CNS [202, 203]. Catechol – O- methyltrans- ferase (COMT) inhibitors are used as the therapeutic agents in the treatment of Parkinson’s disease. COMT is also prominent inhibitor in both the peripheral and central nervous system, where it metabolites various catechols, particularly dopamine, adrenaline and noradrenaline. Some extent shows due to limitations using COMT inhibitors as therapeutic agents are knotty. Hepatic dysfunction will be developed in association with the tolcapone therapy, in which development of COMT inhibitors is still needed [204, 205].
The neurological disorder associated with the choreoathetosis, spasticity is recognized by the hyper- uricemia and excessive uric acid synthesis. The drug azathioprine [6-(1’-methyl-4’-nitro-5’-imidazolyl) thiopurine], an immuno suppressive agent is reported to inhibit the excessive purine synthesis found in some patients with neurological disorder consisting of choreoathetosis [206]. Histone deacetylases are the kind of enzymes that deacetylate the lysine residues from histones, various nuclear, cytoplasmic as well as mitochondrial non-histone proteins. The role of HDAC is studied with the help of cell processes based on the phenotypic changes after isoform-specific knockdown. Histone deacetylase inhibitors contribute to the degradation of the protein aggregation which categorizes various neurodegenerative disorders namely Alzheimer’s, Parkinson and Huntington’s disease [207].
1.4.2. Drugs Availability and its Specificity
Intracellular phospholipase A2 (PLA2) are the group of enzymes that hydrolyze membrane phospholipids into fatty acid and lycophospholipids. Inhibitors of non-neural intracellular PLA2 have been recently discovered. The activities of PLA2 are found in neurological disorders that are associated with inflammation and oxidative stress. The inhibitors of PLA2 cross the blood brain barrier without harm. Non-specific intracellular PLA2 inhibitors namely Quinacrine, heparin, gangliosides, Vitamin E, arachidonyltri- fluoromethyl ketone, bromoenol lactone and cytidine 5-diphosphoamines helps in providing useful information on the tolerance, toxicity and effectiveness for the drug target of intracellular PLA2 [208]. Neuroimmunophilin ligands are the class of compounds that provides great support for the treatment of nerve injuries and neurological disease. These compounds cross the blood brain barrier that is orally effective in the animal models of ischemia, traumatic nerve injury and human neurodegenerative disorders. These neuroimmunophilin ligands act through the unique receptor which is correlated to the classical neurotrophicreceptors. The neuro-immunophilin ligands namely cyclosporine and FK-506 have been already used in humans as immuno-suppressant drugs and these drugs demonstrate to have neuro-protective actions [209].
Potassium channels are the tetra-meric membrane proteins that conduct K+ across cellular membrane selectively. The K+ channels make about half of the extended super family of 143-voltage gated ion channels in the human genome and the third largest family of signaling molecules following the G-Protein coupled receptors and the protein kinase. The broadly active K+ channel blockers include quinine, D-tubocurarine, verapamil. These drugs are organic cations and this block open K+ channel by binding in the inner pore. The riluzole activates the K+ channel at various concentrations and this effect contributes to riluzole’s ability to reduce the infrarealism [210].
Two isoforms of cyclooxygenase report that non-steroidal anti-inflammatory drugs are beneficial in devastating neurological conditions. The cyclooxygenases 1 and 2 are expressed under normal conditions or can be induced by physiological or pathological a stimulus which includes CNS cell types including neurons, glia and cerebro. COMT is the enzyme that catalyzes the transfer of methyl group from S-adenosyl methionine to catechols and cetecholamines like neurotransmitter, dopamine, epinephrine and norephinephrine. COMT has implicated in many neurological and psychiatric disorders like schizophrenia, Parkinson’s disease (PD), bipolar disorders. The second generation inhibitors namely the tolcapone and entacapone is used for the treatment of PD. 3-Hydroxy-4-pyridinones and 5-hydroxysss-4-pyrimidinones were identified as the inhibitors of COMT. These inhibitors exhibit potent inhibition of the enzyme and the improved toxicity is observed than the other inhibitors of COMT [204, 211, 212].
Mostly the patients with Parkinson’s disease were treated with dopamine agonist and the older patients with levodopa. The levodopa leads to dyskinesia, dopamine agonists have long plasma half life that probably leads to more continuous dopamine receptor simulation. MAO-B inhibitors can be a good option in the early stage of Parkinson. These Monoamine oxidase inhibitors are one of the targets responsible for the neurological disorders. MAO-A and MAO-B targets have been selected to increase the available amounts of monoamine neurotransmitter in the brain for the treatment of neurological disorders. The compounds namely the coumarins, chromones, isatins, phthalimides, phthalonitriles and quinolinones are shown as the potential inhibitors of MAO. The inhibitory activity of monoamine oxidase (MAO) A and B hasbeen estimated by the design and synthesis of the series of 3-hydroxy-3-phenacyloxindole analogues of isatin. These series of compounds were proved to be potent and selective in inhibiting the activity of MAO. Molecular interactions between the inhibitors and MAO were well studied through the computational approaches [213-215].
CONCLUSION
In spite of many years of efforts, research has been expanded and studied towards the understanding of the mechanism of neurological diseases and the function of the central nervous system. But, the therapeutic interventions and research modalities are still in progress to get the exact result regarding success in the treatment and diagnosis of the diseases. Recent years have seen greatest interest in the developmental strategies of the treatment, but the society is still lagging behind the clear idea how the generalized suppression of neuronal injury response would be beneficial in all the neurological disorders. The use of various inhibitors has provided the evidence of the effects of neuron or the inducible isoforms in neurological disorders. Even though there is a constructive management of the disease, there is no effective therapeutic cure developed that helps in the slowdown of the progression of the disease in a respective manner. In addition, neuronal injury is not only due to the changes in genes or the neurons, but also due to the external factors that affect the neurons.
In the present era, the management of the disease and its treatment has been developed constructively. As of now, various potential drug targets and new neuro-protective strategies have been proposed for the development of the drugs and the treatment of the diseases based on the studies happening around the research society. To the languishing part, some diseases could not be validated still due to the lack of suitable targets and still some pathways are concealed and perplexed. In this scenario, the computational approaches such as the structure based drug design and the ligand based drug design methods have been developed for the identification of the significant biological target and helps in the evaluation of the interaction with lead molecule. In overall, there is rapidly growing evidence stating that how the recent advances and the applications of Structure based and ligand based drug designing ameliorate in the develop- ment of drug discovery. It is clear that the prominence of SBDD were implemented in the recent advances and applications in the drug discovery. Incorporation of the available knowledge of the neurological disease depicts the approach of SBDD carried out in this review. Development of the pharmaceutical compounds for the treatment of neurological disease leads to the impressive challenge in the society.
This review, however, helps in the understanding of the mechanism and pathology of the neurological diseases which indirectly paves way for the development of the suitable inhibitors for the drug targets by virtue of SBDD. As stated, various SBDD techniques available for the development of inhibitors for the drug targets of diseases. This will be supportive for the understanding of the neurological diseases and discloses that the proper understanding of the diseases is recommended for the successful application and implementation of the structure based drug designing for the progress of the drug discovery.
ACKNOWLEDGEMENTS
The authors thank the Alagappa University, Karaikudi for providing the Infrastructure required for this study.
LIST OF ABBREVIATIONS
- AD
Alzheimer Disease
- ALS
Amylotrophic Lateral Sclerosis
- BBB
Blood Brain Barrier
- CADD
Computer Aided Drug Designing
- COMT
Catechol-O-methyltransferase
- HD
Huntington’s Disease
- LBDD
Ligand Based Drug Designing
- MAO
Monoamine oxidase
- PD
Parkinson Disease
- PD
Parkinson Disease
- PLA
Phospholipase A2
- RBD
Rapid Eye Movement Sleep Behaviour Disorder
- REM
Rapid Eye Movement
- SBDD
Structure Based Drug Designing
- SOD
Superoxide Dismutase
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [http://dx.doi.org/ 10.1001/jama.1992.03480090092034]. [PMID: 1538563]. [PubMed] [Google Scholar]
- 2.Kolev O.I., Milanov I. Central nervous system impairment in diabetic patients. Electromyogr. Clin. Neurophysiol. 1999;39(8):479–484. [PMID: 10627933]. [PubMed] [Google Scholar]
- 3.Livnat A., Pippenger N. An optimal brain can be composed of conflicting agents. Proc. Natl. Acad. Sci. USA. 2006;103(9):3198–3202. doi: 10.1073/pnas.0510932103. [http://dx.doi.org/10.1073/pnas.0510932103]. [PMID: 16492775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L., Parvizi J., Hichwa R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [http://dx.doi.org/10.1038/ 79871]. [PMID: 11017179]. [DOI] [PubMed] [Google Scholar]
- 5.Floyd R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999;222(3):236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [http://dx.doi.org/10.1046/j.1525-1373.1999.d01-140.x]. [PMID: 10601882]. [DOI] [PubMed] [Google Scholar]
- 6.Lovinger D.M. Communication networks in the brain: neurons, receptors, neurotransmitters, and alcohol. Alcohol Res. Health. 2008;31(3):196–214. [PMID: 23584863]. [PMC free article] [PubMed] [Google Scholar]
- 7.Graus F., Delattre J.Y., Antoine J.C., Dalmau J., Giometto B., Grisold W., Honnorat J., Smitt P.S., Vedeler Ch., Verschuuren J.J., Vincent A., Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [http://dx.doi.org/10.1136/ jnnp.2003.034447]. [PMID: 15258215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oscar-Berman M., Shagrin B., Evert D.L., Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res. World. 1997;21(1):65–75. [PMID: 15706764]. [PMC free article] [PubMed] [Google Scholar]
- 9.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [http://dx.doi.org/10.2174/157015909787602823]. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosano C., Kuller L.H., Chung H., Arnold A.M., Longstreth W.T., Jr, Newman A.B. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J. Am. Geriatr. Soc. 2005;53(4):649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [http://dx.doi.org/10.1111/j.1532-5415.2005.53214.x]. [PMID: 15817012]. [DOI] [PubMed] [Google Scholar]
- 11.Verhage M., Maia A.S., Plomp J.J., Brussaard A.B., Heeroma J.H., Vermeer H., Toonen R.F., Hammer R.E., van den Berg T.K., Missler M., Geuze H.J., Südhof T.C. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287(5454):864–869. doi: 10.1126/science.287.5454.864. [http://dx.doi.org/10.1126/science.287. 5454.864]. [PMID: 10657302]. [DOI] [PubMed] [Google Scholar]
- 12.Araque A., Navarrete M. Glial cells in neuronal network function. Philos. Trans. R. Soc. Lond. B Biol. Sci . 2010;365(1551): 2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjöstedt E., Fagerberg L., Hallström B.M., Häggmark A., Mitsios N., Nilsson P., Pontén F., Hökfelt T., Uhlén M., Mulder J. Defining the human brain proteome using transcriptomics and antibody-based profiling with a focus on the cerebral cortex. PLoS One. 2015;10(6):e0130028. doi: 10.1371/journal.pone.0130028. [http://dx.doi.org/10.1371/journal. pone.0130028]. [PMID: 26076492]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards T.N., Meinertzhagen I.A. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog. Neurobiol. 2010;90(4):471–497. doi: 10.1016/j.pneurobio.2010.01.001. [http://dx.doi.org/10.1016/j. pneurobio.2010.01.001]. [PMID: 20109517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanguilder H.D., Freeman W.M. The hippocampal neuro-proteome with aging and cognitive decline: past progress and future directions. Front. Aging Neurosci. 2011;3:8. doi: 10.3389/fnagi.2011.00008. [http://dx. doi.org/10.3389/fnagi.2011.00008]. [PMID: 21647399]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy Y., Onuchic J.N. Water mediation in protein folding and molecular recognition. Annu. Rev. Biophys. Biomol. Struct. 2006;35:389–415. doi: 10.1146/annurev.biophys.35.040405.102134. [http://dx.doi.org/10.1146/annurev.biophys.35.040405. 102134]. [PMID: 16689642]. [DOI] [PubMed] [Google Scholar]
- 17.Ross C.A., Poirier M.A. Protein aggregation and neuro-degenerative disease. Nat. Med. 2004;10(Suppl.):S10–S17. doi: 10.1038/nm1066. [http:// dx.doi.org/10.1038/nm1066]. [PMID: 15272267]. [DOI] [PubMed] [Google Scholar]
- 18.Kuntz I.D. Structure-based strategies for drug design and discovery. Science. 1992;257(5073):1078–1082. doi: 10.1126/science.257.5073.1078. [http://dx.doi.org/ 10.1126/science.257.5073.1078]. [PMID: 1509259]. [DOI] [PubMed] [Google Scholar]
- 19.Hahn C., Erb K.J. The preclinical testing strategy for the development of novel chemical entities for the treatment of asthma. Curr. Drug Targets. 2008;9(6):443–451. doi: 10.2174/138945008784533552. [http://dx.doi.org/10. 2174/138945008784533552]. [PMID: 18537583]. [DOI] [PubMed] [Google Scholar]
- 20.Mavromoustakos T., Durdagi S., Koukoulitsa C., Simcic M., Papadopoulos M.G., Hodoscek M., Grdadolnik S.G. Strategies in the rational drug design. Curr. Med. Chem. 2011;18(17):2517–2530. doi: 10.2174/092986711795933731. [http://dx.doi.org/10.2174/092986711795933731]. [PMID: 21568895]. [DOI] [PubMed] [Google Scholar]
- 21.Knopman D.S., Boeve B.F., Petersen R.C. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin. Proc. 2003;78(10):1290–1308. doi: 10.4065/78.10.1290. [http://dx.doi.org/10.4065/78.10.1290]. [PMID: 14531488]. [DOI] [PubMed] [Google Scholar]
- 22.Seegmiller J.E., Rosenbloom F.M., Kelley W.N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [http://dx.doi.org/10.1126/science.155.3770.1682]. [PMID: 6020292]. [DOI] [PubMed] [Google Scholar]
- 23.Kihara T., Shimohama S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. (Warsz.) 2004;64(1):99–105. doi: 10.55782/ane-2004-1495. [PMID: 15190684]. [DOI] [PubMed] [Google Scholar]
- 24.Kayed R., Lasagna-Reeves C.A. Molecular mechanisms of amyloid oligomers toxicity. J. Alzheimers Dis. 2013;33(Suppl. 1):S67–S78. doi: 10.3233/JAD-2012-129001. [http://dx.doi.org/10.3233/JAD-2012-129001]. [PMID: 22531422]. [DOI] [PubMed] [Google Scholar]
- 25.Lesne S.E. Toxic oligomer species of amyloid-β in Alzheimer’s disease, a timing issue. Swiss Med. Wkly. 2014;144:w14021. doi: 10.4414/smw.2014.14021. [PMID: 25375761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayden E.Y., Teplow D.B. Amyloid β-protein oligomers and Alzheimer’s disease. Alzheimers Res. Ther. 2013;5(6):60. doi: 10.1186/alzrt226. [http://dx.doi.org/10.1186/alzrt226]. [PMID: 24289820]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H.Y. One-compound-multiple-targets strategy to combat Alzheimer’s disease. FEBS Lett. 2005;579(24):5260–5264. doi: 10.1016/j.febslet.2005.09.006. [http:// dx.doi.org/10.1016/j.febslet.2005.09.006]. [PMID: 16194540]. [DOI] [PubMed] [Google Scholar]
- 28.Agnati L.F., Bjelke B., Fuxe K. Volume transmission in the Brain. Am. Sci. 1992;80:362–373. [Google Scholar]
- 29.Lahiri D.K., Sambamurti K., Bennett D.A. Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer’s disease. Neurobiol. Aging. 2004;25(5):651–660. doi: 10.1016/j.neurobiolaging.2003.12.024. [http://dx. doi.org/10.1016/j.neurobiolaging.2003.12.024]. [PMID: 15172744]. [DOI] [PubMed] [Google Scholar]
- 30.Turk B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Discov. 2006;5(9):785–799. doi: 10.1038/nrd2092. [http:// dx.doi.org/10.1038/nrd2092]. [PMID: 16955069]. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt S., Klaver C., Saunders A., Postel E., De La Paz M., Agarwal A., Small K., Udar N., Ong J., Chalukya M., Nesburn A., Kenney C., Domurath R., Hogan M., Mah T., Conley Y., Ferrell R., Weeks D., de Jong P.T., van Duijn C., Haines J., Pericak-Vance M., Gorin M. A pooled case-control study of the apolipoprotein E (APOE) gene in age-related maculopathy. Ophthalmic Genet. 2002;23(4):209–223. doi: 10.1076/opge.23.4.209.13883. [http://dx.doi.org/ 10.1076/opge.23.4.209.13883]. [PMID: 12567264]. [DOI] [PubMed] [Google Scholar]
- 32.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [http://dx.doi.org/10.1126/science. 1072994]. [PMID: 12130773]. [DOI] [PubMed] [Google Scholar]
- 33.Licastro F., Candore G., Lio D., Porcellini E., Colonna-Romano G., Franceschi C., Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun. Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [http://dx.doi.org/10.1186/1742-4933-2-8]. [PMID: 15904534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sano M., Ernesto C., Thomas R.G., Klauber M.R., Schafer K., Grundman M., Woodbury P., Growdon J., Cotman C.W., Pfeiffer E., Schneider L.S., Thal L.J. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997;336(17):1216–1222. doi: 10.1056/NEJM199704243361704. [http://dx.doi.org/10.1056/ NEJM199704243361704]. [PMID: 9110909]. [DOI] [PubMed] [Google Scholar]
- 35.Refolo L.M., Pappolla M.A., LaFrancois J., Malester B., Schmidt S.D., Thomas-Bryant T., Tint G.S., Wang R., Mercken M., Petanceska S.S., Duff K.E. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2001;8(5):890–899. doi: 10.1006/nbdi.2001.0422. [http://dx.doi.org/10.1006/nbdi.2001.0422]. [PMID: 11592856]. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie K., Lovestone S. The dementias. Lancet. 2002;360(9347):1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [http://dx.doi.org/10.1016/S0140-6736(02)11667-9]. [PMID: 12480441]. [DOI] [PubMed] [Google Scholar]
- 37.Bobkova N., Vorobyov V. The brain compensatory mechanisms and Alzheimer’s disease progression: a new protective strategy. Neural Regen. Res. 2015;10(5):696–697. doi: 10.4103/1673-5374.156954. [http://dx.doi.org/ 10.4103/1673-5374.156954]. [PMID: 26109935]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanier M.T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [http://dx.doi.org/10.1186/1750-1172-5-16]. [PMID: 20525256]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wraith J.E., Baumgartner M.R., Bembi B., Covanis A., Levade T., Mengel E., Pineda M., Sedel F., Topçu M., Vanier M.T., Widner H., Wijburg F.A., Patterson M.C. Recommendations on the diagnosis and management of Niemann-Pick disease type C. Mol. Genet. Metab. 2009;98(1-2):152–165. doi: 10.1016/j.ymgme.2009.06.008. [http://dx.doi.org/ 10.1016/j.ymgme.2009.06.008]. [PMID: 19647672]. [DOI] [PubMed] [Google Scholar]
- 40.Rivinus T.M., Jamison D.L., Graham P.J. Childhood organic neurological disease presenting as psychiatric disorder. Arch. Dis. Child. 1975;50(2):115–119. doi: 10.1136/adc.50.2.115. [http://dx.doi.org/10.1136/adc.50.2. 115]. [PMID: 1130816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fink J.K., Filling-Katz M.R., Sokol J., Cogan D.G., Pikus A., Sonies B., Soong B., Pentchev P.G., Comly M.E., Brady R.O., Barton N.W. Clinical spectrum of Niemann-Pick disease type C. Neurology. 1989;39(8):1040–1049. doi: 10.1212/wnl.39.8.1040. [http://dx.doi.org/10.1212/ WNL.39.8.1040]. [PMID: 2761697]. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki K., Parker C.C., Pentchev P.G., Katz D., Ghetti B., D’Agostino A.N., Carstea E.D. Neurofibrillary tangles in Niemann-Pick disease type C. Acta Neuropathol. 1995;89(3):227–238. doi: 10.1007/BF00309338. [http://dx.doi.org/10.1007/BF00309338]. [PMID: 7754743]. [DOI] [PubMed] [Google Scholar]
- 43.Vanier M.T. Lipid changes in Niemann-Pick disease type C brain: personal experience and review of the literature. Neurochem. Res. 1999;24(4):481–489. doi: 10.1023/a:1022575511354. [http://dx.doi.org/10.1023/A:1022575511354]. [PMID: 10227680]. [DOI] [PubMed] [Google Scholar]
- 44.Griffin L.D., Gong W., Verot L., Mellon S.H. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 2004;10(7):704–711. doi: 10.1038/nm1073. [http://dx.doi.org/10.1038/nm1073]. [PMID: 15208706]. [DOI] [PubMed] [Google Scholar]
- 45.Patterson M.C., Vecchio D., Prady H., Abel L., Wraith J.E. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6(9):765–772. doi: 10.1016/S1474-4422(07)70194-1. [http:// dx.doi.org/10.1016/S1474-4422(07)70194-1]. [PMID: 17689147]. [DOI] [PubMed] [Google Scholar]
- 46.Pineda M., Wraith J.E., Mengel E., Sedel F., Hwu W-L., Rohrbach M., Bembi B., Walterfang M., Korenke G.C., Marquardt T., Luzy C., Giorgino R., Patterson M.C. Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol. Genet. Metab. 2009;98(3):243–249. doi: 10.1016/j.ymgme.2009.07.003. [http://dx.doi.org/10.1016/j.ymgme.2009.07.003]. [PMID: 19656703]. [DOI] [PubMed] [Google Scholar]
- 47.Wraith J.E., Imrie J. New therapies in the management of Niemann-Pick type C disease: clinical utility of miglustat. Ther. Clin. Risk Manag. 2009;5:877–887. doi: 10.2147/tcrm.s5777. [http://dx.doi.org/10.2147/ TCRM.S5777]. [PMID: 19956552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly D.A., Portmann B., Mowat A.P., Sherlock S., Lake B.D. Niemann-Pick disease type C: diagnosis and outcome in children, with particular reference to liver disease. J. Pediatr. 1993;123(2):242–247. doi: 10.1016/s0022-3476(05)81695-6. [http://dx.doi.org/10.1016/S0022-3476(05)81695-6]. [PMID: 7688422]. [DOI] [PubMed] [Google Scholar]
- 49.Rieger D., Auerbach S., Robinson P., Gropman A. Neuroimaging of lipid storage disorders. Dev. Disabil. Res. Rev. 2013;17(3):269–282. doi: 10.1002/ddrr.1120. [http://dx.doi.org/10.1002/ddrr.1120]. [PMID: 23798015]. [DOI] [PubMed] [Google Scholar]
- 50.Sleat D.E., Wiseman J.A., El-Banna M., Price S.M., Verot L., Shen M.M., Tint G.S., Vanier M.T., Walkley S.U., Lobel P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. USA. 2004;101(16):5886–5891. doi: 10.1073/pnas.0308456101. [http://dx.doi.org/10.1073/pnas. 0308456101]. [PMID: 15071184]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ottinger E.A., Kao M.L., Carrillo-Carrasco N., Yanjanin N., Shankar R.K., Janssen M., Brewster M., Scott I., Xu X., Cradock J., Terse P., Dehdashti S.J., Marugan J., Zheng W., Portilla L., Hubbs A., Pavan W.J., Heiss J., Vite C.H., Walkley S.U., Ory D.S., Silber S.A., Porter F.D., Austin C.P., McKew J.C. Collaborative development of 2-hydroxypropyl-β-cyclodextrin for the treatment of Niemann-Pick type C1 disease. Curr. Top. Med. Chem. 2014;14(3):330–339. doi: 10.2174/1568026613666131127160118. [http://dx.doi.org/10.2174/ 1568026613666131127160118]. [PMID: 24283970]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbaum A.I., Maxfield F.R. Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J. Neurochem. 2011;116(5):789–795. doi: 10.1111/j.1471-4159.2010.06976.x. [http://dx.doi.org/10.1111/ j.1471-4159.2010.06976.x]. [PMID: 20807315]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns M.P., Duff K. Brain on steroids resists neurodegeneration. Nat. Med. 2004;10(7):675–676. doi: 10.1038/nm0704-675. [http://dx.doi.org/10.1038/ nm0704-675]. [PMID: 15229510]. [DOI] [PubMed] [Google Scholar]
- 54.CarmenVazquez M., Balboa E., Alvarez A.R., Zanlungo S. Oxidative stress: A pathogenic mechanism for Niemann-Pick typeC disease. Oxid. Med. Cell. Longev. 2012 doi: 10.1155/2012/205713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beitz J.M. Parkinson’s disease: a review. Front. Biosci. (Schol. Ed.) 2014;6:65–74. doi: 10.2741/s415. [http://dx.doi.org/10.2741/S415]. [PMID: 24389262]. [DOI] [PubMed] [Google Scholar]
- 56.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [http://dx.doi.org/10.1001/jama.2014.3654]. [PMID: 24756517]. [DOI] [PubMed] [Google Scholar]
- 57.Ravina B., Marder K., Fernandez H.H., Friedman J.H., McDonald W., Murphy D., Aarsland D., Babcock D., Cummings J., Endicott J., Factor S., Galpern W., Lees A., Marsh L., Stacy M., Gwinn-Hardy K., Voon V., Goetz C. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov. Disord. 2007;22(8):1061–1068. doi: 10.1002/mds.21382. [http://dx.doi.org/10.1002/mds.21382]. [PMID: 17266092]. [DOI] [PubMed] [Google Scholar]
- 58.Bromberg-Martin E.S., Matsumoto M., Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [http://dx.doi.org/10.1016/j.neuron.2010. 11.022]. [PMID: 21144997]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulbricht C. Parkinson’s Disease: An Integrative Approach: A Natural Standard Monograph. Altern. Complement. Ther. 2011;17:175–180. [http://dx.doi.org/10.1089/act.2011.17308]. [Google Scholar]
- 60.Braak H., Ghebremedhin E., Rüb U., Bratzke H., Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [http://dx.doi.org/10.1007/ s00441-004-0956-9]. [PMID: 15338272]. [DOI] [PubMed] [Google Scholar]
- 61.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [http://dx.doi.org/10.1136/jnnp.2007.131045]. [PMID: 18344392]. [DOI] [PubMed] [Google Scholar]
- 62.de Lau L.M., Breteler M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [http://dx.doi.org/10.1016/ S1474-4422(06)70471-9]. [PMID: 16713924]. [DOI] [PubMed] [Google Scholar]
- 63.Nuytemans K., Theuns J., Cruts M., Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum. Mutat. 2010;31(7):763–780. doi: 10.1002/humu.21277. [http://dx.doi.org/ 10.1002/humu.21277]. [PMID: 20506312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.West A.B., Moore D.J., Choi C., Andrabi S.A., Li X., Dikeman D., Biskup S., Zhang Z., Lim K.L., Dawson V.L., Dawson T.M. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 2007;16(2):223–232. doi: 10.1093/hmg/ddl471. [http://dx.doi.org/10.1093/hmg/ddl471]. [PMID: 17200152]. [DOI] [PubMed] [Google Scholar]
- 65.Paisán-Ruíz C., Lang A.E., Kawarai T., Sato C., Salehi-Rad S., Fisman G.K., Al-Khairallah T., St George-Hyslop P., Singleton A., Rogaeva E. LRRK2 gene in Parkinson disease: mutation analysis and case control association study. Neurology. 2005;65(5):696–700. doi: 10.1212/01.wnl.0000167552.79769.b3. [http://dx.doi.org/10.1212/01.WNL.0000167552. 79769.b3]. [PMID: 16157901]. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y., Gehrke S., Imai Y., Huang Z., Ouyang Y., Wang J.W., Yang L., Beal M.F., Vogel H., Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl. Acad. Sci. USA. 2006;103(28):10793–10798. doi: 10.1073/pnas.0602493103. [http:// dx.doi.org/10.1073/pnas.0602493103]. [PMID: 16818890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimura H., Hattori N., Kubo Si., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000;25(3):302–305. doi: 10.1038/77060. [http:// dx.doi.org/10.1038/77060]. [PMID: 10888878]. [DOI] [PubMed] [Google Scholar]
- 68.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [http://dx.doi. org/10.1038/33416]. [PMID: 9560156]. [DOI] [PubMed] [Google Scholar]
- 69.Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev. Mol. Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [http://dx.doi.org/10.1017/S1462399409001148]. [PMID: 19631006]. [DOI] [PubMed] [Google Scholar]
- 70.Wood-Kaczmar A., Gandhi S., Wood N.W. Understanding the molecular causes of Parkinson’s disease. Trends Mol. Med. 2006;12(11):521–528. doi: 10.1016/j.molmed.2006.09.007. [http://dx.doi.org/10.1016/j.molmed.2006.09.007]. [PMID: 17027339]. [DOI] [PubMed] [Google Scholar]
- 71.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [http://dx.doi.org/ 10.1016/S0896-6273(03)00568-3]. [PMID: 12971891]. [DOI] [PubMed] [Google Scholar]
- 72.Koller W.C., Rueda M.G. Mechanism of action of dopaminergic agents in Parkinson’s disease. Neurology. 1998;50(6) Suppl. 6:S11–S14. doi: 10.1212/wnl.50.6_suppl_6.s11. [http://dx.doi.org/10.1212/WNL.50.6_Suppl_6.S11]. [PMID: 9633680]. [DOI] [PubMed] [Google Scholar]
- 73.Xu J., Kao S.Y., Lee F.J., Song W., Jin L.W., Yankner B.A. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002;8(6):600–606. doi: 10.1038/nm0602-600. [http://dx.doi.org/10.1038/nm0602-600]. [PMID: 12042811]. [DOI] [PubMed] [Google Scholar]
- 74.Dietz V. Spinal cord pattern generators for locomotion. Clin. Neurophysiol. 2003;114(8):1379–1389. doi: 10.1016/s1388-2457(03)00120-2. [http://dx.doi.org/10. 1016/S1388-2457(03)00120-2]. [PMID: 12888019]. [DOI] [PubMed] [Google Scholar]
- 75.Samantaray S., Knaryan V.H., Shields D.C., Banik N.L. Critical role of calpain in spinal cord degeneration in Parkinson’s disease. J. Neurochem. 2013;127(6):880–890. doi: 10.1111/jnc.12374. [http://dx.doi.org/10.1111/ jnc.12374]. [PMID: 23875735]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schenck C.H. REM sleep behaviour disorder. Sleep Med. 2015;•••:391–405. doi: 10.1016/j.sleep.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Iranzo A., Santamaria J., Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med. Rev. 2009;13(6):385–401. doi: 10.1016/j.smrv.2008.11.003. [http://dx.doi.org/10.1016/j.smrv.2008.11.003]. [PMID: 19362028]. [DOI] [PubMed] [Google Scholar]
- 78.Gagnon J.F., Postuma R.B., Mazza S., Doyon J., Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neuro-degenerative diseases. Lancet Neurol. 2006;5(5):424–432. doi: 10.1016/S1474-4422(06)70441-0. [http:// dx.doi.org/10.1016/S1474-4422(06)70441-0]. [PMID: 16632313]. [DOI] [PubMed] [Google Scholar]
- 79.Boeve B.F., Silber M.H., Saper C.B., Ferman T.J., Dickson D.W., Parisi J.E., Benarroch E.E., Ahlskog J.E., Smith G.E., Caselli R.C., Tippman-Peikert M., Olson E.J., Lin S-C., Young T., Wszolek Z., Schenck C.H., Mahowald M.W., Castillo P.R., Del Tredici K., Braak H. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(Pt 11):2770–2788. doi: 10.1093/brain/awm056. [http://dx.doi.org/10.1093/ brain/awm056]. [PMID: 17412731]. [DOI] [PubMed] [Google Scholar]
- 80.Pivik R.T., Broughton R.J., Coppola R., Davidson R.J., Fox N., Nuwer M.R. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30(6):547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [http://dx.doi.org/10. 1111/j.1469-8986.1993.tb02081.x]. [PMID: 8248447]. [DOI] [PubMed] [Google Scholar]
- 81.Iranzo A., Molinuevo J.L., Santamaría J., Serradell M., Martí M.J., Valldeoriola F., Tolosa E. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5(7):572–577. doi: 10.1016/S1474-4422(06)70476-8. [http://dx.doi.org/10.1016/S1474-4422(06)70476-8]. [PMID: 16781987]. [DOI] [PubMed] [Google Scholar]
- 82.Postuma R.B., Gagnon J.F., Vendette M., Fantini M.L., Massicotte-Marquez J., Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [http://dx.doi.org/ 10.1212/01.wnl.0000340980.19702.6e]. [PMID: 19109537]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boeve B.F., Silber M.H., Ferman T.J. REM sleep behavior disorder in Parkinson’s disease and dementia with Lewy bodies. J. Geriatr. Psychiatry Neurol. 2004;17(3):146–157. doi: 10.1177/0891988704267465. [http://dx. doi.org/10.1177/0891988704267465]. [PMID: 15312278]. [DOI] [PubMed] [Google Scholar]
- 84.Anderson K.N., Shneerson J.M. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J. Clin. Sleep Med. 2009;5(3):235–239. [PMID: 19960644]. [PMC free article] [PubMed] [Google Scholar]
- 85.Santamaria J., Iranzo A. Sleep disorders matter in neurology. Lancet Neurol. 2014;13(1):18–20. doi: 10.1016/S1474-4422(13)70273-4. [http://dx.doi.org/10.1016/ S1474-4422(13)70273-4]. [PMID: 24331789]. [DOI] [PubMed] [Google Scholar]
- 86.Gugger J.J., Wagner M.L. Rapid eye movement sleep behavior disorder. Ann. Pharmacother. 2007;41(11):1833–1841. doi: 10.1345/aph.1H587. [http://dx. doi.org/10.1345/aph.1H587]. [PMID: 17925503]. [DOI] [PubMed] [Google Scholar]
- 87.Boene B.F. REM sleep behaviour disorder: Updated review of the core features, the RBD-Neurodegenerative disease association, evolving concepts, controversies and future directions. Ann. N. Y. Acad. Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [http://dx.doi.org/10.1111/j.1749-6632.2009.05115.x]. [PMID: 20146689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gagnon J.F., Postuma R.B., Mazza S., Doyon J., Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neuro-degenerative diseases. Lancet Neurol. 2006;5(5):424–432. doi: 10.1016/S1474-4422(06)70441-0. [http:// dx.doi.org/10.1016/S1474-4422(06)70441-0]. [PMID: 16632313]. [DOI] [PubMed] [Google Scholar]
- 89.Gagnon J.F., Vendette M., Postuma R.B., Desjardins C., Massicotte-Marquez J., Panisset M., Montplaisir J. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann. Neurol. 2009;66(1):39–47. doi: 10.1002/ana.21680. [http://dx.doi.org/10.1002/ana.21680]. [PMID: 19670440]. [DOI] [PubMed] [Google Scholar]
- 90.Ince P.G., Lowe J., Shaw P.J. Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol. Appl. Neurobiol. 1998;24(2):104–117. doi: 10.1046/j.1365-2990.1998.00108.x. [http://dx.doi.org/10.1046/j.1365-2990.1998.00108.x]. [PMID: 9634206]. [DOI] [PubMed] [Google Scholar]
- 91.Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 2006;7(9):710–723. doi: 10.1038/nrn1971. [http://dx.doi.org/10.1038/nrn1971]. [PMID: 16924260]. [DOI] [PubMed] [Google Scholar]
- 92.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., Rahmani Z., Krizus A., Mckenna-Yasek D., Cayabyab A., Gaston S.M., Berger R., Tanzi R.P., Halperin J.J., Herzfeldt B. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [http://dx.doi.org/10.1038/362059a0]. [PMID: 8446170]. [DOI] [PubMed] [Google Scholar]
- 93.Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y., Kinoshita Y., Kamada M., Nodera H., Suzuki H., Komure O., Matsuura S., Kobatake K., Morimoto N., Abe K., Suzuki N., Aoki M., Kawata A., Hirai T., Kato T., Ogasawara K., Hirano A., Takumi T., Kusaka H., Hagiwara K., Kaji R., Kawakami H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223–226. doi: 10.1038/nature08971. [http://dx.doi.org/10. 1038/nature08971]. [PMID: 20428114]. [DOI] [PubMed] [Google Scholar]
- 94.Lee E.B., Lee V.M., Trojanowski J.Q. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 2011;13(1):38–50. doi: 10.1038/nrn3121. [PMID: 22127299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bruijn L.I., Miller T.M., Cleveland D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [http://dx.doi.org/10.1146/ annurev.neuro.27.070203.144244]. [PMID: 15217349]. [DOI] [PubMed] [Google Scholar]
- 96.Miller R.G., Mitchell J.D., Lyon M., Moore D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2007;(1):CD001447. doi: 10.1002/14651858.CD001447.pub2. [PMID: 17253460]. [DOI] [PubMed] [Google Scholar]
- 97.LaMonte B.H., Wallace K.E., Holloway B.A., Shelly S.S., Ascaño J., Tokito M., Van Winkle T., Howland D.S., Holzbaur E.L. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34(5):715–727. doi: 10.1016/s0896-6273(02)00696-7. [http://dx.doi.org/10.1016/S0896-6273(02)00696-7]. [PMID: 12062019]. [DOI] [PubMed] [Google Scholar]
- 98.Rothstein J.D. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann. Neurol. 2009;65(Suppl. 1):S3–S9. doi: 10.1002/ana.21543. [http://dx.doi.org/10.1002/ana.21543]. [PMID: 19191304]. [DOI] [PubMed] [Google Scholar]
- 99.Lee M.J. Neuronal death in amyotrophic lateral sclerosis is apoptosis: Possible contribution of a programmed cell death mechanism. J. Neuropathol. Exp. Neurol. 1999;58:407–569. doi: 10.1097/00005072-199905000-00005. [PMID: 10331430]. [DOI] [PubMed] [Google Scholar]
- 100.Stifanese R., Averna M., De Tullio R., Pedrazzi M., Milanese M., Bonifacino T., Bonanno G., Salamino F., Pontremoli S., Melloni E. Role of calpain-1 in the early phase of experimental ALS. Arch. Biochem. Biophys. 2014;562:1–8. doi: 10.1016/j.abb.2014.08.006. [http://dx.doi.org/ 10.1016/j.abb.2014.08.006]. [PMID: 25151305]. [DOI] [PubMed] [Google Scholar]
- 101.Vosler P.S., Brennan C.S., Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008;38(1):78–100. doi: 10.1007/s12035-008-8036-x. [http://dx.doi.org/10.1007/s12035-008-8036-x]. [PMID: 18686046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strong M.J., Kesavapany S., Pant H.C. The pathobiology of amyotrophic lateral sclerosis: a proteinopathy? J. Neuropathol. Exp. Neurol. 2005;64(8):649–664. doi: 10.1097/01.jnen.0000173889.71434.ea. [http://dx.doi.org/10.1097/ 01.jnen.0000173889.71434.ea]. [PMID: 16106213]. [DOI] [PubMed] [Google Scholar]
- 103.Bakwin R.M., Bakwin H. Psychologic aspects of pediatrics: epilepsy. J. Pediatr. 1951;39(6):776–784. doi: 10.1016/s0022-3476(51)80243-9. [http://dx.doi.org/10. 1016/S0022-3476(51)80243-9]. [PMID: 14898408]. [DOI] [PubMed] [Google Scholar]
- 104.Jacoby A., Snape D., Baker G.A. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol. 2005;4(3):171–178. doi: 10.1016/S1474-4422(05)01014-8. [http://dx.doi.org/10.1016/S1474-4422(05)70020-X]. [PMID: 15721827]. [DOI] [PubMed] [Google Scholar]
- 105.Jensen F.E. Epilepsy as a spectrum disorder: Implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(Suppl. 1):1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [http://dx.doi.org/10.1111/j.1528-1167.2010.02904.x]. [PMID: 21214533]. [DOI] [PubMed] [Google Scholar]
- 106.Scott D.F. Psychiatric aspects of epilepsy. Br. J. Psychiatry. 1978;132:417–430. [http://dx.doi.org/10.1192/bjp.132.5.417]. [PMID: 350325]. [PubMed] [Google Scholar]
- 107.Thomas R.H., Berkovic S.F. The hidden genetics of epilepsy-a clinically important new paradigm. Nat. Rev. Neurol. 2014;10(5):283–292. doi: 10.1038/nrneurol.2014.62. [http://dx.doi.org/10.1038/nrneurol.2014.62]. [PMID: 24733163]. [DOI] [PubMed] [Google Scholar]
- 108.Scheuer M.L., Pedley T.A. The evaluation and treatment of seizures. N. Engl. J. Med. 1990;323(21):1468–1474. doi: 10.1056/NEJM199011223232107. [http:// dx.doi.org/10.1056/NEJM199011223232107]. [PMID: 2233919]. [DOI] [PubMed] [Google Scholar]
- 109.Kumar K.P., Bhowmik D. Chiranjib, Shukhla, S.; Chandira, M.R. Recent advances in epilepsy drug therapy and management disease. Int. J. Pharm. Tech. Res. 2010;2:981–988. [Google Scholar]
- 110.Bell B.D., Giovagnoli A.R. Recent innovative studies of memory in temporal lobe epilepsy. Neuropsychol. Rev. 2007;17(4):455–476. doi: 10.1007/s11065-007-9049-3. [http://dx.doi.org/10.1007/s11065-007-9049-3]. [PMID: 18026841]. [DOI] [PubMed] [Google Scholar]
- 111.Shin C., McNamara J.O. Mechanism of epilepsy. Annu. Rev. Med. 1994;45:379–389. doi: 10.1146/annurev.med.45.1.379. [http://dx.doi.org/10.1146/annurev.med. 45.1.379]. [PMID: 8198389]. [DOI] [PubMed] [Google Scholar]
- 112.Lecis C., Segatto M. Cholesterol homeostasis imbalance and brain functioning: Neurological disorders and behavioral consequences. J. Neuro. Neurol. Disord. 2014;1:1–14. [Google Scholar]
- 113.Björkhem I., Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [http://dx.doi.org/10.1161/01.ATV.0000120374.59826.1b]. [PMID: 14764421]. [DOI] [PubMed] [Google Scholar]
- 114.Orth M., Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012 doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martins I.J., Berger T., Sharman M.J., Verdile G., Fuller S.J., Martins R.N. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009;111(6):1275–1308. doi: 10.1111/j.1471-4159.2009.06408.x. [http://dx.doi.org/10.1111/j.1471-4159.2009.06408.x]. [PMID: 20050287]. [DOI] [PubMed] [Google Scholar]
- 116.Björkhem I., Leoni V., Meaney S. Genetic connections between neurological disorders and cholesterol metabolism. J. Lipid Res. 2010;51(9):2489–2503. doi: 10.1194/jlr.R006338. [http://dx.doi.org/10.1194/jlr.R006338]. [PMID: 20466796]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klett E.L., Patel S. Genetic defenses against noncholesterol sterols. Curr. Opin. Lipidol. 2003;14(4):341–345. doi: 10.1097/01.mol.0000083763.66245.18. [http://dx.doi. org/10.1097/00041433-200308000-00001]. [PMID: 12865730]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Upadhyay R.K. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids, 2015 doi: 10.1155/2015/971453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dietschy J.M. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 2009;390(4):287–293. doi: 10.1515/BC.2009.035. [http://dx.doi.org/10.1515/BC.2009.035]. [PMID: 19166320]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hasse B., Bosse F., Müller H.W. Proteins of peripheral myelin are associated with glycosphingolipid/cholesterol-enriched membranes. J. Neurosci. Res. 2002;69(2):227–232. doi: 10.1002/jnr.10287. [http://dx.doi. org/10.1002/jnr.10287]. [PMID: 12111804]. [DOI] [PubMed] [Google Scholar]
- 121.Dietschy J.M., Turley S.D. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45(8):1375–1397. doi: 10.1194/jlr.R400004-JLR200. [http://dx.doi.org/10.1194/jlr.R400004-JLR200]. [PMID: 15254070]. [DOI] [PubMed] [Google Scholar]
- 122.Brown R.C., Lockwood A.H., Sonawane B.R. Neurodegenerative diseases: an overview of environmental risk factors. Environ. Health Perspect. 2005;113(9):1250–1256. doi: 10.1289/ehp.7567. [http://dx.doi.org/ 10.1289/ehp.7567]. [PMID: 16140637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bové J., Prou D., Perier C., Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2(3):484–494. doi: 10.1602/neurorx.2.3.484. [http://dx.doi.org/10.1602/neurorx.2.3.484]. [PMID: 16389312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh R., Gautam N., Mishra A., Gupta R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011;43(3):246–253. doi: 10.4103/0253-7613.81505. [http://dx.doi.org/10.4103/0253-7613.81505]. [PMID: 21713085]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chin-Chan M., Navarro-Yepes J., Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [http://dx.doi.org/10.3389/fncel.2015.00124]. [PMID: 25914621]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Esch T., Stefano G.B., Fricchione G.L., Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinol. Lett. 2002;23(3):199–208. [PMID: 12080279]. [PubMed] [Google Scholar]
- 127.Gilad G.M., Gilad V.H. Strain, stress, neurodegeneration and longevity. Mech. Ageing Dev. 1995;78(2):75–83. doi: 10.1016/0047-6374(94)01529-u. [http://dx.doi. org/10.1016/0047-6374(94)01529-U]. [PMID: 7596198]. [DOI] [PubMed] [Google Scholar]
- 128.Mizoguchi K., Kunishita T., Chui D.H., Tabira T. Stress induces neuronal death in the hippocampus of castrated rats. Neurosci. Lett. 1992;138(1):157–160. doi: 10.1016/0304-3940(92)90495-s. [http://dx.doi.org/10.1016/0304-3940(92)90495-S]. [PMID: 1407656]. [DOI] [PubMed] [Google Scholar]
- 129.Joseph R. The neurology of traumatic “dissociative” amnesia: commentary and literature review. Child Abuse Negl. 1999;23(8):715–727. doi: 10.1016/s0145-2134(99)00048-4. [PMID: 10477233]. [DOI] [PubMed] [Google Scholar]
- 130.Negrão A.B., Deuster P.A., Gold P.W., Singh A., Chrousos G.P. Individual reactivity and physiology of the stress response. Biomed. Pharmacother. 2000;54(3):122–128. doi: 10.1016/S0753-3322(00)89044-7. [http://dx.doi.org/ 10.1016/S0753-3322(00)89044-7]. [PMID: 10840588]. [DOI] [PubMed] [Google Scholar]
- 131.Archelos J.J., Hartung H.P. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23(7):317–327. doi: 10.1016/s0166-2236(00)01575-7. [http://dx.doi.org/10.1016/S0166-2236(00)01575-7]. [PMID: 10856942]. [DOI] [PubMed] [Google Scholar]
- 132.Medana I.M., Esiri M.M. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126(Pt 3):515–530. doi: 10.1093/brain/awg061. [http://dx.doi.org/10.1093/brain/awg061]. [PMID: 12566274]. [DOI] [PubMed] [Google Scholar]
- 133.Bains J.S., Shaw C.A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Brain Res. Rev. 1997;25(3):335–358. doi: 10.1016/s0165-0173(97)00045-3. [http://dx.doi. org/10.1016/S0165-0173(97)00045-3]. [PMID: 9495562]. [DOI] [PubMed] [Google Scholar]
- 134.Schulz J.B., Lindenau J., Seyfried J., Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000;267(16):4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [http://dx.doi.org/10.1046/j.1432-1327.2000. 01595.x]. [PMID: 10931172]. [DOI] [PubMed] [Google Scholar]
- 135.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [http://dx.doi.org/10.2174/ 157015909787602823]. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kapetanovic I.M. Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem. Biol. Interact. 2008;171(2):165–176. doi: 10.1016/j.cbi.2006.12.006. [http://dx.doi.org/ 10.1016/j.cbi.2006.12.006]. [PMID: 17229415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song C.M., Lim S.J., Tong J.C. Recent advances in computer-aided drug design. Brief. Bioinform. 2009;10(5):579–591. doi: 10.1093/bib/bbp023. [http://dx.doi.org/10.1093/bib/bbp023]. [PMID: 19433475]. [DOI] [PubMed] [Google Scholar]
- 138.Sahu M., Sinha S.K., Pandey K.K. Computer Aided Drug design: The Most fundamental Goal is to predict whether a given molecule will bind to a target and if so how strongly. . Comp. Engineer. Intell. Syst. 2013 [Google Scholar]
- 139.Sliwoski G., Kothiwale S., Meiler J., Lowe E.W., Jr Computational methods in drug discovery. Pharmacol. Rev. 2013;66(1):334–395. doi: 10.1124/pr.112.007336. [http://dx.doi.org/10.1124/pr.112.007336]. [PMID: 24381236]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Selvaraj C., Omer A., Singh P., Singh S.K. Molecular insights of protein contour recognition with ligand pharmacophoric sites through combinatorial library design and MD simulation in validating HTLV-1 PR inhibitors. Mol. Biosyst. 2015;11(1):178–189. doi: 10.1039/c4mb00486h. [http://dx.doi.org/10.1039/C4MB00486H]. [PMID: 25335799]. [DOI] [PubMed] [Google Scholar]
- 141.Tripathi S.K., Singh S.K. Insights into the structural basis of 3,5-diaminoindazoles as CDK2 inhibitors: prediction of binding modes and potency by QM-MM interaction, MESP and MD simulation. Mol. Biosyst. 2014;10(8):2189–2201. doi: 10.1039/c4mb00077c. [http://dx.doi.org/10.1039/ C4MB00077C]. [PMID: 24909777]. [DOI] [PubMed] [Google Scholar]
- 142.Csermely P., Korcsmáros T., Kiss H.J., London G., Nussinov R. Structure and dynamics of molecular networks: a novel paradigm of drug discovery: a comprehensive review. Pharmacol. Ther. 2013;138(3):333–408. doi: 10.1016/j.pharmthera.2013.01.016. [http://dx.doi.org/10.1016/j.pharmthera. 2013.01.016]. [PMID: 23384594]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kastritis P.L., Rodrigues J.P., Bonvin A.M. HADDOCK(2P2I): a biophysical model for predicting the binding affinity of protein-protein interaction inhibitors. J. Chem. Inf. Model. 2014;54(3):826–836. doi: 10.1021/ci4005332. [http://dx.doi.org/10.1021/ci4005332]. [PMID: 24521147]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stjernschantz E., Oostenbrink C. Improved ligand-protein binding affinity predictions using multiple binding modes. Biophys. J. 2010;98(11):2682–2691. doi: 10.1016/j.bpj.2010.02.034. [http://dx.doi.org/10.1016/j.bpj.2010. 02.034]. [PMID: 20513413]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lounnas V., Ritschel T., Kelder J., McGuire R., Bywater R.P., Foloppe N. Current progress in structure-based rational drug design marks a new mindset in drug discovery. Comput. Struct. Biotechnol. J. 2013;5:e201302011. doi: 10.5936/csbj.201302011. [http://dx.doi.org/10.5936/ csbj.201302011]. [PMID: 24688704]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng M., Liu X., Xu Y., Li H., Luo C., Jiang H. Computational methods for drug design and discovery: focus on China. Trends Pharmacol. Sci. 2013;34(10):549–559. doi: 10.1016/j.tips.2013.08.004. [http://dx. doi.org/10.1016/j.tips.2013.08.004]. [PMID: 24035675]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Babine R.E., Bender S.L. Molecular recognition of protein-Ligand complexes: Applications to drug design. Chem. Rev. 1997;97(5):1359–1472. doi: 10.1021/cr960370z. [http://dx.doi.org/10.1021/cr960370z]. [PMID: 11851455]. [DOI] [PubMed] [Google Scholar]
- 148.Anderson A.C. The process of structure-based drug design. Chem. Biol. 2003;10(9):787–797. doi: 10.1016/j.chembiol.2003.09.002. [http://dx.doi.org/10.1016/j.chembiol. 2003.09.002]. [PMID: 14522049]. [DOI] [PubMed] [Google Scholar]
- 149.Andreoli F., Del Rio A. Computer-aided molecular design of compounds targeting histone modifying enzymes. Comput. Struct. Biotechnol. J. 2015;13:358–365. doi: 10.1016/j.csbj.2015.04.007. [http://dx.doi.org/10.1016/ j.csbj.2015.04.007]. [PMID: 26082827]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang X., Chen H., Yang F., Gong J., Li S., Pei J., Liu X., Jiang H., Lai L., Li H. iDrug: a web-accessible and interactive drug discovery and design platform. J. Cheminform. 2014;6:28. doi: 10.1186/1758-2946-6-28. [http://dx.doi.org/10.1186/1758-2946-6-28]. [PMID: 24955134]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tan J.J., Cong X.J., Hu L.M., Wang C.X., Jia L., Liang X.J. Therapeutic strategies underpinning the development of novel techniques for the treatment of HIV infection. Drug Discov. Today. 2010;15(5-6):186–197. doi: 10.1016/j.drudis.2010.01.004. [http://dx.doi.org/10.1016/j.drudis.2010. 01.004]. [PMID: 20096804]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Veselovsky A.V., Ivanov A.S. Strategy of computer-aided drug design. Curr. Drug Targets Infect. Disord. 2003;3(1):33–40. doi: 10.2174/1568005033342145. [http://dx.doi.org/10.2174/1568005033342145]. [PMID: 12570731]. [DOI] [PubMed] [Google Scholar]
- 153.Liao C., Sitzmann M., Pugliese A., Nicklaus M.C. Software and resources for computational medicinal chemistry. Future Med. Chem. 2011;3(8):1057–1085. doi: 10.4155/fmc.11.63. [http://dx.doi.org/10.4155/fmc.11.63]. [PMID: 21707404]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reddy M.R., Erion M.D. Computer-aided drug design strategies used in the discovery of fructose 1, 6-bisphosphatase inhibitors. Curr. Pharm. Des. 2005;11(3):283–294. doi: 10.2174/1381612053382160. [http://dx.doi.org/10. 2174/1381612053382160]. [PMID: 15723626]. [DOI] [PubMed] [Google Scholar]
- 155.Reddy R.N., Mutyala R., Aparoy P., Reddanna P., Reddy M.R. Computer aided drug design approaches to develop cyclooxygenase based novel anti-inflammatory and anti-cancer drugs. Curr. Pharm. Des. 2007;13(34):3505–3517. doi: 10.2174/138161207782794275. [http://dx. doi.org/10.2174/138161207782794275]. [PMID: 18220787]. [DOI] [PubMed] [Google Scholar]
- 156.Aparoy P., Reddy K.K., Reddanna P. Structure and ligand based drug design strategies in the development of novel 5-LOX inhibitors. Curr. Med. Chem. 2012;19(22):3763–3778. doi: 10.2174/092986712801661112. [http://dx. doi.org/10.2174/092986712801661112]. [PMID: 22680930]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Capriotti E., Fariselli P., Casadio R. A neural-network-based method for predicting protein stability changes upon single point mutations. Bioinformatics. 2004;20(Suppl. 1):i63–i68. doi: 10.1093/bioinformatics/bth928. [http://dx. doi.org/10.1093/bioinformatics/bth928]. [PMID: 15262782]. [DOI] [PubMed] [Google Scholar]
- 158.Chitta S., Larif M., Ghamali M., Bouachrine M., Lakhlifi T. Quantitative structure activity relationship studies of dibenzo [a,d]cycloalkenimine derivatives for non-competitive antagonists of N-methyl-D-aspartate based on density functional theory with electronic and topological descriptors. JTUSCI. 2015;9:143–154. [Google Scholar]
- 159.Li A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today. 2001;6(7):357–366. doi: 10.1016/s1359-6446(01)01712-3. [http://dx. doi.org/10.1016/S1359-6446(01)01712-3]. [PMID: 11267922]. [DOI] [PubMed] [Google Scholar]
- 160.Kuntz I.D. Structure-based strategies for drug design and discovery. Science. 1992;257(5073):1078–1082. doi: 10.1126/science.257.5073.1078. [http://dx.doi.org/ 10.1126/science.257.5073.1078]. [PMID: 1509259]. [DOI] [PubMed] [Google Scholar]
- 161.Meng X.Y., Zhang H.X., Mezei M., Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011;7(2):146–157. doi: 10.2174/157340911795677602. [http://dx.doi.org/ 10.2174/157340911795677602]. [PMID: 21534921]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kroemer R.T. Structure-based drug design: docking and scoring. Curr. Protein Pept. Sci. 2007;8(4):312–328. doi: 10.2174/138920307781369382. [http://dx.doi.org/ 10.2174/138920307781369382]. [PMID: 17696866]. [DOI] [PubMed] [Google Scholar]
- 163.Martí-Renom M.A., Stuart A.C., Fiser A., Sánchez R., Melo F., Sali A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [http://dx.doi.org/10.1146/annurev.biophys.29.1.291]. [PMID: 10940251]. [DOI] [PubMed] [Google Scholar]
- 164.Singh S., Malik B.K., Sharma D.K. Molecular drug targets and structure based drug design: A holistic approach. Bioinformation. 2006;1(8):314–320. doi: 10.6026/97320630001314. [http://dx.doi.org/10.6026/97320630001314]. [PMID: 17597912]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Klebe G. Recent developments in structure-based drug design. J. Mol. Med. (Berl.) 2000;78(5):269–281. doi: 10.1007/s001090000084. [http://dx.doi.org/10.1007/ s001090000084]. [PMID: 10954199]. [DOI] [PubMed] [Google Scholar]
- 166.Blundell T.L., Jhoti H., Abell C. High-throughput crystallo-graphy for lead discovery in drug design. Nat. Rev. Drug Discov. 2002;1(1):45–54. doi: 10.1038/nrd706. [http://dx.doi.org/10.1038/nrd706]. [PMID: 12119609]. [DOI] [PubMed] [Google Scholar]
- 167.Davis A.M., Teague S.J., Kleywegt G.J. Application and limitations of X-ray crystallographic data in structure-based ligand and drug design. Angew. Chem. Int. Ed. Engl. 2003;42(24):2718–2736. doi: 10.1002/anie.200200539. [http://dx.doi.org/10.1002/anie.200200539]. [PMID: 12820253]. [DOI] [PubMed] [Google Scholar]
- 168.Landles C., Bates G.P. Huntingtin and the molecular pathogenesis of Huntington’s disease. Fourth in molecular medicine review series. EMBO Rep. 2004;5(10):958–963. doi: 10.1038/sj.embor.7400250. [http://dx.doi.org/10. 1038/sj.embor.7400250]. [PMID: 15459747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ducker C.E., Stettler E.M., French K.J., Upson J.J., Smith C.D. Huntingtin interacting protein 14 is an oncogenic human protein: palmitoyl acyltransferase. Oncogene. 2004;23(57):9230–9237. doi: 10.1038/sj.onc.1208171. [PMID: 15489887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kleppner S.R., Tobin A.J. GABA signalling: therapeutic targets for epilepsy, Parkinson’s disease and Huntington’s disease. Expert Opin. Ther. Targets. 2001;5(2):219–239. doi: 10.1517/14728222.5.2.219. [http://dx.doi.org/ 10.1517/14728222.5.2.219]. [PMID: 15992178]. [DOI] [PubMed] [Google Scholar]
- 171.Glass M., Dragunow M., Faull R.L. The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97(3):505–519. doi: 10.1016/s0306-4522(00)00008-7. [http://dx.doi.org/10.1016/ S0306-4522(00)00008-7]. [PMID: 10828533]. [DOI] [PubMed] [Google Scholar]
- 172.Keane H., Ryan B.J., Jackson B., Whitmore A., Wade-Martins R. Protein-protein interaction networks identify targets which rescue the MPP+ cellular model of Parkinson’s disease. Sci. Rep. 2015;5:17004. doi: 10.1038/srep17004. [http://dx.doi.org/10.1038/srep17004]. [PMID: 26608097]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ombrato R., Cazzolla N., Mancini F., Mangano G. Structure based discovery of 1H-Indazole-3-Carboxamides as a novel structure class of Human GSK-3-Inhibitors. J. Chem. Inf. Model. 2015;55(12):2540–2551. doi: 10.1021/acs.jcim.5b00486. [http://dx.doi.org/10.1021/acs.jcim. 5b00486]. [PMID: 26600430]. [DOI] [PubMed] [Google Scholar]
- 174.Scotti L., Scotti M.T. Computer aided drug design studies in the discovery of secondary metabolites targeted against Age-related neurodegenerative diseases. Curr. Top. Med. Chem. 2015;15(21):2239–2252. doi: 10.2174/1568026615666150610143510. [http://dx.doi.org/10.2174/1568026615666150610143510]. [PMID: 26059353]. [DOI] [PubMed] [Google Scholar]
- 175.Sheridan R.P., Venkataraghavan R. New methods in computer aided drug design. . ACS., . 1987; 20:322–329. [Google Scholar]
- 176.Khoddami M., Nadri H., Moradi A., Sakhteman A. Homology modeling, molecular dynamic simulation, and docking based binding site analysis of human dopamine (D4) receptor. J. Mol. Model. 2015;21(2):36. doi: 10.1007/s00894-015-2579-3. [http://dx.doi.org/10.1007/s00894-015-2579-3]. [PMID: 25650117]. [DOI] [PubMed] [Google Scholar]
- 177.Mølck C., Harpsøe K., Gloriam D.E., Mathiesen J.M., Nielsen S.M., Bräuner-Osborne H. mGluR5: exploration of orthosteric and allosteric ligand binding pockets and their applications to drug discovery. Neurochem. Res. 2014;39(10):1862–1875. doi: 10.1007/s11064-014-1248-8. [http://dx.doi.org/10.1007/s11064-014-1248-8]. [PMID: 24493625]. [DOI] [PubMed] [Google Scholar]
- 178.Xu L., Zhou S., Yu K., Gao B., Jiang H., Zhen X., Fu W. Molecular modeling of the 3D structure of 5-HT(1A)R: discovery of novel 5-HT(1A)R agonists via dynamic pharmacophore-based virtual screening. J. Chem. Inf. Model. 2013;53(12):3202–3211. doi: 10.1021/ci400481p. [http://dx.doi.org/10.1021/ci400481p]. [PMID: 24245825]. [DOI] [PubMed] [Google Scholar]
- 179.Wing L.K., Behanna H.A., Van Edik L.J., Watterson D.M., RalayRanalvo H. Denovo and molecular target-independent discovey of orally bioavailable lead compounds for neurological disorders. Curr. Alzheimer Res. 2006;3:205–214. doi: 10.2174/156720506777632844. [http://dx. doi.org/10.2174/156720506777632844]. [PMID: 16842097]. [DOI] [PubMed] [Google Scholar]
- 180.Zhu M., De Simone A., Schenk D., Toth G., Dobson C.M., Vendruscolo M. Identification of small-molecule binding pockets in the soluble monomeric form of the Aβ42 peptide. J. Chem. Phys. 2013;139(3):035101. doi: 10.1063/1.4811831. [http://dx.doi.org/10.1063/1.4811831]. [PMID: 23883055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jiang F., Kim S.H. “Soft docking”: matching of molecular surface cubes. J. Mol. Biol. 1991;219(1):79–102. doi: 10.1016/0022-2836(91)90859-5. [http://dx.doi.org/10. 1016/0022-2836(91)90859-5]. [PMID: 2023263]. [DOI] [PubMed] [Google Scholar]
- 182.Rayalu D.J., Selvaraj C., Singh S.K., Ganeshan R., Kumar N.U., Seshapani P. Homology modeling, active site prediction, and targeting the anti hypertension activity through molecular docking on endothelin - B receptor domain. Bioinformation. 2012;8(2):81–86. doi: 10.6026/97320630008081. [http://dx.doi.org/10.6026/97320630008081]. [PMID: 22359440]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sivakamavalli J., Tripathi S.K., Singh S.K., Vaseeharan B. Homology modeling, molecular dynamics, and docking studies of pattern-recognition transmembrane protein-lipopolysaccharide and β-1,3 glucan-binding protein from Fenneropenaeus indicus. J. Biomol. Struct. Dyn. 2015;33(6):1269–1280. doi: 10.1080/07391102.2014.943807. [http://dx.doi.org/10. 1080/07391102.2014.943807]. [PMID: 25139673]. [DOI] [PubMed] [Google Scholar]
- 184.Reddy K.K., Singh P., Singh S.K. Blocking the interaction between HIV-1 integrase and human LEDGF/p75: mutational studies, virtual screening and molecular dynamics simulations. Mol. Biosyst. 2014;10(3):526–536. doi: 10.1039/c3mb70418a. [http://dx.doi.org/10.1039/ c3mb70418a]. [PMID: 24389668]. [DOI] [PubMed] [Google Scholar]
- 185.Huang B., Schroeder M. LIGSITEcsc: predicting ligand binding sites using the Connolly surface and degree of conservation. BMC Struct. Biol. 2006;6:19. doi: 10.1186/1472-6807-6-19. [http://dx.doi.org/10.1186/1472-6807-6-19]. [PMID: 16995956]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reddy M.R., Parrill A.L. Overview of rational drug design. . ACS., . 1997;1 :1–11. [Google Scholar]
- 187.Mavromoustakos T., Durdagi S., Koukoulitsa C., Simcic M., Papadopoulos M.G., Hodoscek M., Grdadolnik S.G. Strategies in the rational drug design. Curr. Med. Chem. 2011;18(17):2517–2530. doi: 10.2174/092986711795933731. [http://dx.doi.org/10.2174/092986711795933731]. [PMID: 21568895]. [DOI] [PubMed] [Google Scholar]
- 188.Mandal S., Moudgil M., Mandal S.K. Rational drug design. Eur. J. Pharmacol. 2009;625(1-3):90–100. doi: 10.1016/j.ejphar.2009.06.065. [http://dx.doi.org/10.1016/ j.ejphar.2009.06.065]. [PMID: 19835861]. [DOI] [PubMed] [Google Scholar]
- 189.Verlinde C.L., Hol W.G. Structure-based drug design: progress, results and challenges. Structure. 1994;2(7):577–587. doi: 10.1016/s0969-2126(00)00060-5. [http://dx. doi.org/10.1016/S0969-2126(00)00060-5]. [PMID: 7922037]. [DOI] [PubMed] [Google Scholar]
- 190.Kroemer R.T. Structure-based drug design: docking and scoring. Curr. Protein Pept. Sci. 2007;8(4):312–328. doi: 10.2174/138920307781369382. [http://dx.doi.org/10. 2174/138920307781369382]. [PMID: 17696866]. [DOI] [PubMed] [Google Scholar]
- 191.Reddy K.K., Singh S.K., Tripathi S.K., Selvaraj C. Identification of potential HIV-1 integrase strand transfer inhibitors: in silico virtual screening and QM/MM docking studies. SAR QSAR Environ. Res. 2013;24(7):581–595. doi: 10.1080/1062936X.2013.772919. [http://dx.doi.org/10.1080/ 1062936X.2013.772919]. [PMID: 23521430]. [DOI] [PubMed] [Google Scholar]
- 192.Lipinski C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000;44(1):235–249. doi: 10.1016/s1056-8719(00)00107-6. [http://dx.doi.org/10.1016/S1056-8719(00) 00107-6]. [PMID: 11274893]. [DOI] [PubMed] [Google Scholar]
- 193.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46(1-3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [http://dx.doi.org/10.1016/ S0169-409X(00)00129-0]. [PMID: 11259830]. [DOI] [PubMed] [Google Scholar]
- 194.Smith J., Stein V. SPORCalc: A development of a database analysis that provides putative metabolic enzyme reactions for ligand-based drug design. Comput. Biol. Chem. 2009;33(2):149–159. doi: 10.1016/j.compbiolchem.2008.11.002. [http://dx.doi.org/10.1016/j.compbiolchem.2008.11.002]. [PMID: 19157988]. [DOI] [PubMed] [Google Scholar]
- 195.Taylor J.P., Hardy J., Fischbeck K.H. Toxic proteins in neurodegenerative disease. Science. 2002;296(5575):1991–1995. doi: 10.1126/science.1067122. [http://dx.doi.org/10.1126/science.1067122]. [PMID: 12065827]. [DOI] [PubMed] [Google Scholar]
- 196.Jones D.A., Fitzpatrick F.A. Genomics and the discovery of new drug targets. Curr. Opin. Chem. Biol. 1999;3(1):71–76. doi: 10.1016/s1367-5931(99)80013-1. [http:// dx.doi.org/10.1016/S1367-5931(99)80013-1]. [PMID: 10021407]. [DOI] [PubMed] [Google Scholar]
- 197.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8(2):141–146. doi: 10.1038/sj.embor.7400897. [http://dx. doi.org/10.1038/sj.embor.7400897]. [PMID: 17268505]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E.S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W.G., Lazzarini A.M., Duvoisin R.C., Di Iorio G., Golbe L.I., Nussbaum R.L. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [http://dx.doi.org/ 10.1126/science.276.5321.2045]. [PMID: 9197268]. [DOI] [PubMed] [Google Scholar]
- 199.Kang S.G., Roh Y.M., Lau A., Westaway D., McKenzie D., Aiken J., Kim Y.S., Yoo H.S. Establishment and characterization of Prnp knockdown neuroblastoma cells using dual microRNA-mediated RNA interference. Prion. 2011;5(2):93–102. doi: 10.4161/pri.5.2.15621. [http://dx. doi.org/10.4161/pri.5.2.15621]. [PMID: 21494092]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shao J., Diamond M.I. Polyglutamine diseases: emerging concepts in pathogenesis and therapy. Hum. Mol. Genet. 2007;16(Spec No. 2):R115–R123. doi: 10.1093/hmg/ddm213. [DOI] [PubMed] [Google Scholar]
- 201.Zuccato C., Valenza M., Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol. Rev. 2010;90(3):905–981. doi: 10.1152/physrev.00041.2009. [http://dx.doi.org/10.1152/physrev. 00041.2009]. [PMID: 20664076]. [DOI] [PubMed] [Google Scholar]
- 202.Beinfeld M.C. Cholecystokinin in the central nervous system: a minireview. Neuropeptides. 1983;3(6):411–427. doi: 10.1016/0143-4179(83)90032-x. [http://dx. doi.org/10.1016/0143-4179(83)90032-X]. [PMID: 6141537]. [DOI] [PubMed] [Google Scholar]
- 203.Klein C., Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2(1):a008888. doi: 10.1101/cshperspect.a008888. [http://dx. doi.org/10.1101/cshperspect.a008888]. [PMID: 22315721]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jatana N., Apoorva N., Malik S., Sharma A., Latha N. Inhibitors of catechol-O-methyltransferase in the treatment of neurological disorders. Cent. Nerv. Syst. Agents Med. Chem. 2013;13(3):166–194. doi: 10.2174/1871524913666140109113341. [http://dx.doi.org/10.2174/1871524913666140109113341]. [PMID: 24450388]. [DOI] [PubMed] [Google Scholar]
- 205.Antonini A., Abbruzzese G., Barone P., Bonuccelli U., Lopiano L., Onofrj M., Zappia M., Quattrone A. COMT inhibition with tolcapone in the treatment algorithm of patients with Parkinson’s disease (PD): relevance for motor and non-motor features. Neuropsychiatr. Dis. Treat. 2008;4(1):1–9. doi: 10.2147/ndt.s2404. [http://dx.doi.org/ 10.2147/NDT.S2404]. [PMID: 18728767]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lallana E.C., Fadul C.E. Toxicities of immunosuppressive treatment of autoimmune neurologic diseases. Curr. Neuropharmacol. 2011;9(3):468–477. doi: 10.2174/157015911796557939. [http://dx.doi.org/10.2174/157015911796557939]. [PMID: 22379461]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kazantsev A.G., Thompson L.M. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug Discov. 2008;7(10):854–868. doi: 10.1038/nrd2681. [http://dx.doi. org/10.1038/nrd2681]. [PMID: 18827828]. [DOI] [PubMed] [Google Scholar]
- 208.Farooqui A.A., Litsky M.L., Farooqui T., Horrocks L.A. Inhibitors of intracellular phospholipase A2 activity: their neurochemical effects and therapeutical importance for neurological disorders. Brain Res. Bull. 1999;49(3):139–153. doi: 10.1016/s0361-9230(99)00027-1. [http:// dx.doi.org/10.1016/S0361-9230(99)00027-1]. [PMID: 10435777]. [DOI] [PubMed] [Google Scholar]
- 209.Gold B.G. Neuroimmunophilin ligands: evaluation of their therapeutic potential for the treatment of neurological disorders. Expert Opin. Investig. Drugs. 2000;9(10):2331–2342. doi: 10.1517/13543784.9.10.2331. [http://dx. doi.org/10.1517/13543784.9.10.2331]. [PMID: 11060810]. [DOI] [PubMed] [Google Scholar]
- 210.Wulff H., Zhorov B.S. + channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem. Rev. 2008;108(5):1744–1773. doi: 10.1021/cr078234p. [http://dx.doi.org/10.1021/cr078234p]. [PMID: 18476673]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yermakova A., O’Banion M.K. Cyclooxygenases in the central nervous system: implications for treatment of neurological disorders. Curr. Pharm. Des. 2000;6(17):1755–1776. doi: 10.2174/1381612003398672. [http://dx. doi.org/10.2174/1381612003398672]. [PMID: 11203433]. [DOI] [PubMed] [Google Scholar]
- 212.Harrison S.T., Poslusney M.S., Mulhearn J.J., Zhao Z., Kett N.R., Schubert J.W., Melamed J.Y., Allison T.J., Patel S.B., Sanders J.M., Sharma S., Smith R.F., Hall D.L., Robinson R.G., Sachs N.A., Hutson P.H., Wolkenberg S.E., Barrow J.C. Synthesis and Evaluation of Heterocyclic catechol mimics as inhibitors of catechool-o-methyltransferases (COMT). ACS Med. Chem. Lett. 2015;6(3):318–323. doi: 10.1021/ml500502d. [http://dx.doi.org/10.1021/ ml500502d]. [PMID: 25815153]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Farooqui A.A., Ong W.Y., Horrocks L.A. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol. Rev. 2006;58(3):591–620. doi: 10.1124/pr.58.3.7. [http://dx.doi.org/10. 1124/pr.58.3.7]. [PMID: 16968951]. [DOI] [PubMed] [Google Scholar]
- 214.Singh R. Geetanjali; Sharma, N. Monoamine oxidase inhibitors for neurological disorders: A review. Chem. Biol. Lett. 2014;1:40–103. [Google Scholar]
- 215.Tripathi R.K., Krishnamurthy S., Ayyannan S.R. Discovery of 3-Hydroxy-3-Phenacyloxindole analogues of Isatin as potential monoamine oxidase inhibitors. ChemMedChem. 2015;11:119–132. doi: 10.1002/cmdc.201500443. [PMID: 26592797]. [DOI] [PubMed] [Google Scholar]