Abstract
Background:
The treatment of schizophrenia is challenging due to the wide range of symptoms (positive, negative, cognitive) associated with the disease. Typical antipsychotics that antagonize D2 receptors are effective in treating positive symptoms, but extrapyramidal side-effects (EPS) are a common occurrence. Atypical antipsychotics targeting 5-HT2A and D2 receptors are more effective at treating cogni-tive and negative symptoms compared to typical antipsychotics, but these drugs also result in side-effects such as metabolic syndromes.
Objective:
To identify evidence in the literature that elucidates the pharmacological profile of aripiprazole.
Methods:
We searched PubMed for peer reviewed articles on aripiprazole and its clinical efficacy, side-effects, pharmacolo-gy, and effects in animal models of schizophrenia symptoms.
Results:
Aripiprazole is a newer atypical antipsychotic that displays a unique pharmacological profile, including partial D2 agonism and functionally selective properties. Aripiprazole is effective at treating the positive symptoms of schizophrenia and has the potential to treat negative and cognitive symptoms at least as well as other atypical antipsychotics. The drug has a favorable side-effect profile and has a low propensity to result in EPS or metabolic syndromes. Animal models of schizophrenia have been used to determine the efficacy of aripiprazole in symptom management. In these instanc-es, aripiprazole resulted in the reversal of deficits in extinction, pre-pulse inhibition, and social withdrawal. Because aripipra-zole requires a greater than 90% occupancy rate at D2 receptors to be clinically active and does not produce EPS, this suggests a functionally selective effect on intracel-lular signaling pathways.
Conclusion:
A combination of factors such as dopamine system stabilization via partial agonism, functional selectivity at D2 receptors, and serotonin-dopamine system interaction may contribute to the ability of aripiprazole to successfully manage schizophrenia symptoms. This review examines these mechanisms of action to further clarify the pharmacological actions of aripiprazole.
Keywords: Dopamine receptors, schizophrenia, antipsychotics, partial agonism, functional selectivity, serotonin receptors, MK-801
1. Introduction
Aripiprazole, originally known as 7-{4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butyloxy}-3,4-dihydro-2(1H)-quinolinone (OPC-145967), marketed under the name Abilify, is a derivative of Quinolinone, a dopamine (DA) autoreceptor agonist [1, 2]. Aripiprazole is considered an atypical antipsychotic and has shown efficacy in the treatment of schizophrenia and acute manic episodes associated with bipolar disorder [1]. Aripiprazole is thought to stabilize DA and serotonin (5-HT) activity [3, 4] within the nucleus accumbens (NAc), ventral tegmental area (VTA), and frontal cortex (FC), resulting in the management of positive, negative, and cognitive symptoms in schizophrenia [5-9]. The drug is generally considered to be a partial agonist at 5-HT1A and DA D2 receptors, and an antagonist at the 5-HT2A [10]. However, there is evidence that aripiprazole has a unique pharmacological profile at D2 receptors that expands beyond partial agonism to include functionally selective effects on intracellular signaling pathways [7, 8, 11, 12], suggesting that the pharmacological profile of aripiprazole is more complicated than originally thought. Other reviewers have reached similar conclusions regarding the functionally selective actions of aripiprazole [13]. This review will provide a comparison of aripiprazole to other antipsychotic drugs in terms of DA release within the mesocorticolimbic system and effects on locomotor activity and DA receptor sensitization. This review will also highlight the ability of aripiprazole to reverse MK-801 induced deficits in an animal model of schizophrenia symptoms, and how this highlights the unique pharmacology of aripiprazole. The final sections will discuss three potential ways aripiprazole may result in therapeutic efficacy, including, stabilization of the DA system, functional selectivity, and 5-HT-DA system interactions.
2. SCHIZOPHRENIA
Schizophrenia is a debilitating heterogeneous disorder that affects approximately 1% of the population [14]. Schizophrenia is generally characterized as a thought disorder punctuated by illogical thinking, lack of reasoning, and a distortion of reality [15]. The symptoms of schizophrenia can be chronic, consist of relapses (an exaggeration of symptoms) and remissions, and change over time [15]. A prodromal phase precludes a diagnosis of schizophrenia, and consists of nonspecific symptoms that can last a period of weeks or months, including loss of interest, work avoidance, and irritability [15].
A convenient classification scheme has defined schizophrenia based on the prevalence of positive, negative, and cognitive symptoms [16]. The more dramatic, and common symptoms of schizophrenia are the positive symptoms, which include delusions, hallucinations, disorganized speech, and bizarre behavior [16]. The negative symptoms are characterized by a decline in everyday functioning, including reduced communication, avolition, flattened affect, and anhedonia [16]. Cognitive symptoms of schizophrenia can include executive function deficits [17], and general deficits in declarative and working memory, language function, and set shifting (leading to a tendency to perseverate) [18, 19]. Cognitive function is usually maintained during the initial phase of the disease, but cognitive dysfunction may progress over time [15].
Individuals with schizophrenia often have difficulty maintaining employment, legal problems, social difficulties [14] and a 2 to 3 fold increase in mortality [20]. The disease typically presents itself during late adolescence/early adulthood and continues throughout life [14, 21]. Men tend to be in their early twenties during their first hospital admission for schizophrenia, whereas women tend to be in their late twenties [22, 23].
2.1. Pharmacological Treatments
The range of symptoms associated with schizophrenia has proven to be a challenge for the development of pharmacological treatments that are effective in alleviating all symptom subtypes without resulting in unwanted side effects. Indeed, the negative and cognitive symptoms have been the most difficult to treat with current pharmacotherapies [21, 24-28]. The first antipsychotics (typical) were effective at alleviating positive symptoms, such as hallucinations and delusions, but were relatively ineffective in alleviating the negative and cognitive symptoms [24, 28, 29]. Newer antipsychotics (atypical) were developed as an attempt to minimize side-effects and treat all symptom classes of schizophrenia [24, 28, 29]. Atypical antipsychotics are considered effective in treating positive, negative, and cognitive symptoms, but treatment efficacy varies across the different pharmacological agents [27-32]. For example: treatment with the atypical antipsychotic olanzapine for 18 months results in less neurocognitive improvement when compared to the typical antipsychotic perphenazine [32].
2.1.1. Typical Antipsychotics and the Dopamine Hypothesis
The hallmark hypothesis relating to the etiology of schizophrenia is that the disease results from alterations within the DA system [33, 34]. Support for the DA hypothesis of schizophrenia came from the study of DA receptor antagonists (Neuroleptics) and the drug-induced behavioral profiles [33]. Administration of these drugs was shown to result in a decrease in the positive symptoms of schizophrenia via D2 receptor antagonism. The fact that D2 blockade decreased the occurrence of positive symptoms provided evidence that DA receptor overstimulation may be, at least in part, responsible for the etiology of the disease [35]. Evidence for the involvement of DA in schizophrenia symptoms also resulted from studying the effects of amphetamine on healthy individuals. The primary action of amphetamine is to increase catecholamine release, although amphetamine can also inhibit MAO activity and catecholamine uptake [35]. Repeated high doses of amphetamine have been shown to result in psychosis in non-schizophrenic patients and exacerbate symptoms in individuals suffering from schizophrenia [36]. Historically, amphetamine use even led to individuals being misdiagnosed with schizophrenia [37]. Due to the resemblance of amphetamine-induced psychosis to schizophrenia, it was suggested that an increase in catecholamine release may be present in individuals with schizophrenia, especially in association with the positive symptoms [35].
The mesocortical and mesolimbic DA pathways are the most relevant in the etiology of schizophrenia [21, 33, 34, 38]. The mesocortical DA pathway consists of neurons that project from the VTA to the FC and the mesolimbic DA pathway consists of neurons that project from the VTA to NAc [38]. In schizophrenia, an increase in DA release in the NAc has been hypothesized to play a role in the positive symptoms [33, 35], whereas a decrease in DA release in the medial prefrontal cortex (mPFC) has been hypothesized to play a role in the negative and cognitive symptoms [34, 39, 40].
D1 class receptors show the highest density in the mesolimbic and mesocortical DA systems while the highest density of D2 class receptors are located in the mesolimbic and nigrostriatal DA systems [38]. Within the NAc, D1 and D2 receptors are the most typical DA receptor subtype found on the gamma-aminobutyric acid (GABA)ergic medium spiny neurons (MSNs) [38]. Both D1 and D2-like receptors are found within the PFC but D1 receptors show a higher density than D2 receptors [41]. In the PFC, D1 and D2 receptors are predominately found on pyramidal cells with some evidence for DA receptors on GABAergic interneurons and on the terminals of DA projections from the VTA to the PFC, which are likely D2 autoreceptors [41]. Antipsychotics that increase DA activity at D1 receptors within the PFC have been shown in improve cognitive symptoms of schizophrenia [42-44]. There is also evidence that a decrease in D1 activity within the PFC can result in overactivity at D2 receptors within the NAc [42, 45-47]. This highlights the importance of balancing DA neurotransmission throughout the mesocorticolimbic DA system to result in optimal symptom management.
D1 and D2 receptors have differential effects on downstream signaling targets [38], hence the importance of balancing DA activity at both receptor types. The D1 class of receptors (D1 and D5) are coupled to Gαs/olf proteins which excite adenylate cyclase (AC) stimulating cyclic adenosine monophosphate (cAMP) and the D2 class of receptors (D2, D3, and D4) are coupled to Gαi/o proteins which inhibit AC, thus inhibiting cAMP production [38]. When D1 and/or D2 receptors are inactive, the α-subunit is bound to guanosine diphosphate (GDP) and a βγ-complex, which forms a trimeric protein complex [38]. When a DA receptor is activated, the Gα subunit excites/inhibits the production of the second messenger cAMP and the separated βγ-complex can engage in signaling activities such as activation of G-protein-regulated inwardly rectifying potassium channels (GIRK) [38]. When an agonist is bound to the D1 or D2 receptor, it results in GDP release and guanosine triphosphate (GTP) binding to the α-subunit, resulting in the dissociation of the α-subunit from the βγ-complex [38]. There are two isoforms of the D2 receptor the D2-short (D2S) and D2-Long (D2L) receptors [48, 49]. The D2S and D2L receptors differ in the addition of 29 amino acids, they also have different pharmacological properties [48, 49]. D2 receptors are located both pre and postsynaptically, with D2S receptors being the predominant DA autoreceptor within the brain, whereas the D2L receptor is mostly found postsynaptically [50, 51]. The D2 autoreceptor is involved in presynaptic regulation of DA, firing rate, synthesis, and release and plays an important role in the treatment efficacy of antipsychotics [38, 51].
Typical antipsychotics are effective in treating the positive symptoms of schizophrenia, however, these drugs are less effective at treating cognitive and negative symptoms [24, 25]. In addition, extrapyramidal motor side effects (EPS) are a common occurrence, such as akinesia [24, 25]. Unwanted side-effects, such as EPS, affect up to 50% of the patient population and can develop within the first few days of treatment with Haloperidol [26]. Typical antipsychotics may also exacerbate or cause cognitive symptoms by antagonizing the mesocortical DA system. For example, treatment with haloperidol has been shown to decrease cognitive abilities, such as impaired ability to sustain attention, reduced reaction time, and diminished information processing capability [25]. Given the low efficacy for negative and cognitive symptoms, and high potential for side effects, newer atypical antipsychotics were developed based on the 5-HT hypothesis of schizophrenia [24].
2.1.2. Atypical Antipsychotics and the Serotonin Hypothesis
Newer treatments for schizophrenia symptom management focus on both the DA and the 5-HT systems in an attempt to alleviate positive, negative and cognitive symptoms [28, 52]. The 5-HT hypothesis of schizophrenia arose from toxicologic explanations of mental illness that were popular in the 1950s [53]. Toxicologic explanations received their inspiration from the observation that exogenous substances could produce effects that resemble certain signs and symptoms of mental illness, such as hallucinations [53]. The 5-HT hypothesis arose based on the observation that hallucinogenic-effects, such as those seen with LSD administration, are mediated by 5-HT agonism, and hence, schizophrenia symptoms likely arise from a similar mechanism [54]. However, in the 1970s, the 5-HT hypothesis of schizophrenia was almost completely replaced by the DA hypothesis, only making a comeback later with the proven effectiveness of the atypical antipsychotics, such as clozapine [54].
While the main action of typical antipsychotics is on the D2 receptor, atypical antipsychotics show enhanced activity at 5-HT receptors with a low affinity for D2 receptors [26]. Atypical antipsychotics are more effective in reducing cognitive and negative symptoms and result in fewer EPS than the typical antipsychotics [55]. The high binding affinity at 5-HT receptors and the treatment efficacy of atypical antipsychotics suggests an important role in the 5-HT system in schizophrenia. The 5-TH system consists of cell bodies that are located within the distinct raphe nuclei in the brain stem [56]. The medulla and spinal cord are innervated by projections from the dorsal raphe nucleus (DRN), and the diencephalon and telencephalon receive projections from the median raphe nuclei (MRN) [56]. The DA nuclei within the midbrain, cerebral cortex, thalamus, and striatum are innervated by the DRN and the hippocampus and septum are innervated by the MRN [56]. The innervation of DA neurons by 5-HT highlights a potential role for DA-5-HT interactions in schizophrenia and antipsychotic treatments.
The highest density of 5-HT1A and 5-HT2A receptors are within the hippocampus, lateral septum, cortical areas, DRN, and MRN [56], which are targets of antipsychotic agents. The 5-HT1A and 5-HT2A receptors are found on both pyramidal glutamatergic neurons and GABAergic interneurons within the cortex and hippocampus [31]. 5-HT1A receptors are located pre-synaptically on 5-HT neurons and postsynaptically within the forebrain [56]. 5-HT2A receptors are positively coupled to intracellular calcium via G-protein receptors and to phospholipase C [56]. Atypical antipsychotics are also agonists at the 5-HT1A receptor, which, due to their function as an autoreceptor, may decrease overall 5-HT output [57-60]. The 5-HT1A receptor is coupled to a Gαi/o protein and when activated, results in an inhibition of adenylyl cyclase [56] and can also increase potassium conductance resulting in hyperpolarization of the cell [61].
Atypical antipsychotics are often the first line treatment for schizophrenia, but efficacy and tolerability varies greatly between drugs [21, 27, 28] and some result in severe side-effects [27, 28, 55]. Clozapine has been shown to be superior to haloperidol at treating both positive and negative symptoms [55, 62]. When patients are switched from typical antipsychotics to the atypical antipsychotics olanzapine and risperidone, improvements in working memory performance have been noted [63]. Unfortunately, these drugs, particularly olanzapine and clozapine, have been shown to result in metabolic syndrome, which are a cluster of side effects including weight gain and insulin resistance, often being so serious as to result in non-adherence to drug regimens [26, 27].
Newer atypical antipsychotics have been developed in an attempt to increase treatment efficacy and reduce side effects. Newer atypical antipsychotics include brexpiprazole, cariprazine, and aripiprazole, which bind to 5-HT receptors and also act as partial agonists at the D2 receptor [64-66]. These drugs differ from each other in DA and 5-HT receptor binding affinities, treatment efficacy, and side-effect profiles [66]. Carpiprazine has a stronger affinity for the D2 and 5-HT1A receptors compared to aripiprazole and brexpiprazole [64]. When schizophrenia patients are assessed on the Positive and Negative syndrome Scale (PANSS), aripiprazole treatment results in the greatest reduction of scores compared to brexpiprazole and cariprazine [67]. Aripiprazole has been demonstrated to have the lowest rate of EPS among Brexpiprazole and carpiprazine, whereas EPS is one of the most common symptoms with carpiprazine treatment [66]. The unique pharmacological profile of aripiprazole, and binding affinities for both DA and 5-HT receptors, may be an important factor in the clinical efficacy and side-effect profile of the drug [1, 27, 68].
3. ARIPIPRAZOLE
The goal in the development of aripiprazole was to regulate DA neurotransmission with minimal motor and metabolic side effects, which result from most typical and atypical antipsychotic treatments [1]. The United States Food and Drug Administration (FDA) approved the use of aripiprazole for the treatment of schizophrenia in November 2002 and approved aripiprazole in the treatment of acute mania in bipolar disorder in September 2004. The European Commission approved the use of Aripiprazole in June 2004, and in Canada, aripiprazole was approved for the treatment of schizophrenia symptoms and acute mania in July 2009.
3.1. Therapeutic Efficacy
Aripiprazole can be administered orally (5-30 mg/kg/day) or administered as a depot injection to facilitate patient compliance (400 mg/month) [69] with both methods resulting in similar steady-state serum concentration and symptom management [70, 71]. In humans, the half-life of aripiprazole after oral administration is approximately 60 hours [70]. With once-daily oral administration (usually at doses between 15 and 30 mg/day), steady serum-level concentrations can be reached following a 14-day period [70]. Following depot injection of aripiprazole, plasma concentrations gradually rise, reaching maximum in 5-7 days [2]. Serum concentrations between 150 and 300 ng/ml result in symptom improvement with minimal side effects [72]. The human cytochrome P450 (CYP) isozymes CYP3A4 and CYP2D6 are involved in the metabolism of aripiprazole to dehydroaripiprazole [2] via dehydrogenation, hydroxylation, and N-dealkylation [73]. The metabolism of aripiprazole can be reduced by the combination of aripiprazole with selective serotonin reuptake inhibitor (SSRI) treatments, such as paroxetine and fluvoxamine, due to the ability of these drugs to inhibit CYP2D and CYP3A4 [74]. These drugs have been shown to reduce clearance of aripiprazole, which is important because aripiprazole is often used in conjunction with SSRIs in the treatment of psychotic depression and mania in bipolar disorder [2]. Dose reductions have been recommended in individuals who are naturally CYP2D6 poor metabolizers and individuals receiving CYP2D6 or CYP3A4 inhibiting agents [2] but aripiprazole has been considered generally safe to use in conjunction with SSRI treatments [74].
Aripiprazole is effective in treating the positive and negative symptoms of schizophrenia when administered as an acute therapy or as maintenance therapy [75, 76]. Over the course of 4 weeks, aripiprazole (20-30mg/kg/day) is significantly more effective in reducing both positive and negative symptoms compared to placebo [75]. In maintenance therapy following acute relapse, aripiprazole is more effective in treating negative symptoms than haloperidol, but similarly effective in treating positive symptoms [77]. As a maintenance treatment for chronic schizophrenia symptoms (26-52 week trials), patients receiving aripiprazole show a lower rate of relapse than placebo, a longer time between relapses, and a higher survival rate [78]. When patients are switched to aripiprazole from other antipsychotics, such as haloperidol, thioridazine, olanzapine, or risperidone, symptom management (positive and negative) continues, and the drug is well tolerated [79]. Patients switched from oral aripiprazole to once-monthly injections have even shown signs of symptom improvement [80]. Aripiprazole has also been shown to be highly effective for management of positive and negative symptoms in treatment-resistant schizophrenia at 30 mg/day [81].
The efficacy of aripiprazole in the treatment of cognitive symptoms has been studied in comparison to olanzapine in 26-week trials [82, 83]. Several measures such as, the Wisconsin Card Sorting Task, the Benton Visual Retention Test, the California Verbal Learning Test, and the Grooved Pegboard were used to assess cognitive functions. Cornblatt and colleagues (2002) conducted a trial to study the effects of aripiprazole (30 mg/day) and Olanzapine (10 mg/day) on the cognitive symptoms of schizophrenia [82]. In terms of general cognitive functioning, the aripiprazole and olanzapine groups showed significant symptom improvement following 8 weeks of treatment compared to baseline measures, but this effect disappeared at 26 weeks. No significant differences were observed in the Wisconsin Card Sorting Task, but there was a significant improvement in secondary verbal memory at week 8 and 26 in aripiprazole treated patients compared to placebo and olanzapine [82]. A study by Kern and colleagues (2006) conducted a similar trial to study the effects of aripiprazole (30 mg/day) and Olanzapine (15 mg/day) on the cognitive symptoms of schizophrenia [83]. Both groups had improved general cognitive functioning above baseline scores that remained stable over the 26-week trial. In terms of executive function, the groups did not improve above baseline over the course of the trial. For verbal learning, the aripiprazole group improved above baseline and compared to olanzapine at week 8 and compared to baseline at week 26. The authors concluded that aripiprazole has at least a similar treatment efficacy as olanzapine in treating cognitive deficits in schizophrenia [83].
3.2. Side Effects
Aripiprazole displays a favorable side-effect profile compared to both typical and atypical antipsychotics, which often cause EPS or metabolic syndromes [84]. The drug is associated with a low incidence of EPS, weight gain, cardiovascular abnormalities, hyperprolactinemia, hypercholesterolemia, and glucose dysregulation [60, 73, 75, 84, 85]. A meta-analysis by Marder and colleagues (2003) determined that common side-effects that occur from aripiprazole administration include somnolence, nausea, vomiting, akathisia, and lightheadedness [84]. None of these adverse effects are considered common and do not appear to be dose dependent [84]. In terms of EPS, aripiprazole does not appear to differ from placebo at any dose, but the combination of haloperidol and aripiprazole may result in a slight increase in EPS incidence [84]. However, in children and adolescents aripiprazole has been associated with an increase in acute EPS, with an incidence of 17% [86]. There have also been single case reports of acute dystonia after a single dose of aripiprazole, but this was in patients suffering from cocaine dependence [87]. While at doses of 20 and 30 mg/day, aripiprazole is associated with a minimal increase in body weight of less than 1 kg, it is not considered clinically significant because it is not 7% greater than placebo [84]. Aripiprazole does not significantly alter serum prolactin concentrations and results in minimal changes in fasting glucose concentration [84]. Finally, aripiprazole is not associated with an increased risk of metabolic syndrome, as evidenced by two, 26-week, double blind, randomized control trials [84]. The long-acting injectable form of aripiprazole shares a similar side-effect profile, with generally mild side-effects [69].
3.3. Dopamine Receptor Activity
The ability of aripiprazole to bind to its target receptor is represented by the affinity of the drug, which can be represented numerically with Ki, where a lower value indicates higher affinity [3]. Aripiprazole has a low affinity for D1 and D5 receptors [10], a moderate affinity for D4 receptors [88, 89], and a relatively high affinity for D2 and D3 receptors [3, 10, 88]. The majority of antipsychotics, other than aripiprazole, are clinically active at approximately 65% D2 receptor occupancy and result in motor side effects when D2 receptor occupancy is greater than 80% [90, 91]. For aripiprazole to be clinically active, a D2 receptor occupancy of greater than ~ 90% is required, but the drug does not induce catalepsy at this occupancy [90], suggesting aripiprazole has a unique pharmacology compared to other marketed antipsychotics [90, 92]. The drug has been shown to have antagonistic activity at D1 receptors and a unique pharmacological profile at D2 receptors. Aripiprazole binds to both pre and postsynaptic D2 receptors, has been shown to display agonistic and antagonistic activity at these receptors, and is generally considered a partial D2 agonist, but may display functionally selective properties [3, 8, 88, 93-95]. As a partial D2 agonist Aripiprazole has a high affinity for the receptor, but the intrinsic activity of the drug is less than the natural ligand DA [1]. Partial D2 agonism would explain the high D2 occupancy required for symptom management and the low possibility of EPS. The partial agonism of aripiprazole at D2 receptors may be responsible for effective management of positive, negative, and cognitive symptoms of schizophrenia.
3.3.1. Evidence for Partial Dopamine Receptor Agonism
3.3.1.1. Locomotor Activity
The effectiveness of antipsychotics in managing the positive symptoms of schizophrenia has been attributed to D2 receptor antagonism [61]. Aripiprazole has demonstrated D2 receptor partial agonism behaviorally via reducing drug-induced hyperlocomotion, which can be used as a preclinical animal model of positive symptoms and mania [96, 97]. Aripiprazole has been shown to reverse hyperlocomotion induced by acute amphetamine administration (1.5 mg/kg) dose-dependently at 0.3, 1, and 3 mg/kg i.p. [98]. D-amphetamine has also been used to model behavioral symptoms of mania when given at a dose of 0.5 mg/kg i.p [99]. In this mania model, acute administration of aripiprazole (0.75, 1.5, and 2.5 mg/kg i.p.) reversed the d-amphetamine induced hyperlocomotion. Chronic administration of aripiprazole over 7 days (1.5 mg/kg i.p.) has also been shown to counteract hyperlocomotion induced by d-amphetamine [99]. When given orally (0.5, 1, 2, 4 and 8 mg/kg), which mimics the route of administration in humans, 8 hrs. prior to apomorphine (1.5 mg/kg s.c.), aripiprazole reduced apomorphine-induced hyperlocomotion in a dose-dependent manner [11]. The drug has also been shown to reduce locomotor activity induced by cocaine (5 mg/kg) at doses ranging from 0.1-1 mg/kg [100].
Administration of most typical and atypical antipsychotics results in a dose-dependent decrease in locomotor activity due to blockade of post synaptic D2 receptors within the striatum [101]. For the most part, aripiprazole does not affect locomotor activity in rodents, and when it does produce an effect, the result is not consistent with typical D2 receptor antagonists, such as haloperidol. In studies where aripiprazole was administered chronically at doses of 0.75 mg/kg 3 x day or 1.5 mg/kg 1 x day, aripiprazole did not significantly alter locomotor activity [102, 103]. Similarly, acute administration of aripiprazole in mice (0.1, 1, and 10 mg/kg i.p.) did not significantly affect locomotor activity [104]. A study by Zocchi and colleagues (2005) used acute aripiprazole administration (0.3 and 3 mg/kg not 0.1 and 30 mg/kg) and demonstrated hypolocomotion but only at the moderate doses [105]. This suggests that aripiprazole has a lower intrinsic efficacy for D2 receptor, because if it were simply an antagonist, aripiprazole should dose-dependently decrease locomotor activity on its own, in all instances.
3.3.1.2. Dopamine Receptor Sensitization
Treatment with typical antipsychotics has been shown to result in sensitization of DA receptors, via D2 antagonism, and result in an increase in D2 receptor density and autoreceptor sensitivity [106, 107]. The typical antipsychotic haloperidol, a potent D2 receptor antagonist, has been consistently shown to induce super sensitivity at D2 receptors following chronic administration [106]. Sensitization of D2 receptors may result from continued inhibition of adenylate cyclase activity, decreased formation of cAMP, and stimulation of GTPase activity, which is coupled to intracellular effector systems [106, 108, 109].
The ability of aripiprazole to induce D2 receptor sensitization/upregulation has produced different results depending on the age of the animal and length of administration, and has provided evidence that aripiprazole acts as either a D2 antagonist [103, 110] or partial agonist [111, 112]. Tadokoro and colleagues (2012) administered aripiprazole (1.5 mg/kg/day), haloperidol (0.75 mg/kg/day), or saline chronically for 14 or 28 days using a minipump and tested rats for locomotor activity following administration of methamphetamine [103]. Rats were also evaluated for D2 receptor binding 7 days following treatment with either aripiprazole or Haloperidol [103]. Following 14 days of treatment, there were no significant differences in locomotor activity (with the methamphetamine challenge) or striatal D2 receptor density between the saline and aripiprazole treated rats. Haloperidol treatment resulted in a marked increase in locomotor activity following the methamphetamine challenge and an increase in striatal D2 receptor density [103]. Koener and colleagues found a similar result where repeated administration (3 weeks) of aripiprazole at doses of 10 and 30 mg/kg did not result in upregulation or hyperfunctionality of D2 receptors within the striatum [110]. In the same study, aripiprazole administration did not alter sniffing and licking behaviors, whereas pure D2 agonists resulted in an increase, providing further evidence for the unique pharmacology of aripiprazole [113].
A study by Seeman and colleagues (2008) demonstrated an increase in high affinity DA D2 receptors following 7 days of subcutaneous aripiprazole (1.5 mg/kg) treatment; although, the increase in D2 receptors was more pronounced in Haloperidol treated rats [111]. The authors concluded that aripiprazole may not display antagonistic properties at the D2 receptor, but rather display partial agonist activity [111]. Because young patients have been shown to be more susceptible to aripiprazole induced side effects, such as a higher incidence of EPS [114], studies have been undertaken in young rats to determine D2 receptor up-regulation and DA super sensitivity using a challenge injection of amphetamine at 1, 4, or 8 days following 10 days of aripiprazole treatment (10 mg/kg/day) [112]. Chronic treatment with aripiprazole and haloperidol resulted in supersensitive behaviors (locomotor activity) when rats were given a high (4 mg/kg) dose of amphetamine, although the effect with haloperidol was more robust. Chronic treatment with haloperidol and aripiprazole combined also increased D2 receptor density [112].
3.3.1.3. Effect on DA Release
The acute effects of typical and atypical antipsychotics on DA release within the mesocorticolimbic and nigrostriatal DA systems have been studied using in vivo microdialysis (Table 1). Moghaddam and Bunney (1990) studied the effect of sulpiride (20, 50, and 100 mg/kg i.v.), haloperidol (0.1 and 0.5 mg/kg i.v.), and clozapine (5 and 10 mg/kg) on DA release within the PFC, NAc, and striatum in the rat [115]. The typical antipsychotics sulpiride and haloperidol increased DA release in the striatum and NAc. In the mPFC, sulpiride did not significantly affect extracellular DA, but haloperidol increased DA release at the 0.5 mg/kg dose. Clozapine increased the extracellular concentration of DA in the striatum, NAc, and mPFC, with a more pronounced increase in the mPFC compared to the typical antipsychotics [115]. A study by Imperato and Chiara (1985) found similar results when studying typical antipsychotics, where haloperidol (0.012 - 0.5 mg/kg s.c.) and sulpiride (2.5 -100 mg/kg s.c.) increased DA release within the striatum [116]. The authors also found an increase in DA metabolites, DOPAC and HVA, following administration of haloperidol and sulpiride [116].
Table 1.
DA release following antipsychotic administration compared to aripiprazole.
The effect of aripiprazole on DA release within the mesocorticolimbic and nigrostriatal DA systems, as evidenced by microdialysis studies, differs from DA release that results from typical and atypical antipsychotic administration. In the striatum and NAc, DA is decreased at 3 and 40 mg/kg but does not change at 2.5 mg/kg and lower, although DA metabolites have been shown to increase at lower doses [12, 117, 118]. In the mPFC, DA is increased at 0.3 and 0.5 mg/kg, whereas a higher dose of 10 mg/kg has been associated with a decrease [105, 117, 118]. Semba and colleagues (1995) attributed the difference between the DA metabolites and DA release to the ability of aripiprazole to have both agonist and antagonistic properties at pre and post synaptic DA receptors [12]. It is plausible that the decrease in DA release within the NAc and PFC following higher doses of aripiprazole results from partial agonism at DA receptors, and that the increase in DA release at lower doses results from activity at 5-HT1A receptors, which will be discussed later.
3.3.2. Effects of Aripiprazole in an MK-801 Animal Model of Schizophrenia Symptoms
The efficacy of aripiprazole in treating positive, negative, and cognitive symptoms may be attributed to stabilization of the DA system, which has been assessed in an MK-801 animal model of schizophrenia. Administration of MK-801 has been used to provide a putative model of schizophrenia symptoms via NMDA receptor hypofunction, which is one of the hypotheses surrounding the etiology of the disease [119]. MK-801 is a non-competitive NMDA receptor antagonist that binds to the PCP site within the ion channel [120]. MK-801 has been shown to increase DA output [121] in the NAc [122, 123], Striatum [124], and PFC [125]. The increase in DA is suggested to result from increased firing in mesolimbic DA neurons in the VTA [126]. Behaviorally, MK-801 can be used to produce positive symptoms (hyperlocomotion, PPI), negative symptoms (social withdrawal), and cognitive dysfunction (executive function deficits) [122, 123, 127, 128].
Rats treated with MK-801 prior to operant extinction show a persistently elevated bar pressing response in the absence of reward compared to controls; a response that has been argued to be similar to a deficit in executive function [123, 127, 128]. Acute administration of 1 mg/kg, but not 0.3 or 3 mg/kg, of aripiprazole significantly reduced the MK-801-induced elevated bar pressing during extinction to that of saline controls [129]. Aripiprazole (1 and 3 mg/kg) also decreased the MK-801-induced hyperlocomotion, which is consistent with previous reports that aripiprazole can reduce drug-induced hyperlocomotion [129]. The fact that the moderate dose of MK-801 reduced bar pressing and hyperlocomotion suggests aripiprazole has the potential to alleviate both positive and cognitive symptoms at the same dose. This may occur via stabilization of the DA system by increasing DA within the mPFC and decreasing DA within the NAc.
Aripiprazole has also shown promise in reducing MK-801-induced deficits in pre-pulse inhibition (PPI) [130]. PPI is a behavioral procedure that measures sensorimotor gating in rodents [131]. Generally, a burst of noise will result in a muscle twitch defined as an acoustic startle response, which can be reduced when the noise is preceded by a milder stimulus call a pre-pulse, which does not result in a startle response on its own [131]. Patients with schizophrenia are observed to have PPI deficits, where the startle response is higher after a pre-pulse in patients compared to controls [132, 133]. Treatment with MK-801 has been shown to result in a higher startle response compared to saline controls, suggesting MK-801 induces PPI deficits [130]. Aripiprazole, at a dose of 4.0 mg/kg, but not at lower doses, has been shown to significantly reverse the MK-801-induced PPI deficit in rats [130]. Aripiprazole, on its own, has not been shown to affect baseline PPI responding [134]. In rodents, atypical, but not typical, antipsychotics have been shown to reverse PPI deficits induced by MK-801 [135, 136]. This may be the result of drug activity at both 5-HT1A receptors and D2 receptors, which are suggested to be involved in sensorimotor gating in schizophrenia patients [137]. Aripiprazole may reverse MK-801-induced PPI deficits via action at 5-HT and/or DA receptors due to its similar actions at 5-HT receptors compared to atypical antipsychotics.
Social withdrawal is a common negative symptom of schizophrenia and generally manifests with patients living alone, and having few social contacts or social interactions [138]. MK-801 and PCP, another NMDA receptor antagonist, have been used to produce social withdrawal in rodents, argued to reflect negative symptoms [139-141]. In MK-801 and PCP treated rats, social interaction can be measured by defining the proximity between two animals, which is the time spent at a defined distance apart (e.g. within 20 cm) [139]. Social withdrawal is represented in MK-801 or PCP treated animals that spend less time in close proximity to another rodent compared to saline treated controls [139]. Aripiprazole, at doses of 0.04 mg/kg and 0.16 mg/kg i.p., has been shown to significantly reverse social interaction deficits induced by PCP and increase time spent in close proximity [141]. This improvement in social interaction was reversed by the use of WAY100635, a 5-HT1A antagonist, suggesting the importance of 5-HT1A receptors in the treatment efficacy of aripiprazole [141]. Snigdha and colleagues (2008) also found that aripiprazole (5 mg/kg s.c.) could reverse PCP induced deficits in social behaviors, including avoidance, which is increased following PCP [142]. Deiana and colleagues (2015) investigated the effect of aripiprazole on MK-801 induced social withdrawal and deficits in social recognition memory [140]. Aripiprazole, at doses of 2 and 10 mg/kg i.p., did not affect MK-801 induced social interaction but improved the MK-801-induced decrease in social recognition. Social recognition was defined as time spent near a clear pyramid housing a stranger rat, which previously held a different rat for the social interaction test [140]. In the same study, olanzapine, an atypical antipsychotic had no effect on social interaction or social recognition [140]. The authors suggested that the combined activity of aripiprazole on 5-HT1A receptors and D2 receptors may contribute to the improvements in social deficits [140, 141].
Three main pharmacological hypotheses have been developed to explain how aripiprazole treats positive, negative, and cognitive symptoms with minimal side-effects. The hypotheses focus on DA system stabilization via partial agonism, functional selectivity at the postsynaptic D2 receptor, and DA-5-HT system interactions.
3.3.3. Dopamine System Stabilization
The effect of aripiprazole on locomotor activity and animal models of schizophrenia provides evidence of DA system stabilization. Stabilization within the DA system from antipsychotic treatments can be accomplished by balancing the activity of pre and postsynaptic D2 receptors, but stabilization may also depend on binding affinities, distribution, density, and receptor coupling [6]. DA system stabilizers are suggested to bind to D2 receptors and have either an agonist or antagonist effect depending on the levels of exogenous DA [3, 6]. As a DA system stabilizer, aripiprazole would act to reduce DA effects in regions with high DA activity and elevate DA effects in regions with low DA activity. The ability of a drug to stabilize the DA system in patients with schizophrenia is an optimal goal [5, 6].
Supporting evidence for the DA system stabilization effects of aripiprazole comes from PET studies in humans. In a study by Ito and colleagues (2012), individuals were given a single oral dose of aripiprazole (3, 6 or 9 mg) and PET images were collected using 11C raclopride and L-(β-11C) DOPA under resting conditions [143]. Regions of interest included the cerebellar cortex, putamen, caudate head, and occipital cortex. Aripiprazole occupancy of D2 receptors increased in a dose-dependent fashion but there were no significant changes in DA synthesis and no relationship between D2 receptor occupancy and DA synthesis [143]. There was a negative correlation between baseline DA synthesis and aripiprazole, which suggests aripiprazole can cause an increase in DA synthesis with a low baseline DA and a decrease DA synthesis with high baseline DA [143]. The authors suggested that the results indicate the therapeutic effects of aripiprazole may result from stabilization of DA neurotransmission [143]. A previous report using PET studies also suggested that partial DA agonism may explain minimal EPS in patients treated with aripiprazole [144].
There is evidence that aripiprazole functions as both a presynaptic D2 agonist and post synaptic D2 antagonist [7, 99, 100]. Presynaptic D2 autoreceptors may play a vital role in the ability of aripiprazole to act as a DA system stabilizer, but at higher doses (such as the ones used to treat schizophrenia), aripiprazole also has a significant binding affinity at post synaptic D2 receptors [145]. A study by Ma and colleagues (2015) investigated the agonist and antagonist effects of aripiprazole on D2 like receptors by measuring DA synthesis [7]. Under basal conditions, aripiprazole acted as a D2 autoreceptor agonist at 10nM as evidenced by the ability to inhibit DA synthesis in a similar manner to quinpirole [7]. Aripiprazole has also been successful at reducing reserpine induced increases in DOPA accumulation in the rodent forebrain, and at the same dose was able to inhibit apomorphine induced hyperlocomotion [11]. Semba and colleagues (1995) found a similar result where aripiprazole decreased the DA increase induced by the DA autoreceptor antagonist (+)-AJ76 and at the same dose, decreased apomorphine induced hyperlocomotion [12]. Kikuchi and colleagues (1995) suggested that aripiprazole acts as a DA agonist at presynaptic autoreceptors decreasing DA release, and as an antagonist at postsynaptic D2 receptors at higher doses. Presynaptic agonist activity was evidenced by a decrease in tyrosine hydroxylase activity following aripiprazole administration and the drug displayed post synaptic D2 antagonism by inhibiting apomorphine induced hyperlocomotion [11]. These results suggest aripiprazole may act as a presynaptic D2 agonist and a postsynaptic D2 antagonist, which could account for the high treatment efficacy in the absence of significant EPS [7, 11, 12].
Receptor density may play a role in the ability of aripiprazole to act as a presynaptic receptor agonist and a post synaptic receptor antagonist [3]. Evidence for this comes from in vitro studies where aripiprazole affected cAMP accumulation differentially depending on DA receptor location and density [3, 88]. A study by Lawler and colleagues (1999) investigated differences in cAMP accumulation following administration of DA and aripiprazole in CHO vs C-6 cell lines containing D2L receptors [88]. The study employed the use of forskolin to increase cAMP levels in DA transfected cells in the CHO cell line. This was followed by DA or aripiprazole administration to determine any changes in cAMP levels [88]. In the CHO cell line, DA, on its own, produced a 50% inhibition in cAMP levels, whereas aripiprazole did not inhibit cAMP induced by forskolin, suggesting antagonistic activity [88]. In the C-6 cell line, where isoproterenol was used to stimulate cAMP, aripiprazole appeared to display partial agonist activity by partially reducing cAMP synthesis [88]. The two main differences between the cell lines were the density of D2L receptors, which was three to four times higher in the CHO cells compared to the C-6 cells, and the use of forskolin in the CHO cell line and isoproterenol in the C-6 line to stimulate cAMP [88]. The authors suggested the density of D2L may be a factor in the actions of aripiprazole [88]. In a similar study, Burris and colleagues (2002) investigated the effect of aripiprazole on cAMP formation in CHO cells containing D2L receptors [3]. Aripiprazole inhibited cAMP accumulation induced by forskolin, which did not occur in Lawler et al., 1999 [3, 88]. Aripiprazole displayed partial agonist activity evidenced by inhibition of cAMP, and the blockade of DA action as aripiprazole concentration was increased [3]. The ability of aripiprazole to reduce cAMP accumulation was reduced by a decreased receptor reserve, suggesting receptor reserve plays a role in the pharmacological actions of aripiprazole [3]. The authors suggested that when receptor reserve is low, partial agonists, such as aripiprazole, act as antagonists and that when receptor reserves are high, partial agonists display high efficacy values [3]. A higher receptor density for D2S receptors would have aripiprazole acting as an agonist at presynaptic autoreceptors and a lower receptor density for D2L receptors would have aripiprazole acting as an antagonist at post synaptic receptors [146]. This would coincide with Meller and colleagues (1987, 1991) and Enz and colleagues (1990), where it was reported that presynaptic D2 autoreceptors have a large receptor reserve and post synaptic D2 receptors are lacking reserves [147-149].
The ability of aripiprazole to manage positive, negative, and cognitive symptoms with minimum side-effects may result from stabilization within the mesolimbic and mesocortical DA systems [5, 6]. Since aripiprazole is a partial D2 agonist, it would decrease DA activity within the mesolimbic system by binding primarily to autoreceptors as an agonist and secondarily, as an antagonist on postsynaptic receptors. This would, in effect, reduce the positive symptoms in schizophrenia. At the same time, the drug would increase DA activity in regions with decreased DA activity, such as the mesocortical DA system in schizophrenia, by acting as a partial agonist [5, 6]. Enhanced activation in the dorsolateral PFC has been shown using fMRI techniques following acute aripiprazole administration [150] This could, in effect, reduce the negative and cognitive symptoms in schizophrenia.
3.3.4. Functional Selectivity
It has been suggested that aripiprazole displays functional selectivity at the post-synaptic D2 receptor [151]. Functionally selective drugs have the ability to activate intracellular pathways and decrease symptoms without activating pathways that result in detrimental side effects [152]. The most prominent hypothesis for the etiology of schizophrenia has been that the disease results from a dysfunction within the DA system [34], which is targeted with antipsychotics drugs. However, intracellular signaling pathways that are the target of numerous receptors have shown alterations in schizophrenia [153-155]. For example: decreased expression and phosphorylation of mitogen-activated protein kinases (MAPK) and cAMP associated signaling molecules has been demonstrated in postmortem cortical analyses from schizophrenia patients [154].
Urban and colleagues (2007) investigated the functionally selective actions of aripiprazole on D2 receptor mediated pathways, specifically the D2L receptor, which primarily consists of postsynaptic D2 receptors, and its binding properties [95] (Fig. 1) (Table 2). Downstream, intracellular effects of D2 receptor mediated actions including MAPK phosphorylation, liberation of arachidonic acid (AA), and D2 receptor internalization were examined. Aripiprazole (10µm) partially activated the MAPK and AA pathways, and unlike typical agonists, did not result in D2L internalization [8]. A study by Brust and colleagues (2015) provided further evidence for the functional selectivity of aripiprazole at the D2 receptor by showing that aripiprazole can have agonistic and antagonistic effects on DA receptor signaling pathways [156]. The study explored the ability of aripiprazole to activate/inactivate G(α) signaling, which is involved in the inhibition of cAMP [38] and G(βγ) signaling, which can affect calcium and potassium channels and also activate/deactivate cAMP [38]. Aripiprazole acted as a partial agonist for G(α) activation, resulting in 48% of the response of the natural DA ligand. G(βγ) signaling was not affected by aripiprazole administration, and when given with DA, aripiprazole inhibited DA induced G(βγ) signaling, suggesting the drug acted as an antagonist [156].
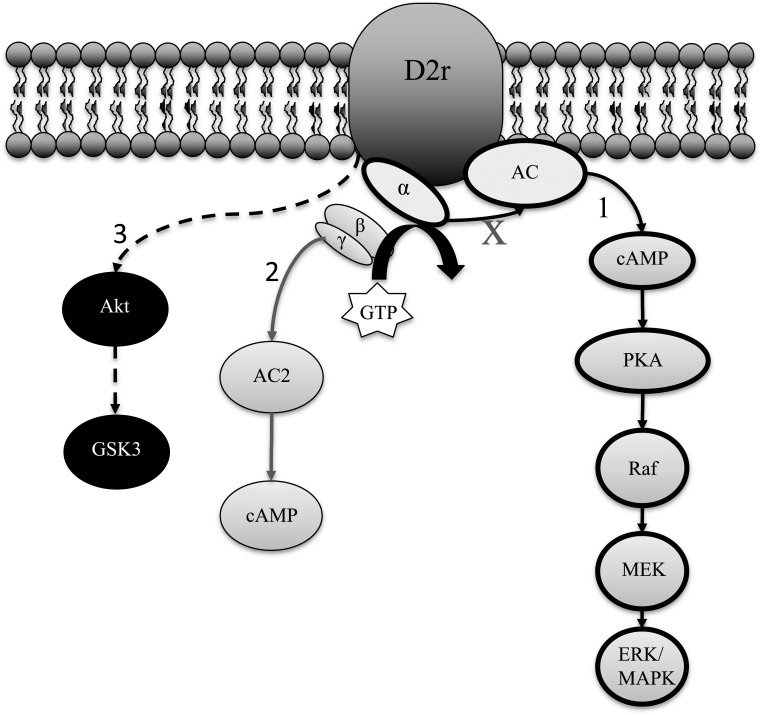
Fig. (1).
Examples of aripiprazole functional selectivity at the D2r: 1) Aripiprazole partially inhibits the G(α) pathway [156], 2) acts as an antagonist at the G(βγ) pathway [156], 3) and acts as an agonist by increasing phosphorylation of GSK3 in the PFC [151].
Table 2.
Effect of aripiprazole on D2 receptor signaling.
Antipsychotic treatments also target G-protein independent signaling pathways, which have a slower onset and longer duration compared to G-protein coupled signaling [157]. For example: the 5-HT1A receptor and D2 receptor target GSK3 [158, 159], a protein kinase which was first associated with psychiatric disease in the 90’s when it was shown to be inhibited by lithium [160]. Current antipsychotics such as clozapine and olanzapine have been shown to increase phosphorylation of Glycogen Synthase Kinase 3 (GSK3) in a mouse brain [161,162]. Agonist activity at both D2 and 5-HT1A receptors has been shown to increase GSK3 in the cortex, whereas antagonism results in a decrease [156, 158, 161, 163]. Agonist activity at D1 and 5-HT2A receptors has the opposite effect where GSK3 activity is inhibited in the FC [158, 161, 164]. An example of a GSK3 pathway is the G-protein independent signaling pathway involving β-arrestins, protein kinase B (Akt), and GSK3 [165]. When active, the protein kinase Akt can phosphorylate GSK3, which is necessary for inhibition of GSK3, a kinase that is naturally active [165]. In schizophrenia, lower levels of GSK3 activity have been found during postmortem studies in the cortex [166, 167].
Altering levels of GSK3 have been shown to affect behavior in animal models, particularly by affecting drug induced hyperlocomotion [163, 168-170]. Mice with a GSK3 knockout show a reduced locomotor response to both cocaine and Apomorphine but display normal baseline locomotor activity [168]. Inhibitors of GSK3 have been tested in DAT-knockout mice and amphetamine treated animals where they have been shown to reduce locomotor hyperactivity [163, 169]. GSK-3β heterozygote mice are less responsive to amphetamine suggesting an involvement of GSK-3 in DA related behaviors [163]. Further, mice that are mutants lacking an inhibitory GSK-3β phosphorylation site develop locomotor hyperactivity similar to that seen in DAT-knockout mice [170].
Further support for the functional selectivity hypothesis of aripiprazole comes from studies that compared the effect of aripiprazole (0.75 mg/kg i.p.) on the downstream targets of DA receptor signaling [151]. Levels of cAMP, PKA, Akt, and GSK3(β) were examined in tissue from the VTA, PFC, NAc, substantia nigra (SN), and caudate putamen (CPu). Within the PFC and NAc aripiprazole significantly increased levels of GSK3(β) [151, 171], but did not significantly alter cAMP, protein kinase A (PKA), or Akt [151]. Within the CPu, aripiprazole significantly elevated PKA expression [151, 172] and GSK3(β), whereas within the VTA, aripiprazole increased PKA expression, but not GSK3(β) expression [151]. In the SN, aripiprazole increased Akt levels and GSK3(β) phosphorylation, but did not affect other downstream targets [151]. GSK3 has also been found to be increased in human cell lines infused with aripiprazole, such as SH-SY5Y [167]. There were no significant effects on cAMP accumulation recorded in any brain region following aripiprazole administration. The authors suggested that aripiprazole may have a low intrinsic activity at the postsynaptic D2 receptor, and display functionally selective properties, as there were differential effects depending on brain region [151, 172]. One interesting side note from these findings is that GSK3 has been reported to be reduced in the PFC of patients with schizophrenia [166, 167]. Since aripiprazole has been shown to result in elevated GSK3 expression within the PFC [151, 171], it may contribute to the management of negative and cognitive symptoms.
The functional selectivity hypothesis suggests that it may be beneficial to develop therapeutic agents that target specific intracellular signaling pathways. For example: GSK3 has been suggested as a potential therapeutic target for future antipsychotic agents [173]. However, Lithium has limited effects on schizophrenia symptoms suggesting that abnormal GSK3 may only play a role in a subset of symptoms [153]. More studies into the role of intracellular signaling pathways in antipsychotic treatments may be necessary for improved treatment efficacy with minimal side-effects.
3.3.5. Serotonin Modulation of Dopamine
Like atypical antipsychotics, the pharmacological actions of aripiprazole are not limited to DA receptors. Aripiprazole occupancy at clinically effective doses for 5-HT receptors is considerably lower compared to D2 occupancy (~90%) at 54-60% for 5-HT2A receptors and 16% for 5-HT1A receptors [174]. Aripiprazole has the highest binding affinity for the 5-HT2B receptor, a significant binding affinity for the 5-HT1A, 5-HT2A, 5-HT2C, and 5-HT7 receptors, and a relatively low affinity for the 5-HT1B, 5-HT3, 5-HT5A, and 5-HT6 receptors [10, 73]. Aripiprazole is a partial agonist at the 5-HT1A receptor and an antagonist at the 5-HT2A, 5-HT2B, and 5-HT6 receptors [10]. The partial agonistic activity of aripiprazole at the 5-HT1A receptor has been suggested to play a role in modulating DA release, suggesting aripiprazole may be a DA-5-HT system stabilizer [3, 4].
Some of the ability of atypical antipsychotics to manage negative and cognitive symptoms has been attributed to 5-HT receptor activity, possible due to actions within the PFC [31] 5-HT receptors, in particular the 5-HT1A and 5-HT2A receptors, have the ability to modulate DA release in the cortex [175]. The 5-HT2A and 5-HT1A receptors are in high concentrations in the cortex [176] and are found on pyramidal glutamatergic neurons [177, 178] as well as GABAergic interneurons [179]. Antipsychotics that are 5-HT1A agonists, such as clozapine, have been shown to increase DA release in the mPFC, an effect that has been diminished by use of the 5-HT1A antagonist WAY100635 [175]. Since there is a high concentration of 5-HT receptors in the PFC [176], the atypical antipsychotics may have the ability to increase DA concentration via 5-HT receptors [31, 175].
It has been suggested that the DA increase in the mPFC following systemic administration of low doses of aripiprazole (0.3 – 0.5 mg/kg) may be mediated by 5-HT1A receptors. 5-HT itself has not been shown to increase in the PFC following systemic aripiprazole administration (0.1 – 30 mg/kg) [105]. To explore the role of the 5-HT1A receptor in the action of aripiprazole, studies have co-administered the 5-HT1A antagonist WAY100635 in an attempt to reverse the aripiprazole induced DA increase seen in the mPFC [117]. Following co-administration of aripiprazole (0.3-0.5 mg.kg) and WAY100635, DA release in the mPFC was reduced compared to aripiprazole administration on its own [117, 118]. This suggests that the agonistic activity of aripiprazole at the 5-HT1A receptor may be responsible for the DA increase in the PFC, and perhaps the alleviation of cognitive and negative symptoms [31].
CONCLUSION
Symptom management in schizophrenia is difficult due to the wide range of symptoms that can accompany the disease. Previous typical antipsychotics have a poor treatment efficacy for negative and cognitive symptoms and have the potential to result in severe side-effects, such as EPS. Atypical antipsychotics have an improved capacity to treat all aspects of schizophrenia, but also have the potential for EPS and metabolic syndromes. The efficacy of aripiprazole in treating the positive, negative, and cognitive symptoms in schizophrenia, with minimal side effects, is due to a unique pharmacological profile. Stabilization of the DA system, functional selectivity at the postsynaptic D2 receptor, and DA-5-HT interactions have all been implicated in the efficacy of the drug. A combination of these factors, and appropriate dosing, may all contribute to the successful management of schizophrenia symptoms.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTs
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hirose T., Kikuchi T. Aripiprazole, a novel antipsychotic agent: dopamine D2 receptor partial agonist. J. Med. Invest. 2005;52(Suppl.):284–290. doi: 10.2152/jmi.52.284. [http://dx.doi.org/10.2152/jmi.52.284]. [PMID: 16366516]. [DOI] [PubMed] [Google Scholar]
- 2.(aripiprazole), A. M. U. Full Prescribing Information, Otsuka Pharmaceutical Co. Ltd, Tokyo, Japan. [Google Scholar]
- 3.Burris K.D., Molski T.F., Xu C., Ryan E., Tottori K., Kikuchi T., Yocca F.D., Molinoff P.B. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 2002;302(1):381–389. doi: 10.1124/jpet.102.033175. [http://dx. doi.org/10.1124/jpet.102.033175]. [PMID: 12065741]. [DOI] [PubMed] [Google Scholar]
- 4.Jordan S., Koprivica V., Chen R., Tottori K., Kikuchi T., Altar C.A. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur. J. Pharmacol. 2002;441(3):137–140. doi: 10.1016/s0014-2999(02)01532-7. [http://dx.doi.org/10.1016/S0014-2999(02)01532-7]. [PMID: 12063084]. [DOI] [PubMed] [Google Scholar]
- 5.Stahl S.M. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J. Clin. Psychiatry. 2001;62(12):923–924. doi: 10.4088/jcp.v62n1201. [http://dx.doi. org/10.4088/JCP.v62n1201]. [PMID: 11780870]. [DOI] [PubMed] [Google Scholar]
- 6.Stahl S.M. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J. Clin. Psychiatry. 2001;62(11):841–842. doi: 10.4088/jcp.v62n1101. [http://dx.doi.org/10.4088/JCP.v62n1101]. [PMID: 11775041]. [DOI] [PubMed] [Google Scholar]
- 7.Ma G.F., Raivio N., Sabria J., Ortiz J. Agonist and antagonist effects of aripiprazole on D2-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tonE. Int. J. Neuropsychopharmacol. 2015;18(4):1–9. doi: 10.1093/ijnp/pyu046. [http://dx.doi.org/10.1093/ ijnp/pyu046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban J.D., Vargas G.A., von Zastrow M., Mailman R.B. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32(1):67–77. doi: 10.1038/sj.npp.1301071. [http://dx.doi.org/10.1038/sj.npp.1301071]. [PMID: 16554739]. [DOI] [PubMed] [Google Scholar]
- 9.Davies M.A., Sheffler D.J., Roth B.L. Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 2004;10(4):317–336. doi: 10.1111/j.1527-3458.2004.tb00030.x. [http://dx.doi.org/10.1111/ j.1527-3458.2004.tb00030.x]. [PMID: 15592581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro D.A., Renock S., Arrington E., Chiodo L.A., Liu L-X., Sibley D.R., Roth B.L., Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi: 10.1038/sj.npp.1300203. [http://dx. doi.org/10.1038/sj.npp.1300203]. [PMID: 12784105]. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi T., Tottori K., Uwahodo Y., Hirose T., Miwa T., Oshiro Y., Morita S. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl] butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J. Pharmacol. Exp. Ther. 1995;274(1):329–336. [PMID: 7616416]. [PubMed] [Google Scholar]
- 12.Semba J., Watanabe A., Kito S., Toru M. Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology. 1995;34(7):785–791. doi: 10.1016/0028-3908(95)00059-f. [http://dx.doi.org/10.1016/0028-3908(95)00059-F]. [PMID: 8532145]. [DOI] [PubMed] [Google Scholar]
- 13.de Bartolomeis A., Tomasetti C., Iasevoli F. Update on the mechanism of action of aripiprazole: Translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs. 2015;29(9):773–799. doi: 10.1007/s40263-015-0278-3. [http://dx.doi.org/10.1007/s40263-015-0278-3]. [PMID: 26346901]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rössler W., Salize H.J., van Os J., Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 2005;15(4):399–409. doi: 10.1016/j.euroneuro.2005.04.009. [http://dx.doi.org/10.1016/ j.euroneuro.2005.04.009]. [PMID: 15925493]. [DOI] [PubMed] [Google Scholar]
- 15.The ICD-10 classification of mental and behavioural disorders. Int. Classif. 1992;10:1–267. [Google Scholar]
- 16.DSM-V. Diagnostic and Statistical Manual of mental disorders. 5th ed. 2013. [DOI] [PubMed] [Google Scholar]
- 17.Owoso A., Carter C.S., Gold J.M., MacDonald A.W., III, Ragland J.D., Silverstein S.M., Strauss M.E., Barch D.M. Cognition in schizophrenia and schizo-affective disorder: impairments that are more similar than different. Psychol. Med. 2013;43(12):2535–2545. doi: 10.1017/S0033291713000536. [http://dx.doi.org/10.1017/S0033291713000536]. [PMID: 23522057]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichenberg A. The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin. Neurosci. 2010;12(3):383–392. doi: 10.31887/DCNS.2010.12.3/areichenberg. [PMID: 20954432]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shallice T., Burgess P., Robertson I. The domain of supervisory processes and temporal organization of behaviour. 1996. [DOI] [PubMed]
- 20.McGrath J., Saha S., Chant D., Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [http://dx.doi.org/10.1093/epirev/ mxn001]. [PMID: 18480098]. [DOI] [PubMed] [Google Scholar]
- 21.Tandon R., Nasrallah H.A., Keshavan M.S. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr. Res. 2010;122(1-3):1–23. doi: 10.1016/j.schres.2010.05.025. [http://dx.doi.org/10.1016/ j.schres.2010.05.025]. [PMID: 20655178]. [DOI] [PubMed] [Google Scholar]
- 22.Häfner H., an der Heiden W., Behrens S., Gattaz W.F., Hambrecht M., Löffler W., Maurer K., Munk-Jørgensen P., Nowotny B., Riecher-Rössler A., Stein A. Causes and consequences of the gender difference in age at onset of schizophrenia. Schizophr. Bull. 1998;24(1):99–113. doi: 10.1093/oxfordjournals.schbul.a033317. [http://dx.doi.org/10.1093/ oxfordjournals.schbul.a033317]. [PMID: 9502549]. [DOI] [PubMed] [Google Scholar]
- 23.Angermeyerl M.C., Kiihn L. Sciences Gender Differences in Age at Onset of Schizophrenia. Eur. Arch. Psychiatry Neurol. Sci. 1988;237:351–364. doi: 10.1007/BF00380979. [http://dx.doi.org/10.1007/BF00380979]. [PMID: 3053193]. [DOI] [PubMed] [Google Scholar]
- 24.Tandon R., Jibson M.D. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Ann. Clin. Psychiatry. 2002;14(2):123–129. doi: 10.1023/a:1016811222688. [http://dx.doi.org/10.3109/ 10401230209149099]. [PMID: 12238737]. [DOI] [PubMed] [Google Scholar]
- 25.Saeedi H., Remington G., Christensen B.K. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr. Res. 2006;85(1-3):222–231. doi: 10.1016/j.schres.2006.03.033. [http://dx.doi.org/10.1016/j.schres. 2006.03.033]. [PMID: 16679001]. [DOI] [PubMed] [Google Scholar]
- 26.Divac N., Prostran M., Jakovcevski I., Cerovac N. Generation Antipsychotics and Extrapyramidal Adverse Effects. Biomed Res. Int. 2014:1–6. doi: 10.1155/2014/656370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leucht S., Cipriani A., Spineli L., Mavridis D., Orey D., Richter F., Samara M., Barbui C., Engel R.R., Geddes J.R., Kissling W., Stapf M.P., Lässig B., Salanti G., Davis J.M. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [http://dx.doi.org/10.1016/S0140-6736(13)60733-3]. [PMID: 23810019]. [DOI] [PubMed] [Google Scholar]
- 28.Leucht S., Corves C., Arbter D., Engel R.R., Li C., Davis J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [http://dx.doi.org/10.1016/S0140-6736(08)61764-X]. [PMID: 19058842]. [DOI] [PubMed] [Google Scholar]
- 29.Bailey K.P. Pharmacologic agents for the treatment of schiozophrenia: similarities and differences. J. Am. Psychiatr. Nurses Assoc. 1996;2(5):181–185. [http://dx.doi.org/10.1177/ 107839039600200511]. [Google Scholar]
- 30.Meltzer H.Y. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology (Berl.) 1989;99(S1) Suppl.:S18–S27. doi: 10.1007/BF00442554. [http://dx.doi.org/10.1007/BF00442554]. [PMID: 2682729]. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer H.Y., Li Z., Kaneda Y., Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(7):1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [http://dx. doi.org/10.1016/j.pnpbp.2003.09.010]. [PMID: 14642974]. [DOI] [PubMed] [Google Scholar]
- 32.Keefe R.S., Bilder R.M., Davis S.M., Harvey P.D., Palmer B.W., Gold J.M., Meltzer H.Y., Green M.F., Capuano G., Stroup T.S., McEvoy J.P., Swartz M.S., Rosenheck R.A., Perkins D.O., Davis C.E., Hsiao J.K., Lieberman J.A. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [http://dx.doi.org/10.1001/archpsyc.64.6.633]. [PMID: 17548746]. [DOI] [PubMed] [Google Scholar]
- 33.Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1(2):133–152. doi: 10.1002/syn.890010203. [http://dx.doi.org/ 10.1002/syn.890010203]. [PMID: 2905529]. [DOI] [PubMed] [Google Scholar]
- 34.Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr. Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [http://dx.doi.org/10.1093/schbul/sbp006]. [PMID: 19325164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meltzer H.Y., Stahl S.M. The dopamine hypothesis of schizophrenia: a review. Schizophr. Bull. 1976;2(1):19–76. doi: 10.1093/schbul/2.1.19. [http://dx. doi.org/10.1093/schbul/2.1.19]. [PMID: 779020]. [DOI] [PubMed] [Google Scholar]
- 36.Young D., Scoville W. Paranoid psychosis in narcolepsy and the possible danger of benzedrine treatment. Med. Clin. North Am. 1938;22:637–646. [http://dx.doi.org/10.1016/S0025-7125(16) 37027-4]. [Google Scholar]
- 37.Groves P.M., Rebec G.V. Biochemistry and behavior: some central actions of amphetamine and antipsychotic drugs. Annu. Rev. Psychol. 1976;27(27):91–127. doi: 10.1146/annurev.ps.27.020176.000515. [http://dx.doi.org/10.1146/annurev. ps.27.020176.000515]. [PMID: 773267]. [DOI] [PubMed] [Google Scholar]
- 38.Beaulieu J.M., Gainetdinov R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [http://dx.doi.org/10.1124/pr.110.002642]. [PMID: 21303898]. [DOI] [PubMed] [Google Scholar]
- 39.Carlsson A., Waters N., Carlsson M.L. Neurotransmitter interactions in schizophrenia--therapeutic implications. Biol. Psychiatry. 1999;46(10):1388–1395. doi: 10.1016/s0006-3223(99)00117-1. [http://dx.doi.org/10.1016/S0006-3223 (99)00117-1]. [PMID: 10578453]. [DOI] [PubMed] [Google Scholar]
- 40.Tamminga C.A., Holcomb H.H. Phenotype of schizophrenia: a review and formulation. Mol. Psychiatry. 2005;10(1):27–39. doi: 10.1038/sj.mp.4001563. [http://dx.doi.org/10.1038/sj.mp.4001563]. [PMID: 15340352]. [DOI] [PubMed] [Google Scholar]
- 41.Tzschentke T.M. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog. Neurobiol. 2001;63(3):241–320. doi: 10.1016/s0301-0082(00)00033-2. [http://dx.doi.org/10.1016/S0301-0082(00)00033-2]. [PMID: 11115727]. [DOI] [PubMed] [Google Scholar]
- 42.Goldman-Rakic P.S., Castner S.A., Svensson T.H., Siever L.J., Williams G.V. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl.) 2004;174(1):3–16. doi: 10.1007/s00213-004-1793-y. [http://dx.doi.org/10.1007/s00213-004-1793-y]. [PMID: 15118803]. [DOI] [PubMed] [Google Scholar]
- 43.Gessa G.L., Devoto P., Diana M., Flore G., Melis M., Pistis M. Dissociation of haloperidol, clozapine, and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology. 2000;22(6):642–649. doi: 10.1016/S0893-133X(00)00087-7. [http://dx.doi.org/10.1016/S0893-133X(00)00087-7]. [PMID: 10788763]. [DOI] [PubMed] [Google Scholar]
- 44.Meltzer H.Y., McGurk S.R. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr. Bull. 1999;25(2):233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [http://dx.doi.org/10.1093/oxfordjournals. schbul.a033376]. [PMID: 10416729]. [DOI] [PubMed] [Google Scholar]
- 45.Laruelle M., Kegeles L.S., Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann. N. Y. Acad. Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [http://dx.doi.org/10.1196/ annals.1300.063]. [PMID: 14684442]. [DOI] [PubMed] [Google Scholar]
- 46.Okubo Y., Suhara T., Suzuki K., Kobayashi K., Inoue O., Terasaki O., Someya Y., Sassa T., Sudo Y., Matsushima E., Iyo M., Tateno Y., Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634–636. doi: 10.1038/385634a0. [http://dx.doi.org/10.1038/385634a0]. [PMID: 9024661]. [DOI] [PubMed] [Google Scholar]
- 47.Abi-Dargham A., Mawlawi O., Lombardo I., Gil R., Martinez D., Huang Y., Hwang D.R., Keilp J., Kochan L., Van Heertum R., Gorman J. M., Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. . J. Neurosci. 2002:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giros B., Sokoloff P., Martres M.P., Riou J.F., Emorine L.J., Schwartz J.C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342(6252):923–926. doi: 10.1038/342923a0. [http://dx.doi.org/10.1038/342923a0]. [PMID: 2531847]. [DOI] [PubMed] [Google Scholar]
- 49.Monsma F.J., McVittie L.D., Gerfen C.R., Mahan L.C., Sibley D.R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(28):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- 50.Usiello A., Baik J-H., Rouge-Pont F., Picetti R., Dierich A., LeMeur M., Piazza P.V., Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408(9):199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 51.De Mei C., Ramos M., Iitaka C., Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr. Opin. Pharmacol. 2009;9(1):53–58. doi: 10.1016/j.coph.2008.12.002. [http://dx.doi.org/10.1016/j.coph. 2008.12.002]. [PMID: 19138563]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leucht S., Wahlbeck K., Hamann J., Kissling W. New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet. 2003;361(9369):1581–1589. doi: 10.1016/S0140-6736(03)13306-5. [http://dx.doi.org/10.1016/S0140-6736(03) 13306-5]. [PMID: 12747876]. [DOI] [PubMed] [Google Scholar]
- 53.Osmond H. A review of the clinical effects of psychotomimetic agents. Ann. N. Y. Acad. Sci. 1957;66(3):418–434. doi: 10.1111/j.1749-6632.1957.tb40738.x. [http://dx.doi. org/10.1111/j.1749-6632.1957.tb40738.x]. [PMID: 13425232]. [DOI] [PubMed] [Google Scholar]
- 54.Baumeister A.A., Hawkins M.F. The serotonin hypothesis of schizophrenia: a historical case study on the heuristic value of theory in clinical neuroscience. J. Hist. Neurosci. 2004;13(3):277–291. doi: 10.1080/09647040490510560. [http://dx.doi.org/10.1080/09647040490510560]. [PMID: 15370312]. [DOI] [PubMed] [Google Scholar]
- 55.Davis J.M., Chen N., Glick I.D. A meta-analysis of the efficacy of second-generation antipsychotics. Arch. Gen. Psychiatry. 2003;60(6):553–564. doi: 10.1001/archpsyc.60.6.553. [http://dx.doi.org/10.1001/archpsyc.60.6.553]. [PMID: 12796218]. [DOI] [PubMed] [Google Scholar]
- 56.Amato D. Serotonin in antipsychotic drugs action. Behav. Brain Res. 2015;277:125–135. doi: 10.1016/j.bbr.2014.07.025. [http://dx.doi.org/10.1016/j.bbr.2014.07. 025]. [PMID: 25078293]. [DOI] [PubMed] [Google Scholar]
- 57.Hoyer D., Pazos A., Probst A., Palacios J.M. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 1986;376(1):97–107. doi: 10.1016/0006-8993(86)90903-0. [http://dx.doi.org/10.1016/0006-8993(86)90903-0]. [PMID: 2941113]. [DOI] [PubMed] [Google Scholar]
- 58.Bortolozzi A., Masana M., Díaz-Mataix L., Cortés R., Scorza M.C., Gingrich J.A., Toth M., Artigas F. Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT(1A) receptors but not 5-HT(2A) receptors. Int. J. Neuropsychopharmacol. 2010;13(10):1299–1314. doi: 10.1017/S146114571000009X. [http://dx.doi.org/10. 1017/S146114571000009X]. [PMID: 20158933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meltzer H.Y., Massey B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011;11(1):59–67. doi: 10.1016/j.coph.2011.02.007. [http://dx.doi.org/10.1016/j.coph.2011.02.007]. [PMID: 21420906]. [DOI] [PubMed] [Google Scholar]
- 60.Meltzer H.Y., Li Z., Kaneda Y., Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(7):1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [http://dx. doi.org/10.1016/j.pnpbp.2003.09.010]. [PMID: 14642974]. [DOI] [PubMed] [Google Scholar]
- 61.Newman-Tancredi A., Kleven M.S. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology (Berl.) 2011;216(4):451–473. doi: 10.1007/s00213-011-2247-y. [http://dx.doi.org/10.1007/s00213-011-2247-y]. [PMID: 21394633]. [DOI] [PubMed] [Google Scholar]
- 62.Zedkova I., Dudova I., Urbanek T., Hrdlicka M. Onset of action of atypical and typical antipsychotics in the treatment of adolescent schizophrenic psychoses. Neuroendocrinol. Lett. 2011;32(5):667–670. [PMID: 22167144]. [PubMed] [Google Scholar]
- 63.Gorwood P. Meeting everyday challenges: antipsychotic therapy in the real world. Eur. Neuropsychopharmacol. 2006;16(Suppl. 3):S156–S162. doi: 10.1016/j.euroneuro.2006.06.002. [http://dx.doi.org/10.1016/j.euroneuro.2006.06.002]. [PMID: 16872807]. [DOI] [PubMed] [Google Scholar]
- 64.Citrome L. Which role for brexpiprazole, a new dopamine D2 partial agonist, in the treatment of schizophrenia? Evid. Based Ment. Health. 2016;19(2):e6–e6. doi: 10.1136/eb-2015-102143. [http://dx.doi.org/10.1136/eb-2015-102143]. [PMID: 27048849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veselinović T., Paulzen M., Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev. Neurother. 2013;13(11):1141–1159. doi: 10.1586/14737175.2013.853448. [http://dx.doi.org/10.1586/ 14737175.2013.853448]. [PMID: 24175719]. [DOI] [PubMed] [Google Scholar]
- 66.Frankel J.S., Schwartz T.L. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther. Adv. Psychopharmacol. 2017;7(1):29–41. doi: 10.1177/2045125316672136. [http://dx.doi.org/10.1177/2045125316672136]. [PMID: 28101322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Citrome L. The ABC’s of dopamine receptor partial agonists - aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int. J. Clin. Pract. 2015;69(11):1211–1220. doi: 10.1111/ijcp.12752. [http://dx.doi.org/10.1111/ijcp.12752]. [PMID: 26477545]. [DOI] [PubMed] [Google Scholar]
- 68.Hirose T., Uwahodo Y., Yamada S., Miwa T., Kikuchi T., Kitagawa H., Burris K.D., Altar C.A., Nabeshima T. Mechanism of action of aripiprazole predicts clinical efficacy and a favourable side-effect profile. J. Psychopharmacol. (Oxford) 2004;18(3):375–383. doi: 10.1177/026988110401800308. [http://dx.doi.org/10.1177/026988110401800308]. [PMID: 15358981]. [DOI] [PubMed] [Google Scholar]
- 69.Potkin S.G., Preda A. Aripiprazole once-monthly long-acting injectable for the treatment of schizophrenia. Expert Opin. Pharmacother. 2016;17(3):395–407. doi: 10.1517/14656566.2015.1114100. [http://dx.doi.org/10.1517/ 14656566.2015.1114100]. [PMID: 26864352]. [DOI] [PubMed] [Google Scholar]
- 70.Mallikaarjun S., Salazar D.E., Bramer S.L. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J. Clin. Pharmacol. 2004;44(2):179–187. doi: 10.1177/0091270003261901. [http://dx.doi.org/10.1177/0091270003261901]. [PMID: 14747427]. [DOI] [PubMed] [Google Scholar]
- 71.Raoufinia A., Baker R.A., Eramo A., Nylander A-G., Landsberg W., Kostic D., Larsen F. Initiation of aripiprazole once-monthly in patients with schizophrenia. Curr. Med. Res. Opin. 2015;31(3):583–592. doi: 10.1185/03007995.2015.1006356. [http://dx.doi.org/10.1185/03007995.2015.1006356]. [PMID: 25586294]. [DOI] [PubMed] [Google Scholar]
- 72.Kirschbaum K.M., Müller M.J., Malevani J., Mobascher A., Burchardt C., Piel M., Hiemke C. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J. Biol. Psychiatry. 2008;9(3):212–218. doi: 10.1080/15622970701361255. [http://dx.doi.org/10.1080/ 15622970701361255]. [PMID: 17853280]. [DOI] [PubMed] [Google Scholar]
- 73.DeLeon A., Patel N.C., Crismon M.L. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin. Ther. 2004;26(5):649–666. doi: 10.1016/s0149-2918(04)90066-5. [http://dx.doi.org/10.1016/ S0149-2918(04)90066-5]. [PMID: 15220010]. [DOI] [PubMed] [Google Scholar]
- 74.Azuma J., Hasunuma T., Kubo M., Miyatake M., Koue T., Higashi K., Fujiwara T., Kitahara S., Katano T., Hara S. The relationship between clinical pharmacokinetics of aripiprazole and CYP2D6 genetic polymorphism: effects of CYP enzyme inhibition by coadministration of paroxetine or fluvoxamine. Eur. J. Clin. Pharmacol. 2012;68(1):29–37. doi: 10.1007/s00228-011-1094-4. [http://dx.doi.org/10.1007/s00228-011-1094-4]. [PMID: 21739267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Potkin S.G., Saha A.R., Kujawa M.J., Carson W.H., Ali M., Stock E., Stringfellow J., Ingenito G., Marder S.R. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch. Gen. Psychiatry. 2003;60(7):681–690. doi: 10.1001/archpsyc.60.7.681. [http://dx. doi.org/10.1001/archpsyc.60.7.681]. [PMID: 12860772]. [DOI] [PubMed] [Google Scholar]
- 76.Swainston Harrison T., Perry C.M. Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64(15):1715–1736. doi: 10.2165/00003495-200464150-00010. [http://dx.doi.org/10.2165/00003495-200464150-00010]. [PMID: 15257633]. [DOI] [PubMed] [Google Scholar]
- 77.Kasper S., Lerman M.N., McQuade R.D., Saha A., Carson W.H., Ali M., Archibald D., Ingenito G., Marcus R., Pigott T. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int. J. Neuropsychopharmacol. 2003;6(4):325–337. doi: 10.1017/S1461145703003651. [http://dx.doi. org/10.1017/S1461145703003651]. [PMID: 14609439]. [DOI] [PubMed] [Google Scholar]
- 78.Mortimer A.M., Carson W., Saha A. Aripiprazole is effective for relapse prevention in people with chronic stable schizophrenia. Evid. Based Ment. Health. 2004;7(2):41. doi: 10.1136/ebmh.7.2.41. [http://dx.doi.org/ 10.1136/ebmh.7.2.41]. [PMID: 15107337]. [DOI] [PubMed] [Google Scholar]
- 79.Casey D.E., Carson W.H., Saha A.R., Liebeskind A., Ali M.W., Jody D., Ingenito G.G. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology (Berl.) 2003;166(4):391–399. doi: 10.1007/s00213-002-1344-3. [http://dx.doi. org/10.1007/s00213-002-1344-3]. [PMID: 12610718]. [DOI] [PubMed] [Google Scholar]
- 80.Peters-Strickland T., Zhao C., Perry P.P., Eramo A., Salzman P.M., McQuade R.D., Johnson B.R., Sanchez R. Effects of aripiprazole once-monthly in patients with schizophrenia switched from oral antipsychotics to aripiprazole once-monthly. CSN spectrums., . 2016:1–6. doi: 10.1017/S1092852916000365. [DOI] [PubMed] [Google Scholar]
- 81.Kane J.M., Meltzer H.Y., Carson W.H., Jr, McQuade R.D., Marcus R.N., Sanchez R. Aripiprazole for treatment-resistant schizophrenia: results of a multicenter, randomized, double-blind, comparison study versus perphenazine. J. Clin. Psychiatry. 2007;68(2):213–223. [http://dx.doi.org/10.4088/JCP.v68n0206]. [PMID: 17335319]. [PubMed] [Google Scholar]
- 82.Cornblatt B., Kern R., Carson W., Ali M., Luo X., Green M. Neurocognitive effects of aripiprazole versus olanzapine in stable psychosis. Neuropsychopharmacology. 2002;5(Suppl. 1):S185. [Google Scholar]
- 83.Kern R.S., Green M.F., Cornblatt B.A., Owen J.R., McQuade R.D., Carson W.H., Ali M., Marcus R. The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacology (Berl.) 2006;187(3):312–320. doi: 10.1007/s00213-006-0428-x. [http://dx. doi.org/10.1007/s00213-006-0428-x]. [PMID: 16810506]. [DOI] [PubMed] [Google Scholar]
- 84.Marder S.R., McQuade R.D., Stock E., Kaplita S., Marcus R., Safferman A.Z., Saha A., Ali M., Iwamoto T. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr. Res. 2003;61(2-3):123–136. doi: 10.1016/s0920-9964(03)00050-1. [http://dx.doi.org/10.1016/S0920-9964(03)00050-1]. [PMID: 12729864]. [DOI] [PubMed] [Google Scholar]
- 85.Kane J.M., Carson W.H., Saha A.R., McQuade R.D., Ingenito G.G., Zimbroff D.L., Ali M.W. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J. Clin. Psychiatry. 2002;63(9):763–771. doi: 10.4088/jcp.v63n0903. [http://dx.doi.org/10.4088/JCP.v63n0903]. [PMID: 12363115]. [DOI] [PubMed] [Google Scholar]
- 86.Bernagie C., Danckaerts M., Wampers M., De Hert M. Aripiprazole and Acute Extrapyramidal Symptoms in Children and Adolescents: A Meta-Analysis. CNS Drugs. 2016;30(9):807–818. doi: 10.1007/s40263-016-0367-y. [http://dx.doi.org/10.1007/s40263-016-0367-y]. [PMID: 27395403]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henderson J.B., Labbate L., Worley M., Labbate L., Worley M. A case of acute dystonia after single dose of aripiprazole in a man with cocaine dependence. Am. J. Addict. 2007;16(3):244. doi: 10.1080/10550490701375343. [http:// dx.doi.org/10.1080/10550490701375343]. [PMID: 17612831]. [DOI] [PubMed] [Google Scholar]
- 88.Lawler C.P., Prioleau C., Lewis M.M., Mak C., Jiang D., Schetz J.A., Gonzalez A.M., Sibley D.R., Mailman R.B. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20(6):612–627. doi: 10.1016/S0893-133X(98)00099-2. [http://dx.doi.org/10.1016/S0893-133X(98) 00099-2]. [PMID: 10327430]. [DOI] [PubMed] [Google Scholar]
- 89.Marona-Lewicka D., Nichols D.E. Aripiprazole (OPC-14597) fully substitutes for the 5-HT1A receptor agonist LY293284 in the drug discrimination assay in rats. Psychopharmacology (Berl.) 2004;172(4):415–421. doi: 10.1007/s00213-003-1677-6. [http://dx.doi.org/10.1007/s00213-003-1677-6]. [PMID: 14647959]. [DOI] [PubMed] [Google Scholar]
- 90.Natesan S., Reckless G.E., Nobrega J.N., Fletcher P.J., Kapur S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology. 2006;31(9):1854–1863. doi: 10.1038/sj.npp.1300983. [http://dx.doi.org/10.1038/sj.npp.1300983]. [PMID: 16319908]. [DOI] [PubMed] [Google Scholar]
- 91.Kapur S., Remington G., Zipursky R.B., Wilson A.A., Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci. 1995;57(10):PL103–PL107. doi: 10.1016/0024-3205(95)02037-j. [http://dx.doi.org/10.1016/0024-3205(95) 02037-J]. [PMID: 7543969]. [DOI] [PubMed] [Google Scholar]
- 92.Yokoi F., Gründer G., Biziere K., Stephane M., Dogan A.S., Dannals R.F., Ravert H., Suri A., Bramer S., Wong D.F. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248–259. doi: 10.1016/S0893-133X(02)00304-4. [http://dx.doi.org/10.1016/ S0893-133X(02)00304-4]. [PMID: 12093598]. [DOI] [PubMed] [Google Scholar]
- 93.Oshiro Y., Sato S., Kurahashi N., Tanaka T., Kikuchi T., Tottori K., Uwahodo Y., Nishi T. Novel antipsychotic agents with dopamine autoreceptor agonist properties: synthesis and pharmacology of 7-[4-(4-phenyl-1-piperazinyl)butoxy]-3,4-dihydro-2(1H)-quinolinone derivatives. J. Med. Chem. 1998;41(5):658–667. doi: 10.1021/jm940608g. [http://dx.doi.org/10.1021/jm940608g]. [PMID: 9513593]. [DOI] [PubMed] [Google Scholar]
- 94.Aihara K., Shimada J., Miwa T., Tottori K., Burris K.D., Yocca F.D., Horie M., Kikuchi T. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9–17. doi: 10.1016/j.brainres.2003.09.082. [http://dx.doi.org/10.1016/ j.brainres.2003.09.082]. [PMID: 15019558]. [DOI] [PubMed] [Google Scholar]
- 95.Urban J.D., Clarke W.P., von Zastrow M., Nichols D.E., Kobilka B., Weinstein H., Javitch J.A., Roth B.L., Christopoulos A., Sexton P.M., Miller K.J., Spedding M., Mailman R.B. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [http://dx.doi.org/ 10.1124/jpet.106.104463]. [PMID: 16803859]. [DOI] [PubMed] [Google Scholar]
- 96.Jones C.A., Watson D.J., Fone K.C. Animal models of schizophrenia. Br. J. Pharmacol. 2011;164(4):1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [http://dx.doi. org/10.1111/j.1476-5381.2011.01386.x]. [PMID: 21449915]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yee B.K., Singer P. A conceptual and practical guide to the behavioural evaluation of animal models of the symptomatology and therapy of schizophrenia. Cell Tissue Res. 2013;354(1):221–246. doi: 10.1007/s00441-013-1611-0. [http://dx.doi.org/10.1007/s00441-013-1611-0]. [PMID: 23579553]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bäckström P., Etelälahti T.J., Hyytiä P. Attenuation of reinforcing and psychomotor stimulant effects of amphetamine by aripiprazole. Addict. Biol. 2011;16(1):55–63. doi: 10.1111/j.1369-1600.2010.00223.x. [http://dx.doi.org/10.1111/ j.1369-1600.2010.00223.x]. [PMID: 21158016]. [DOI] [PubMed] [Google Scholar]
- 99.Mavrikaki M., Nomikos G.G., Panagis G. Efficacy of the atypical antipsychotic aripiprazole in d-amphetamine-based preclinical models of mania. Int. J. Neuropsychopharmacol. 2010;13(4):541–548. doi: 10.1017/S1461145709991143. [http://dx.doi.org/10.1017/S1461145709991143]. [PMID: 20047715]. [DOI] [PubMed] [Google Scholar]
- 100.Leite J.V., Guimarães F.S., Moreira F.A. Aripiprazole, an atypical antipsychotic, prevents the motor hyperactivity induced by psychotomimetics and psychostimulants in mice. Eur. J. Pharmacol. 2008;578(2-3):222–227. doi: 10.1016/j.ejphar.2007.09.016. [http://dx.doi.org/10.1016/j.ejphar.2007. 09.016]. [PMID: 18021764]. [DOI] [PubMed] [Google Scholar]
- 101.Simón V.M., Parra A., Miñarro J., Arenas M.C., Vinader-Caerols C., Aguilar M.A. Predicting how equipotent doses of chlorpromazine, haloperidol, sulpiride, raclopride and clozapine reduce locomotor activity in mice. Eur. Neuropsychopharmacol. 2000;10(3):159–164. doi: 10.1016/s0924-977x(00)00070-5. [http://dx.doi.org/10.1016/S0924-977X(00) 00070-5]. [PMID: 10793317]. [DOI] [PubMed] [Google Scholar]
- 102.De Santis M., Pan B., Lian J., Huang X.F., Deng C. Different effects of bifeprunox, aripiprazole, and haloperidol on body weight gain, food and water intake, and locomotor activity in rats. Pharmacol. Biochem. Behav. 2014;124:167–173. doi: 10.1016/j.pbb.2014.06.004. [http://dx.doi.org/ 10.1016/j.pbb.2014.06.004]. [PMID: 24933333]. [DOI] [PubMed] [Google Scholar]
- 103.Tadokoro S., Okamura N., Sekine Y., Kanahara N., Hashimoto K., Iyo M. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr. Bull. 2012;38(5):1012–1020. doi: 10.1093/schbul/sbr006. [http://dx. doi.org/10.1093/schbul/sbr006]. [PMID: 21402722]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Viana T.G., Almeida-Santos A.F., Aguiar D.C., Moreira F.A. Effects of aripiprazole, an atypical antipsychotic, on the motor alterations induced by acute ethanol administration in mice. Basic Clin. Pharmacol. Toxicol. 2013;112(5):319–324. doi: 10.1111/bcpt.12036. [http://dx.doi. org/10.1111/bcpt.12036]. [PMID: 23157340]. [DOI] [PubMed] [Google Scholar]
- 105.Zocchi A., Fabbri D., Heidbreder C.A. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci. Lett. 2005;387(3):157–161. doi: 10.1016/j.neulet.2005.06.035. [http://dx. doi.org/10.1016/j.neulet.2005.06.035]. [PMID: 16023290]. [DOI] [PubMed] [Google Scholar]
- 106.Olianas M.C., Onali P. Supersensitivity of striatal D2 dopamine receptors mediating inhibition of adenylate cyclase and stimulation of guanosine triphosphatase following chronic administration of haloperidol in mice. Neurosci. Lett. 1987;78(3):349–354. doi: 10.1016/0304-3940(87)90386-7. [http:// dx.doi.org/10.1016/0304-3940(87)90386-7]. [PMID: 2819791]. [DOI] [PubMed] [Google Scholar]
- 107.Vogelsang G.D., Piercey M.F. The supersensitivity of dopaminergic neurons to apomorphine in rats following chronic haloperidol. Eur. J. Pharmacol. 1985;110(2):267–269. doi: 10.1016/0014-2999(85)90222-5. [http://dx.doi.org/ 10.1016/0014-2999(85)90222-5]. [PMID: 3987817]. [DOI] [PubMed] [Google Scholar]
- 108.Kuno T., Shirakawa O., Tanaka C. Selective decrease in the affinity of D2 dopamine receptor for agonist induced by islet-activating protein, pertussis toxin, associated with ADP-ribosylation of the specific membrane protein of bovine striatum. Biochem. Biophys. Res. Commun. 1983;115(1):325–330. doi: 10.1016/0006-291x(83)91007-0. [http://dx.doi. org/10.1016/0006-291X(83)91007-0]. [PMID: 6225433]. [DOI] [PubMed] [Google Scholar]
- 109.Olianas M.C., Onali P. Pertussis toxin attenuates D2 inhibition and enhances D1 stimulation of adenylate cyclase by dopamine in rat striatum. J. Neurochem. 1987;48(5):1443–1447. doi: 10.1111/j.1471-4159.1987.tb05683.x. [http://dx. doi.org/10.1111/j.1471-4159.1987.tb05683.x]. [PMID: 2951497]. [DOI] [PubMed] [Google Scholar]
- 110.Koener B., Goursaud S., Van De Stadt M., Calas A.G., Jeanjean A.P., Maloteaux J.M., Hermans E. Pharmacological blockade of dopamine D2 receptors by aripiprazole is not associated with striatal sensitization. Naunyn Schmiedebergs Arch. Pharmacol. 2011;383(1):65–77. doi: 10.1007/s00210-010-0577-7. [http://dx.doi.org/10.1007/s00210-010-0577-7]. [PMID: 21061116]. [DOI] [PubMed] [Google Scholar]
- 111.Seeman P. Dopamine D2(High) receptors moderately elevated by bifeprunox and aripiprazole. Synapse. 2008;62(12):902–908. doi: 10.1002/syn.20557. [http://dx.doi.org/10.1002/syn.20557]. [PMID: 18792990]. [DOI] [PubMed] [Google Scholar]
- 112.Varela F.A., Der-Ghazarian T., Lee R.J., Charntikov S., Crawford C.A., McDougall S.A. Repeated aripiprazole treatment causes dopamine D2 receptor up-regulation and dopamine supersensitivity in young rats. J. Psychopharmacol. (Oxford) 2014;28(4):376–386. doi: 10.1177/0269881113504016. [http://dx.doi.org/10.1177/0269881113504016]. [PMID: 24045880]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Minematsu N. Behavioral effects of chronic apomorphine, and D-1/D-2 dopamine receptor activities in rats. Nihon Shinkei Seishin Yakurigaku Zasshi. 1995;15(3):247–252. [PMID: 7584718]. [PubMed] [Google Scholar]
- 114.Doey T. Aripiprazole in pediatric psychosis and bipolar disorder: a clinical review. J. Affect. Disord. 2012;138(Suppl.):S15–S21. doi: 10.1016/j.jad.2012.02.031. [http://dx.doi.org/10.1016/j.jad.2012.02.031]. [PMID: 22406333]. [DOI] [PubMed] [Google Scholar]
- 115.Moghaddam B., Bunney B.S. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J. Neurochem. 1990;54(5):1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [http:// dx.doi.org/10.1111/j.1471-4159.1990.tb01230.x]. [PMID: 1969939]. [DOI] [PubMed] [Google Scholar]
- 116.Imperato A., Di Chiara G. Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. J. Neurosci. 1985;5(2):297–306. doi: 10.1523/JNEUROSCI.05-02-00297.1985. [PMID: 2857776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Z., Ichikawa J., Dai J., Meltzer H.Y. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur. J. Pharmacol. 2004;493(1-3):75–83. doi: 10.1016/j.ejphar.2004.04.028. [http://dx.doi.org/10.1016/j.ejphar.2004.04. 028]. [PMID: 15189766]. [DOI] [PubMed] [Google Scholar]
- 118.Tanahashi S., Yamamura S., Nakagawa M., Motomura E., Okada M. Dopamine D2 and serotonin 5-HT1A receptors mediate the actions of aripiprazole in mesocortical and mesoaccumbens transmission. Neuropharmacology. 2012;62(2):765–774. doi: 10.1016/j.neuropharm.2011.08.031. [http:// dx.doi.org/10.1016/j.neuropharm.2011.08.031]. [PMID: 21925189]. [DOI] [PubMed] [Google Scholar]
- 119.Olney J.W., Newcomer J.W., Farber N.B. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [http://dx.doi.org/10.1016/S0022-3956(99)00029-1]. [PMID: 10628529]. [DOI] [PubMed] [Google Scholar]
- 120.Wong E.H., Knight A.R., Woodruff G.N. [3H]MK-801 labels a site on the N-methyl-D-aspartate receptor channel complex in rat brain membranes. J. Neurochem. 1988;50(1):274–281. doi: 10.1111/j.1471-4159.1988.tb13260.x. [http:// dx.doi.org/10.1111/j.1471-4159.1988.tb13260.x]. [PMID: 2826686]. [DOI] [PubMed] [Google Scholar]
- 121.Mathé J.M., Nomikos G.G., Hildebrand B.E., Hertel P., Svensson T.H. Prazosin inhibits MK-801-induced hyperlocomotion and dopamine release in the nucleus accumbens. Eur. J. Pharmacol. 1996;309(1):1–11. doi: 10.1016/0014-2999(96)00315-9. [http://dx.doi.org/10.1016/0014-2999(96)00315-9]. [PMID: 8864686]. [DOI] [PubMed] [Google Scholar]
- 122.Holahan M.R., Madularu D., McConnell E.M., Walsh R., DeRosa M.C. Intra-accumbens injection of a dopamine aptamer abates MK-801-induced cognitive dysfunction in a model of schizophrenia. PLoS One. 2011;6(7):e22239. doi: 10.1371/journal.pone.0022239. [http://dx.doi.org/10. 1371/journal.pone.0022239]. [PMID: 21779401]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Holahan M.R., Westby E.P., Albert K. Comparison of the MK-801-induced appetitive extinction deficit with pressing for reward and associated pERK1/2 staining in prefrontal cortex and nucleus accumbens. Behav. Brain Res. 2012;228(1):194–202. doi: 10.1016/j.bbr.2011.11.044. [http:// dx.doi.org/10.1016/j.bbr.2011.11.044]. [PMID: 22182675]. [DOI] [PubMed] [Google Scholar]
- 124.Ali S.F., Newport G.D., Bracha H.S. Phencyclidine and (+)-MK-801-induced circling preference: correlation with monoamine levels in striatum of the rat brain. Neurotoxicol. Teratol. 1994;16(4):335–342. doi: 10.1016/0892-0362(94)90021-3. [http://dx.doi.org/10.1016/0892-0362(94)90021-3]. [PMID: 7968937]. [DOI] [PubMed] [Google Scholar]
- 125.Padilla-de la Torre M., Franco-Pérez J., Santamaría A., Galvan S., González E., Paz C. Effect of acetaldehyde on behavioral and neurochemical changes induced by MK-801 in rats. Ann. N. Y. Acad. Sci. 2008;1139:259–267. doi: 10.1196/annals.1432.038. [http://dx.doi.org/10.1196/annals. 1432.038]. [PMID: 18991871]. [DOI] [PubMed] [Google Scholar]
- 126.Zhang J., Chiodo L.A., Freeman A.S. Electrophysiological effects of MK-801 on rat nigrostriatal and mesoaccumbal dopaminergic neurons. Brain Res. 1992;590(1-2):153–163. doi: 10.1016/0006-8993(92)91091-r. [http:// dx.doi.org/10.1016/0006-8993(92)91091-R]. [PMID: 1422830]. [DOI] [PubMed] [Google Scholar]
- 127.Davis-MacNevin P.L., Dekraker J., LaDouceur L., Holahan M.R. Comparison of the MK-801-induced increase in non-rewarded appetitive responding with dopamine agonists and locomotor activity in rats. J. Psychopharmacol. (Oxford) 2013;27(9):854–864. doi: 10.1177/0269881113492029. [http://dx.doi.org/10.1177/0269881113492029]. [PMID: 23761388]. [DOI] [PubMed] [Google Scholar]
- 128.Holahan M.R., Clarke M.J., Hines D.D. Dopamine-mediated MK-801-induced elevation in food-based extinction responding in rats and associated changes in region-specific phosphorylated ERK. Psychopharmacology (Berl.) 2010;212(3):393–403. doi: 10.1007/s00213-010-1959-8. [http://dx. doi.org/10.1007/s00213-010-1959-8]. [PMID: 20652538]. [DOI] [PubMed] [Google Scholar]
- 129.Tuplin E. W., Stocco M. R., Holahan M. R. Attenuation of MK-801-induced behavioral perseveration by typical and atypical antipsychotic pretreatment in rats. 2015. [DOI] [PubMed]
- 130.Ishii D., Matsuzawa D., Kanahara N., Matsuda S., Sutoh C., Ohtsuka H., Nakazawa K., Kohno M., Hashimoto K., Iyo M., Shimizu E. Effects of aripiprazole on MK-801-induced prepulse inhibition deficits and mitogen-activated protein kinase signal transduction pathway. Neurosci. Lett. 2010;471(1):53–57. doi: 10.1016/j.neulet.2010.01.010. [http:// dx.doi.org/10.1016/j.neulet.2010.01.010]. [PMID: 20083164]. [DOI] [PubMed] [Google Scholar]
- 131.Sandner G., Canal N.M. Relationship between PPI and baseline startle response. Cogn Neurodyn. 2007;1(1):27–37. doi: 10.1007/s11571-006-9008-3. [http://dx. doi.org/10.1007/s11571-006-9008-3]. [PMID: 19003494]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Geyer M.A., Krebs-Thomson K., Braff D.L., Swerdlow N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. 2001. [DOI] [PubMed]
- 133.Swerdlow N.R., Braff D.L., Geyer M.A., Koob G.F. Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biol. Psychiatry. 1986;21(1):23–33. doi: 10.1016/0006-3223(86)90005-3. [http://dx.doi.org/10.1016/0006-3223(86)90005-3]. [PMID: 3080033]. [DOI] [PubMed] [Google Scholar]
- 134.Auclair A.L., Galinier A., Besnard J., Newman-Tancredi A., Depoortère R. Putative antipsychotics with pronounced agonism at serotonin 5-HT1A and partial agonist activity at dopamine D2 receptors disrupt basal PPI of the startle reflex in rats. Psychopharmacology (Berl.) 2007;193(1):45–54. doi: 10.1007/s00213-007-0762-7. [http://dx.doi.org/10.1007/ s00213-007-0762-7]. [PMID: 17393144]. [DOI] [PubMed] [Google Scholar]
- 135.Lipina T., Labrie V., Weiner I., Roder J. Modulators of the glycine site on NMDA receptors, D-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology (Berl.) 2005;179(1):54–67. doi: 10.1007/s00213-005-2210-x. [http://dx.doi.org/10.1007/s00213-005-2210-x]. [PMID: 15759151]. [DOI] [PubMed] [Google Scholar]
- 136.Yamada S., Harano M., Annoh N., Nakamura K., Tanaka M. Involvement of serotonin 2A receptors in phencyclidine-induced disruption of prepulse inhibition of the acoustic startle in rats. Biol. Psychiatry. 1999;46(6):832–838. doi: 10.1016/s0006-3223(98)00356-4. [http://dx.doi.org/10.1016/ S0006-3223(98)00356-4]. [PMID: 10494453]. [DOI] [PubMed] [Google Scholar]
- 137.Gogos A., Bogeski M., van den Buuse M. Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav. Pharmacol. 2008;19(5-6):548–561. doi: 10.1097/FBP.0b013e32830cd822. [http://dx.doi.org/10.1097/FBP.0b013e32830cd822]. [PMID: 18690109]. [DOI] [PubMed] [Google Scholar]
- 138.Ellenbroek B.A., Cools A.R. Animal models for the negative symptoms of schizophrenia. Behav. Pharmacol. 2000;11(3-4):223–233. doi: 10.1097/00008877-200006000-00006. [http://dx.doi.org/10.1097/00008877-200006000-00006]. [PMID: 11103877]. [DOI] [PubMed] [Google Scholar]
- 139.Rung J.P., Carlsson A., Rydén Markinhuhta K., Carlsson M.L. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(5):827–832. doi: 10.1016/j.pnpbp.2005.03.004. [http://dx.doi.org/ 10.1016/j.pnpbp.2005.03.004]. [PMID: 15916843]. [DOI] [PubMed] [Google Scholar]
- 140.Deiana S., Watanabe A., Yamasaki Y., Amada N., Kikuchi T., Stott C., Riedel G. MK-801-induced deficits in social recognition in rats: reversal by aripiprazole, but not olanzapine, risperidone, or cannabidiol. . Behav. Pharmacol. 2015:748–765. doi: 10.1097/FBP.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 141.Bruins Slot L.A., Kleven M.S., Newman-Tancredi A. Effects of novel antipsychotics with mixed D(2) antagonist/5-HT(1A) agonist properties on PCP-induced social interaction deficits in the rat. Neuropharmacology. 2005;49(7):996–1006. doi: 10.1016/j.neuropharm.2005.05.013. [http://dx.doi.org/ 10.1016/j.neuropharm.2005.05.013]. [PMID: 16009387]. [DOI] [PubMed] [Google Scholar]
- 142.Snigdha S., Neill J.C. Improvement of phencyclidine-induced social behaviour deficits in rats: involvement of 5-HT1A receptors. Behav. Brain Res. 2008;191(1):26–31. doi: 10.1016/j.bbr.2008.03.018. [http://dx.doi.org/10.1016/ j.bbr.2008.03.018]. [PMID: 18453008]. [DOI] [PubMed] [Google Scholar]
- 143.Ito H., Takano H., Arakawa R., Takahashi H., Kodaka F., Takahata K., Nogami T., Suzuki M., Suhara T. Effects of dopamine D2 receptor partial agonist antipsychotic aripiprazole on dopamine synthesis in human brain measured by PET with L-[β-11C]DOPA. PLoS One. 2012;7(9):e46488. doi: 10.1371/journal.pone.0046488. [http://dx.doi.org/10.1371/journal. pone.0046488]. [PMID: 23029533]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takahata K., Ito H., Takano H., Arakawa R., Fujiwara H., Kimura Y., Kodaka F., Sasaki T., Nogami T., Suzuki M., Nagashima T., Shimada H., Kato M., Mimura M., Suhara T. Striatal and extrastriatal dopamine D2 receptor occupancy by the partial agonist antipsychotic drug aripiprazole in the human brain: a positron emission tomography study with [11C]raclopride and [11C]FLB457. Psychopharmacology (Berl.) 2012;222(1):165–172. doi: 10.1007/s00213-011-2633-5. [http://dx.doi.org/10.1007/s00213-011-2633-5]. [PMID: 22237854]. [DOI] [PubMed] [Google Scholar]
- 145.Feenstra M.G., Sumners C., Goedemoed J.H., de Vries J.B., Rollema H., Horn A.S. A comparison of the potencies of various dopamine receptor agonists in models for pre- and postsynaptic receptor activity. Naunyn Schmiedebergs Arch. Pharmacol. 1983;324(2):108–115. doi: 10.1007/BF00497015. [http://dx.doi.org/10.1007/BF00497015]. [PMID: 6646238]. [DOI] [PubMed] [Google Scholar]
- 146.Tadori Y., Miwa T., Tottori K., Burris K.D., Stark A., Mori T., Kikuchi T. Aripiprazole’s low intrinsic activities at human dopamine D2L and D2S receptors render it a unique antipsychotic. Eur. J. Pharmacol. 2005;515(1-3):10–19. doi: 10.1016/j.ejphar.2005.02.051. [http://dx.doi.org/10.1016/ j.ejphar.2005.02.051]. [PMID: 15894311]. [DOI] [PubMed] [Google Scholar]
- 147.Meller E., Bohmaker K., Namba Y., Friedhoff A.J., Goldstein M. Relationship between receptor occupancy and response at striatal dopamine autoreceptors. Mol. Pharmacol. 1987;31(6):592–598. [PMID: 2885734]. [PubMed] [Google Scholar]
- 148.Meller E., Puza T., Miller J.C., Friedhoff A.J., Schweitzer J.W. Receptor reserve for D2 dopaminergic inhibition of prolactin release in vivo and in vitro. J. Pharmacol. Exp. Ther. 1991;257(2):668–675. [PMID: 1674531]. [PubMed] [Google Scholar]
- 149.Enz A., Goldstein M., Meller E. Dopamine agonist-induced elevation of striatal acetylcholine: relationship between receptor occupancy and response in normal and denervated rat striatum. Mol. Pharmacol. 1990;37(4):560–565. [PMID: 1970116]. [PubMed] [Google Scholar]
- 150.Murphy A., Dursun S., McKie S., Elliott R., Deakin J.F. Dopamine agonist-induced elevation of striatal acetylcholine: relationship between receptor occupancy and response in normal and denervated rat striatum. Mol. Pharmacol. 1990;37(4):560–565. [http://dx.doi.org/10.1007/s00213-016-4234-9]. [PubMed] [Google Scholar]
- 151.Pan B., Chen J., Lian J., Huang X-F., Deng C. Unique effects of acute aripiprazole treatment on the dopamine D2 receptor downstream cAMP-PKA and Akt-GSK3β signalling pathways in rats. PLoS One. 2015;10(7):1–20. doi: 10.1371/journal.pone.0132722. [http://dx.doi.org/10.1371/journal. pone.0132722]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mailman R.B. GPCR functional selectivity has therapeutic impact. Trends Pharmacol. Sci. 2007;28(8):390–396. doi: 10.1016/j.tips.2007.06.002. [http://dx.doi. org/10.1016/j.tips.2007.06.002]. [PMID: 17629962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jope R.S., Roh M-S. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr. Drug Targets. 2006;7(11):1421–1434. doi: 10.2174/1389450110607011421. [http://dx.doi.org/10.2174/ 1389450110607011421]. [PMID: 17100582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Funk A.J., McCullumsmith R.E., Haroutunian V., Meador-Woodruff J.H. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology. 2012;37(4):896–905. doi: 10.1038/npp.2011.267. [http://dx.doi.org/10.1038/npp.2011.267]. [PMID: 22048463]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lovestone S., Killick R., Di Forti M., Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30(4):142–149. doi: 10.1016/j.tins.2007.02.002. [http://dx.doi.org/10.1016/j.tins.2007.02.002]. [PMID: 17324475]. [DOI] [PubMed] [Google Scholar]
- 156.Brust T.F., Hayes M.P., Roman D.L., Watts V.J. New functional activity of aripiprazole revealed: Robust antagonism of D2 dopamine receptor-stimulated Gβγ signaling. Biochem. Pharmacol. 2015;93(1):85–91. doi: 10.1016/j.bcp.2014.10.014. [http://dx.doi.org/10.1016/j.bcp.2014.10.014]. [PMID: 25449598]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beaulieu J.M., Gainetdinov R.R., Caron M.G. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 2007;28(4):166–172. doi: 10.1016/j.tips.2007.02.006. [http://dx.doi.org/10.1016/j.tips.2007. 02.006]. [PMID: 17349698]. [DOI] [PubMed] [Google Scholar]
- 158.Li X., Zhu W., Roh M.S., Friedman A.B., Rosborough K., Jope R.S. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29(8):1426–1431. doi: 10.1038/sj.npp.1300439. [http://dx.doi.org/10.1038/ sj.npp.1300439]. [PMID: 15039769]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Masri B., Salahpour A., Didriksen M., Ghisi V., Beaulieu J-M., Gainetdinov R.R., Caron M.G. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA. 2008;105(36):13656–13661. doi: 10.1073/pnas.0803522105. [http://dx.doi.org/10.1073/pnas.0803522105]. [PMID: 18768802]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Klein P.S., Meltont D.A. 2016. [Google Scholar]
- 161.Li X., Rosborough K.M., Friedman A.B., Zhu W., Roth K.A. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int. J. Neuropsychopharmacol. 2007;10(1):7–19. doi: 10.1017/S1461145706006547. [http://dx.doi.org/10.1017/S1461145706006547]. [PMID: 16672106]. [DOI] [PubMed] [Google Scholar]
- 162.Roh M-S., Seo M.S., Kim Y., Kim S.H., Jeon W.J., Ahn Y.M., Kang U.G., Juhnn Y.S., Kim Y.S. Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp. Mol. Med. 2007;39(3):353–360. doi: 10.1038/emm.2007.39. [http://dx.doi.org/10.1038/emm.2007.39]. [PMID: 17603289]. [DOI] [PubMed] [Google Scholar]
- 163.Beaulieu J-M., Sotnikova T.D., Yao W-D., Kockeritz L., Woodgett J.R., Gainetdinov R.R., Caron M.G. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/ glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA. 2004;101(14):5099–5104. doi: 10.1073/pnas.0307921101. [http://dx.doi.org/10.1073/ pnas.0307921101]. [PMID: 15044694]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gil M., Zhen X., Friedman E. Prenatal cocaine exposure alters glycogen synthase kinase-3beta (GSK3beta) pathway in select rabbit brain areas. Neurosci. Lett. 2003;349(3):143–146. doi: 10.1016/s0304-3940(03)00852-8. [http://dx. doi.org/10.1016/S0304-3940(03)00852-8]. [PMID: 12951189]. [DOI] [PubMed] [Google Scholar]
- 165.Freyberg Z., Ferrando S.J., Javitch J.A. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am. J. Psychiatry. 2010;167(4):388–396. doi: 10.1176/appi.ajp.2009.08121873. [http://dx. doi.org/10.1176/appi.ajp.2009.08121873]. [PMID: 19917593]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kozlovsky N., Belmaker R.H., Agam G. Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophr. Res. 2001;52(1-2):101–105. doi: 10.1016/s0920-9964(00)00174-2. [http://dx.doi.org/10.1016/S0920-9964(00) 00174-2]. [PMID: 11595396]. [DOI] [PubMed] [Google Scholar]
- 167.Park S.W., Lee J.G., Ha E.K., Choi S.M., Cho H.Y., Seo M.K., Kim Y.H. Differential effects of aripiprazole and haloperidol on BDNF-mediated signal changes in SH-SY5Y cells. Eur. Neuropsychopharmacol. 2009;19(5):356–362. doi: 10.1016/j.euroneuro.2008.12.012. [http://dx.doi.org/10.1016/ j.euroneuro.2008.12.012]. [PMID: 19196496]. [DOI] [PubMed] [Google Scholar]
- 168.Gainetdinov R.R., Premont R.T., Bohn L.M., Lefkowitz R.J., Caron M.G. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 2004;27(1):107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [http://dx.doi.org/10.1146/annurev.neuro.27.070203.144206]. [PMID: 15217328]. [DOI] [PubMed] [Google Scholar]
- 169.Gould T.D., Einat H., Bhat R., Manji H.K. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int. J. Neuropsychopharmacol. 2004;7(4):387–390. doi: 10.1017/S1461145704004535. [http://dx.doi.org/10.1017/S1461145704004535]. [PMID: 15315719]. [DOI] [PubMed] [Google Scholar]
- 170.Prickaerts J., Moechars D., Cryns K., Lenaerts I., van Craenendonck H., Goris I., Daneels G., Bouwknecht J.A., Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J. Neurosci. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [http://dx.doi.org/10.1523/JNEUROSCI.5216-05.2006]. [PMID: 16943560]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pan B., Huang X-F., Deng C. Aripiprazole and haloperidol activate GSK3β-dependent signalling pathway differentially in various brain regions of rats. Int. J. Mol. Sci. 2016;17(4):459. doi: 10.3390/ijms17040459. [http:// dx.doi.org/10.3390/ijms17040459]. [PMID: 27043526]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pan B., Lian J., Huang X.F., Deng C. Aripiprazole Increases the PKA Signalling and Expression of the GABAA Receptor and CREB1 in the Nucleus Accumbens of Rats. J. Mol. Neurosci. 2016;59(1):36–47. doi: 10.1007/s12031-016-0730-y. [http://dx.doi.org/10.1007/s12031-016-0730-y]. [PMID: 26894264]. [DOI] [PubMed] [Google Scholar]
- 173.Li Y.C., Gao W.J. GSK-3β activity and hyperdopamine-dependent behaviors. Neurosci. Biobehav. Rev. 2011;35(3):645–654. doi: 10.1016/j.neubiorev.2010.08.001. [http://dx.doi.org/10.1016/j.neubiorev.2010.08.001]. [PMID: 20727368]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mamo D., Graff A., Mizrahi R., Shammi C.M., Romeyer F., Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am. J. Psychiatry. 2007;164(9):1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [http://dx.doi.org/10.1176/appi.ajp.2007.06091479]. [PMID: 17728427]. [DOI] [PubMed] [Google Scholar]
- 175.Ichikawa J., Ishii H., Bonaccorso S., Fowler W.L., O’Laughlin I.A., Meltzer H.Y. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J. Neurochem. 2001;76(5):1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [http://dx.doi.org/ 10.1046/j.1471-4159.2001.00154.x]. [PMID: 11238736]. [DOI] [PubMed] [Google Scholar]
- 176.Hoyer D., Pazos A., Probst A., Palacios J.M. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 1986;376(1):97–107. doi: 10.1016/0006-8993(86)90903-0. [http://dx.doi.org/10.1016/0006-8993(86)90903-0]. [PMID: 2941113]. [DOI] [PubMed] [Google Scholar]
- 177.Hirose A., Sasa M., Akaike A., Takaori S. Inhibition of hippocampal CA1 neurons by 5-hydroxytryptamine, derived from the dorsal raphe nucleus and the 5-hydroxytryptamine1A agonist SM-3997. Neuropharmacology. 1990;29(2):93–101. doi: 10.1016/0028-3908(90)90048-v. [http://dx.doi.org/ 10.1016/0028-3908(90)90048-V]. [PMID: 1970426]. [DOI] [PubMed] [Google Scholar]
- 178.Jakab R.L., Goldman-Rakic P.S. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc. Natl. Acad. Sci. USA. 1998;95(2):735–740. doi: 10.1073/pnas.95.2.735. [http://dx.doi.org/10.1073/pnas.95.2.735]. [PMID: 9435262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Willins D.L., Deutch A.Y., Roth B.L. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27(1):79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [http://dx.doi.org/10.1002/ (SICI)1098-2396(199709)27:1<79:AID-SYN8>3.0.CO;2-A]. [PMID: 9268067]. [DOI] [PubMed] [Google Scholar]