Abstract
A tenth of all pediatric liver transplantations (LTs) are performed for unresectable liver malignancies, especially the more common hepatoblastoma (HBL). Less understood are outcomes after LT for the rare hepatocellular carcinoma, nonhepatoblastoma embryonal tumors (EMBs), and slow growing metastatic neuroendocrine tumors of childhood. Pediatric LT is increasingly performed for rare unresectable liver malignancies other than HBL. We performed a retrospective review of outcomes after LT for malignancy in the multicenter US Scientific Registry of Transplant Recipients (SRTR; n = 677; 1987‐2015). We then reviewed the Children's Hospital of Pittsburgh (CHP; n = 74; 1981‐2014) experience focusing on LT for unresectable hepatocellular cancer (HCC), EMBs, and metastatic liver tumors (METS). HBL was included to provide reference statistics. In the SRTR database, LT for HCC and HBL increased over time (P < 0.001). Compared with other malignancies, the 149 HCC cases received fewer segmental grafts (P < 0.001) and also experienced 10‐year patient survival similar to 15,710 adult HCC LT recipients (51.6% versus 49.6%; P = 0.848, not significant [NS], log‐rank test). For 22 of 149 cases with incidental HCC, 10‐year patient survival was higher than 127 primary HCC cases (85% [95% confidence interval (CI), 70.6%‐100%] versus 48.3% [95% CI, 38%‐61%]; P = 0.168, NS) and similar to 3392 biliary atresia cases (89.9%; 95% CI, 88.7%‐91%). Actuarial 10‐year patient survival for 17 EMBs, 10 METS, and 6 leiomyosarcoma patients exceeded 60%. These survival outcomes were similar to those seen for HBL. At CHP, posttransplant recurrence‐free and overall survival among 25 HCC, 17 (68%) of whom had preexisting liver disease, was 16/25 or 64%, and 9/25 or 36%, respectively. All 10 patients with incidental HCC and tumor‐node‐metastasis stage I and II HCC survived recurrence‐free. Only vascular invasion predicted poor survival in multivariate analysis (P < 0.0001). A total of 4 of 5 EMB patients (80%) and all patients with METS (neuroendocrine‐2, pseudopapillary pancreatic‐1) also survived recurrence‐free. Among children, LT can be curative for unresectable HCC confined to the liver and without vascular invasion, incidental HCC, embryonal tumors, and metastatic neuroendocrine tumors. Liver Transplantation 23 1577–1588 2017 AASLD.
Abbreviations
- AFP
alpha‐fetoprotein
- BA
biliary atresia
- BDCA
bile duct carcinoma
- CHP
Children's Hospital of Pittsburgh
- CI
confidence interval
- CIT
cold ischemia time
- EMB
nonhepatoblastoma embryonal tumor
- HBL
hepatoblastoma
- HCC
hepatocellular carcinoma
- LD
living donor
- LT
liver transplantation
- METS
metastatic liver tumors
- NA
not applicable
- NS
not significant
- PTLD
posttransplant lymphoproliferative disease
- PV
portal vein
- SARC
leiomyosarcoma
- SEC
sinusoidal endothelial cell
- SRTR
Scientific Registry of Transplant Recipients
- TACE
transarterial chemoembolization
- WIT
warm ischemia time
Although malignant liver tumors in children make up 1% of all pediatric tumors, those liver malignancies which are unresectable account for a tenth of all liver transplantations (LTs) performed in children in the United States.1, 2 Three‐fourths of these LTs are performed for hepatoblastoma (HBL), the most common liver cancer during childhood.2, 3 Indications for the remainder are diverse, and include hepatocellular carcinoma (HCC), metastatic neuroendocrine tumors confined to the liver after removal of the primary tumor, and nonhepatoblastoma embryonal tumors (EMBs), such as rhabdoid sarcoma, embryonal sarcoma, and angiosarcoma. Survival after LT for HBL approaches 80% in multiple reports including those from our center.2, 3, 4 Less is known about longterm outcomes after LT for these other hepatic malignancies because of their rarity. The reported experience is mostly based on modest numbers from single‐center or multicenter reports including ours.5, 6, 7, 8, 9, 10, 11, 12, 13
To better understand the outcomes of LT for hepatocellular cancer and non‐HBL liver malignancies, we first describe the United States multicenter experience with LT for pediatric liver malignancy. We then re‐evaluate and update our single‐center experience by focusing on outcomes of LT for hepatocellular cancer and non‐HBL liver malignancies. In both data sets, HBL, the reference malignancy requiring pediatric LT, is included only to assess relative outcomes of non‐HBL malignancies requiring LT.
Patients and Methods
SCIENTIFIC REGISTRY OF TRANSPLANT RECIPIENTS 1987‐2015
The Scientific Registry of Transplant Recipients (SRTR) database contains data on donors, recipients, and listed candidates from all centers in the United States.9 For subjects < 21 years with LT for liver malignancy in the United States, demographics and graft and patient characteristics impacting survival were obtained from the SRTR. Details of tumor histology, stage, and chemotherapy are not available in this database. To provide a reference for survival outcomes, the SRTR survival analysis also included children who received LT for biliary atresia (BA), the most common nonmalignant indication for LT. “Incidental” HCC was presumed when HCC was listed as the posttransplant diagnosis but not the pretransplant diagnosis.
CHILDREN'S HOSPITAL OF PITTSBURGH EXPERIENCE 1981‐2014
A retrospective chart review of 74 children with unresectable liver malignancy who received LT was conducted after approval from the institutional review board of the University of Pittsburgh (institutional review board protocol PRO15100064). Described previously, parameters recorded were age, sex, ethnicity, tumor size, margins, histology, necrosis, vascular invasion, lymph node invasion, and alpha‐fetoprotein (AFP).2, 5 Treatment parameters recorded were clinical tumor‐node‐metastasis stage as described in the seventh edition of the American Joint Committee on Cancer Staging Manual, chemotherapy, graft and patient characteristics impacting survival such as donor and graft type, preexisting liver disease, explant histology, and posttransplant recurrence. HCC discovered incidentally in the liver explanted for preexisting nonmalignant liver disease was termed incidental HCC.
STATISTICAL ANALYSIS
Descriptive and inferential statistics included distribution of nonparametric and parametric variables. Fisher's exact test was used for the comparison of small groups, and Kruskal‐Wallis test was used to detect differences in the rank value among 3 or more tumor categories for continuous data, which did not follow normal distribution. Kaplan‐Meier survival analysis was used for time‐to‐event data. Log‐rank test was used to detect differences in the survival distributions between categories. A P value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS, version 22 (IBM, Armonk, NY) and with R, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria) using “survival” and “KMsurv” packages.14, 15
SRTR RESULTS
Demographics are detailed in Supporting Table 1. Bile duct carcinoma (BDCA) and leiomyosarcoma (SARC) represented 2 additional categories analyzed in the SRTR data for 677 LT recipients that were not present in our single‐center experience. Mean age at LT for malignancy was 5.5 years and was lower for HBL (2.9 years) compared with HCC (12.8 years) or other categories of malignancy (range, 8.4‐13.4 years). Male:female sex distribution was equal in HCC (78:71), skewed toward male distribution in HBL (304:186) and sarcoma (SARC, 4:2) and female distribution in BDCA (2:3), metastatic tumors (4:6), and embryonal sarcoma (5:12). Children were predominantly Caucasian. Allografts were predominantly cadaveric, with living donor (LD) grafts constituting a tenth of LT in each cancer subcategory. Overall, roughly 65% of affected children received whole liver grafts, and 35% received segmental grafts (440 versus 237 patients). Compared with other categories, more segmental grafts than whole liver grafts were used in HBL (294:196 or 60%:40%) and metastatic tumors (6:4 or 60%:40%). LT for HCC and HBL roughly doubled in the most recent 10‐year era compared with the preceding 9‐year era, to 70 HCC cases from 42 cases in the previous era, and to 305 HBL cases from 141 cases in the previous era (P < 0.001). As with HBL, more segmental grafts were also used in the reference population of BA (whole:segmental, 1838:1558 or 54%:46%) reflecting similar mean age at presentation (2.9 versus 2 years, respectively) and the scarcity of size‐matched cadaveric whole liver allografts from pediatric donors in the same age groups.
SURVIVAL
Ten‐year graft and patient survival was significantly different after LT for the various types of malignancies and were lower among children with HCC and BDCA, compared with HBL and other types of cancer (Fig. 1). Survival trends can be better understood by using (BA), the dominant nonmalignant indication for LT, as a reference liver disease and focusing initially on HCC and HBL as prototypical liver malignancies because of reasonable numbers of subjects in these 3 categories: 3396 versus 149 versus 490, respectively.
Figure 1.
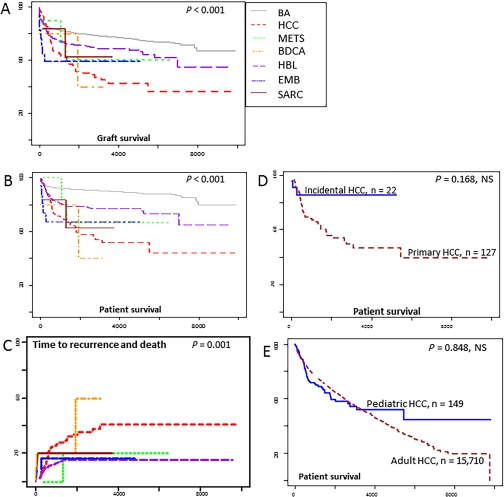
(A) Graft and (B) patient survival after LT for pediatric liver malignancies in the SRTR data set (1987‐2015). Also included are respective survival curves for BA, the most common nonmalignant indication for pediatric LT. (C) Time to recurrence‐related death after LT for the various liver malignancies. (D) Differences in patient survival for children in whom HCC is diagnosed in the explant (blue line, incidental HCC) versus children in whom HCC is the primary diagnosis at the time of LT (red lines), and (E) patient survival for children (blue lines) and adults (red lines) who received LT for HCC. Between‐group comparisons are performed with log‐rank tests using Kaplan‐Meier analysis.
One‐year graft survival for BA, HCC, and HBL was similar at 85.5%, 85%, and 81.5%, respectively, with overlapping confidence intervals (CIs; Table 1). At 5 and 10 years after LT, the decrease in graft survival in the BA (from 80.4% to 77.5%) and HBL (from 71.6% to 69%) subcategories are relatively smaller compared with a continued decline in the HCC group, from 85% at 1 year to 51.7% at 5 years, and 42.7% at 10 years. At 5 and 10 years after LT, CIs for graft survival show no overlap for the BA, HCC, and HBL subcategories (P < 0.001). Survival trends for BDCA were similar to those for HCC, whereas trends for metastatic liver tumors (METS), EMB, and SARC were similar to those for HBL, as suggested by overlapping CIs. Patient survival trends mirrored those for graft survival for all subcategories (Table 1).
Table 1.
Graft and Patient Survival at 1, 5, and 10 Years After LT for HCC, BDCA, HBL, EMB, SARC, and METS
| 95% CI | 95% CI | 95% CI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | n | 1 Year | Lower | Upper | 5 Years | Lower | Upper | 10 Years | Lower | Upper | P Value |
| Graft survival | <0.001 | ||||||||||
| BA | 3396 | 0.855 | 0.843 | 0.867 | 0.804 | 0.790 | 0.818 | 0.775 | 0.759 | 0.790 | |
| HCC | 149 | 0.850 | 0.792 | 0.911 | 0.517 | 0.433 | 0.618 | 0.332 | 0.547 | ||
| BDCA | 5 | 0.800 | 0.516 | 1 | 0.800 | 0.516 | 1 | 0.400 | 0.094 | 1 | |
| HBL | 490 | 0.815 | 0.780 | 0.851 | 0.716 | 0.674 | 0.760 | 0.690 | 0.644 | 0.739 | |
| EMB | 17 | 0.588 | 0.395 | 0.876 | 0.588 | 0.395 | 0.876 | 0.588 | 0.395 | 0.876 | |
| SARC | 6 | 0.833 | 0.583 | 1 | 0.625 | 0.320 | 1 | 0.625 | 0.320 | 1 | |
| SEC | 10 | 0.900 | 0.732 | 1 | 0.600 | 0.329 | 1 | 0.600 | 0.329 | 1 | |
| Patient survival | <0.001 | ||||||||||
| BA | 3392 | 0.937 | 0.928 | 0.945 | 0.914 | 0.904 | 0.924 | 0.899 | 0.887 | 0.910 | |
| HCC | 149 | 0.890 | 0.838 | 0.944 | 0.592 | 0.505 | 0.695 | 0.516 | 0.417 | 0.637 | |
| BDCA | 5 | 0.800 | 0.516 | 1 | 0.800 | 0.516 | 1 | 0.400 | 0.094 | 1 | |
| HBL | 490 | 0.874 | 0.844 | 0.906 | 0.788 | 0.748 | 0.830 | 0.771 | 0.728 | 0.816 | |
| EMB | 17 | 0.672 | 0.473 | 0.956 | 0.672 | 0.473 | 0.956 | 0.672 | 0.473 | 0.956 | |
| SARC | 6 | 0.833 | 0.583 | 1 | 0.625 | 0.320 | 1 | 0.625 | 0.320 | 1 | |
| SEC | 10 | 0.999 | / | 1 | 0.667 | 0.379 | 1 | 0.667 | 0.379 | 1 | |
NOTE: BA, the most common nonmalignant indication for LT in children, serves as the reference group. SRTR, 1988‐2014.
The HCC subcategory was analyzed further to address whether LT results in better outcomes in children affected with HCC compared with adults. No such differences were demonstrated for graft survival between 149 children < 21 years and 15,714 adults ≥ 21 years (P = 0.402, not significant [NS]) and patient survival between 149 children and 15,710 adults (P = 0.848, NS; Table 2). We next asked whether “incidental” HCC in livers removed for another primary disease (n = 22) was associated with better survival compared with children who presented for LT with a pretransplant diagnosis of HCC (n = 127). In the small subset with incidental HCC, the numerically higher 10‐year graft (64.1% versus 40.5%; P = 0.441, NS) and patient (85% versus 48.3%; P = 0.168, NS) survival was NS when compared with survival in the primary HCC subset. Notably, however, the CIs for patient survival for incidental HCC (70.6%‐100%) show no overlap with those for primary HCC (38.1%‐61.3%) suggesting that these differences may achieve significance as sample size increases. It is also noteworthy that 10‐year patient survival for incidental HCC (85%; 95% CI, 70.6%‐100%) was similar to that for BA (89.9%; 95% CI, 88.7%‐91%).
Table 2.
Patient Survival After LT for HCC in Children <21 Years and Adults ≥ 21 Years, and in Children With Primary HCC and Children Found to Have HCC in the Explant
| 95% CI | 95% CI | 95% CI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All HCC Cases | N | 1 Year | Lower | Upper | 5 Years | Lower | Upper | 10 Years | Lower | Upper | P Value |
| Age < 21 years | 149 | 0.890 | 0.838 | 0.944 | 0.592 | 0.505 | 0.695 | 0.516 | 0.417 | 0.637 | 0.848, NS |
| Pre‐LT diagnosis: non‐HCC | 22 | 0.850 | 0.706 | 1 | 0.850 | 0.706 | 1 | 0.850 | 0.706 | 1 | 0.168, NS |
| Pre‐LT diagnosis: HCC | 127 | 0.897 | 0.844 | 0.954 | 0.564 | 0.471 | 0.675 | 0.483 | 0.381 | 0.613 | |
| Age ≥ 21 years | 15,710 | 0.880 | 0.875 | 0.885 | 0.665 | 0.656 | 0.874 | 0.496 | 0.481 | 0.511 | |
NOTE: SRTR data 1988‐2014.
DEATH AND GRAFT LOSS
Death occurred in 153 of 677 total recipients (22.6%) with unresectable liver cancer. Recurrence, the most common cause of death, was more likely in HCC (35/149, 23.5%) and BDCA (2/5, 40%) compared with HBL (51/490, 10.4%), METS (1/10, 10%), EMB (2/17, 11.8%), and SARC (1/6; 16.7%; P = 0.001; Supporting Table 2). Mortality due to hemorrhage, sepsis, single organ failure, cardiovascular events, and unknown/other causes ranged in incidence from <1% to 5% and was similar between groups. Graft loss requiring retransplantation occurred in 60 of 677 (9%) children. Vascular thrombosis was the most common cause of graft loss, with higher incidence in HBL (26/490, 5.3%), EMB (1/17, 5.9%), and METS (1/10, 10%) and lower incidence in HCC (4/149, 2.7%) and other tumors (P = 0.706, NS). Additional causes of graft loss requiring retransplantation, such as primary graft failure, rejection, biliary complications, and recurrence, occurred in 2% or fewer children in any cancer category (Supporting Table 2). The risk of death due to recurrence persisted longer after LT for HCC and BDCA compared with other types of cancer (Fig. 1).
CHILDREN'S HOSPITAL OF PITTSBURGH RESULTS
A total of 74 children, 39 males and 35 females, received LT for unresectable liver malignancies during a 33‐year period (1981‐2014) at the Children's Hospital of Pittsburgh (CHP). Nonhepatoblastoma malignancies were HCC in 25 children (Table 3; Fig. 2), EMB (embryonal sarcoma, 3; angiosarcoma, 1; rhabdoid sarcoma, 1) in 5 children, and METS in 3 children. These secondary tumors represented slow‐growing metastases in the liver discovered after resection of the primary tumor and consisted of 2 neuroendocrine tumors and 1 pseudopapillary tumor of the pancreas. HBL accounted for 42 LTs in 41 children, and updates our previous analysis of 35 of these children.2 A total of 63 children received cadaveric grafts (56 whole grafts and 7 reduced liver left lateral segment grafts) and 11 children received LD left lateral segmental grafts (Table 3). Mean patient follow‐up was 1180 days in METS, 1396 days in EMB, 2750 days in HCC, and 3075 days in HBL cases, and actuarial 10‐year post‐LT patient survival was significantly lower for HCC, compared with other liver malignancies (P = 0.011, Kaplan‐Meier test; Supporting Table 3).
Table 3.
Patient Demographics: CHP Experience
| HCC (n = 25) | EMB (n = 5) | METS (n = 3) | HBL (n = 41) | P Value | |
|---|---|---|---|---|---|
| Age, years, mean (range) | 9.6 (1‐25) | 3.6 (1‐7) | 16.3 (14‐19) | 2.4 (0‐6) | <0.001 |
| Sex, n | |||||
| Female | 14 | 2 | 1 | 19 | 0.787 |
| Male | 11 | 3 | 2 | 22 | |
| Weight, mean (range) | 29.8 (8.3‐93.8) | 17.2 (10.0‐27.6) | 73.8 (47.0‐103.8) | 13.8 (7.1‐46.0) | <0.001 |
| Organ type, n | 0.114 | ||||
| Reduced | 2 | 1 | 1 | 14 | |
| Whole | 23 | 4 | 2 | 27 | |
| Donor type, n | 0.205 | ||||
| Cadaveric | 24 | 4 | 3 | 32 | |
| Living | 1 | 1 | 0 | 9 | |
| CIT, minutes | 568.8 | 440.6 | 505.7 | 430.9 | 0.238 |
| WIT, minutes | 46.6 | 40.4 | 46 | 48.7 | 0.243 |
| Donor age, years | 11.0 | 4.8 | 17.3 | 13.6 | 0.393 |
| Pretreatment AFP, mean | 259 | 23 | 4 | 105,433 | 0.001 |
| AFT at the time of transplant, mean | 67.5 | NA | NA | 58,251 | 0.077 |
| Overall survival | 9 (36%) | 4 (80%) | 3 (100%) | 34 (83%) | 0.001 |
| Death due to recurrence | 9 (36%) | 1 (20%) | 0 (0%) | 5 (12%) | 0.100 |
Figure 2.
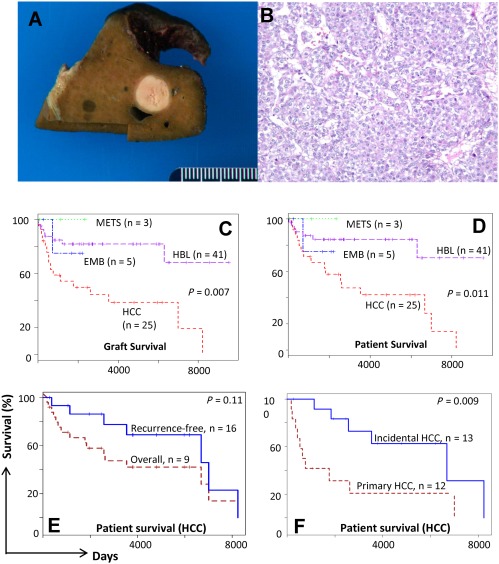
HCC and survival: (A) Liver explant from a child with familial intrahepatic cholestasis shows a discrete 2‐cm HCC nodule in a cholestatic background. (B) Histologic section shows well‐differentiated HCC. Broad trabeculae contain tumor cells with large vesicular nuclei and prominent nucleoli (×200). (C) Graft and (D) patient survival after LT for all liver malignancies at CHP. (E) Overall and recurrence‐free patient survival after LT for HCC. (F) Patient survival after LT for primary HCC and incidental HCC (discovered in the explant).
Hepatocellular Carcinoma
A total of 25 children, 11 boys and 14 girls, received LT for HCC with whole liver cadaveric allografts (n = 23), left lateral segmental cadaveric (n = 1) and LD grafts (n = 1; Table 3). Mean age at LT was 9.6 years (range, 1‐25 years). Tumor characteristics are shown in Table 4. The tumor was stage 1 or 2 in 16 children, stage 3 in 5 children, and stage 4 in 4 children. A total of 17 of 25 (68%) children also had preexisting nonmalignant liver disease requiring LT. Pre‐existing diseases were tyrosinemia in 6 patients, familial cholestasis in 2 patients, cirrhosis due to cholestatic liver disease/uncertain etiology in 5 patients, hepatitis B in 2 patients, and metabolic disorder in 2 patients. Six children had solitary tumors, 3 had 2 lesions, and 16 had multiple (3 or more, 64%) lesions. Other salient findings were associated cirrhosis in 15 (64%) patients, and tumors discovered incidentally in the explant after LT for nonmalignant liver disease in 10 (40%) patients. AFP levels were elevated in 13 of 18 (72%) children in whom they were measured. At LT and in explants, regional node involvement was seen in 4 patients. Margins were tumor‐free in 23 patients and involved in 2 patients. Microvascular invasion occurred in 11 patients, 5 of whom showed compression or occlusion of major intrahepatic veins on imaging studies. Two other children with vascular occlusion on imaging did not demonstrate microvascular invasion in the explant.
Table 4.
Tumor Characteristics of 25 HCC Cases
| Patient Number | Diagnosis at LT | Preexisitng disease | Age (years), Sex | Cirrhosis | Milan Criteria | Size of the Largest HCC Lesion, cm | Lobea | Countb | Nodes | METS | Microvascular Involvement | Margins | Stage | Outcomec | AFP | Microvascular Occlusion or Compression on Imaging | Chemotherapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | Incidental HCC | Biliary artresia | 7, Female | No | NA | 7 × 5×3 | 1 | 1 | No | No | No | No | I | D‐S | |||
| 11 | Incidental HCC | Alagille | 25, Female | Yes | NA | 2 | 1 | 1 | No | No | No | No | I | D‐S | |||
| 24 | Incidental HCC | Tyrosinemia | 6.7, Male | Yes | NA | 1.5 | 1 | 1 | No | No | No | No | I | A | |||
| 1 | Incidental HCC | Cirrhosis | 4, Female | Yes | NA | 3 | 2 | ≥3 | No | No | Yes | No | II | A | |||
| 2 | Incidental HCC | PFIC | 9, Male | Yes | NA | 2.5 × 1.5 × 0.8 | 1 | 2 | No | No | No | No | II | D‐CR | |||
| 10 | Incidental HCC | Niemann pick | 10, Female | Yes | NA | 2.0;1.5 | 1 | 2 | No | No | No | No | II | D‐S | High | ||
| 13 | Incidental HCC | Giant cell hepatitis | 0.7, Male | Yes | NA | 0.7 | 2 | ≥3 | No | No | Yes | No | II | A | High | Systemic | |
| 15 | Incidental HCC | Tyrosinemia | 3, Female | Yes | NA | 2.5 | 2 | ≥3 | No | No | No | No | II | A | |||
| 21 | Incidental HCC | Tyrosinemia | 4, Female | Yes | NA | 2.5 | 2 | ≥3 | No | No | No | No | II | A | High | ||
| 25 | Incidental HCC | Tyrosinemia | 1.5, Female | Yes | NA | 0.9 | 2 | ≥3 | No | No | No | No | II | A | |||
| 18 | Pretransplant diagnosis of HCC | HCC | 11, Female | No | Outside | 13.5 × 12×9 | 1 | 1 | No | No | No | No | I | D‐U | Normal | Left PV absent | Systemic |
| 9 | Pretransplant diagnosis of HCC | Ceroid Lipofuscinosis | 7, Female | Yes | Outside | 6 | 1 | 1 | No | No | No | No | I | D‐U | Normal | Refused | |
| 7 | Pretransplant diagnosis of HCC | PFIC | 12, Female | Yes | Within | 2.2 | 1 | 1 | No | No | No | No | I | A | High | Systemic | |
| 16 | Pretransplant diagnosis of HCC | HCC | 14, Female | No | Outside | 15 × 5.5 × 5 | 1 | 1 | No | No | No | Yes | II | A | High | Systemic, TACE | |
| 3 | Pretransplant diagnosis of HCC | Cirrhosis | 15, Male | Yes | Outside | 3 | 1 | ≥3 | No | No | No | No | II | A | High | PV absent | |
| 22 | Pretransplant diagnosis of HCC | Tyrosinemia | 2, Female | Yes | Outside | 2 | 2 | >3 | No | No | No | No | II | D‐PTLD | High | ||
| 14 | Pretransplant diagnosis of HCC | Rec HCCd | 12, Male | No | Within | 2.5 | 2 | 2 | No | No | Yes | No | IIIB | D‐R | Normal | Systemic, arterial chemotherapy | |
| 20 | Pretransplant diagnosis of HCC | Hepatitis B/Rec HCCd | 13, Male | No | Outside | 2 | 2 | >3 | No | No | Yes | No | IIIB | D‐R | High | Systemic | |
| 23 | Pretransplant diagnosis of HCC | Primary HCC | 9, Male | No | Outside | >5 cm | 2 | ≥3 | No | No | Yes | No | IIIA | D‐R | High | Systemic, arterial chemotherapy | |
| 17 | Pretransplant diagnosis of HCC | Tyrosinemia | 6, Male | Yes | Outside | 3 | 1 | ≥3 | No | No | Yes | No | IIIB | D‐R | High | Left PV Occlusion | Systemic–posttransplant chemotherapy only |
| 19 | Pretransplant diagnosis of HCC | Hepatitis B | 10, Male | Yes | Outside | 5.8 × 4.3 × 5.0 | 1 | 2 | No | No | Yes | No | IIIB | D‐R | High | PV occlusion | Systemic |
| 4 | Pretransplant diagnosis of HCC | Primary HCC | 12, Female | No | Outside | 11.5 × 8.0 × 6.0 | 1 | ≥3 | Yes | No | Yes | No | IVA | D‐R | Normal | IVC compression | Arterial chemotherapy, Systemic–posttransplant chemotherapy only |
| 8 | Pretransplant diagnosis of HCC | Rec HCCd | 4, Male | No | Outside | 9 | 2 | ≥3 | Yes | No | Yes | No | IVA | D‐R | High | Systemic–posttransplant chemotherapy only | |
| 12 | Pretransplant diagnosis of HCC | Rec HCCd | 18, Male | Yes | Outside | >5 cm | 2 | >3 | Yes | No | Yes | Yes | IVA | D‐R | Normal | PV occlusion | Systemic, TACE |
| 6 | Pretransplant diagnosis of HCC | Primary HCC | 17, Female | No | Outside | 4.8 × 3.2 × 2.5 | 1 | 1 | Yes | Yes | Yes | No | IVB | D‐R | High | PV occlusion | Systemic, TACE |
NOTE: Patient numbers 4 and 12 had fibrolamellar HCC. Milan criteria for LT: solitary lesion < 5 cm, largest of 3 lesions < 3 cm, and no major vascular involvement.
Lobe + number of liver lobes involved.
Number of HCC lesions.
D, death; R, recurrence; S, sepsis; CR, chronic rejection.
HCC which recurred in the residual liver after trisegmentectomy.
A total of 13 children received chemotherapy, 11 before and 2 after LT. Systemic chemotherapy agents included varying combinations of Adriamycin, cisplatin, 5‐fluoro‐uracil, vincristine, and interferon. Six children also received transarterial chemotherapy with Adriamycin and cisplatin, which included transarterial chemoembolization (TACE) with gelfoam in 2 and microspheres in 1. TACE resulted in 100% tumor necrosis in 1 and tumor shrinkage in 2 patients. Systemic chemotherapy produced minimal or no response.
SURVIVAL OUTCOMES
The actual overall survival was 9/25 (36%) with a mean survival of 142.4 months (standard error, 23.9 months). A total of 16 children died: 9 due to recurrence/metastatic disease, 3 due to infection, 1 due to posttransplant lymphoproliferative disease (PTLD), 1 due to chronic rejection, and 2 due to unknown causes. Actual recurrence‐free survival was 16/25 or 64%. Among tumor factors contributing to death (Table 5), tumor stage (P < 0.001), vascular invasion (P < 0.001), lymph node involvement (P = 0.022), and incidental HCC (P = 0.011) were significant in univariate analysis. The respective mean patient survival time for stages 1, 2, 3, and 4 tumors was 187, 230, 14, and 44 months (P < 0.001). Recurrence accounted for all 4 deaths in stage 4 and all 5 deaths in stage 3 HCC. Nonrecurrence causes accounted for all 4 deaths in stage 1 and all 3 deaths in stage 2. Patient survival was also significantly better for patients in whom HCC was associated with preexisting nonmalignant liver disease compared with primary tumors (174 versus 83 months; P = 0.053), and for HCC detected incidentally in explants compared with HCC diagnosed before LT (mean, 223 versus 91 months; P = 0.011; Fig. 2). In stepwise logistic regression analysis incorporating the abovementioned variables, vascular invasion emerged as a significant determinant of poor survival (odds ratio = 13.3, P = 0.028). Of 17 children with pretransplant AFP measurements, AFP was elevated in 6 children who died of recurrence and 6 who were recurrence‐free. AFP was normal in 3 children who died of recurrence and 2 who were recurrence‐free.
Table 5.
Factors Affecting Survival After LT for HCC
| Patients | Total (n = 25) | Survival, n (%) | Mean Survival, Months | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 14 | 6 (42.9) | 180.2 | 0.027, NS |
| Male | 11 | 3 (27.3) | 89.2 | |
| Preexisting disease | ||||
| No | 8 | 1 (12.5) | 83.2 | 0.053, NS |
| Yes | 17 | 8 (47.1) | 173.6 | |
| Cirrhosis | ||||
| No | 10 | 1 (10) | 97.5 | 0.065, NS |
| Yes | 15 | 8 (53.3) | 178.1 | |
| Location | ||||
| Unilobar | 13 | 4 (30.8) | 154.5 | 0.885, NS |
| Bilobar | 12 | 5 (41.7) | 125.2 | |
| Number | ||||
| Single | 8 | 3 (37.5) | 179.8 | 0.436, NS |
| Multiple | 17 | 6 (35.3) | 123.8 | |
| Nodes | ||||
| No | 21 | 9 (42.9) | 163.1 | 0.022 |
| Yes | 4 | 0 (0.0) | 43.6 | |
| Vascular invasion | ||||
| No | 14 | 8 (57.1) | 218.9 | <0.001 |
| Yes | 11 | 1 (9.1) | 46.6 | |
| Metastasis | ||||
| No | 25 | 9 (36.0) | 142.4 | NA |
| Yes | 0 | 0 (0.0) | NA | |
| Margin | ||||
| Negative | 23 | 8 (34.8) | 142.7 | 0.951, NS |
| Positive | 2 | 1 (50) | 72.0 | |
| Incidental | ||||
| No | 15 | 3 (20) | 91.1 | 0.011 |
| Yes | 10 | 6 (60) | 222.8 | |
| Stage I | 6 | 2 (33.3) | 186.8 | <0.001 |
| Stage II | 10 | 7 (70) | 229.9 | |
| Stage III | 5 | 0 (0.0) | 14.3 | |
| Stage IV | 4 | 0 (0.0) | 43.6 |
Nonhepatoblastoma Embryonal Tumors
Of 5 such children, all 3 with primary embryonal sarcoma of liver, and 1 with rhabdoid sarcoma of the liver showed excellent response to chemotherapy and are alive (4/5, 80%) without recurrences after LT. The fifth child with angiosarcoma died due to recurrent disease at 24 months after LT.
Metastatic Liver Tumors
Three children underwent transplantation for slow growing multiple liver metastases due to neuroendocrine tumors (n = 2) and pseudopapillary (n = 1) tumors. All children are alive and disease‐free (100%).
Discussion
The multicenter SRTR data set supports the highly favorable outcomes of incidental HCC, while questioning the longstanding belief that pediatric HCC might behave differently from and have a better prognosis after LT than HCC in adults.5, 12 Among 149 children who received LT for HCC, 10‐year patient survival in 22 children, in whom HCC was diagnosed after LT (85%; 95% CI, 70.6%‐100%) was better than that seen in 127 children with primary HCC (48.3%; 95% CI, 38.1%‐61.3%; P = 0.168; Tables 1 and 2; Fig. 1), but was similar to that seen in children with BA (89.9%; 95% CI, 88.7%‐91%; P = NS). BA is the reference nonmalignant indication for LT in children. Furthermore, graft survival (P = 0.402, NS) and patient survival (P = 0.848, NS) were not different between 149 children and 15,710 adults who received LT for HCC. This can be explained in part by HCC recurrences, which continued to occur for a significantly longer period after LT, compared with other tumors in our single‐center experience (Fig. 2). The exception was BDCA, for which patient and graft survival and time to recurrence were similar to HCC.
Our single‐center review of LT for liver malignancy shows that two‐thirds of children with HCC (16/25) survived recurrence‐free (Tables 3 and 4). Overall survival was lower because of nonrecurrence‐related death such as technical factors, PTLD, sepsis, or unknown causes in 7 (28%) additional children (Table 4). The higher overall death rate in the CHP cohort is accounted for by higher nonrecurrence‐related deaths, compared with the SRTR cohort (7/25 versus 16/149, respectively; P = 0.027, Fishers’ exact). This difference reflects the earlier part of our experience when patient selection and immunosuppressive regimens were still in evolution. All deaths occurred between 1981 and 1987, a time period that antedates the SRTR database which begins in 1988. In contrast, recurrence‐related deaths were not different between the SRTR and CHP cohorts (9/25 versus 35/149; P = 0.215, NS, Fisher's exact). Additional era‐based contributions to survival were difficult to estimate in the modest cohort of 25 HCC LTs accrued over 34 years. Significant predictors of recurrence were tumor stage and determinants of tumor stage, such as vascular invasion and lymph node involvement (Table 3). All 9 children with stages 3 and 4 HCC died of recurrence. Four of these deaths occurred within 2 years after LT in those children in whom LT was performed to remove recurrence in the residual liver after trisegmentectomy for large tumors. In contrast, none of the 7 children who died after LT for stage 1 and 2 HCC died of recurrence. Therefore, early detection remains the key to curing children with unresectable HCC with LT.
Early detection of HCC is possible in children because two‐thirds or 17 of 25 cases occurred in the setting of preexisting nonmalignant liver disease requiring LT (Table 4). These children demonstrated better survival compared with those with primary HCC (median, 223 versus 91 months; P = 0.011), likely because the tumor was removed at an earlier stage in its development. No recurrences were seen among 10 of these 17 children in whom HCC was discovered incidentally in the explant, an outcome that has also been reported from other case series.11 Compared with the European multicenter Childhood Liver Tumor Strategy Group of the Société Internationale d'Oncologie Pédiatrique experience, in which a third of all HCC cases occurred with preexisting liver disease, our single‐center series had twice as many such cases.16 This high incidence can be attributed in part to our inclusion of incidental tumors, a common presentation for HCC arising in a diseased liver. A previous study reported incidental HCC in explants from 12 of the 16 patients (75%) undergoing LT for hereditary tyrosinemia.10 Although AFP can be elevated with cirrhotic liver disease, progressive increases can facilitate early detection and lead to early LT in children with preexisting liver disease. AFP levels were elevated in >70% of all tumors including 3 children with Niemann‐Pick disease, giant cell hepatitis, and tyrosinemia (Table 4). In these children, HCC was suspected before LT, but it could not be confirmed despite repeat imaging in 2 patients and biopsy in 1 patient. All 3 children demonstrated HCC in the explant. Imaging techniques have improved markedly during the 3 decades of the CHP experience. These advances should permit pretransplant detection of smaller lesions, as well as a diffuse segmental HCC tumor, which was detected incidentally in a 7‐cm caudate lobe in the early part of our experience.
Because of the rarity of unresectable pediatric HCC, individualized assessment of LT candidacy will remain the preferred approach. This task is challenging because traditional tumor biology must be inferred from clinical findings and the limited published experience. To illustrate, we applied the Milan criteria, a solitary tumor <5 cm, largest of up to 3 lesions < 3 cm, and absence of major vascular involvement, to 15 of 25 children in whom the diagnosis of HCC was made before LT (Table 4). Of 13 children outside the Milan criteria, 5 experienced recurrence‐free survival and would have been denied lifesaving LT if the Milan criteria had been enforced. Of 2 children within Milan criteria, 1 experienced death due to recurrence, questioning whether the criteria originally identified in adult patients with cirrhosis with HCC can be applied to children. In multivariate analysis, which has the obvious limitation of modest cohort size, recurrence was only associated with microvascular invasion, which is usually discovered in the explant. Major venous compression or occlusion on pretransplant imaging may be an important guide, because 5 of 7 children with abnormal imaging demonstrated microvascular invasion. Interestingly, microvascular invasion was also present in 2 children with incidental HCC, both of whom survived (Table 4). Potential explanations include the smaller tumor burden in incidental tumors compared with the remaining HCC cases, as evidenced by the median size of the largest lesion in respective groups, 2.25 cm (range, 0.9‐7 cm) versus 5.8 cm (range, 2‐15 cm; P = 0.003). Another explanation may be that 1 of these children also received 4 cycles of posttransplant chemotherapy with Adriamycin and cisplatin. Tumor chemosensitivity is another important marker of tumor biology. Intra‐arterial chemotherapy reduced the size of recurrent HCC lesions after trisegmentectomy in a 12‐year‐old boy. He died of recurrence after LT. TACE was followed by tumor shrinkage in all 3 patients (Table 4). A 14‐year‐old girl with a 15‐cm HCC tumor experienced 100% tumor necrosis with TACE and is alive after LT. In a 15‐year‐old female, the tumor shrank from 8 cm at diagnosis to 4.8 cm after TACE, and extrahepatic nodal involvement discovered at staging laparotomy resolved with radiation therapy. She survived for 84 months after LT but died of recurrence eventually. An 18‐year‐old male developed recurrent HCC after a trisegmentectomy and experienced modest reduction in tumor size with TACE. He died of recurrence within 2 years after LT. Whether sorafenib added to pretransplant chemotherapy with cisplatin and Adriamycin is more effective at downstaging HCC will require additional experience.17
Excellent outcomes have been reported after LT for embryonal liver tumors other than HBL in small cases series (Table 1; Fig. 1).18, 19, 20 In the SRTR data set, 15 of 17 or 88% of children with embryonal liver tumors and 5 of 6 or 83% children with SARC experienced recurrence‐free survival. At our center, all 3 children with embryonal sarcoma and 1 with rhabdoid sarcoma are alive and disease‐free 5 years after LT, corroborating multicenter results (Table 3). The only mortality was a child transplanted for angiosarcoma of the liver, for which the prognosis remains poor historically.16, 21, 22 Survival after LT for metastatic neuroendocrine tumors was similarly excellent. Nine of 10 children in the SRTR cohort, and all 3 children in the CHP cohort survived recurrence‐free (Table 1; Supporting Table 3). The durability of these results must be confirmed with additional follow‐up, because of variable overall survival of 52% and disease‐free survival of 30% in 1 series, and 90% overall survival with a high rate of recurrence in another.23, 24 A previous analysis of predominantly adult recipients with LT for METS in the United Network for Organ Sharing database revealed 5‐year survival of 49%.25
In summary, LT for HCC in children can be curative with lower stage tumors, if vascular invasion has not occurred or if HCC is discovered incidentally or in the setting of preexisting nonmalignant liver disease. Tumor surveillance with serial AFP measurements and imaging may lead to early detection of HCC in the chronically diseased liver. Recurrences are related to tumor burden indicated by disease stage and lymph node invasion, rescue LT, as well as vascular invasion. With the exception of angiosarcoma, for which LT should be avoided, unresectable rare embryonal tumors have highly favorable outcomes and therefore merit consideration for LT if the disease is confined to the liver, as with HBL. Metastatic neuroendocrine liver tumors also demonstrate excellent posttransplant outcomes, as do SARCs.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information
Acknowledgments
We thank Ms. Zecca for manuscript preparation. We acknowledge funding support from the Hillman Foundation of Pittsburgh and the Szalay Family foundation.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Dickinson DM, Arrington CJ, Fant G, Levine GN, Schaubel DE, Pruett TL, et al. SRTR program‐specific reports on outcomes: a guide for the new reader. Am J Transplant 2008;8(pt 2):1012‐1026. [DOI] [PubMed] [Google Scholar]
- 2. Cruz RJ Jr, Ranganathan S, Mazariegos G, Soltys K, Nayyar N, Sun Q, et al. Analysis of national and single‐center incidence and survival after liver transplantation for hepatoblastoma: new trends and future opportunities. Surgery 2013;153:150‐159. [DOI] [PubMed] [Google Scholar]
- 3. Sakamoto S, Kasahara M, Mizuta K, Kuroda T, Yagi T, Taguchi T, et al. Nationwide survey of the outcomes of living donor liver transplantation for hepatoblastoma in Japan. Liver Transpl 2014;20:333‐346. [DOI] [PubMed] [Google Scholar]
- 4. Austin MT, Leys CM, Feurer ID, Lovvorn HN 3rd, O'Neill JA Jr, Pinson CW, Pietsch JB. Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 2006;41:182‐186. [DOI] [PubMed] [Google Scholar]
- 5. Reyes JD, Carr B, Dvorchik I, Kocoshis S, Jaffe R, Gerber D, et al. Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 2000;136:795‐804. [PubMed] [Google Scholar]
- 6. Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 2006;44:478‐486. [DOI] [PubMed] [Google Scholar]
- 7. Beaunoyer M, Vanatta JM, Ogihara M, Strichartz D, Dahl G, Berquist WE, et al. Outcomes of transplantation in children with primary hepatic malignancy. Pediatr Transplant 2007;11:655‐660. [DOI] [PubMed] [Google Scholar]
- 8. Hadžić N, Quaglia A, Portmann B, Paramalingam S, Heaton ND, Rela M, et al. Hepatocellular carcinoma in biliary atresia: King's College Hospital experience. J Pediatr 2011;159:617‐622. [DOI] [PubMed] [Google Scholar]
- 9. Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, Aronson D, et al.; for Liver Tumors Study Group of the International Society of Pediatric Oncology . Hepatocellular carcinoma in children: results of the first prospective Study of the International Society of Pediatric Oncology group. J Clin Oncol 2002;20:2798‐2804. [DOI] [PubMed] [Google Scholar]
- 10. Seda Neto J, Leite KM, Porta A, Fonseca EA, Feier FH, Pugliese R, et al. HCC prevalence and histopathological findings in liver explants of patients with hereditary tyrosinemia type 1. Pediatr Blood Cancer 2014;61:1584‐1589. [DOI] [PubMed] [Google Scholar]
- 11. Romano F, Stroppa P, Bravi M, Casotti V, Lucianetti A, Guizzetti M, et al. Favorable outcome of primary liver transplantation in children with cirrhosis and hepatocellular carcinoma. Pediatr Transplant 2011;15:573‐579. [DOI] [PubMed] [Google Scholar]
- 12. Ismail H, Broniszczak D, Kaliciński P, Markiewicz‐Kijewska M, Teisseyre J, Stefanowicz M, et al. Liver transplantation in children with hepatocellular carcinoma. Do Milan criteria apply to pediatric patients? Pediatr Transplant 2009;13:682‐692. [DOI] [PubMed] [Google Scholar]
- 13. Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Liu‐Mares W, Douglass EC, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group intergroup study. J Clin Oncol 2002;20:2789‐2797. [DOI] [PubMed] [Google Scholar]
- 14. Klein JP, Moeschberger ML, Therneau T. A package for survival analysis in S. version 2.38. http://CRAN.R-project.org/package=survival. Accessed March 1, 2017.
- 15. Klein JP, Moeschberger ML, Yan J. KMsurv: data sets from Klein and Moeschberger (1997), survival analysis. http://CRAN.R-project.org/package=KMsurv. Accessed March 1, 2017.
- 16. Maluf D, Cotterell A, Clark B, Stravitz T, Kauffman HM, Fisher RA. Hepatic angiosarcoma and liver transplantation: case report and literature review. Transplant Proc 2005;37:2195‐2199. [DOI] [PubMed] [Google Scholar]
- 17. Schmid I, Häberle B, Albert MH, Corbacioglu S, Fröhlich B, Graf N, et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer 2012;58:539‐544. [DOI] [PubMed] [Google Scholar]
- 18. Walther A, Geller J, Coots A, Towbin A, Nathan J, Alonso M, et al. Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl 2014;20:191‐199. [DOI] [PubMed] [Google Scholar]
- 19. Plant AS, Busuttil RW, Rana A, Nelson SD, Auerbach M, Federman NC. A single‐institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol 2013;35:451‐455. [DOI] [PubMed] [Google Scholar]
- 20. Kelly MJ, Martin L, Alonso M, Altura RA. Liver transplant for relapsed undifferentiated embryonal sarcoma in a young child. J Pediatr Surg 2009;44:e1‐3. [DOI] [PubMed] [Google Scholar]
- 21. Bonaccorsi‐Riani E, Lerut JP. Liver transplantation and vascular tumours. Transpl Int 2010;23:686‐691. [DOI] [PubMed] [Google Scholar]
- 22. Orlando G, Adam R, Mirza D, Soderdahl G, Porte RJ, Paul A, et al. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation‐‐the European Liver Transplant Registry experience. Transplantation 2013;95:872‐877. [DOI] [PubMed] [Google Scholar]
- 23. Le Treut YP, Grégoire E, Klempnauer J, Belghiti J, Jouve E, Lerut J, et al.; for ELITA . Liver transplantation for neuroendocrine tumors in Europe‐results and trends in patient selection: a 213‐case European liver transplant registry study. Ann Surg 2013;257:807‐815. [DOI] [PubMed] [Google Scholar]
- 24. Rossi RE, Burroughs AK, Caplin ME. Liver transplantation for unresectable neuroendocrine tumor liver metastases. Ann Surg Oncol 2014;21:2398‐2405. [DOI] [PubMed] [Google Scholar]
- 25. Gedaly R, Daily MF, Davenport D, McHugh PP, Koch A, Angulo P, Hundley JC. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg 2011;146:953‐958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information


