ABSTRACT
Aim
Higher dosages of erythropoiesis‐stimulating agents (ESAs) have been associated with adverse effects. Intravenous iron is used to optimize ESA response and reduces ESA doses in haemodialysis patients; this meta‐analysis evaluates the magnitude of this effect.
Methods
A literature search was performed using MEDLINE, Embase and the Cochrane Collaboration Central Register of Clinical Trials from inception until December 2014, to identify randomized controlled trials of intravenous iron and ESA, in patients undergoing haemodialysis for end‐stage kidney disease. Dosing of IV iron in concordance with the Kidney Disease Improving Global Outcomes guidelines was considered optimal iron therapy.
Results
Of the 28 randomized controlled trials identified, seven met the criteria for inclusion in the meta‐analysis. Results of random‐effects meta‐analysis show a statistically significant weighted mean (95% CI) difference of −1733 [−3073, −392] units/week in ESA dose for optimal iron versus suboptimal iron. The weighted average change in ESA dose was a reduction of 23% (range −7% to −55%) attributable to appropriate dosing of intravenous iron. A comparison of intravenous iron versus oral iron/no iron (five trials) showed a greater reduction in ESA dose, although this did not reach statistical significance (weighted mean difference, 95% CI: −2,433 [−5183, 318] units/week). The weighted average change in ESA dose across the five trials was a reduction of 31% (range −8% to −55%).
Conclusion
Significant reductions in ESA dosing may be achieved with optimal intravenous iron usage in the haemodialysis population, and suboptimal iron use may require higher ESA dosing to manage anaemia.
Keywords: chronic kidney disease, epoetin, ESA, haemodialysis, intravenous iron, meta‐analysis
Summary at a Glance
This review manuscript evaluated the magnitude of ESA dose reduction in relation to optimal or suboptimal iron treatment. Optimal iron therapy should allow reduction of ESA dosages, allowing a possible cost saving.
Iron deficiency anaemia is common in patients with chronic kidney disease (CKD), and this may be exacerbated by the use of erythropoiesis‐stimulating agents (ESAs).1 Patients with CKD requiring haemodialysis (HD) are at increased risk of iron deficiency anaemia as they are subject to repeated blood loss due to retention of blood in the dialyser and blood lines, frequent blood sampling for laboratory testing, and blood loss from surgical procedures, as well as reduced iron absorption due to inflammation and hepcidin upregulation, exacerbated by concomitant medications such as gastric acid inhibitors and phosphate binders.2, 3
There have been recent concerns around the toxicity of injectable ESAs (thromboembolic events and recurrent malignancies), which could be related to higher dosages of ESA used and consequently increased circulating blood levels of the ESA, rather than the target haemoglobin achieved.4 Moreover, iron deficiency is a common cause of hyporesponsiveness to ESAs, whether it is absolute or functional in nature. Beyond this, there has been a call for more conservative dosing strategies5 due to concerns with ESA‐associated cardiovascular morbidity.6, 7, 8, 9
As such, international treatment guidelines10, 11, 12, 13 strongly recommend the monitoring of iron status and the treatment of iron deficiency with intravenous (IV) iron ± ESA in patients undergoing chronic HD. Although it is generally accepted that the treatment of iron deficiency with IV iron allows a reduction in ESA dose, quantification of the magnitude of the reduction is challenging, because of heterogeneity in study designs that could allow this assessment.
Three systematic reviews of the use of IV iron in patients with CKD have been identified in the medical literature. In 2008, Rozen‐Zvi et al. assessed trials of IV iron versus oral iron in patients with CKD, including those undergoing dialysis.14 The same group have recently repeated this exercise in the light of more recent literature.15 A Cochrane review comparing IV iron with oral iron in adults and children with CKD was published in 2012, 1 and a separate meta‐analysis was published in 2014 to assess the safety and efficacy of IV iron in HD patients with functional iron deficiency.16 The percentage reduction in ESA dose from baseline, however, was not reported in any of these reviews.
The aim of this meta‐analysis was to evaluate the magnitude of the effect (reduction) on ESA dosing when IV iron doses, (consistent with the Kidney Disease Improving Global Outcomes (KDIGO) guidelines), were administered in an adult HD population.
METHODS
Search strategy
A meta‐analysis of the available literature was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement.17 Published studies indexed in MEDLINE, Embase and the Cochrane Collaboration Central Register of Clinical Trials, Cochrane database of Systematic Reviews and Cochrane Database of Reviews of Effect were searched from inception to December 2014 using the search strategy described in Appendix I. A manual search for additional studies using references from retrieved articles was also performed.
Inclusion criteria
A systematic literature review was performed to identify randomized controlled trials (RCTs) investigating the efficacy and safety of IV iron, in conjunction with an ESA, in patients undergoing HD for end‐stage kidney failure. The participants, interventions, comparators, outcomes and study design (PICOS) approach was used. The complete PICOS inclusion and exclusion criteria are reported in Table 1. Adult patients undergoing HD were included regardless of gender, or race, provided that they were treated with IV iron in conjunction with an ESA.
Table 1.
Participants, interventions, comparators, outcomes and study design criteria for systematic literature review
| P – Patients | I – Interventions | C – Comparators | O – Outcomes | S – Study design | |
|---|---|---|---|---|---|
| Inclusion criteria | Adult patients on stable HD for CKD, regardless of gender, and race |
■ IV iron, including but not limited to ferric carboxymaltose, ferric chloride, ferric gluconate, iron dextran, iron dextrin, iron polymaltose, iron sucrose ■ Erythropoiesis stimulating agents, including but not limited to epoetin alfa, epoetin beta, darbepoetin alfa |
■ IV iron, oral iron ■ Placebo ■ No iron therapy |
ESA dose | Placebo‐controlled and active‐controlled RCTs with at least one arm randomized to an intervention of interest |
| Exclusion criteria | Patients on peritoneal dialysis or not stabilized on HD, patients with iron overload | Studies that do not include a treatment arm with IV iron and an ESA | Studies that do not include a treatment arm with any of the selected comparators of interest | Studies lacking relevant data on any clinical efficacy, safety and tolerability outcomes of interest | Studies that are not written in the English language; animal, in vitro, pharmacokinetic, or pharmacodynamic studies; reviews; letters to the editor; opinions; studies without abstracts; conference proceedings; pooled analyses or meta‐analyses; non‐randomized studies |
CKD, chronic kidney disease; ESA, erythropoiesis stimulating agent; HD, haemodialysis; IV, intravenous; RCT, randomized controlled trial.
Study eligibility was independently determined by two consultants from Commercial Eyes Pty Ltd. Differing decisions were resolved by mutual consensus.
Study validity assessment
The methodological quality of each study was assessed using the Cochrane Handbook for Systematic Reviews of Interventions.18 The presence of bias was assessed by using the ‘risk of bias’ table (Results).
Data extraction
After application of the PICOS criteria, a standardized data collection form was used to record the following information: last name of the first author, year of publication, study design, duration of follow up, baseline iron studies, sample size, intervention, target iron indices, ESA dose and outcomes measured. A feasibility assessment was subsequently performed to determine which trials could be included in a meta‐analysis. A subset of trials was excluded because of populations, outcomes or interventions that were not amenable to quantitative comparison.
Statistical analysis
Review Manager 5.2 software (RevMan) from the Cochrane Collaboration (Oxford, UK) was used for data analysis. The mean and standard deviation of the baseline and endpoint values were entered into RevMan, and meta‐estimates were generated. Standard deviations were estimated from standard errors where necessary, using the RevMan internal calculators. This provided the meta‐estimate for the mean difference (MD) or standardized mean difference between the intervention and control arms, as well as a 95% confidence interval (CI), a forest plot and a test for overall homogeneity. Heterogeneity between studies was measured using the I 2 statistic.19
RESULTS
The search strategy yielded 208 potentially relevant articles. Of these, 58 were duplicate records, leaving 150 citations for assessment. After application of the PICOS criteria described in Table 1, 23 met the criteria for inclusion in the systematic literature review (Fig. 1). Studies were excluded because they were not RCTs, did not include the population, intervention or outcome of interest or were proceedings abstracts, guidelines or reviews, or not written in English. There were no unresolved conflicts in the review process in terms of studies included or excluded.
Figure 1.
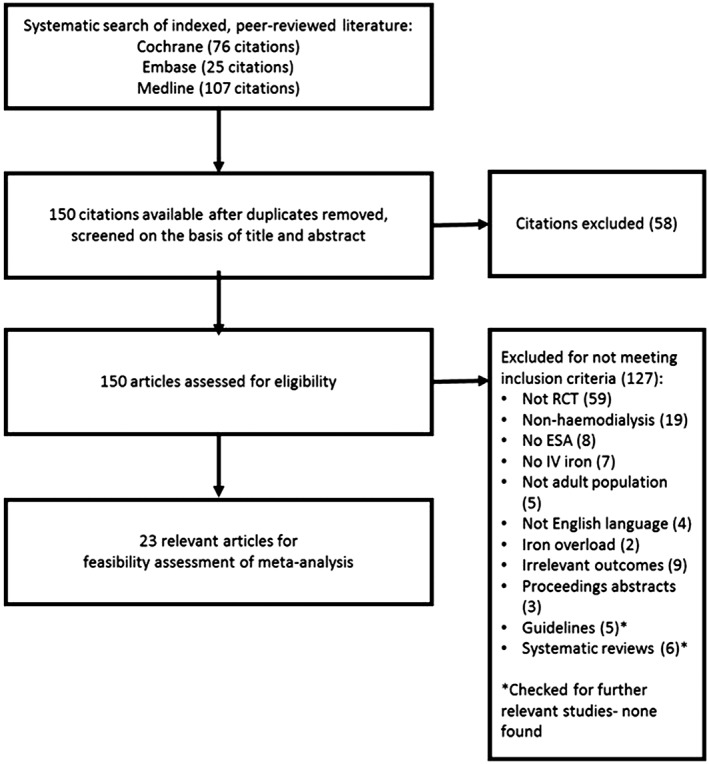
Study selection flow diagram. ESA, erythropoietin stimulating agent; IV, intravenous; RCT, randomized controlled trial.
Feasibility assessment
A feasibility assessment of the 23 studies was subsequently conducted, for inclusion in a meta‐analysis. As the primary aim of the meta‐analysis was to determine the effect of IV iron on ESA dose, studies which mandated that the ESA dose remain fixed throughout were clearly unable to inform the primary research question. Hence, a total of 12 studies were excluded from the analysis because they did not allow a reduction in ESA dose, with the exception of a reduction for safety reasons only. An additional study was excluded because it was an observational extension to one of the preceding 12 studies. The remaining 10 studies assessed in the feasibility review had variable protocols for dosing both IV iron and ESA therapy. Studies which compared anaemia management protocols, and/or included IV iron in both treatment arms, could only be included if there was a meaningful difference in the dose of IV iron delivered to each group across the study duration. Accordingly, three further studies were excluded from the analysis. Table 2 describes the detailed characteristics of the seven included studies.
Table 2.
List of studies included in meta‐analysis
| Study | Intervention | Dose per week of IV iron | Control | Dose per week of IV iron |
|---|---|---|---|---|
| DeVita et al.20 | n = 19; IV iron dextran up to 1 g to maintain SF 400 ng/mL; ESA to maintain Hct 32.5–36% | Mean (SD) total iron dose
1650.0 (981.2) mg Given as 100 mg per dialysis session |
n = 17; IV iron dextran up to 1 g to maintain SF 200 ng/mL; ESA to maintain Hct 32.5–36% | Mean (SD) total iron dose
906.7 (953.1) mg Given as 100 mg/r dialysis session |
| Fishbane et al.21 | n = 64; IV iron dextran to maintain SF ≥ 100 μg/L and TSAT ≥20%; ESA to maintain Hct 33–36% | Mean (SD) dose per wk
47.7 (35.5) mg Given as 100 mg per dialysis session |
n = 74; IV iron to maintain CHr ≥ 30 pg; ESA to maintain Hct 33–36% | Mean (SD) dose per wk
22.9 (20.5) mg Given as 100 mg per dialysis session |
| Fishbane et al.22 | n = 20; IV iron dextran 100 mg twice per wk; ESA to maintain Hct 30–34% |
Not reported Given as 200 mg/wk |
n = 32; oral iron; ESA to maintain Hct 30–34 % | Oral iron |
| Kaneko et al.23 | n = 100; IV iron colloid 40 mg 3 x/wk if TSAT <20 %; ESA to maintain Hct 29.5–32.5 % | Mean (SD) total iron at wk 16
377.5 (361.6) mg Given as 120 mg/wk |
n = 97 IV iron colloid 40 mg three times per wk if Chr < 32.5 pg; ESA to maintain Hct 29.5–32.5% | Mean (SD) total iron at wk 16
267.7 (353.2) mg Given as 120 mg/wk |
| Kotaki et al.24 | n = 15; IV iron (not specified) 100 mg/wk; ESA to maintain Hct within three points of baseline |
Not reported Given as 100 mg/wk |
n = 16; oral iron; ESA to maintain Hct within three points of baseline | Oral iron |
| Li and Wang25 | n = 70; IV iron sucrose 100 mg twice/wk; ESA reduced by 25% if Hb ≥ 11.0 g/dL |
Not reported Given as 100–200 mg/wk |
n = 66; oral iron; ESA reduced by 25% if Hb ≥ 11.0 g/dL | Oral iron |
| Macdougall et al.26 | n = 12; IV iron dextran 250 mg every 2 weeks; ESA reduced by 33% if Hb > 12.0 g/dL, otherwise ESA dose increased by 100% if Hb increased <1 g/dL at 8 weeks |
Not reported Given as 250 mg every 2 weeks |
n = 13; oral iron; ESA reduced by 33% if Hb > 12.0 g/dL, otherwise ESA dose increased by 100% if Hb increased <1 g/dL at 8 weeks n = 12; no iron; ESA reduced by 33% if Hb >12.0 g/dL, otherwise ESA dose increased by 100% if Hb increased <1 g/dL at 8 weeks | Oral iron or no iron |
CHr, reticulocyte haemoglobin content; ESA, erythropoietin stimulating agent; Hb, haemoglobin, Hct, haematocrit; HD, haemodialysis; IV, intravenous; SF, serum ferritin; TSAT, transferrin saturation; wk, week.
Assessment of quality of included studies
A summary of the measures taken to minimize bias across the included studies is presented in Table 3. Although all studies reported that randomization had taken place, few provided details on the method used. Almost all studies were open‐label; however, as the primary outcomes in most cases were laboratory variables (iron indices and other clinical parameters), the risk of bias from this feature is low. Almost half the studies did not use intention‐to‐treat analysis methods, with these studies reporting data only for patients who completed the treatment protocol; a high potential for bias could result.
Table 3.
Summary of measures undertaken to minimize bias
| Study ID | Sample size | Randomization | Blinding patients | Blinding investigator | Blinding assessors | Basis for analysis |
|---|---|---|---|---|---|---|
| DeVita et al.20 | n = 36 | Yes, NS | NS, lab outcomes | NS, lab outcomes | NS, lab outcomes | ITT |
| Fishbane et al.21 | n = 157 | Yes, NS | Yes | Yes | NS, lab outcomes | Other, 12% excluded |
| Fishbane et al.22 | n = 52 | Yes, NS | NS, lab outcomes | NS, lab outcomes | NS, lab outcomes | Other, 20–36% excluded |
| Kaneko et al.23 | n = 197 | Yes, NS | NS, lab outcomes | NS, lab outcomes | NS, lab outcomes | ITT |
| Kotaki et al.24 | n = 37 | Yes, NS | NS, lab outcomes | NS, lab outcomes | NS, lab outcomes | Other, 16% excluded |
| Li and Wang25 | n = 136 | Yes, A | NS, lab outcomes | NS, lab outcomes | NS, lab outcomes | ITT |
| Macdougall et al.26 | n = 38 | Yes, NS | NS, lab outcomes | NS, lab outcomes | NS, lab outcomes | Other, 3% excluded |
ITT, intention to treat; NS, not specified; PP, per protocol.
Effect of IV iron on erythropoiesis‐stimulating agent dose
The intervention arms from the RCTs were categorized as optimal (100–200 mg IV iron per week) and suboptimal (lower dose IV iron (<100 mg/week)), with respect to IV iron supplementation. Where both arms of the trial received IV iron (e.g. according to different protocols), the treatment arm receiving the highest dose of iron was considered to be the ‘optimal’ arm. The results of the oral iron and no iron arms of Macdougall 1996 were pooled for the comparison of optimal iron and suboptimal iron.26 All included studies used epoetin alfa as the ESA; however, the searches were not limited by the type of ESA.
Results of random‐effects meta‐analysis showed a statistically significant weighted mean (95% CI) difference of −1733 (−3073, −392) units (U)/week in ESA dose at endpoint in favour of optimal iron (Fig. 2). A simple weighted average of the difference for the change from baseline indicates that this difference represents a 23% dose reduction for optimal iron versus suboptimal iron prescription.
Figure 2.
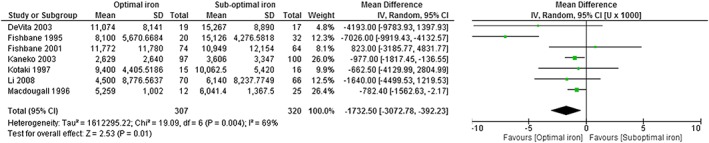
Forest plot of meta‐analysis of ESA dose at endpoint, optimal versus suboptimal iron. Based on all seven included studies.20, 21, 22, 23, 24, 25, 26 Optimal iron: intravenous iron used within a protocol consistent with KDIGO, treat to target with 100 to 200 mg/week; suboptimal iron: oral iron, no iron or lower dose intravenous iron (<100 mg IV iron per week).
Limiting the analysis to the four RCTs of IV iron versus oral iron provides a weighted mean (95% CI) difference of −2344 (−5292, 604) U/week in ESA dose, which did not reach statistical significance, although the point estimate favours IV iron (Fig. 3). A simple weighted average of the difference for the change from baseline indicates that this difference represents a 31% dose reduction for optimal iron versus suboptimal iron.
Figure 3.
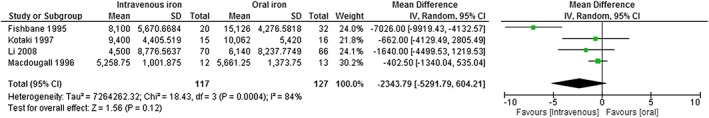
Forest plot of meta‐analysis of ESA dose at endpoint, IV versus oral iron. Based on four studies of IV iron versus oral iron.22, 24, 25, 26 Optimal iron: intravenous iron used within a protocol consistent with KDIGO, treat to target with 100 to 200 mg/week; suboptimal iron: oral iron, no iron or lower dose intravenous iron (<100 mg IV iron per week).
Combining the oral iron and no iron arms of Macdougall 199626 into the previous analysis had minimal impact: a weighted mean (95% CI) difference of −2433 [−5183, 318] U/week in ESA dose (Fig. 4). This trial did not present baseline data, and therefore, a percentage reduction from baseline could not be calculated for this comparison, although it may be concluded that it falls within the range of 23% to 31% based on previous estimates.
Figure 4.
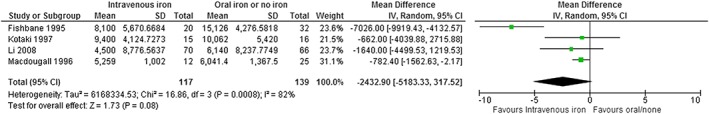
Forest plot of meta‐analysis of ESA dose at endpoint, IV versus oral Iron/no iron. Based on four studies of IV iron versus oral/no iron.22, 24, 25, 26 Optimal iron: intravenous iron used within a protocol consistent with KDIGO, treat to target with 100 to 200 mg/week; suboptimal iron: oral iron, no iron or lower dose intravenous iron (<100 mg IV iron per week).
The weighted average change in ESA dose across the six included studies with baseline data was a reduction of 23% (range −7% to −55%) attributable to appropriate dosing of IV iron (Table 4).
Table 4.
Weighted average percentage reduction in ESA dose per week
| Study | Optimal iron | Suboptimal iron | ||||
|---|---|---|---|---|---|---|
| % Change | N | % Change | N | Weighting | Difference (%) | |
| DeVita et al.20 | −49 | 19 | −13 | 17 | 0.06 | −36 |
| Fishbane et al.21 | −11 | 64 | −4 | 74 | 0.23 | −7 |
| Fishbane et al.22 | −43 | 20 | 12 | 32 | 0.09 | −55 |
| Kaneko et al.23 | −36 | 97 | −12 | 100 | 0.33 | 23 |
| Kotaki et al.24 | 10 | 15 | 18 | 16 | 0.05 | −8 |
| Li and Wang25 | −27 | 70 | 0 | 66 | 0.23 | −27 |
| Weighted average change in ESA dose attributable to IV iron: (Optimal iron vs suboptimal iron) | −23% | |||||
| Weighted average change in ESA dose attributable to IV iron: (Intravenous iron vs oral iron/no iron) | −31% | |||||
ESA, erythropoiesis stimulating agent; IV, intravenous.
Effect of IV iron on ferritin
Whilst transferrin saturation (TSAT) was not analyzed as part of this meta‐analysis, improvements in serum ferritin levels were observed for the optimal dose group with a mean difference of 231.3 ng/mL (−31.5, 494.1) and 420.9 ng/mL (175.6, 666.2) when compared with suboptimal iron dosing or oral iron/no iron, respectively.
DISCUSSION
This meta‐analysis suggests that the use of optimal IV iron can reduce ESA requirements by 23% when compared with suboptimal IV iron usage in adult HD patients. The magnitude of reduction is more profound when IV iron is compared to oral iron or no concomitant iron use, with a 31% reduction observed. These findings equate to a potential reduction in ESA dose of between 1733 and 2343 U/week of epoetin alfa or equivalent. The meta‐estimates of the comparison between optimal and suboptimal dosing were statistically significant in favour of optimal dosing. The comparison between IV iron and oral/no iron did not reach statistical significance, likely due to the reduced power of the smaller pooled sample size, although the direction of effect continued to favour IV iron.
Optimal iron dosing is also associated with improvements in iron indices. The 2012 KDIGO guidelines state that if an increase in haemoglobin and/or a reduction in ESA therapy is desired, then the evidence supports giving IV iron when the TSAT ≤30% and ferritin ≤500 ng/mL.10 Although studies in this meta‐analysis were completed prior to the introduction of these guidelines, in general, the ‘optimal’ dosing arms are consistent with the KDIGO guidelines as they now exist. TSAT was not examined, but serum ferritin increased.
In patients undergoing HD, it is estimated that iron losses are ~1000–2000 mg/year and possibly as great as 8000 mg/year.27, 28 Because of dietary restrictions and increased hepcidin levels the amount of iron that HD patients receive (from dietary sources) is likely to be less than 50 mg/month, resulting in an iron deficiency. Patients from studies included in this meta‐analysis were iron‐deficient at commencement and therefore required higher doses, that is, 100–200 mg iron a week, to address the ongoing losses as well as to replete stores. These doses have been termed ‘optimal’ in our report. After repletion of the initial iron deficit, lower doses and/or less frequent administration of iron may be appropriate to maintain optimal iron parameters (i.e. serum ferritin and TSAT). Therefore, while currently available data limit knowledge of optimal concomitant IV iron and ESA dose, this analysis suggests that initial doses of ~100–200 mg IV iron per week (when used in combination with minimized ESA dosing) permits correction of anaemia. Post‐repletion of iron stores, the optimal monthly IV iron dosing, could be envisaged to decrease to levels of approximately 100–200 mg/month, consistent with the yearly losses, to maintain serum ferritin and TSAT levels within targets as set by KDIGO guidelines.
Several strategies exist for IV iron delivery, including multiple low doses potentially with dosing during each dialysis (e.g. 10–50 mg iron with each session), single weekly doses (of 100–200 mg iron) or even larger doses on a monthly or possibly quarterly basis (i.e. 500–1000 mg iron). The doses vary significantly between institutes, although the frequency is usually aligned with dialysis sessions.
In relation to ESA usage, KDIGO recommends that ESA therapy should not be used to maintain a haemoglobin above 11.5 g/dL, and not to intentionally increase the haemoglobin above 13.0 g/dL.10 Across the included studies, optimal iron was associated with a significant improvement in haemoglobin/haematocrit when compared with suboptimal iron.
Previous systematic reviews1, 14, 15, 16 have reported on both efficacy and safety of IV iron in both dialysis and non‐dialysis CKD patients. These studies had similar findings and observed decreased ESA requirements when concomitant IV iron was administered.
The Cochrane review1 demonstrated that haemoglobin was significantly increased by IV iron compared with oral iron (22 studies, 1862 patients; MD 0.90 g/dL, 95% CI 0.44 to 1.37) in all patients and in the subgroup of dialysis patients (13 studies, 828 patients; MD 1.16 g/dL, 95 % CI 0.30 to 2.02). The review identified a significant reduction in ESA requirements in patients treated with IV iron with a standardized MD of −0.76 [−1.22 to −0.30], not further quantified, and found no significant difference in mortality. There were fewer patients who reported adverse effects with IV iron, but the result did not achieve significance: (12 studies, 1488 patients; risk difference −0.09, 95% CI 0.19 to 0.00; I 2 = 85%).
Avni et al. in a meta‐analysis not restricted to CKD patients did not find that intravenous iron was associated with increased rates of serious adverse events or infections.29 Susantitaphong et al. presented an exploratory analysis restricted to two RCTs of IV iron (359 analyzable patients) indicating IV iron therapy was not associated with an increased incidence of any adverse event (RR = 0.88; 95% CI: 0.72–1.08; P = 0.23).16 Additionally, IV iron resulted in significant increases in haemoglobin of 0.54 g/dL (95% CI: 0.32–0.75 g/dL; P < 0.001), haematocrit of 1.45% (95% CI: 0.05–2.84%; P = 0.04), serum ferritin level of 126 ng/mL (95% CI: 63–190 ng/mL; P < 0.001), TSAT of 9.6% (95% CI: 6.9–12.2%; P < 0.001). Results of random‐effects meta‐analysis of single arm studies, and arms from RCTs (22 studies, 2147 patients) indicated a mean change in EPO dose of −1,428 (95% CI: −2511, −345; P = 0.01) U/week. Limiting the analysis to RCTs (two studies, 224 patients) provided a non‐significant result: mean change in EPO dose of −4,850 (95% CI: −13,113, 3,142; P = 0.25) U/week.
The review by Rozen‐ Zvi et al.14 concluded that compared with oral iron, there was a significantly greater haemoglobin concentration in dialysis patients treated with IV iron (weighted mean difference, 0.83 g/dL; 95% CI: 0.09 to 1.57). Meta‐regression showed a positive association between haemoglobin level increase and IV iron dose administered and a negative association with baseline haemoglobin concentration. Data for all‐cause mortality (pooled data for dialysis and CKD patients) were sparse, but no difference was noted (RR = 0.28; 95% CI: 0.02–5.22), and there was no difference in adverse events between the IV‐treated and oral‐treated patients (RR = 0.80; 95% CI: 0.46–1.41). The observed reduction in ESA dose was reported as a weighted mean difference of −28.21 U/kg/week (95% CI: −42.12, −14.3). Lastly, the most recent meta‐analysis by Shepshelovich et al. reported that HD patients treated with IV verses oral iron were more likely to reach a haemoglobin response >1 g/dL (risk ratio 2.14 [95% CI, 1.68–2.72]. The safety analysis showed similar rates of mortality and both serious and any adverse effects.15
The meta‐estimate presented in this report provides a mean difference in ESA dose which is also directly applicable to the costs incurred in managing uraemic anaemia, whether in developed or under‐developed healthcare systems. Intravenous iron may also aid with overcoming ESA resistance that has been observed when an ESA has been used without concomitant IV iron. Furthermore, the reduction in ESA dose may reduce the risk of known ESA‐related adverse effects (thromboembolic risk, strokes and recurrent neoplasia) that have been observed, especially with more aggressive ESA dosing to attempt to achieve higher target haemoglobin concentrations.8
A number of limitations of the current study should be noted. There was high heterogeneity between the pooled studies, in terms of effect size, as shown by the high I 2, as well as heterogeneous treatment protocols between the included RCTs. The studies generally had a high risk of bias (as seen in the quality assessment). The sample sizes of the included RCTs were relatively small, and the search was limited to English language studies only. Additionally, the inclusion of older studies, not aligned with KDIGO guidelines and/or having protocol‐driven ESA dosing, may further decrease the external validity of the pooled result.
In conclusion, this meta‐analysis demonstrates that significant reductions in ESA dosing may be achieved with optimal IV iron usage in the HD population being concurrently treated with ESAs. In addition, suboptimal iron use may require undesirably higher ESA dosing to manage anaemia. The optimal balance between ESA dosing and IV iron dosing remains unclear pending data on the long‐term safety of IV iron, administered at higher dosages. This may be clarified by the largest RCT of intravenous iron in HD patients (PIVOTAL).30 It is an event‐driven trial comparing the incidence of hard clinical endpoints of death, heart attack, stroke and heart failure in over 2000 patients randomized to either a proactive regimen of 400 mg IV iron sucrose per month (with a safety cut‐off) or a minimalistic approach to IV iron (aiming to keep the serum ferritin just above 200 ng/mL and TSAT >20%). One of the secondary endpoints in this trial is the relative ESA dose requirements in the two arms over the 2–4 years period of follow up. At the present time, however, we do know that the use of higher amounts of IV iron supplementation in dialysis patients allows lower doses of ESA therapy to be used, which could potentially have positive effects on thromboembolic risk, assuming that there are no negative effects from IV iron using this approach.
AUTHOR CONTRIBUTION
All authors have added to the meta‐analysis in terms of the following criteria: (i) substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; (ii) drafting the work or revising it critically for important intellectual content; (iii) final approval of the version to be published; and (iv) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
The meta‐analysis including all statistical analysis was performed by Commercial Eyes Pty Ltd. Study eligibility was independently determined by two consultants from Commercial Eyes Pty Ltd (Sinead Garrett, Kim Magner) with differing decisions resolved by mutual consensus. The final analysis was independently verified by the Statistical Consulting Centre at the University of Melbourne. Funding for the project was provided by Vifor Pharma. Simon D Roger has received speakers' fees, honoraria and consultancy fees from several manufacturers of ESAs and IV iron, including Amgen, Hoffmann‐La Roche, Janssen‐Cilag, Novartis, Sandoz, Takeda and Vifor Pharma. Martin Tio has no competing interests. Hyeong‐Cheon Park has received speakers' fees, honoraria and consultancy fees from JW Pharmaceutical, Boryung Pharmaceutical, Kyowa Kirin and Chong Kun Dang. Hui Lin Choong has no competing interests. Bakleong Goh has no competing interests. Timothy R Cushway and Vanessa Stevens are employed by Vifor Pharma. Iain C Macdougall has received speakers' fees, honoraria and consultancy fees from several manufacturers of ESAs and IV iron, including Affymax, AMAG, Amgen, Ortho Biotech, Pharmacosmos, Hoffmann‐La Roche, Takeda and Vifor Pharma.
Search terms*
exp Renal Dialysis/
hemodialysis.ti,kw,sh.
haemodialysis.ti,kw,sh.
1 or 2 or 3
Ferric Compounds
Iron Compounds
Iron.ti,kw,sh.
5 or 6 or 7
exp Infusions, intravenous/
exp Injections, intravenous/
intravenous$.ti,kw,sh.
iv.ti,kw,sh.
9 or 10 or 11 or 12
exp random allocation/
exp randomized controlled trial/
exp Double‐Blind Method/ or exp double‐blind procedure/
random$.ti,ab,kw,sh.
random$.ti,ab,kw,sh.
14 or 15 or 16 or 17 or 18
4 and 8 and 13 and 19
limit 20 to humans
exp meta analysis/
meta analy$.ti,ab,kw,sh.
systematic$ adj2 review$
guideline$.ti,ab,kw,sh.
exp practice guideline/
practice guideline.pt.
22 or 23 or 24 or 25 or 26 or 27
4 and 8 and 13 and 28
21 not 29
limit 30 to “review articles”
30 not 31
32 or 29 or 31
*Databases: Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Cochrane Database of Reviews of Effect, EMBASE, MEDLINE.
REFERENCES
- 1. Albaramki J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst. Rev. 2012; (1) Art. No.: CD007857. DOI: 10.1002/14651858.CD007857.pub2. [DOI] [PubMed] [Google Scholar]
- 2. Fishbane S, Maesaka JK. Iron management in end‐stage renal disease. Am. J. Kidney Dis. 1997; 29: 319–333. [DOI] [PubMed] [Google Scholar]
- 3. Zaritsky J, Young B, Wang HJ et al. Hepcidin – a potential novel biomarker for iron status in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009; 4: 1051–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solomon SD, Uno H, Lewis EF et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N. Engl. J. Med. 2010; 363: 1146–1155. [DOI] [PubMed] [Google Scholar]
- 5. FDA Drug Safety Communication . Modified dosing recommendations to improve the safe use of erythropoiesis‐stimulating agents (ESAs) in chronic kidney disease; c2011 [updated 2011 Jun 26; cited 2016 Mar 20]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm
- 6. Drüeke TB, Locatelli F, Clyne N et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N. Engl. J. Med. 2006; 355: 2071–2084. [DOI] [PubMed] [Google Scholar]
- 7. Singh AK, Szczech L, Tang KL et al. Correction of anemia with epoetin alfa in chronic kidney disease. N. Engl. J. Med. 2006; 355: 2085–2098. [DOI] [PubMed] [Google Scholar]
- 8. Pfeffer MA, Burdmann EA, Chen CY et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 2009; 361: 2019–2032. [DOI] [PubMed] [Google Scholar]
- 9. Koulouridis I, Alfayez M, Trikalinos TA et al. Dose of erythropoiesis‐stimulating agents and adverse outcomes in CKD: a meta‐regression analysis. Am. J. Kidney Dis. 2013; 61: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group . KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012; 2: 279–335. [Google Scholar]
- 11. McMahon LP, Macginley R. KHA‐CARI guideline. Biochemical and haematological targets: haemoglobin concentrations in patients using erythropoietin‐stimulating agents. Nephrology 2012; 17: 17–19. [DOI] [PubMed] [Google Scholar]
- 12. Macginley R, Walker R, Irving M. KHA‐CARI Guideline. Use of iron in chronic kidney disease patients. Nephrology 2013; 18: 747–749. [DOI] [PubMed] [Google Scholar]
- 13. National Institute for Health and Care Excellence . Chronic kidney disease: managing anaemia; c2015 [published June 2015; cited 2016 Mar 20]. Available from: https://www.nice.org.uk/guidance/ng8 .
- 14. Rozen‐Zvi B, Gafter‐Gvili A, Paul M et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta‐analysis. Am. J. Kidney Dis. 2008; 52: 897–906. [DOI] [PubMed] [Google Scholar]
- 15. Shepshelovich D, Rozen‐Zvi B, Avni T et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta‐analysis. Am. J. Kidney Dis. 2016; pii DOI: 10.1053/j.ajkd.2016.04.018 [Epub ahead of print].S0272‐6386(16)30125‐1 [DOI] [PubMed] [Google Scholar]
- 16. Susantitaphong P, Alqahtani F, Jaber BL. Efficacy and safety of intravenous iron therapy for functional iron deficiency anemia in hemodialysis patients: a meta‐analysis. Am. J. Nephrol. 2014; 39: 130–141. [DOI] [PubMed] [Google Scholar]
- 17. Liberati ADG, Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0. [Updated March 2011]. Cambridge: The Cochrane Collaboration, 2011. .Available from: http://handbook.cochrane.org/ [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeVita MV, Frumkin D, Mittal S et al. Targeting higher ferritin concentrations with intravenous iron dextran lowers erythropoietin requirement in hemodialysis patients. Clin. Nephrol. 2003; 60: 335–340. [DOI] [PubMed] [Google Scholar]
- 21. Fishbane S, Shapiro W, Dutka P et al. A randomized trial of iron deficiency testing strategies in hemodialysis patients. Kidney Int. 2001; 60: 2406–2411. [DOI] [PubMed] [Google Scholar]
- 22. Fishbane S, Frei GL, Maesaka J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am. J. Kidney Dis. 1995; 26: 41–46. [DOI] [PubMed] [Google Scholar]
- 23. Kaneko Y, Miyazaki S, Hirasawa Y et al. Transferrin saturation versus reticulocyte hemoglobin content for iron deficiency in Japanese hemodialysis patients. Kidney Int. 2003; 63: 1086–1093. [DOI] [PubMed] [Google Scholar]
- 24. Kotaki M, Uday K, Henriquez M et al. Maintenance therapy with intravenous iron in hemodialysis patients receiving erythropoietin. Clin. Nephrol. 1997; 48: 63–64. [PubMed] [Google Scholar]
- 25. Li H, Wang SX. Intravenous iron sucrose in Chinese hemodialysis patients with renal anemia. Blood Purif. 2007; 26: 151–156. [DOI] [PubMed] [Google Scholar]
- 26. Macdougall IC, Tucker B, Thompson J et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int. 1996; 50: 1694–1699. [DOI] [PubMed] [Google Scholar]
- 27. Eschbach JW, Cook JD, Scribner BH, Finch CA. Iron balance in hemodialysis patients. Ann. Intern. Med. 1977; 87: 710–713. [DOI] [PubMed] [Google Scholar]
- 28. Macdougall IC, Bircher AJ, Eckardt KU et al. Iron management in chronic kidney disease: conclusions from a ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) controversies conference. Kidney Int. 2016; 89: 23–39. [DOI] [PubMed] [Google Scholar]
- 29. Avni T, Bieber A, Grossman A, Green Het al. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clin. Proc. 2015; 90: 12–23. [DOI] [PubMed] [Google Scholar]
- 30. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐002267‐25/ [cited 2016 Jul 9]


