ABSTRACT
Aim
Iron deficiency stimulates fibroblast growth factor 23 (FGF23) transcription. This study aimed to determine whether oral ferrous iron (Fe2+) reduces the serum FGF23 levels of iron‐deficient maintenance haemodialysis (MHD) patients in the same way as oral ferric iron (Fe3+)
Methods
Thirty‐one MHD patients with iron deficiency were enrolled in this prospective study. Patients who had taken iron supplements during the 8 weeks before the study were excluded. The patients’ iron stores and their serum FGF23, phosphate, intact parathyroid hormone (iPTH), albumin, C‐reactive protein (CRP), and albumin‐adjusted calcium (Ca) levels were examined at the baseline and after 3 months’ treatment with sodium ferrous citrate (Fe2+).
Results
The patients’ transferrin saturation values and serum iron and ferritin levels were significantly increased after 3 months’ treatment (P < 0.01), as were their serum albumin levels (P < 0.05). Conversely, their serum intact FGF23 (iFGF23) [1820 (342–4370) vs 1240 (214–2940) pg/mL, P < 0.05], C‐terminal FGF23 (cFGF23) [309 (120–1211) vs 259 (99–600) pg/mL, P < 0.05)], and CRP levels (P < 0.01) were significantly reduced after 3 months’ treatment. No changes were detected in the patients’ serum iFGF23:cFGF23 ratios or their serum phosphate, Ca, or iPTH levels. The changes in the patients’ serum iFGF23 and cFGF23 levels induced by sodium ferrous citrate supplementation were shown to be attributable to changes in their serum ferritin levels (P < 0.05).
Conclusion
Short‐term oral iron supplementation with sodium ferrous citrate replenished the iron stores and reduced the serum iFGF23 and cFGF23 levels of MHD patients with iron deficiency without affecting their serum phosphate, Ca, or iPTH levels.
Keywords: bone metabolism, haemodialysis, iron
Summary at a Glance
There is increasing interest in FGF23 and its role in mineral bone disorder and cardiovascular disease in chronic kidney disease. FGF23 is also associated with iron metabolism, with higher levels in iron deficiency. This prospective study evaluates the effect of short‐term oral iron supplementation, with sodium ferrous citrate, on FGF23 and other markers of mineral metabolism in haemodialysis patients.
There is a pervasive view that elevated serum fibroblast growth factor 23 (FGF23) levels are responsible for cardiovascular disease and death in haemodialysis (HD) patients.1, 2 However, FGF23 cannot actually be said to be a “risk factor” for cardiovascular disease because to date no causative link between FGF23 and cardiovascular disease has been demonstrated. In addition, no interventional studies have suggested that simply reducing FGF23 levels improves the outcomes of HD patients. Furthermore, little is known about FGF23 regulation in HD patients. It is often assumed that hyperphosphataemia is the primary stimulator of elevated FGF23 levels in chronic kidney disease (CKD), but increases in FGF23 levels are seen before changes in parathyroid hormone (PTH) and phosphate levels in CKD.3, 4, 5 It is also reported that elevated 1,25(OH)2 vitamin D and PTH levels stimulate FGF23 production.4, 5
Recently, an animal study suggested that iron deficiency stimulates FGF23 transcription in osteocytes.6 Furthermore, it was reported that the short‐term use of iron‐based phosphate binders, such as ferric citrate and sucroferric oxyhydroxide, replenished the iron stores and reduced the serum phosphate and FGF23 levels of patients with CKD and HD patients with iron deficiency.7, 8, 9, 10, 11 Both ferric citrate and sucroferric oxyhydroxide contain ferric iron (Fe3+), whereas sodium ferrous citrate (Ferromia; Eisai Co., Ltd. Tokyo, Japan) contains ferrous iron (Fe2+). The package insert for Ferromia is shown in the supplemental file. In general, ferric iron (Fe3+) results in lower iron absorption than ferrous iron (Fe2+),11, 12 and ferric iron (Fe3+) retains its phosphate binding capacity.11 The co‐administration of antacid and iron tablets results in a marked reduction in iron absorption, and so sodium ferrous citrate (C12H10FeNa4O14; Fe2+) was developed to increase patients’ serum iron levels in a manner that was not affected by gastric acid secretion (see the supplemental file). It was reported that the increase in the serum iron concentration brought about by the administration of sodium ferrous citrate was enhanced by the addition of ascorbic acid, whereas the increase associated with the administration of ferrous sulfate (FeSO4•xH2O; Fe2+) was not affected.12
The 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines for anaemia in CKD recommend that iron supplements should be administered to patients that exhibit serum transferrin saturation (TSAT) values of ≤30% and serum ferritin concentrations of ≤500 ng/mL.13 However, the Japanese Society for Dialysis Therapy (JSDT) guidelines state that iron should be given to HD patients with TSAT values of ≤20% and serum ferritin levels of ≤100 ng/mL.14 According to data for 2012–2014 from the United States (US) RENAL DATA STSTEM (https://www.usrds. org/2015/view/v2_03.aspx?zoom_), 13% of US HD patients had TSAT values of <20%, and 12% had ferritin concentrations of ≤200 ng/mL. In addition, data for 2012 from the UK renal registry indicated that the median ferritin concentration of UK HD patients was 431 µg/L.15 Furthermore, a statistical survey conducted by the JSDT at the end of 2012 revealed that 36.2% of Japanese HD patients had TSAT values of <20%, and 58.3% had ferritin concentrations of <100 ng/mL.16 Thus, iron deficiency is relatively common in Japanese HD patients.
The aim of this study was to determine whether oral ferrous iron (Fe2+) supplementation with sodium ferrous citrate reduces the serum FGF23 levels of maintenance HD (MHD) patients with iron deficiency in the same way as oral ferric iron (Fe3+).7, 8, 9, 10
METHODS
This study was a prospective, open‐label interventional study, in which eligible patients were given one sodium ferrous citrate tablet containing 50 mg of iron a day for 3 months at Ichiyokai Harada Hospital in Japan. It did not involve a matching placebo group. The inclusion criteria were adult patients who underwent MHD for at least 2 months and had serum ferritin levels of ≤100 ng/mL, TSAT values of ≤20%, and haemoglobin levels of <12.0 g/dL. All of the patients underwent routine HD three times a week (4 h per session) using standard high‐flux dialysis membranes. The exclusion criteria included the use of intravenous or oral iron supplementation within 8 weeks of screening, anaemia of any cause other than iron deficiency or CKD, symptomatic gastrointestinal bleeding, or bowel disease. Continued use of the same phosphate binders, the same active vitamin D supplements, and cinacalcet were allowed, but the dosages of active vitamin D, phosphate binders, cinacalcet, and dialysate were not changed during the study period. The patients were seen by doctors during every HD session. For safety purposes, the patients’ data (except their serum FGF23 and ferritin level data) were reviewed once a month throughout the study. The Committee on Human Research at Ichiyokai Hospital approved this study (authorization No. 201319), and all of the patients provided written informed consent. All blood samples were collected before the beginning of the first session of the week both at the baseline (0 months) and after 3 months’ treatment with sodium ferrous citrate. The clinical biochemical analyses were performed at our hospital's laboratory, but the patients’ serum ferritin, intact PTH (iPTH), intact FGF23 (iFGF23), and C‐terminal FGF23 (cFGF23) levels were measured by SRL Inc. (Tokyo, Japan). The patients’ serum iFGF23 (pg/mL) levels were measured using a sandwich ELISA kit (Kainos Laboratories, Tokyo, Japan), as were their serum cFGF23 (pmol/L) levels (Biomedica Medizinprodukte GmbH & Co KG, Vienna, Austria). The unit conversion of the cFGF23 data was performed using the following equation: 0.133 pmol/L = 1 pg/mL (molecular weight: 7.52 kDa). The geriatric nutritional risk index (GNRI) was calculated using the equation [1.489 × albumin (g/dL)] + [41.7 × (body weight/ideal body weight)],17 and the erythropoiesis‐stimulating agent resistance (ESA) index was calculated using the following equation: weekly ESA dose per kg body weight divided by the haematocrit level.18 The examined outcomes, all of which were assessed at the baseline and after 3 months’ sodium ferrous citrate treatment, included the patients’ TSAT, GNRI, ESA index, Kt/Vurea, and normalized protein equivalent of nitrogen appearance (nPNA) values,19 haemoglobin levels, random plasma glucose levels, serum iFGF23:cFGF23 ratios, and serum iron, ferritin, iFGF23, cFGF23, phosphate, magnesium, iPTH, C‐reactive protein (CRP), albumin, and albumin‐adjusted calcium (Ca) levels.
STATISTICAL ANALYSIS
The data were analyzed using the statistical software JMP ver. 10 (SAS Institute, Inc., Cary, NC, USA). The parameters that demonstrated normal distributions are expressed as mean ± standard deviation values and were analyzed with the paired t‐test. Non‐parametric variables are expressed as median values and interquartile ranges and were analyzed with the Wilcoxon‐signed rank test. Multivariate analysis of variance (MANOVA) was used to evaluate whether the differences between the FGF23 levels observed at the baseline and after 3 months’ treatment were dependent and attributable to changes in the serum ferritin (or TSAT), phosphate, Ca, or iPTH level. The patients’ iPTH, iFGF23, and cFGF23 values, which exhibited non‐parametric distributions, were subjected to logarithmic (log) transformation to achieve normality prior to the MANOVA.
RESULTS
A total of 33 MHD patients were enrolled in this study. Two patients were withdrawn, one due to the exacerbation of dermatitis and one because of death from an acute myocardial infarction, which was not considered to be related to the administration of sodium ferrous citrate, and so 31 patients were included in the analysis. No other adverse events occurred during the study, although all patients reported black or dark‐coloured stools while receiving sodium ferrous citrate.
The baseline characteristics of the study subjects are shown in Table 1. The subjects’ mean age was 67 ± 13 years, and 25.8% of the subjects were female. They had been receiving dialysis for a median period of 67 (5‐91) months, and 64.5% of them exhibited urinary volumes of <100 mL/day. The subjects’ mean dry weight and median body mass index were 54.7 ± 10.9 kg and 21.3 (18.7–23.6) kg/m2, respectively. In addition, their mean systolic blood pressure and diastolic blood pressure at the start of the baseline HD session were 153 ± 24 mmHg and 80 ± 15 mmHg, respectively. As for comorbid conditions, 18/31 (58.1%) patients were diabetic, 11/31 (35.5%) had coronary heart disease, and 13/31 (41.9%) had congestive heart failure. Active vitamin D was administered in the form of intravenous maxacalcitol, oral calcitriol, and oral alfacalcidol to 10/31 (32.2%), 5/31 (16.1%), and 5/31 (16.1%) patients, respectively, but the doses of these drugs were not changed during the study period. During the period from 24–8 weeks prior to the trial, iron supplementation was administered to 17/31 (54.8%) patients. It was administered in the form of intravenous saccharated ferric oxide, oral sodium ferrous citrate, and sucroferric oxyhydroxide to 8/31 (25.8%), 7/31(22.6%), and 2/31 (6.5%) patients, respectively, although patients that received iron supplementation within 8 weeks of the trial were excluded from the study.
Table 1.
Subjects’ baseline characteristics
| n = 31 | |
|---|---|
| Demographics | |
| Age (years) | 67 ± 13 |
| Female sex (%) | 8/31 (25.8) |
| Dialysis vintage (months) | 67 (5‐91) |
| Urine volume of <100 mL/day (%) | 20/31 (64.5) |
| Dry weight (kg) | 54.7 ± 10.9 |
| BMI (kg/m2) | 21.3 (18.7–23.6) |
| Systolic blood pressure (mmHg)* | 153 ± 24 |
| Diastolic blood pressure (mmHg)* | 80 ± 15 |
| Comorbid conditions | |
| Diabetes (%) | 18/31 (58.1) |
| Coronary heart disease (%) | 11/31 (35.5) |
| Congestive heart failure (%) | 13/31 (41.9) |
| Use of active vitamin D (%) | 20/31 (64.5) |
| Intravenous active vitamin D: Maxacalcitol (%) | 10/31 (32.2) |
| Oral active vitamin D: Calcitriol (%) | 5/31 (16.1) |
| Oral active vitamin D: Alfacalcidol (%) | 5/31 (16.1) |
| Non‐user (%) | 11/31 (35.5) |
| Use of cinacalcet (%) | 9/31 (29.0) |
| Iron supplementation at 24–8 weeks prior to the trial** (%) | 17/31 (54.8) |
| Intravenous iron: Saccharated ferric oxide (%) | 8/31 (25.8) |
| Oral iron: Sodium ferrous citrate (%) | 7/31 (22.6) |
| Oral iron: Sucroferric oxyhydroxide (%) | 2/31 (6.4) |
| Non‐user (%) | 14/31 (45.2) |
Continuous variables are shown as the mean ± standard deviation or median (interquartile range). Categorical variables are shown as absolute values (percentages).
Blood pressure at the start of the baseline hemodialysis session
Iron supplement use during the period from 24 to 8 weeks prior to the trial
Patients that received iron supplementation within 8 weeks of the trial were excluded from this study.
BMI, body mass index.
Compared with their baseline (0 months) values, the patients’ TSAT values (13.8 ± 4.7 vs 38.8 ± 24.6%, P < 0.0001) and serum iron (37.4 ± 12.8 vs 94.6 ± 67.8 µg/dL, P < 0.0001) and ferritin (37.6 ± 24.4 vs 87.0 ± 90.6 ng/mL, P < 0.01) levels were significantly increased after 3 months’ sodium ferrous citrate supplementation (Fig. 1).
Figure 1.
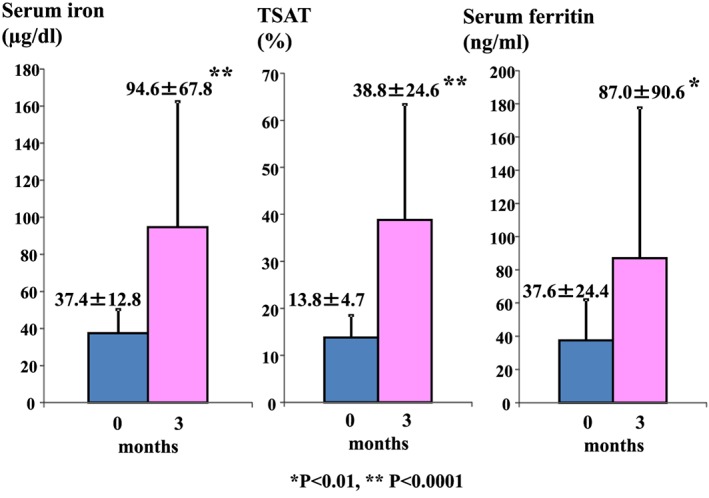
Subjects’ transferrin saturation (TSAT) values and serum iron and ferritin levels at the baseline and after 3 months’ sodium ferrous citrate treatment. Data are expressed as mean ± standard deviation values. *P < 0.01, ** P < 0.0001 compared with the baseline values.
Both the patients’ serum iFGF23 [1820 (342–4370) vs 1240 (214–2940) pg/mL, P < 0.05] and cFGF23 [309 (120–1211) vs 259 (99‐600) pg/mL, P < 0.05] levels were significantly reduced after 3 months’ sodium ferrous citrate supplementation, but their serum iFGF23:cFGF23 ratios were not significantly altered [5.6 (2.7–8.6) vs 5.9 (2.0–7.9)] (Fig. 2).
Figure 2.
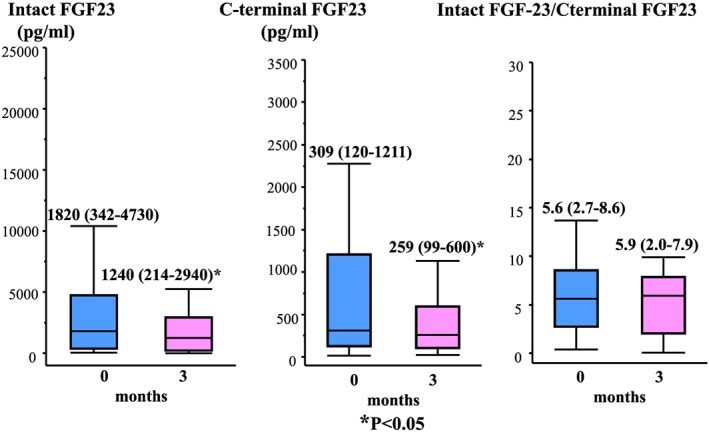
Subjects’ serum intact FGF23 and C‐terminal FGF23 levels and intact FGF23:C‐terminal FGF23 ratios at the baseline and after 3 months’ sodium ferrous citrate treatment. The iFGF23 (pg/mL) and cFGF23 (pmol/L) measurements were obtained simultaneously, and the unit conversion of the cFGF23 data was performed using the following equation: 0.133 pmol/L = 1 pg/mL. The data are expressed as median values and interquartile ranges. *P < 0.05 compared with the baseline values.
The patients’ other laboratory data at the baseline and after 3 months’ oral sodium ferrous citrate supplementation are shown in Table 2. While their serum haemoglobin levels were not significantly altered after 3 months’ treatment, their ESA index [3.5 (2.1–5.1) vs 0.8 (0.0–3.8) U/week/ kg/haematocrit, P < 0.001] values were significantly reduced. The patients’ serum CRP levels were significantly reduced after 3 months’ treatment [2.7 (0.7–6.1) vs 1.8 (0.4–4.4) mg/dL, P < 0.01]. Conversely, their serum albumin (33.3 ± 5.7 vs 34.1 ± 5.4 g/L) and GNRI (87.7 ± 10.6 vs 89.0 ± 10.4) values were significantly (P < 0.01) increased after 3 months’ treatment. No significant changes in the patients’ serum phosphate, Ca, magnesium, or iPTH levels, random plasma glucose levels, or Kt/Vurea or nPNA values were seen after 3 months’ oral sodium ferrous citrate treatment.
Table 2.
Subjects’ laboratory data at the baseline and after 3 months’ sodium ferrous citrate supplementation
| 0 months (baseline) | 3 months | P‐value | |
|---|---|---|---|
| Haemoglobin (g/L) | 112 ± 13 | 115 ± 15 | ns |
| ESA index (U/week/kg/haematocrit) | 3.5 (2.1–5.1) | 0.8 (0–3.8) | <0.001 |
| Serum phosphate (mmol/L) | 1.68 ± 0.42 | 1.58 ± 0.48 | ns |
| Serum magnesium (mmol/L) | 1.07 ± 0.18 | 1.07 ± 0.15 | ns |
| Serum iPTH (pg/ml) | 92 (64–166) | 129 (87–175) | ns |
| Albumin‐adjusted serum Ca (mmol/L) | 2.30 ± 0.18 | 2.30 ± 0.13 | ns |
| Serum C‐reactive protein (mg/L) | 2.7 (0.7–6.1) | 1.8 (0.4–4.4) | <0.01 |
| Serum albumin (g/L) | 33.3 ± 5.7 | 34.1 ± 5.4 | <0.01 |
| Geriatric nutritional risk index | 87.7 ± 10.6 | 89.0 ± 10.4 | <0.01 |
| Random plasma glucose (mmol/L) | 7.19 ± 1.86 | 7.18 ± 1.86 | ns |
| Kt/Vurea (/dialysis session) | 1.48 ± 0.40 | 1.52 ± 0.39 | ns |
| nPNA (g/kg/day) | 0.91 ± 0.23 | 0.90 ± 0.16 | ns |
Values are shown as the mean ± standard deviation or median (interquartile range).
Ca, calcium; ESA index, erythropoiesis‐stimulating agent resistance index; iPTH, intact parathyroid hormone; nPNA, normalized protein equivalent of nitrogen appearance.
The results of an analysis of the variables related to the differences between the log serum iFGF23 levels seen at the baseline and after 3 months’ sodium ferrous citrate supplementation are shown in Table 3. Only the difference between the serum ferritin levels observed at the baseline and after 3 months’ treatment was found to be significantly related to the post‐treatment variation in the log serum iFGF23 level (F = 7.04, P = 0.013) (Table 3, Model 2).
Table 3.
Variables contributing to the differences between the log serum intact FGF23 levels seen at the baseline and after 3 months’ sodium ferrous citrate supplementation
| Model 1 | ||
|---|---|---|
| Variables | F | P‐value |
| Differences in TSAT | 0.99 | 0.330 |
| Differences in serum phosphate | 0.09 | 0.771 |
| Differences in albumin‐adjusted serum Ca | 0.10 | 0.754 |
| Differences in log serum iPTH | 0.09 | 0.772 |
| Model 2 | ||
| Variables | F | P‐value |
| Differences in serum ferritin | 7.04 | 0.013 |
| Differences in serum phosphate | 2.62 | 0.118 |
| Differences in albumin‐adjusted serum Ca | 0.05 | 0.823 |
| Differences in log serum iPTH | 0.15 | 0.670 |
The analysis was performed using repeated measures, multivariate analysis of variance (MANOVA), and the results of the between‐subjects analyses are shown.
Differences: differences between the levels seen at the baseline and after 3 months’ sodium ferrous citrate supplementation
Ca, calcium; iPTH, intact parathyroid hormone; TSAT, transferrin saturation.
The results of an analysis of the variables related to the differences between the log serum cFGF23 levels observed at the baseline and after 3 months’ sodium ferrous citrate supplementation are shown in Table 4. The differences between the serum ferritin levels (F = 10.78, P = 0.003) or serum phosphate levels (F = 8.56, P = 0.007) recorded at the baseline and after 3 months’ treatment were found to be significantly related to the post‐treatment changes in the log serum cFGF23 level (Table 4, Model 2).
Table 4.
Variables contributing to the differences between the log serum c‐terminal FGF23 levels seen at the baseline and after 3 months’ sodium ferrous citrate supplementation
| Model 1 | ||
|---|---|---|
| Variables | F | P‐value |
| Differences in TSAT | 0.99 | 0.330 |
| Differences in serum phosphate | 0.09 | 0.771 |
| Differences in albumin‐adjusted serum Ca | 0.10 | 0.754 |
| Differences in log serum iPTH | 0.09 | 0.772 |
| Model 2 | ||
| Variables | F | P‐value |
| Differences in serum ferritin | 10.78 | 0.003 |
| Differences in serum phosphate | 8.56 | 0.007 |
| Differences in albumin‐adjusted serum Ca | 0.29 | 0.595 |
| Differences in log serum iPTH | 0.04 | 0.847 |
The analysis was performed using repeated measures, multivariate analysis of variance (MANOVA), and the results of the between‐subjects analyses are shown.
Differences: differences between the levels seen at the baseline and after 3 months’ sodium ferrous citrate supplementation
Ca, calcium; iPTH, intact parathyroid hormone; TSAT, transferrin saturation.
DISCUSSION
Circulating FGF23 levels are regulated at two distinct levels, FGF23 production and FGF23 cleavage, both of which occur within osteocytes. Low serum iron levels are associated with elevated FGF23 levels in autosomal dominant hypophosphataemic rickets (ADHR), but in healthy humans, low serum iron levels are associated with elevated cFGF23, but not iFGF23 levels, suggesting that the cleavage of FGF23 helps to maintain homeostasis during increased FGF23 expression.6, 20
Wolf et al. postulated that iron deficiency, which stimulates FGF23 transcription and downregulates FGF23 cleavage, could be one of the mechanisms responsible for elevated FGF23 levels in CKD, as was found in patients with ADHR.4 A recent animal study suggested that inflammation and iron deficiency might contribute to the mechanism responsible for elevated FGF23 levels in CKD.21 Moreover, iron dextran reduced the cFGF23 and iFGF23 levels of women with iron deficiency, whereas ferric carboxylate reduced their cFGF23 levels but increased their iFGF23 levels.22 The authors proposed that intravenous iron supplementation reduces the blood concentration of cFGF23, whereas carbohydrate moieties might simultaneously inhibit FGF23 degradation.22 Another study reported that oral ferric citrate reduces the serum iFGF23 and cFGF23 levels of dialysis patients.9 However, ferric citrate is an iron‐based phosphate binder and might reduce serum FGF23 levels via actions other than iron supplementation. Our study is the first to report that oral iron supplementation with sodium ferrous citrate (Fe2+), which does not exhibit phosphate‐binding activity, significantly replenished the iron stores and significantly reduced the serum iFGF23 and cFGF23 levels of MHD patients with iron deficiency. It is assumed that elevated serum phosphate,23, 24 calcium,25 1,25(OH)2 vitamin D26, and PTH levels23 stimulate FGF23 production.5 Phosphate intake and the use of phosphate binders, active vitamin D, or cinacalcet all influence serum FGF23 levels.5 In our study, sodium ferrous citrate supplementation reduced the patients’ serum iFGF23 and cFGF23levels without affecting their serum phosphate, Ca, or iPTH levels. Active vitamin D was administered to 20 of 31 patients in this study, but the dosages of active vitamin D, phosphate binders, cinacalcet, and dialysate were not changed during the study period. Dietary phosphate intake was not assessed based on interviews or dietary recall, however, the patients’ baseline nPNA values did not differ significantly from those recorded after 3 months’ sodium ferrous citrate treatment, suggesting that their dietary phosphate intake did not change significantly during the 3‐month study period. Furthermore, the fact that the changes in the patients’ serum ferritin values were associated with the changes in their log iFGF23 and log cFGF23 levels after 3 months’ oral iron supplementation supports our assumption that the replenishment of the patients’ iron stores caused the reductions in their iFGF23 and cFGF23 levels. An association was detected between the changes in the patients’ serum phosphate levels and the changes in their log serum cFGF23 levels, but the patients’ serum phosphate levels were not significantly affected by 3 months’ sodium ferrous citrate supplementation.
Intact FGF23 assays measure the levels of full‐length FGF23, and C‐terminal assays assess the levels of both full‐length FGF23 and the C‐terminal fragment.4 The iFGF23:cFGF23 ratio is a measure of the relative frequency of total circulating FGF23 species. If excessive degradation occurs, cFGF23 levels increase markedly, resulting in iFGF23:cFGF23 approaching zero.4 However, the iFGF23:cFGF23 ratio did not change significantly in the current study, suggesting that oral sodium ferrous citrate has less influence on FGF23 degradation. The precise mechanism responsible for the effect of iron on the FGF23 levels of MHD patients is beyond this study's scope, but there is a possibility that sodium ferrous citrate may reduce stimulus of FGF23 transcription in MHD patients with iron deficiency.
In the present study, reductions in the patients’ serum CRP levels and increases in their serum albumin levels were also observed after oral iron supplementation. Both iron deficiency and iron overload adversely affect the immune system.27 Oral iron is generally considered to be a safe, well‐tolerated, and effective form of iron supplementation in long‐term HD patients, but it can cause gastrointestinal side‐effects,27, 28 although none were observed in the current study.
The patients’ mean TSAT value and serum ferritin level after 3 months’ sodium ferrous citrate treatment were 38.8 ± 24.6% and 87.0 ± 90.6 ng/mL, respectively, and the associated improvements in their iron deficiency (without causing iron overload) might have caused their reduced serum CRP levels, which were suggestive of less severe inflammation. Elevated serum CRP levels mainly reflect the presence of infection/inflammation and are associated with hypoalbuminaemia in CKD patients and HD patients.29 We speculated that in the present study oral iron supplementation reduced the patients’ serum CRP levels, which in turn induced significant increases in their serum albumin levels and GNRI values. However, the patients’ iron stores were still suboptimally replete, and some of the patients might have required ongoing iron supplementation, including intravenous iron supplementation. The GNRI is calculated using an equation that includes serum albumin,17 and the increase in the GNRI seen after sodium ferrous citrate treatment in this study might have been related to rising serum albumin levels. A GNRI of <91.2 was found to exhibit high sensitivity and specificity for predicting malnutrition.17 In the current study, GNRI values of 87.7 ± 10.6 and 89.0 ± 10.4 were recorded at the baseline and after 3 months’ treatment, respectively, which suggested that the patients’ malnourished state persisted even after the oral iron supplementation.
The reductions in the patients’ serum iFGF23, cFGF23, CRP levels and the increases in their serum albumin levels were both small, but significant. With regard to the clinical interpretation of our findings, we consider that in small doses oral sodium ferrous citrate supplementation does not have deleterious effects on the risk of infection or serum albumin levels and might be useful for reducing the serum iFGF23 and cFGF23 levels of MHD patients with iron deficiency.
This study is limited by its relatively small sample size, the absence of a placebo control group, its short duration, the absence of precise data regarding non‐compliance with medication, and the fact that it focused on biochemical outcomes. It would be difficult to conduct a placebo‐controlled trial since the JSDT guidelines for renal anaemia recommend iron supplementation for MHD patients with serum ferritin levels of ≤100 ng/mL and TSAT values of ≤20%.14 Another limitation was that we did not measure 1,25‐dihydroxyvitamin D or 25‐hydroxyvitamin D levels. Further large randomized controlled studies are desirable to confirm the effects of oral iron supplementation with sodium ferrous citrate on the serum FGF23 levels of MHD patients with iron deficiency.
In conclusion, short‐term oral iron supplementation with sodium ferrous citrate replenished the iron stores and reduced the serum iFGF23 and cFGF23 levels of MHD patients with iron deficiency without affecting their serum phosphate, Ca, or iPTH levels.
Supporting information
Supporting info item
This study was supported by the private foundation of Ichiyokai Harada Hospital.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
REFERENCES
- 1. Gutiérrez OM, Mannstadt M, Isakova T et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008; 359: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kendrick J, Cheung AK, Kaufman JS et al. FGF‐23 associates with death, cardiovascular events, and initiation of chronic dialysis. J. Am. Soc. Nephrol. 2011; 22: 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Isakova T, Wahl P, Vargas GS et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011; 79: 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolf M, White KE. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr. Opin. Nephrol. Hypertens. 2014; 23: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012; 82: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farrow EG, Yu X, Summers LJ et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor‐23 (Fgf23) knock‐in mice. Proc. Natl. Acad. Sci. U. S. A. 2011; 108: E1146–E1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yokoyama K, Hirakata H, Akiba T et al. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis‐dependent CKD. Clin. J. Am. Soc. Nephrol. 2014; 9: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Block GA, Fishbane S, Rodriguez M et al. A 12‐week, double‐blind, placebo‐controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3‐5. Am. J. Kidney Dis. 2015; 65: 728–736. [DOI] [PubMed] [Google Scholar]
- 9. Iguchi A, Kazama JJ, Yamamoto S et al. Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron 2015; 131: 161–166. [DOI] [PubMed] [Google Scholar]
- 10. Geisser P, Philipp E. PA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clin. Nephrol. 2010; 74: 4–11. [DOI] [PubMed] [Google Scholar]
- 11. Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH. An open‐label, crossover study of a new phosphate‐binding agent in haemodialysis patients: ferric citrate. Nephrol. Dial. Transplant. 2002; 17: 265–270. [DOI] [PubMed] [Google Scholar]
- 12. Motoya T, Miyashita M, Kawachi A, Yamada K. Effects of sodium ferrous citrate, ferrous fumarate and ferrous sulphate on the absorption of cefdinir and cexime. J. Appl. Ther. Res. 1999; 2: 261–268. [Google Scholar]
- 13. Kidney Disease Improving Global Outcomes (KDIGO) Anemia Work Group (2012) . KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Use of iron to treat anemia in CKD. Kidney Int. Suppl. 2012; 2: 292–298. [Google Scholar]
- 14. Tsubakihara Y, Nishi S, Akiba T et al. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther. Apher. Dial. 2010; 14: 240–275. [DOI] [PubMed] [Google Scholar]
- 15. Rao A, Gilg J, Williams A. UK Renal Registry 16th annual report: chapter 10 haemoglobin, ferritin and erythropoietin amongst UK adult dialysis patients in 2012: national and centre‐specific analyses. Nephron Clin. Pract. 2013; 125: 183–208. [DOI] [PubMed] [Google Scholar]
- 16. Nakai S, Hanafusa N, Masakane I et al. Overview of regular dialysis treatment in Japan (as of 31 December 2012). Ther. Apher. Dial. 2014; 18: 535–602. [DOI] [PubMed] [Google Scholar]
- 17. Yamada K, Furuya R, Takita T et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008; 87: 106–113. [DOI] [PubMed] [Google Scholar]
- 18. Penne EL, van der Weerd NC, Grooteman MP et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011; 6: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. K/DOQI, National Kidney Foundation . Clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2000; 35: S17–S104. [DOI] [PubMed] [Google Scholar]
- 20. Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J. Clin. Endocrinol. Metab. 2011; 96: 3541–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. David V, Martin A, Isakova T et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016; 89: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013; 28: 1793–1803. [DOI] [PubMed] [Google Scholar]
- 23. Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010; 61: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutiérrez OM, Wolf M, Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow‐up study. Clin. J. Am. Soc. Nephrol. 2011; 6: 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimada T, Yamazaki Y, Takahashi M et al. Vitamin D receptor‐independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 2005; 289: F1088–F1095. [DOI] [PubMed] [Google Scholar]
- 26. Shimada T, Hasegawa H, Yamazaki Y et al. FGF‐23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004; 19: 429–435. [DOI] [PubMed] [Google Scholar]
- 27. Vaziri ND. Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am. J. Kidney Dis. 2013; 61: 992–1000. [DOI] [PubMed] [Google Scholar]
- 28. Lenga I, Lok C, Marticorena R, Hunter J, Dacouris N, Goldstein M. Role of oral iron in the management of long‐term hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2007; 2: 688–693. [DOI] [PubMed] [Google Scholar]
- 29. Menon V, Wang X, Greene T et al. Relationship between C‐reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am. J. Kidney Dis. 2003; 42: 44–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


