Abstract
A recently discovered peroxygenase from the fungus Marasmius rotula (MroUPO) is able to catalyze the progressive one‐carbon shortening of medium and long‐chain mono‐ and dicarboxylic acids by itself alone, in the presence of H2O2. The mechanism, analyzed using H2 18O2, starts with an α‐oxidation catalyzed by MroUPO generating an α‐hydroxy acid, which is further oxidized by the enzyme to a reactive α‐keto intermediate whose decarboxylation yields the one‐carbon shorter fatty acid. Compared with the previously characterized peroxygenase of Agrocybe aegerita, a wider heme access channel, enabling fatty acid positioning with the carboxylic end near the heme cofactor (as seen in one of the crystal structures available) could be at the origin of the unique ability of MroUPO shortening carboxylic acid chains.
Keywords: carboxylic acids, chain shortening, enzymes, hydrogen peroxide, oxidation
The use of biocatalysts for organic synthesis replacing traditional metal catalysts has several advantages, such as better regio‐ and stereoselectivity, fewer side products, and potentially lower environmental impact. Enzymes that catalyze the transfer of an oxygen atom from peroxide to substrates are classified as peroxygenases (EC.1.11.2). Unspecific peroxygenase (UPO, EC 1.11.2.1) is the most prominent member of this group because of its versatility for oxygen transfer reactions1 that makes it highly attractive as industrial biocatalyst.2, 3, 4
The first UPO was described in the basidiomycetous fungus Agrocybe aegerita (AaeUPO)5 catalyzing reactions formerly assigned only to P450 monooxygenases (P450s). However, unlike P450s that are intracellular enzymes and often require a flavin‐containing auxiliary enzyme or protein domain and a source of reducing power [NAD(P)H], UPO is a secreted protein, therefore far more stable, and only requires H2O2 for activation.2 AaeUPO was shown to catalyze oxygenation reactions on aromatic compounds,6 and later, the action on aliphatic compounds was demonstrated,7, 8 expanding its biotechnological relevance.
Since then, similar UPO proteins have been purified from other basidiomycetes and ascomycetes such as Coprinellus radians,9 Marasmius rotula 10 and Chaetomium globosum;11 and almost 3000 related sequences (from sequenced genomes and environmental samples) are currently available in databases.1, 11 The peroxygenase from M. rotula (MroUPO) shows several special features compared to other UPOs, for example, the inability to oxidize halides, less pronounced capacity to oxygenate aromatics,10 and the unique ability for terminal hydroxylation of n‐alkanes.12
Despite the widespread occurrence of peroxygenases and related heme–thiolate peroxidases, only two molecular structures have been reported to date, corresponding to the classic ascomycete chloroperoxidase (CPO),13 and AaeUPO.14 Fortunately, the crystal structure of MroUPO has just been made available (PDB entries 5FUJ and 5FUK). Interestingly, although MroUPO is a dimeric protein due to an inter‐monomer disulfide bond (Figure 1 B, whereas AaeUPO and CPO are monomers), this fact does not affect the accessibility to the heme channel and cofactor (Figure 1 A).
Figure 1.
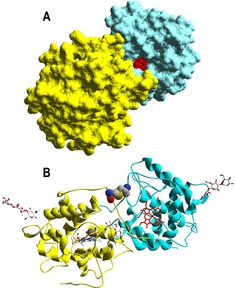
Dimeric MroUPO. A) Solvent‐access surface with one of the heme cofactors (red spheres) visible through an access channel. B) Ribbon‐like model including the C227‐C′227 disulfide bond (CPK‐colored spheres), the two cofactors (red and CPK sticks), and surface glycosylation (CPK sticks). From 5FUJ.
Here, the one‐carbon shortening of carboxylic acids, a fascinating reaction catalyzed by MroUPO, is revealed. This reaction was first evidenced when a dicarboxylic acid (tetradecanedioic acid) was tested as substrate of the enzyme, and the products were analyzed by gas chromatography–mass spectrometry (GC‐MS) (Figures 2 A,B, S1 and S2). High (93 %) substrate (0.1 mm) conversion by MroUPO (≈2 μm) was attained in 24 h, yielding a series of chain‐shortened dicarboxylic acids, such as tridecanedioic (63 % of products) and dodecanedioic (13 %) acids, together with α‐, β‐ and γ‐hydroxyderivatives. The reaction was also studied with AaeUPO (Figure 2 C), which failed to convert the substrate.
Figure 2.

GC‐MS of 1 h (A) and 24 h (B) reactions of tetradecanedioic acid (underlined) with MroUPO, and with AaeUPO after 24 h (C), showing the shortened dicarboxylic acids, and the α‐, β‐, and γ‐hydroxylated and α‐enol derivatives (italics) (see mass spectra in Figures S1 and S2).
When monocarboxylic fatty acids were studied as MroUPO substrates, the shortening reaction seemed to be chain‐length dependent. With tetradecanoic acid, terminal and subterminal oxygenations (forming the dicarboxylic and [ω‐1]‐keto derivatives, respectively) were predominant (Figure 3 A). However, with decanoic acid, a relevant amount of nonanoic acid was generated (Figure 3 B), although the reaction was less selective than with dicarboxylic acids.
Figure 3.

Comparison of tetradecanoic acid (A) and decanoic acid (B) reactions (2 h) with MroUPO showing the remaining substrate (underlined), the shortened monocarboxylic acids, and the α‐hydroxy, (ω‐1)‐keto and dicarboxylic derivatives.
To explore the role of α‐, β‐ and γ‐hydroxy‐derivatives as intermediates in the chain‐shortening mechanism, we studied the reactions of α‐ and β‐hydroxytetradecanoic acids with MroUPO. With the α‐hydroxy‐derivative, tridecanoic acid was one of the main products (Figure S3 A), but no chain shortening was accomplished with the β‐hydroxy‐derivative (Figure S3 B). This confirms that both even and odd chain fatty acids are produced by successive removal of one carbon atom after α‐oxidation (although, under some forced reaction conditions, some two‐carbon shortenings of β‐hydroxy acid could be observed as well; Figure S4).
Despite the difficulties for GC‐MS estimation of initial rates in the above MroUPO reactions, apparent kinetic constants could be obtained for the products: i) resulting in chain‐shortening; and ii) of other oxygenation reactions (Table 1). Concerning shortening, the MroUPO had higher catalytic efficiency (k cat/K m) on C10 than on di‐C14, due to the almost 10‐fold higher catalytic constant (k cat), although it was less selective as shown by the ratios (0.8 and 2.0, respectively) between the catalytic efficiencies of shortening and other reactions. The chain‐shortening of both mono‐ and dicarboxylic acids, and the α‐hydroxylation of carboxylic acids by a peroxygenase are reported here for the first time.
Table 1.
Apparent kinetic constants for tetradecanedioic acid (di‐C14) and decanoic acid (C10) reactions with MroUPO.
| k cat [min−1] | K m [μm] | k cat/K m [min−1 mm −1] | |
|---|---|---|---|
| Chain shortening[a] | |||
| di‐C14 | 32±9 | 557±295 | 58±4 |
| C10 | 293±98 | 703±549 | 420±350 |
| Other oxygenations[b] | |||
| di‐C14 | 7±1 | 239±42 | 28±5 |
| C10 | 337±92 | 649±430 | 520±370 |
[a] After oxygenation at α position. [b] At β, γ, ω and ω‐1 positions.
To get additional insight into the chain shortening mechanism, 18O‐labeling studies with H2 18O2 (90 % isotopic purity) were performed using tetradecanedioic acid as target substrate (Figures S1 and S2). Overall, our data led to the chain‐shortening mechanism depicted in Scheme 1 a. The initial product of MroUPO reaction will be the α‐hydroxy acid, as demonstrated by incorporation of H2 18O2 oxygen to form α‐hydroxytetradecanedioic acid, whose diagnostic fragment (m/z 373, Figure S1 A, top) appeared fully (90 %) 18O‐labeled (m/z 375, Figure S1 A, bottom). Its oxidation will yield a gem‐diol (ketone hydrate) from a second Cα hydroxylation by MroUPO that will be in equilibrium with the ketone by dehydration, and then will react with excess H2O2 decarboxylating and forming a new carboxyl group (chain shortening) as explained below.
Scheme 1.
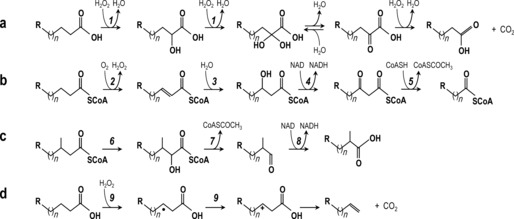
Comparison of fatty‐acid shortening reactions: a) α‐oxidation and decarboxylation by MroUPO; b) usual β‐oxidation (multienzymatic) pathway; c) α‐oxidation (multienzymatic) pathway for β‐methylated acids; and d) decarboxylation and alkene formation by P450; (R, H or COOH; enzymes: 1, MroUPO 2, acyl‐CoA oxidase; 3, enoyl‐CoA hydratase; 4, 3‐hydroxyacyl‐CoA dehydrogenase; 5, 3‐ketoacyl‐CoA thiolase; 6, phytanoyl‐CoA hydroxylase; 7, 2‐hydroxy‐phytanoyl‐CoA lyase; 8, aldehyde dehydrogenase; and 9, P450 fatty‐acid decarboxylase).
Direct evidence for involvement of H2 18O2‐borne oxygen into the gem‐diol/ketone formation yielding α‐ketotetradecanedioic could not be obtained, since α‐keto acids rapidly decarboxylate in the presence of oxidizing agents including H2O2.15 However, evidence of their transient formation was obtained, as the enolic form was detected (Figures 2 A, S3 A and S5 A). 18O‐labeling also illuminated the formation of tridecanedioic acid after incorporation of two or one 18O‐atoms (Figure S1 B, bottom). The co‐existence of single and double 18O‐labeling in the carboxylic group suggests that a gem‐diol, with some hydroxyl exchange with the water (labelling loss), may occur prior to decarboxylation and chain shortening. This second α‐hydroxylation is clearly provoked by the enzyme and not by the H2O2, as revealed by the negative control with α‐hydroxytetradecanoic acid and H2O2 in the absence of enzyme (Figure S6). However, the final reaction step can directly be produced by the H2O2 present in the UPO reaction set‐up, mediated by a hydroperoxide intermediate, as reported for other α‐ketoacids.16
The different reactivity of MroUPO and AaeUPO regarding α‐hydroxylation could be explained by the only recently available crystal structure of MroUPO (PDB entries 5FUJ and 5FUK) compared with the previously reported AaeUPO structure.14 MroUPO is a smaller protein but it has a wider heme‐access channel (Figure 4 A) than AaeUPO (Figure 4 B), the channel of which is flanked by several bulky phenylalanine residues (a narrow access channel also exists in CPO). This wider heme channel directly exposes the reactive Fe=O of H2O2‐activated MroUPO (compound I) to the entering substrate, enabling oxygenation at the α‐position of carboxylic acids.
Figure 4.
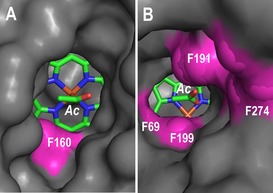
Different sizes of the heme‐access channel in MroUPO (A) and AaeUPO (B), and bulky phenylalanine residues (magenta) (an acetate occupies the substrate‐binding site). From 5FUJ (A) and 2YP1 (B).
Interestingly, one of the MroUPO crystal structures available (5FUK) includes a bound palmitic acid molecule along the heme access channel with one of the carboxylate oxygens at coordination distance of the heme iron (Figure 5 A) (while an acetate occupies this position in 5FUJ, Figure 5 B). Sub‐terminal oxygenation by most UPOs implies fatty‐acid binding with the carboxyl located at the channel entrance. However, the palmitic acid position found in the 5FUK crystal is in agreement with the unique chain‐shortening ability reported here for MroUPO.
Figure 5.
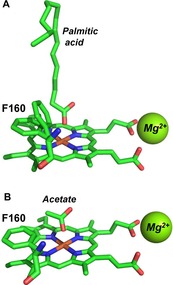
Palmitic acid (A) and acetate (B) ligands in MroUPO crystal structures. Ligands, heme cofactor and neighbor Phe160 are shown as sticks (CPK colors) while Mg2+ cations are shown as spheres (when palmitic acid is present in the crystal, the Phe160 side‐chain adopts two different conformations). From PDB entries 5FUK (A) and 5FUJ (B).
In summary, we show the ability of MroUPO to catalyze the stepwise chain shortening of carboxylic acids through a chemo‐enzymatic reaction cascade (Scheme 1 a). In plants, fungi and animals, the general β‐oxidation pathway, leads to two‐C shorter acids (Scheme 1 b)17 and the alternative α‐oxidation pathway (Scheme 1 c), leading to one‐C shorter fatty acids, typically includes several steps (hydroxylation, activation, cleavage of the C1−C2 bond and aldehyde dehydrogenation) with several enzymes involved.18 However, MroUPO is capable of catalyzing all these reactions self‐sufficiently (i.e. alone), in the presence of H2O2. Bacterial P450s are also known to decarboxylate fatty acids, but in this case n−1 terminal alkenes (Scheme 1 d), instead of chain‐shortened fatty acids, are formed.19
This carbon‐by‐carbon chain‐shortening reaction represents a novel chemistry that may be used in biotechnological applications including the obtainment of tailor‐made acids such as odd‐numbered dicarboxylic or monocarboxylic fatty acids (less abundant in nature than the even‐numbered ones). The “odd‐even” effect on the aqueous solubility of dicarboxylic acids20 could be used for product isolation, and in the synthesis of ad hoc polymers.21
The chain‐shortening reaction described here must be added to the repertoire of reactions that versatile fungal peroxygenases catalyze on linear12, 22 and cyclic aliphatic compounds,23, 24 in addition to aromatic compounds.1, 2 The availability of a heterologous expression system for MroUPO will permit to improve the catalytic properties of this promising enzyme, for example, in chain‐shortening and/or alkane terminal hydroxylation reactions,12 using the protein engineering tools recently applied to AaeUPO.25
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by the EnzOx2 (H2020‐BBI‐PPP‐2015‐2‐1–720297) EU‐project, and the BIORENZYMERY (AGL2014‐53730‐R) and NOESIS (BIO2014‐56388‐R) projects of the Spanish MINECO (co‐financed by FEDER). E.D. Babot (IRNAS) is thanked for experimental help.
A. Olmedo, J. C. d. Río, J. Kiebist, R. Ullrich, M. Hofrichter, K. Scheibner, A. T. Martínez, A. Gutiérrez, Chem. Eur. J. 2017, 23, 16985.
References
- 1. Hofrichter M., Kellner H., Pecyna M. J., Ullrich R., Adv. Exp. Med. Biol. 2015, 851, 341–368. [DOI] [PubMed] [Google Scholar]
- 2. Hofrichter M., Ullrich R., Curr. Opin. Chem. Biol. 2014, 19, 116–125. [DOI] [PubMed] [Google Scholar]
- 3. Bormann S., Baraibar A. G., Ni Y., Holtmann D., Hollmann F., Catal. Sci. Technol. 2015, 5, 2038–2052. [Google Scholar]
- 4. Martínez A. T., Ruiz-Dueñas F. J., Camarero S., Serrano A., Linde D., Lund H., Vind J., Tovborg M., Herold-Majumdar O. M., Hofrichter M., et al., Biotechnol. Adv. 2017, 35, 815–831. [DOI] [PubMed] [Google Scholar]
- 5. Ullrich R., Nuske J., Scheibner K., Spantzel J., Hofrichter M., Appl. Environ. Microbiol. 2004, 70, 4575–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hofrichter M., Ullrich R., Pecyna M. J., Liers C., Lundell T., Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [DOI] [PubMed] [Google Scholar]
- 7. Gutiérrez A., Babot E. D., Ullrich R., Hofrichter M., Martínez A. T., del Río J. C., Arch. Biochem. Biophys. 2011, 514, 33–43. [DOI] [PubMed] [Google Scholar]
- 8. Peter S., Kinne M., Wang X., Ulrich R., Kayser G., Groves J. T., Hofrichter M., FEBS J. 2011, 278, 3667–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anh D. H., Ullrich R., Benndorf D., Svatos A., Muck A., Hofrichter M., Appl. Environ. Microbiol. 2007, 73, 5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gröbe G., Ullrich M., Pecyna M., Kapturska D., Friedrich S., Hofrichter M., Scheibner K., AMB Express 2011, 1, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiebist J., Schmidtke K. U., Zimmermann J., Kellner H., Jehmlich N., Ullrich R., Zänder D., Hofrichter M., Scheibner K., ChemBioChem 2017, 18, 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olmedo A., Aranda C., del Río J. C., Kiebist J., Scheibner K., Martínez A. T., Gutiérrez A., Angew. Chem. Int. Ed. 2016, 55, 12248–12251; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 12436–12439. [Google Scholar]
- 13. Sundaramoorthy M., Terner J., Poulos T. L., Structure 1995, 3, 1367–1377. [DOI] [PubMed] [Google Scholar]
- 14. Piontek K., Strittmatter E., Ullrich R., Gröbe G., Pecyna M. J., Kluge M., Scheibner K., Hofrichter M., Plattner D. A., J. Biol. Chem. 2013, 288, 34767–34776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper A. J. L., Ginos J. Z., Meister A., Chem. Rev. 1983, 83, 321–358. [Google Scholar]
- 16. Vlessis A. A., Bartos D., Trunkey D., Biochem. Biophys. Res. Commun. 1990, 170, 1281–1287. [DOI] [PubMed] [Google Scholar]
- 17. Schulz H. in Biochemistry of Lipids, Lipoproteins and Membranes, Vol. 5, (Eds.: D. E. Vance, J. Vance), Elsevier, Amsterdam: 2008, 131–154. [Google Scholar]
- 18. Jansen G. A., Wanders R. J. A., Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 1403–1412. [DOI] [PubMed] [Google Scholar]
- 19. Dennig A., Kuhn M., Tassoti S., Thiessenhusen A., Gilch S., Bülter T., Haas T., Hall M., Faber K., Angew. Chem. Int. Ed. 2015, 54, 8819–8822; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 8943–8946. [Google Scholar]
- 20. Zhang H., Xie C., Liu Z., Gong J., Bao Y., Zhang M., Hao H., Hou B., Yin Q., Ind. Eng. Chem. Res. 2013, 52, 18458–18465. [Google Scholar]
- 21. Huf S., Krugener S., Hirth T., Rupp S., Zibek S., Eur. J. Lipid Sci. Technol. 2011, 113, 548–561. [Google Scholar]
- 22. Babot E. D., del Río J. C., Kalum L., Martínez A. T., Gutiérrez A., Biotechnol. Bioeng. 2013, 110, 2323. [DOI] [PubMed] [Google Scholar]
- 23. Babot E. D., del Río J. C., Cañellas M., Sancho F., Lucas F., Guallar V., Kalum L., Lund H., Gröbe G., Scheibner K., Ullrich R., Hofrichter M., Martínez A. T., Gutiérrez A., Appl. Environ. Microbiol. 2015, 81, 4130–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lucas F., Babot E. D., del Río J. C., Kalum L., Ullrich R., Hofrichter M., Guallar V., Martínez A. T., Gutiérrez A., Catal. Sci. Technol. 2016, 6, 288–295. [Google Scholar]
- 25. Molina-Espeja P., Canellas M., Plou F. J., Hofrichter M., Lucas F., Guallar V., Alcalde M., ChemBioChem 2016, 17, 341–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


