Abstract
Mesenchymal stem cell (MSC) therapies have been used in clinical trials in various fields. These cells are easily expanded, show low immunogenicity, can be acquired from medical waste, and have multiple functions, suggesting their potential applications in a variety of diseases, including liver disease and inflammatory bowel disease. MSCs help prepare the microenvironment, in response to inflammatory cytokines, by producing immunoregulatory factors that modulate the progression of inflammation by affecting dendritic cells, B cells, T cells, and macrophages. MSCs also produce a large amount of cytokines, chemokines, and growth factors, including exosomes that stimulate angiogenesis, prevent apoptosis, block oxidation reactions, promote remodeling of the extracellular matrix, and induce differentiation of tissue stem cells. According to ClinicalTrials.gov, more than 680 clinical trials using MSCs are registered for cell therapy of many fields including liver diseases (more than 40 trials) and inflammatory bowel diseases (more than 20 trials). In this report, we introduce background and clinical studies of MSCs in liver disease and inflammatory bowel diseases.
Keywords: Mesenchymal stem cell, Liver disease, Inflammatory bowel disease, Cell therapy
Background
The digestive system, which consists of the gastrointestinal tract, liver, pancreas, and biliary tree, functions in digestion, absorption, and metabolism and affects the basis of life. Various diseases, including cancer, inflammatory disease, infection, stones, and ulcers, are studied under the context of gastroenterology. While innovative drugs against Helicobacter pylori [1], hepatitis C virus [2], and inflammatory bowel disease (IBD) [3] have recently been developed, there are still unmet needs in this field, including in acute and chronic liver failure and refractory IBDs. Cell therapy may fulfill these unmet needs, and cell therapies using mesenchymal stem cells (MSCs) have become a major focus in many fields [4]. MSCs are reported to have multiple functions, especially anti-fibrosis and anti-inflammatory effects are focused in acute and chronic liver failure and refractory IBDs. Furthermore, MSCs have low immunogenicity, can expand easily, and can be obtained from medical waste, suggesting their potential to expand regenerative medicine for the treatment of liver diseases and IBDs.
In this paper, we review the current status of clinical trials using autologous/allogeneic MSCs in liver diseases and IBDs.
Characteristics of MSCs
MSCs have recently received attention as potential cell sources for cell therapy due to their ease of expansion and wide range of functions. MSCs can be obtained from not only bone marrow but also medical wastes, such as adipose tissue, umbilical tissue, and dental pulp. MSCs are positive for the common markers CD73, CD90, and CD105; however, they are negative for the endothelial marker CD31 and hematopoietic marker CD45 [4–7]. The expansion of MSCs in culture is relatively easy, and under appropriate conditions, MSCs have trilineage differentiation (osteogenic, chondrogenic, and adipogenic) potential. The effects of MSCs are broadly divided into two mechanisms: (1) recruited MSCs differentiate into functional cells to replace damaged cells, permitting the treatment of bone and cartilage damage; and (2) in response to inflammatory cytokines, MSCs help prepare the microenvironment by producing immunoregulatory factors that modulate the progression of inflammation by affecting dendritic cells, B cells, T cells, and macrophages. MSCs also produce a large amount of cytokines, chemokines, and growth factors, including exosomes, which stimulate angiogenesis, prevent apoptosis, block oxidation reactions, promote remodeling of the extracellular matrix (ECM), and induce the differentiation of tissue stem cells [4, 7, 8]. These latter mechanisms can be applied for many diseases, including liver disease and IBSs. Some studies have reported that the effects of MSCs are determined by host conditions, such as inflammation stage and the use of immunosuppressants.
Although the behaviors of MSCs after administration have been analyzed, and some studies have shown that MSCs migrate to the injured site, MSC behaviors in humans have not been fully elucidated. Some studies have reported that MSCs disappear within a few weeks and do not remain long in the target tissue [5]. Recent studies have reported that only culture-conditioned medium or exosomes induce treatment effects, suggesting that the trophic effect is the most important effect of MSCs [9–11]. Another important characteristic of MSCs is that they generally have low immunogenicity. MSCs have no antigen-presenting properties and do not express major histocompatibility complex class II or costimulatory molecules; thus, injection of autologous or allogeneic MSCs has been employed in clinical studies. Allogeneic MSC therapy has the potential to expand MSC therapy to many patients [4, 7].
Clinical trials using MSCs
Since MSCs can be obtained relatively easily and have multiple functions, more than 680 clinical trials are ongoing according to ClinicalTrials.gov (https://clinicaltrials.gov/); most of these studies are phase I or II trials evaluating the use of MSCs in bone/cartilage, heart, neuron, immune/autoimmune, diabetes/kidney, lung, liver, and gastrointestinal fields. These studies aim to elucidate the safety/effectiveness of MSCs in the treatment of various diseases. In liver diseases, 40 trials are registered, most of which target liver cirrhosis or acute liver diseases (Table 1) [12–21]. The MSCs used in clinical trials of the liver are derived from the bone marrow (55%), umbilical cord tissue (35%), and adipose tissue (8%). Approximately 50% of MSCs are allogeneic. Additionally, while the major administration route is the peripheral blood, approximately 40% of cases are treated via the hepatic artery, reflecting the fact that hepatologists and radiologists often use catheters to treat hepatocellular carcinoma through the hepatic artery [22, 23] (Fig. 1).
Table 1.
Clinical trials in liver diseases
| No. | Start year | Cell source | Autologous/allogeneic | Administration route | Number of cells infused | Etiology | Number of patients | Follow-up period | Phase | Study design | ClinicalTrials.gov identifier | Status | Result | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2013 | Bone marrow | Autologous | Peripheral vein | Unknown | LC | 20 | 48 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT01877759 | Unknown | ||
| 2 | 2009 | Bone marrow | Autologous | Hepatic artery | 5 × 106 cells/patient, 2 times | LC (alcohol) | 11 | 24 weeks | Phase 2 | Non-randomized, single group assignment, open label |
NCT01741090 | Unknown | Histological improvement. Improvement in Child- Pugh score. Decrease in TGFβ1, collagen type I, and α-SMA |
|
| 3 | 2009 | Bone marrow | Autologous | Peripheral vein | 1.0 × 106/kg | LC | 25 | 24 weeks | Unknown | Non-randomized, single group assignment, open label | NCT01499459 | Unknown | Improvement in Alb and MELD scores. | 13 |
| 4 | 2014 | Umbilical cord | Allogeneic | Peripheral vein | 4.0 × 107/patient, 4 times | LC | 320 | 144 weeks | Phase 1–2 | Non-randomized, parallel assignment, open label | NCT01573923 | Unknown | ||
| 5 | 2016 | Adipose tissue | Autologous | Portal vein or hepatic artery | 1.0 × 106/kg via peripheral vein, 3 times or 3.0 × 106/kg via hepatic artery, 3 times | LC (HCV) | 5 | 48 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT02705742 | Recruiting | ||
| 6 | 2007 | Bone marrow | Autologous | Peripheral or portal vein | 30–50 × 106/patient | LC | 8 | 24 weeks | Phase 1–2 | Randomized, single group assignment, single blind | NCT00420134 | Completed | Improvement in liver function and MELD scores. | 14 |
| 7 | 2016 | Bone marrow | Allogeneic | Peripheral vein | 2.0 × 106/kg, 4 times | ACLF | 30 | 96 weeks | Phase 1 | Randomized, parallel assignment, double blind (subject, caregiver, investigator) | NCT02857010 | Recruiting | ||
| 8 | 2009 | Umbilical cord | Allogeneic | Peripheral vein | 5.0 × 105/kg, 3 times | ACLF (HBV) | 43 | 96 weeks | Phase 1–2 | Randomized, parallel assignment, double blind (subject, caregiver) | NCT01218464 | Unknown | Improvement in liver function and MELD scores. | 15 |
| 9 | 2011 | Bone marrow | Allogeneic | Peripheral vein | 2.0 × 105/kg, 4 times or 1.0 × 106/kg, 4 times or 5.0 × 106/kg, 4 times | Liver failure (HBV) | 120 | 48 weeks | Phase 2 | Randomized, parallel assignment, open label | NCT01322906 | Unknown | ||
| 10 | 2010 | Umbilical cord | Allogeneic | Unknown | Unknown | LC | 20 | 48 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01342250 | Completed | ||
| 11 | 2012 | Bone marrow | Allogeneic | Hepatic artery | Unknown | LC (Alcohol) | 40 | 96 weeks | Phase 2 | Randomized, parallel assignment, open label | NCT01591200 | Completed | ||
| 12 | 2012 | Umbilical cord | Allogeneic | Peripheral vein | 1.0 × 105/kg, 4 times | Liver failure (HBV) | 120 | 48 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01724398 | Unknown | ||
| 13 | 2016 | Bone marrow | Autologous | Portal vein | 2.0 × 106/kg | LC | 40 | 24 weeks | Phase 1–2 | Non-randomized, parallel assignment, open label | NCT02943889 | Not yet recruiting | ||
| 14 | 2009 | Umbilical cord | Allogeneic | Portal vein or hepatic artery | Unknown | LC | 200 | 48 weeks | Phase 1–2 | Randomized, parallel assignment, single blind (subject) | NCT01233102 | Suspended | ||
| 15 | 2009 | Bone marrow | Autologous | Portal vein | Unknown | LC (HBV) | 60 | 48 weeks | Phase 2 | Non-randomized, parallel assignment, open label | NCT00993941 | Unknown | ||
| 16 | 2010 | Umbilical cord | Allogeneic | Hepatic artery | Unknown | LC | 50 | 4 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01224327 | Unknown | ||
| 17 | 2013 | Bone marrow | Autologous | Hepatic artery | 1.0 × 106/kg | LC | 30 | 12 weeks | Phase 3 | Non-randomized, single group assignment, open label | NCT01854125 | Enrolling by invitation | ||
| 18 | 2012 | Umbilical cord | Allogeneic | Hepatic artery | 1.0 × 106/kg | LC (HBV) | 240 | 48 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01728727 | Unknown | ||
| 19 | 2013 | Umbilical cord or bone marrow | Allogeneic | Peripheral vein | 1.0 × 105/kg, 1.0 × 106/kg or 1.0 × 107/kg, 8 times | Liver failure (HBV) | 210 | 72 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01844063 | Recruiting | ||
| 20 | 2016 | Umbilical cord | Allogeneic | Peripheral vein | 4 or 8 times | ACLF (HBV) | 261 | 52 weeks | Phase 2 | Randomized, parallel assignment, open label | NCT02812121 | Not yet recruiting | ||
| 21 | 2010 | Menstrual blood | Allogeneic | Peripheral vein | 1.0 × 106/kg, 4 times | LC | 50 | 48 weeks | Phase 1–2 | Randomized, single group assignment, open label | NCT01483248 | Enrolling by invitation | ||
| 22 | 2008 | Bone marrow | Autologous | Hepatic artery | Unknown | LC | 50 | 96 weeks | Phase 2 | Randomized, parallel assignment, single blind (subject) | NCT00976287 | Unknown | ||
| 23 | 2012 | Bone marrow | Autologous | Hepatic artery | 5 × 107/patient, 1 time or 2 times | LC (alcohol) | 72 | 24 weeks | Phase 2 | Randomized, parallel assignment, open label | NCT01875081 | Completed | Histological improvement. Improvement in AST, ALT, ALP, γ-GTP, Child-Pugh score, and MELD score. | 16 |
| 24 | 2014 | Bone marrow | Autologous | Peripheral vein | Unknown | LC | 10 | 24 weeks | Phase 1 | Non-randomized, single group assignment, open label | NCT02327832 | Recruiting | ||
| 25 | 2005 | Bone marrow | Autologous | Hepatic artery | 3.4 × 108/patient | Liver failure (HBV) | 158 | 192 weeks | Phase 1–2 | Case control, retrospective | NCT00956891 | Completed | Improvement in Alb, T-Bil, PT, and MELD score. | |
| 26 | 2009 | Umbilical cord | Allogeneic | Peripheral vein | 5.0 × 105/kg, 3 times | LC | 45 | 48 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01220492 | Unknown | Improvement in Alb, T-Bil, and MELD score. Reduction of ascites. |
17 |
| 27 | 2010 | Bone marrow | Autologous | Portal vein | 1.4–2.5 × 108/patient, 2 times | LC | 2 | 48 weeks | Phase 1 | Non-randomized, single group assignment, open label | NCT01454336 | Completed | Transient improvement in MELD scores. | 18 |
| 28 | 2007 | Bone marrow | Autologous | Peripheral vein | (1.2–2.95 × 108) 1.95 × 108/patient | LC | 27 | 48 weeks | Unknown | Randomized, parallel assignment, double blind (subject, outcomes assessor) |
NCT00476060 | Unknown | No beneficial effect. | 19 |
| 29 | 2011 | Bone marrow | Allogeneic | Hepatic artery and peripheral artery | 1.0 × 106/kg (5.0 × 107 cells via the hepatic artery and the remaining cells via the peripheral vein) | Wilson’s disease | 10 | 24 weeks | Unknown | Non-randomized, single group assignment, open label | NCT01378182 | Completed | ||
| 30 | 2016 | Umbilical cord or bone marrow | Allogeneic | Portal vein or hepatic artery | 2.0 × 107/patient, 4 times | LC | 20 | 48 weeks | Phase 1 | Non-randomized, single group assignment, open label | NCT02652351 | Recruiting | ||
| 31 | 2016 | Bone marrow | Autologous | Hepatic artery | 5 × 107/patient, 1 time or 2 times | LC (alcohol) | 50 | 144 weeks | Phase 2 | Randomized, parallel assignment, open label | NCT02806011 | Enrolling by invitation | ||
| 32 | 2011 | Umbilical cord | Allogeneic | Peripheral vein | 1.0 × 106/kg, 3 times | Liver failure (AIH) | 100 | 96 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01661842 | Unknown | ||
| 33 | 2009 | Adipose tissue | Autologous | Unknown | Unknown | LC | 6 | 24 weeks | Phase 1 | Non-randomized, single group assignment, open label | NCT00913289 | Terminated | ||
| 34 | 2012 | Adipose tissue | Autologous | Hepatic artery | Unknown | LC | 4 | 4 weeks | Unknown | Non-randomized, single group assignment, open label | NCT01062750 | Completed | ||
| 35 | 2016 | Umbilical cord | Allogeneic | Lobe | 5.0 × 108/patient | LC | 40 | 96 weeks | Phase 1–2 | Randomized, parallel assignment, double blind (subject, outcomes assessor) | NCT02786017 | Recruiting | ||
| 36 | 2011 | Bone marrow | Unknown | Peripheral vein | 5.0–50 × 106/kg | LC (PBC) | 20 | 96 weeks | Phase 1 | Randomized, parallel assignment, open label | NCT01440309 | Unknown | ||
| 37 | 2011 | Umbilical cord | Allogeneic | Peripheral vein | 5.0 × 105/kg, 3 times | LC (PBC) | 7 | 48 weeks | Phase 1–2 | Randomized, parallel assignment, open label | NCT01662973 | Unknown | Improvement in Alb, T-Bil, and MELD score. Reduction of ascites. | 20 |
| 38 | 2010 | Bone marrow | Allogeneic | Portal vein or hepatic artery | Unknown | Liver failure (HBV) | 60 | 48 weeks | Phase 2 | Non-randomized, parallel assignment, open label | NCT01221454 | Unknown | ||
| 39 | 2010 | Bone marrow | Allogeneic | Portal vein or hepatic artery | Unknown | LC | 60 | 48 weeks | Phase 2 | Non-randomized, parallel assignment, open label | NCT01223664 | Unknown | ||
| 40 | 2010 | Bone marrow | Autologous | Hepatic artery | (0.25–1.25 × 106) 0.75 × 106/patient | LC (HBV) | 39 | 24 weeks | Phase 2–3 | Non-randomized, parallel assignment, open label | NCT01560845 | Unknown | Decrease in Th-17 cells, RORγt, IL-17, TNF-α, and IL-6. Increase in Tregs and Foxp3. | 21 |
LC liver cirrhosis, ACLF acute-on-chronic liver failure, HBV hepatitis B virus, HCV hepatitis C virus, AIH autoimmune hepatitis, PBC primary biliary cholangitis, MELD Model for End-Stage Liver Disease, AST aspartate transaminase, ALT alanine transaminase, ALP alkaline phosphatase, γ-GTP gamma-glutamyl transpeptidase, Alb albumin, T-bill total bilirubin, PT prothrombin time, PC protein C, ROR RAR-related orphan receptor, Foxp3 forkhead box P3, IL interleukin, Th T helper, SMA smooth muscle actin, TGF transforming growth factor, TNF tumor necrosis factor
Fig. 1.
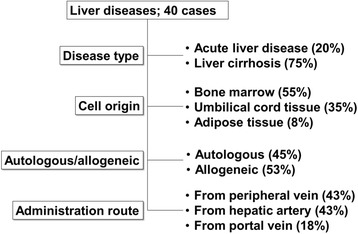
Summary of clinical trials in liver diseases
In IBDs, 26 trials are registered (Table 2), 23 of which are investigating the use of MSCs in Crohn’s disease (CD), and 3 of which are investigating the use of MSCs in ulcerative colitis (UC) [24–33]. More than 60% of trials are employing allogeneic MSCs, and in CD, more than 40% of the trials are evaluating intralesional injection into the fistula, which is the major and refractory complication of CD (Fig. 2).
Table 2.
Clinical trials in inflammatory bowel diseases
| No. | Start year | Cell source | Autologous/allogeneic | Administration route | Number of cells infused | Diseases | Number of patients | Follow-up period | Phase | Study design | ClinicalTrials.gov identifier | Status | Result | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2006 | Bone marrow | Allogeneic | Peripheral vein | 8 × 106 cells/kg, 2 times or 2 × 106 cells/kg, 2 times | Crohn’s disease | 10 | 4 weeks | Phase 2 | Randomized, parallel assignment, open label | NCT00294112 | Completed | ||
| 2 | 2007 | Bone marrow | Allogeneic | Peripheral vein | Total of 6 × 108 cells/patient, 4 times or total of 12 × 108 cells/patient, 4 times |
Crohn’s disease | 98 | 24 weeks | Phase 3 | Randomized, parallel assignment, double blind | NCT00543374 | Completed | ||
| 3 | 2010 | Adipose tissue | Autologous | Unknown | Unknown | Fistulizing Crohn’s disease | 15 | 3 years | Phase 1–2 | Non-randomized, single group assignment, open label | NCT01157650 | Completed | ||
| 4 | 2015 | Umbilical cord | Allogeneic | Peripheral vein | Unknown | Crohn’s disease | 32 | 1 year | Phase 1–2 | Randomized, parallel assignment, open label | NCT02445547 | Completed | ||
| 5 | 2012 | Bone marrow | Allogeneic | Peripheral vein | 2 × 108 cells/patient, more than 4 times | Crohn’s disease | 11 | 4 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT01510431 | Completed | ||
| 6 | 2010 | Bone marrow | Allogeneic | Peripheral vein | 2 × 106 cells/kg, 4 times | Crohn’s disease | 15 | 6 weeks | Phase 2 | Non-randomized, single group assignment, open label | NCT01090817 | Completed | Improvement in CDAI, AQoL score. Decrease in CRP. Endoscopic improvement | 24 |
| 7 | 2012 | Bone marrow | Autologous | Peripheral vein | 2 × 106 cells/kg, 5 × 106 cells/kg, or 1 × 107 cells/kg | Crohn’s disease | 16 | 1 year | Phase 1 | Non-randomized, single group assignment, open label | NCT01659762 | Completed | ||
| 8 | 2010 | Bone marrow | Allogeneic | Intralesional | 1 × 107 cells/patient, 3 × 107 cells/patient, or 9 × 107 cells/patient | Fistulizing Crohn’s disease | 21 | 12 weeks | Phase 1–2 | Randomized, parallel assignment, double blind | NCT01144962 | Completed | Local treatment with MSCs showed promotion of fistula healing. Lower MSC dose seemed superior. | 25 |
| 9 | 2009 | Adipose tissue | Autologous | Intralesional | 3 × 107 cells/patient (in the event of incomplete closure at 8 weeks, a second injection was given that contained 1.5 times more cells than the f irst) | Fistulizing Crohn’s disease | 43 | 8 weeks | Phase 1 | Non-randomized, single group assignment, open label | NCT00992485 | Completed | Local treatment with MSCs showed promotion of fistula healing. | 26 |
| 10 | 2010 | Adipose tissue | Allogeneic | Intralesional | 2 × 107 cells/patient (in the event of incomplete closure at 12 weeks, an additional 4 × 107 cells were administered) |
Fistulizing Crohn’s disease | 24 | 24 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT01372969 | Completed | Local treatment with MSCs showed promotion of fistula healing. | 27 |
| 11 | 2009 | Adipose tissue | Autologous | Intralesional | 1 × 107 cells/patient, 2 × 107 cells/patient, or 4 × 107 cells/patient |
Fistulizing Crohn’s disease | 10 | 4 weeks | Phase 1 | Non-randomized, single group assignment, open label | NCT00992485 | Completed | Local treatment with MSCs showed promotion of fistula healing. All patients with complete healing showed a sustained effect. | 28 |
| 12 | 2009 | Adipose tissue | Allogeneic | Intralesional | 2 × 107 cells/patient (in the event of incomplete closure at 12 weeks, an additional 4 × 107 cells were administered) | Fistulizing Crohn’s disease | 10 | 12 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT00999115 | Completed | Local treatment with MSCs showed promotion of fistula healing; 60% of patients achieved complete healing. | 29 |
| 13 | 2009 | Adipose tissue | Autologous | Intralesional | 1 × 107 cells/cm2 | Fistulizing Crohn’s disease | 43 | 8 weeks | Phase 2 | Non-randomized, single group assignment, open label | NCT01011244 | Completed | In most cases, complete closure after initial treatment was well-sustained over a 24-month period. | 30 |
| 14 | 2007 | Bone marrow | Allogeneic | Peripheral vein | Total of 6 × 108 cells/patient, 4 times or total of 1.2 × 109 cells/patient, 4 times | Crohn’s disease | 330 | 4 weeks | Phase 3 | Randomized, parallel assignment, double blind | NCT00482092 | Active | ||
| 15 | 2012 | Adipose tissue | Allogeneic | Intralesional | 1.2 × 108 cells/patient | Fistulizing Crohn’s disease | 212 | 24 weeks | Phase 3 | Randomized, parallel assignment, double blind | NCT01541579 | Active | Local treatment with MSCs showed promotion of fistula healing. | 31 |
| 16 | 2010 | Bone marrow | Allogeneic | Peripheral vein | 2 × 10^8 cells/patient, 3 times | Crohn’s disease | 120 | 180 days | Phase 3 | Non-randomized, single group assignment, open label | NCT01233960 | Active | ||
| 17 | 2015 | Adipose tissue | Autologous | Intralesional | Unknown | Fistulizing Crohn’s disease | 10 | 62 weeks | Phase 2 | Non-randomized, single group assignment, open label | NCT02403232 | Recruiting | ||
| 18 | 2013 | Bone marrow | Autologous | Intralesional | Unknown | Fistulizing Crohn’s disease | 10 | 16 weeks | Phase 1 | Randomized, parallel assignment, single blind | NCT01874015 | Recruiting | ||
| 19 | 2015 | Adipose tissue | Allogeneic | Peripheral vein | 5 × 107 cells/patient, 7.5 × 107 cells/patient, or 1 × 108 cells/patient | Crohn’s disease | 9 | 4 weeks | Phase 1 | Non-randomized, single group assignment, open label | NCT02580617 | Recruiting | ||
| 20 | 2013 | Umbilical cord | Allogeneic | Peripheral vein | 5 × 107 cells/patient or 1 × 108 cells/patient | Crohn’s disease | 24 | 12 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT02000362 | Recruiting | ||
| 21 | 2013 | Adipose tissue | Autologous | Intralesional | 2 × 107 cells/patient | Fistulizing Crohn’s disease | 20 | 2–24 months | Phase 1 | Non-randomized, single group assignment, open label | NCT01915927 | Recruiting | ||
| 22 | Unknown | Bone marrow | Autologous | Peripheral vein | 1–2 × 106 cells/kg | Crohn’s disease | 10 | 6 weeks | Phase 1 | Unknown | – | – | Three patients showed clinical response (decrease in CDAI).Three patients required surgery due to disease worsening. | 32 |
| 23 | 2016 | Bone marrow | Allogeneic | Intralesional | 2 × 107 cells/patient | Fistulizing Crohn’s disease | 20 | 7, 10, 16 months | Phase 1 | Non-randomized, single group assignment, open label | NCT02677350 | Not yet recruiting | ||
| 24 | 2015 | Umbilical cord | Allogeneic | Peripheral vein | 1 × 106 cells/kg, 3 times | Ulcerative colitis | 30 | 24 weeks | Phase 1–2 | Randomized, parallel assignment, single blind | NCT02442037 | Recruiting | ||
| 25 | 2015 | Adipose tissue | Allogeneic | Through a colonoscope | 6 × 107 cells/patient | Ulcerative colitis | 8 | 12 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT01914887 | Unknown | ||
| 26 | 2015 | Umbilical cord | Allogeneic | First: peripheral vein, second: superior mesenteric artery | First: 3.8 ± 1.6 × 107 cells/patient, second: 1.5 × 107 cells/patient | Ulcerative colitis | 80 | 12 weeks | Phase 1–2 | Non-randomized, single group assignment, open label | NCT01221428 | Unknown | Decrease in the median Mayo score and histology score. Improvement in IBDQ scores. | 33 |
CD Crohn’s disease, CDAI Crohn’s Disease Activity Index, AQoL The Assessment of Quality of Life, CRP C-reactive protein, IBDQ Inflammatory Bowel Disease Questionnaire
Fig. 2.
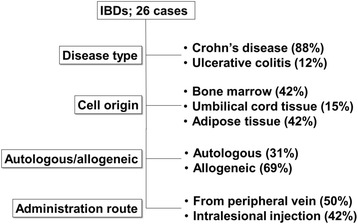
Summary of clinical trials in inflammatory bowel diseases
Clinical trials in liver diseases
Background of liver diseases
Although the liver has high regenerative capacity, acute liver damage caused by viruses, drugs, alcohol, and autoimmune diseases, or chronic liver damage caused by hepatitis B or C virus, alcohol, non-alcoholic steatohepatitis (NASH), autoimmune hepatitis, and primary biliary cholangitis often cause liver failure [34]. The liver has a variety of functions, including metabolism of protein, sugar, and fat; detoxification; production of coagulation factors; and production of bile. Thus, during liver failure, several symptoms, including jaundice, edema, ascites, hepatic encephalopathy, and increased bleeding, can appear at the same time, resulting in life-threatening disease. In addition, during liver failure caused by chronic liver disease, accumulated liver fibrosis (i.e., liver cirrhosis) can cause portal hypertension, which often induces the varices, and long-term liver damage can cause gene abnormalities, leading to liver cancers. The ultimate therapy for liver failure is liver transplantation; however, only a small portion of patients with liver failure can receive liver transplantation due to the shortage of donor organs, invasiveness of operations, and economic reasons [35]. Revolutionary treatments, such as interferon-free treatment for hepatitis C and providing information regarding the importance of the daily lifestyle to prevent alcoholic liver disease and NASH, can potentially decrease the liver diseases; however, unmet needs to treat advanced liver failure will continue.
Advanced acute liver failure and chronic liver failure (liver cirrhosis) can be good targets for cell therapy. Since 2003, Terai et al. initiated autologous bone marrow cell infusion (ABMi) therapy against decompensated liver cirrhosis and confirmed the improvement of liver fibrosis and liver function [36–38]. However, due to the invasiveness of liver transplantation in patients with liver failure, minimally invasive procedures using specific cells, such as MSCs and macrophages [39–41], are now being developed, with a focus on MSCs. In the next section, we will describe recent reported results using MSCs registered at ClinicalTrials.gov.
Effects of MSC therapy in liver disease from published papers
Animal experiments have shown that MSCs can have anti-apoptotic [42] and antioxidant effects in hepatocytes [43], and antifibrotic [44, 45], angiogenic [46], and immunosuppressive effects in T cells, macrophages, and dendritic cells [8]. In human clinical trials, all reports have shown that MSC injection is safe. Although the effects of cell therapy are not uniform, the majority of therapies have some beneficial effects; in contrast, in a few reports, treatment effects were not observed. For example, Kantarcioglu et al. [13] and Mohamadnejad et al. [19] injected bone marrow-derived MSCs into patients with liver cirrhosis and did not observe treatment effects. However, Kharaziha et al. [14] reported phase I–II clinical trials using autologous bone marrow-derived MSCs against liver cirrhosis with a variety of etiologies, and improvement of liver function was confirmed. Jang et al. and Suk et al. [12, 16] reported a pilot study and a phase II study using autologous bone marrow-derived MSCs injected through the hepatic artery against alcoholic liver cirrhosis, and improvement of histological liver fibrosis and liver function was confirmed. Xu et al. [21] reported trials using autologous bone marrow-derived MSCs against hepatitis B virus-associated cirrhosis and confirmed the improvement of liver function, the decrease of Th17 cells, and the increase of regulatory T cells. Xhang et al. [17] and Wang et al. [20] reported trials using allogeneic umbilical cord-derived MSCs in patients with chronic hepatitis B having decompensated liver cirrhosis and primary biliary cirrhosis, respectively. They confirmed improvement of liver function, particularly reduced ascites and recovery of biliary enzymes, respectively. Shi et al. [15] reported a trial investigating acute or chronic liver failure associated with hepatitis B virus and confirmed that MSCs significantly increased survival rates. From these reports, MSCs appeared to improve liver function; however, additional trials are needed to confirm these effects and to elucidate the mechanisms in more detail.
Clinical trials in IBDs
Background of IBDs
IBDs are chronic inflammatory disorders, including UC and CD. The pathogenesis of IBD is thought to be highly complex due to several factors, such as environmental factors, genetic predisposition, and inflammatory abnormalities [47]. UC is characterized by inflammation of the mucosal membrane of the colon continued from the rectum. Type 2 T helper cell (Th2) cytokine profile is associated with the pathogenesis of UC. In contrast, CD is a segmental, transluminal disorder that can arise within the entire gastrointestinal tract from the mouth to the anus. Th1 cells are associated with the pathogenesis of CD [48]. Furthermore, a recent report showed that Th17 cells are present in both UC and CD. Thus, mucosal CD4+ T cells are key mediators of the driving response [49]. Macrophages that produce tumor necrosis factor (TNF)-α have also been reported to be relevant in IBD. Imbalances in other cytokines, such as interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-12, IL-17, IL-23, and transforming growth factor-β (TGF-β), are also detected during diseases [48]. Recent advancements in the development of drugs for IBD include drugs targeting TNF and new candidate drugs, such as antibodies against IL-6 [50] and IL-12/23 [51–53], small molecules including Janus kinase inhibitors [54], antisense oligonucleotides against SMAD7 mRNA [55], and inhibitors of leukocyte trafficking to intestinal sites of inflammation [56, 57]. However, some patients will fail to respond to current medical options, immunosuppressive agents, and anti-TNF biologicals. MSCs may be an effective option in these patients [9, 49]. In the next section, we will describe recently reported results using MSCs registered in ClinicalTrials.gov.
Effects of MSC therapy in IBD from published papers
Eight CD trials and one UC trial have been published in ClinicalTrials.gov. Six papers describing CD are on trials treating fistula, and two papers are trials for luminal CD. Molendijk et al. [25] reported improved healing of refractory perianal fistulas using allogeneic bone marrow-derived MSCs. They administered these allogeneic MSCs locally and concluded that injection of 3 × 107 MSCs appeared to promote the healing of perianal fistula. Panes et al. [31] reported a phase III randomized, double-blind, parallel-group, placebo-controlled study of complex perianal fistula using expanded allogeneic adipose-derived MSCs and confirmed the safety of the MSCs and the healing effects of MSCs on the fistula. Duijvestein et al. [32] reported a phase I study of refractory luminal CD using autologous bone marrow-derived MSCs and confirmed the safety and feasibility of MSC therapy. Forbes et al. [24] reported a phase II study using allogeneic bone marrow-derived MSCs for luminal CD refractory to biologic therapy. They administered 2 × 106 cells/kg weekly for 4 weeks and found that allogeneic MSCs reduced the CD activity index (CDAI) and CD endoscopic index of severity (CDEIS) scores in patients with luminal CD refractory to biologic therapy. Hu et al. [33] reported a phase I/II study for severe UC using umbilical cord-derived allogeneic MSCs by combination injection through the peripheral blood and superior mesenteric artery with a 7-day interval. They confirmed the safety of MSCs and alleviation of diffuse and deep ulcer formation and severe inflammatory mucosa by MSCs.
Safety of the MSC therapy
MSC therapy is associated with some concerns, such as adverse events related to infusion, tumor formation during the treatment of liver cirrhosis, and long-term observations of tumor formation. Regarding adverse events related to the infusion, Lalu et al. performed a meta-analysis of the safety of MSCs in clinical trials and showed that autologous and allogeneic MSC therapies were related to transient fever but not infusion toxicity, organ system complications, infection, death, and malignancies (Table 2) [5]. Regarding tumor formation during the treatment of liver cirrhosis, Peng et al. reported that no severe adverse events or no significant differences in tumor formation were detected compared with those in the control group during autologous bone marrow-derived MSC therapy for liver cirrhosis [58]. Regarding long-term observations of tumor formation derived from MSCs, Bahr et al. reported recent autopsy data from patients in a clinical trial of graft-versus-host disease (GvHD) who received MSC therapy between 2002 and 2007 and revealed no ectopic tissues, neoplasms, or donor-derived DNA [6].
Conclusions
Many clinical trials of autologous and allogeneic MSCs have aimed to elucidate the effects and mechanisms of MSCs. MSCs can expand easily and can be obtained from medical waste, suggesting their applications in regenerative medicine for the treatment of liver diseases and IBDs. Recently, limitations of MSCs have been reported. For example, therapeutic effects were not long term and were affected by inflammatory condition [59, 60]. Thus, the results of ongoing clinical studies will be expected to provide further insights.
Acknowledgements
The authors thank Dr. Takayuki Watanabe and Dr. Suguru Takeuchi for their cooperation.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (B) (26293175) from the Ministry of Education, Science, Technology, Sports, and Culture of Japan and by Highway Program for Realization of Regenerative Medicine from Japan Agency for Medical Research and Development, AMED.
Availability of data and materials
There is no available data except the manuscript and tables.
Author’s contributions
AT and ST wrote the paper. YK, SI, SS, YW, and YK prepared the data and made the tables. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors agreed to publish this work.
Ethics approval and consent to participate
There is no ethics approval and consent to participate due to review.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ABMi
Autologous bone marrow cell infusion
- CD
Crohn’s disease
- CD CDEIS
Endoscopic index of severity
- CDAI
CD activity index
- ECM
Extracellular matrix
- GvHD
Graft-versus-host disease
- IBD
Inflammatory bowel disease
- IL
Interleukin
- MSCs
Mesenchymal stem cells
- NASH
Non-alcoholic steatohepatitis
- TGF-β
Transforming growth factor
- TNF
Tumor necrosis factor
- UC
Ulcerative colitis
References
- 1.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, non-alcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.e1. [DOI] [PMC free article] [PubMed]
- 3.Gecse KB, Lakatos PL. IBD in 2016: biologicals and biosimilars in IBD - the road to personalized treatment. Nat Rev Gastroenterol Hepatol. 2017;14(2):74-76. [DOI] [PubMed]
- 4.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen A, Newsome PN. Mesenchymal stromal cell therapy in liver disease: opportunities and lessons to be learnt? Am J Physiol Gastrointest Liver Physiol. 2015;309:G791–800. doi: 10.1152/ajpgi.00036.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terai S, Tsuchiya A. Status of and candidates for cell therapy in liver cirrhosis: overcoming the “point of no return” in advanced liver cirrhosis. J Gastroenterol. 2017;52(2):129-40. [DOI] [PubMed]
- 8.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–16. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 9.Hawkey CJ, Hommes DW. Is stem cell therapy ready for prime time in treatment of inflammatory bowel diseases? Gastroenterology. 2017;152:389–397. doi: 10.1053/j.gastro.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Katsuda T, Kosaka N, Takeshita F, et al. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–53. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 11.Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–54. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34:33–41. doi: 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 13.Kantarcioglu M, Demirci H, Avcu F, et al. Efficacy of autologous mesenchymal stem cell transplantation in patients with liver cirrhosis. Turk J Gastroenterol. 2015;26:244–50. doi: 10.5152/tjg.2015.0074. [DOI] [PubMed] [Google Scholar]
- 14.Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 15.Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–31. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology. 2016;64(6):2185-97. [DOI] [PubMed]
- 17.Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112–20. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 18.Vosough M, Moossavi S, Mardpour S, et al. Repeated intraportal injection of mesenchymal stem cells in combination with pioglitazone in patients with compensated cirrhosis: a clinical report of two cases. Arch Iran Med. 2016;19:131–6. [PubMed] [Google Scholar]
- 19.Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–6. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28(Suppl 1):85–92. doi: 10.1111/jgh.12029. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Gong Y, Wang B, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29:1620–8. doi: 10.1111/jgh.12653. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya A, Kubota T, Takizawa K, et al. Successful treatment in a case of massive hepatocellular carcinoma with paraneoplastic syndrome. Case Rep Gastroenterol. 2009;3:105–110. doi: 10.1159/000213480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–16. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 24.Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Molendijk I, Bonsing BA, Roelofs H, et al. Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients With Crohn’s disease. Gastroenterology. 2015;149:918–27. doi: 10.1053/j.gastro.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31:2575–81. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 27.de la Portilla F, Alba F, Garcia-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–23. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 28.Cho YB, Lee WY, Park KJ, et al. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22:279–85. doi: 10.3727/096368912X656045. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Arranz M, Dolores Herreros M, Gonzalez-Gomez C, et al. Treatment of Crohn’s-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: a phase I-IIa Clinical Trial. Stem Cells Transl Med. 2016;5(11):1441-46. [DOI] [PMC free article] [PubMed]
- 30.Cho YB, Park KJ, Yoon SN, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4:532–7. doi: 10.5966/sctm.2014-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panes J, Garcia-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–90. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 32.Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–9. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Zhao G, Zhang L, et al. Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp Ther Med. 2016;12:2983–2989. doi: 10.3892/etm.2016.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchiya A, Kamimura H, Takamura M, et al. Clinicopathological analysis of CD133 and NCAM human hepatic stem/progenitor cells in damaged livers and hepatocellular carcinomas. Hepatol Res. 2009;39:1080–90. doi: 10.1111/j.1872-034X.2009.00559.x. [DOI] [PubMed] [Google Scholar]
- 35.Fayek SA, Quintini C, Chavin KD, et al. The current state of liver transplantation in the United States: perspective from American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council. Am J Transplant. 2016;16:3093–3104. doi: 10.1111/ajt.14017. [DOI] [PubMed] [Google Scholar]
- 36.Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–8. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 37.Kim JK, Park YN, Kim JS, et al. Autologous bone marrow infusion activates the progenitor cell compartment in patients with advanced liver cirrhosis. Cell Transplant. 2010;19:1237–46. doi: 10.3727/096368910X506863. [DOI] [PubMed] [Google Scholar]
- 38.Saito T, Okumoto K, Haga H, et al. Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev. 2011;20:1503–10. doi: 10.1089/scd.2011.0074. [DOI] [PubMed] [Google Scholar]
- 39.Thomas JA, Pope C, Wojtacha D, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–15. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 40.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–69. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 41.Moore JK, Mackinnon AC, Wojtacha D, et al. Phenotypic and functional characterization of macrophages with therapeutic potential generated from human cirrhotic monocytes in a cohort study. Cytotherapy. 2015;17:1604–16. doi: 10.1016/j.jcyt.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin S, Li H, Han M, et al. Mesenchymal stem cells with enhanced Bcl-2 expression promote liver recovery in a rat model of hepatic cirrhosis. Cell Physiol Biochem. 2016;40:1117–1128. doi: 10.1159/000453166. [DOI] [PubMed] [Google Scholar]
- 43.Quintanilha LF, Takami T, Hirose Y, et al. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res. 2014;44:E206–17. doi: 10.1111/hepr.12204. [DOI] [PubMed] [Google Scholar]
- 44.Volarevic V, Nurkovic J, Arsenijevic N, et al. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818–23. doi: 10.1002/stem.1818. [DOI] [PubMed] [Google Scholar]
- 45.Dai LJ, Li HY, Guan LX, et al. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2:16–25. doi: 10.1016/j.scr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Xia X, Tao Q, Ma Q, et al. Growth hormone-releasing hormone and its analogues: significance for MSCs-mediated angiogenesis. Stem Cells Int. 2016;2016:8737589. doi: 10.1155/2016/8737589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagaishi K, Arimura Y, Fujimiya M. Stem cell therapy for inflammatory bowel disease. J Gastroenterol. 2015;50:280–6. doi: 10.1007/s00535-015-1040-9. [DOI] [PubMed] [Google Scholar]
- 48.Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci. 2017;38(2):127-42. [DOI] [PubMed]
- 49.Gregoire C, Lechanteur C, Briquet A, et al. Review article: mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:205–221. doi: 10.1111/apt.13864. [DOI] [PubMed] [Google Scholar]
- 50.Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–96. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–41. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–28. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 53.Sandborn W, Gasink C, Blank M, et al. O-001 A multicenter, double-blind, placebo-controlled phase3 study of Ustekinumab, a human IL-12/23P40 mAB, in moderate-service Crohn’s disease refractory to anti-TFNalpha: UNITI-1. Inflamm Bowel Dis. 2016;22(Suppl 1):S1. doi: 10.1097/MIB.0000000000000720. [DOI] [Google Scholar]
- 54.Van Rompaey L, Galien R, van der Aar EM, et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol. 2013;191:3568–77. doi: 10.4049/jimmunol.1201348. [DOI] [PubMed] [Google Scholar]
- 55.Monteleone G, Neurath MF, Ardizzone S, et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015;372:1104–13. doi: 10.1056/NEJMoa1407250. [DOI] [PubMed] [Google Scholar]
- 56.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 57.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 58.Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–8. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 59.Dave M, Jaiswal P, Cominelli F. Mesenchymal stem/stromal cell therapy for inflammatory bowel disease: an updated review with maintenance of remission. Curr Opin Gastroenterol. 2017;33:59–68. doi: 10.1097/MOG.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 60.Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8:54–68. doi: 10.15283/ijsc.2015.8.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no available data except the manuscript and tables.


