Abstract
Purpose
Spine surgeons have increasingly used intraoperative application of topical vancomycin powder (TVP) to prevent surgical site infections (SSIs). The goals of this study were to define the rate of pharmacological adverse reaction to TVP in young patients undergoing posterior spinal surgery and to summarise institutional variation in TVP dosing.
Methods
This retrospective observational study included ten spine centres in the United States and one in Europe. Patients with early onset scoliosis who underwent posterior spine surgery were eligible for inclusion. Age, weight, TVP dose and surgery type were recorded. Surgeries where patient age was > 12 years were excluded. Pharmacological adverse reactions were defined as clinical instances of Red Man Syndrome, rash, nephrotoxicity, proteinuria, hepatotoxicity or ototoxicity. The rate of pharmacological adverse reaction to TVP was calculated. Dosing practices were summarised.
Results
Patient age was in the range of seven months to 12 years (median ten years). Of 1398 observations, there was one possible pharmacological adverse reaction. This was in a ten-year-old, 20.4-kg female patient with neuromuscular sco-liosis undergoing growing rod implantation. She was dosed with 1500 mg of TVP and immediately developed a transient rash without systemic symptoms. This abated over minutes without any medical intervention. There were no other adverse reactions in the sample. The population rate of pharmacological adverse reaction was 0.072% (95% confidence interval 0 to 0.4). Significant variability in dosing practices existed between centres.
Conclusion
Pharmacological adverse reactions to TVP are rare. Future work may establish evidence-based guidelines for TVP dosing based on patient weight and other variables.
Keywords: Vancomycin powder, surgical site infection, pharmacological adverse event, scoliosis
Introduction
Surgical site infections (SSIs) are a source of great morbidity and associated healthcare costs in paediatric spinal deformity surgery. While SSI rates range up to 6.7% for patients with adolescent idiopathic scoliosis (AIS), the risk is higher for adults and children with co-morbidities.1
Reports of topical vancomycin powder (TVP) application at the surgical site in paediatric spine surgery date as far back as 2011.2 Although recent work has questioned its efficacy,3 studies have suggested that TVP use results in therapeutic local drug concentrations4,5 and a fourfold drop in the odds of deep SSI for spine surgeries.6,7 In terms of clinical safety, TVP has been shown to produce low, non-toxic serum drug levels,4,5 suggesting that the systemic pharmacological reactions seen with intravenous (IV) vancomycin are less likely to occur with TVP alone. However, the true rate of pharmacological adverse reaction to TVP has not been previously defined despite hypersensitivity reactions having been reported with the drug.8 This leaves a significant gap in knowledge for surgeons weighing the benefits and risks of TVP use.
Concerns regarding the safety of TVP in spinal deformity surgery are most compelling in the smallest paediatric patients, where standard dosages combined with low volumes of distribution could theoretically lead to dangerously high serum drug concentrations. The major indication for spine surgery in such patients is management of early onset scoliosis (EOS), a condition diagnosed prior to age ten years. These children routinely weigh < 25 kg to 30 kg at surgery.
The literature currently includes no large dedicated assessment of pharmacological adverse reactions to TVP. The goal of this study was to define the rate of pharmacological adverse reaction to TVP in posterior spinal surgery for EOS in a young sample. Secondary goals included assessing the rate of adverse reaction in patients weighing ≤ 25 kg at surgery and summarising institutional variations in dosing practices.
Materials and methods
One European and ten North American high-volume paediatric spinal deformity centres were recruited to participate in this multicentre retrospective observational study. Each hospital’s institutional review board approved the study protocol prior to data abstraction. Each institution had a surgeon site leader and local clinical research personnel who identified cases of TVP application in EOS patients undergoing posterior spine surgery between 01 January 2009 and 09 December 2015. Surgeries where the patient was aged 13 years or older at the procedure were excluded. Although EOS is defined as scoliosis diagnosed before age ten, patients up to age 12 were included in order to capture patients managed surgically after months to years of management (i.e. patients with serial growing rod expansions). Included surgeries were primarily posterior instrumented spinal fusions, vertical expandable prosthetic titanium rib (VEPTR) implantations and expansions and growing rod (GR) implantations and expansions. The medical record was reviewed for any text reports of pharmacological adverse reactions to vancomycin, including instances of Red Man Syndrome, anaphylaxis, rash, nephrotoxicity, proteinuria, hepatotoxicity and ototoxicity up to 90 days post-operatively. Lab values were not reviewed to assess for these outcomes. Baseline patient characteristics including age, weight, EOS subtype (idiopathic, neuromuscular, congenital, syndromic)9 and surgery type were recorded. De-identified data were transmitted to the primary author’s institution, where final analysis was performed. The rate of adverse pharmacological reaction was calculated for the overall group. As the group’s median weight was expected to lie near 25 kg, any reaction in a patient weighing ≤ 25 kg at surgery was specifically noted. Local TVP dosing practices were reported by each surgeon site leader. These data were summarised.
Results
The 11 spinal deformity centres reported 1398 cases of TVP use in posterior spinal surgery for EOS. Table 1 shows the number of consecutive cases reported by each (de-identified) institution, which was in the range of 32 to 298. Patient age was in the range of seven months to 12 years (median ten years). Patient weight was in the range of 7.3 kg to 127.4 kg. The subtypes of EOS treated included neuromuscular (36%), idiopathic (28%), congenital (21%) and syndromic (15%). Surgeries primarily included posterior instrumented spinal fusions (49%), VEPTR implantations or expansions (19%) and GR implan-tations or expansions (30%). Shilla growth guidance procedures, vertebral column resections, pedicle subtraction osteotomies and vertebral tethering procedures together represented approximately 2% of the sample. TVP was routinely placed subfascially or both subfascially and subcutaneously. The medication was only used in a primarily subcutaneous fashion when the fascia was not opened appreciably (i.e. VEPTR surgery). TVP dosing was in the range of 60 mg to 2000 mg (median 500 mg). There were 484 cases where patient weight was reported as ≤ 30 kg and 367 cases with patient weight ≤ 25 kg.
Table 1. Summary of events and dosage guidelines by site.
| Location | Cases (n) | Adverse events (n) | Dosage guidelines | ||
|---|---|---|---|---|---|
| 1 | 50 | 0 | 1000 mg; no adjustment | ||
| 2 | 79 | 0 | 1000 mg; no adjustment | ||
| 3 | 298 | 0 | 1000 mg, adjusted according to incision size | ||
| 4 | 100 | 0 | Surgeon preference, usually 500 to 2000 mg | ||
| 5 | 136 | 0 | Surgeon preference, usually 1000 to 2000 mg | ||
| 6 | 108 | 0 | Children > 25 kg: 1000 mg | Children < 25 kg: 500 mg | |
| 7 | 75 | 0 | Children > 30 kg: 1000 mg | Children < 30 kg: 500 mg | |
| 8 | 107 | 0 | Children > 30 kg: 1000 mg | Children < 30 kg: 500 mg | |
| 9 | 272 | 0 | Children > 30 kg: 500 mg | Children < 30 kg: 16.7 mg/kg | |
| 10 | 141 | 1 | VEPTR or GR expansion: 250 mg/incision | VEPTR or GR implantation: 1000 mg total | PSF: 2000 mg total |
| 11 | 32 | 0 | Same dose that would be given intra-venously based on patient weight | ||
VEPTR, vertical expandable prosthetic titanium rib; GR, growing rod (including magnetic growing rods); PSF, posterior spinal fusion
Only one adverse reaction occurred: a transient rash in a ten-year-old female patient with neuromuscular scoliosis undergoing GR implantation. The patient weighed 20.4 kg and was dosed with 1500 mg of TVP. She had no known drug allergies and did not receive any blood products peri-operatively. Immediately after TVP application, the patient developed erythema of the arms and low back. This rash resolved without medical intervention over minutes to hours and caused no immediate or lasting systemic symptoms. The patient was not otherwise being treated with IV vancomycin but did receive peri-operative IV cefepime. No other adverse reactions or problems were noted in the patient sample.
The sample rate of adverse reaction to TVP was thus 0.072% (1/1398) overall. The 95% confidence interval (CI) for the population rate was 0 to 0.400. No serious (persistent, systemic or life-threatening) reactions were found in any patient, including the one with the transient rash. At least five patients who previously experienced Red Man Syndrome with IV vancomycin had no reaction to TVP.
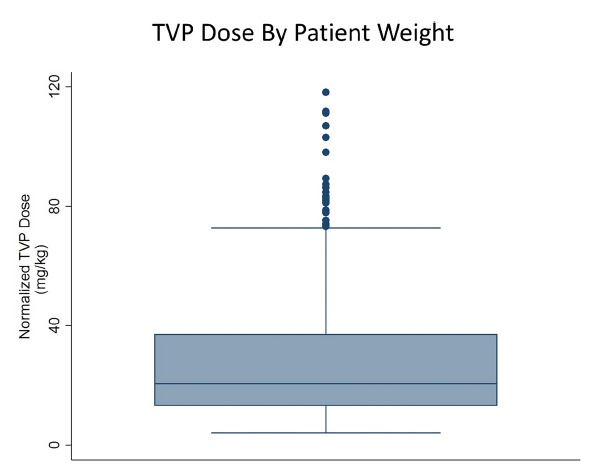
TVP dosing practice varied considerably by spine centre (Table 1). Four sites adjusted dosage based upon patient weight. One site adjusted dosage based upon surgery type, and another adjusted dosage based upon the size of the incision. Two sites reported that dosage varied with surgeon preference. Two more sites reported that they always gave the same standard 1000 mg dose. One site determined TVP dosage based upon patient weight according to guidelines for prophylactic IV vancomycin dosing. Among the observations, TVP dose normalised to patient weight was in the range of 4.1 mg/kg to 118.1 mg/kg (median 20.6 mg/kg). The single patient with an adverse reaction received a dose of 75.5 mg/kg, representing the 97th percentile of normalised TVP dose in this sample. Figure 1 shows the distribution of normalised TVP dosages, illustrating that 2.9% of doses were outliers, including that given to the patient who developed a rash. An outlier was defined here as a value that lay outside the interquartile range (IQR) by > 1.5 IQR.10
Fig. 1. Box plot showing the mean and interquartile range for weight-normalised topical vancomycin powder (TVP) dosing as observed in this study. Note that the distribution is skewed to the right and includes a significant number of outlying values at its higher reaches.
Discussion
SSI can be a devastating complication after paediatric spine surgery, leading to repeat operations, pain, rehospitalisation, high healthcare costs and potentially even death. Previous studies estimate that up to 12% of adult spine surgeries are complicated by SSI,11 while analogous rates for AIS are lower, in the range of 0.5% to 6.7%.1 A recent study of young patients who underwent spinal fusion for scoliosis, kyphosis or spondylolisthesis found that 1.5% of patients developed late SSIs (more than three months post-operatively). These complications resulted in an average hospital stay of 29 days and $154 537 in costs.12 While peri-operative use of IV vancomycin has been used for SSI prevention in paediatric spine centres, this comes with concerns. IV vancomycin administration may delay surgery due to the need for continuous infusion to establish and maintain target drug levels. The United States Centers for Disease Control and Prevention further recommends against the prophylactic use of IV vancomycin due to concerns about increasing antibiotic resistance,13 although this concept has been challenged by a recent investigation.14 Lastly, systemic vancomycin dosing is known to cause various pharmacological adverse reactions, including phlebitis and Red Man Syndrome.
In efforts to attain local antibiotic effects while avoiding concerns associated with IV vancomycin, several centres have adopted an approach used in some neurosurgical cases and joint arthroplasties: intra-operative application of TVP at the surgical site. Although it is still debated,3 previous research has suggested that TVP use effectively lowers the risk of SSI after spine surgery.4-7 Recent work in an in vivo rat model further suggests that TVP produces a higher local drug concentration than expected with IV vancomycin alone, results in an appreciable distribution of antibiotic to adjacent bone and is effectively removed from the body within 48 hours to 96 hours.15 Despite such promising results concerning TVP efficacy and pharmacokinetics, there has previously been no large-scale assessment of the risk of pharmacological adverse reaction to TVP or current variations in dosing practice in young children undergoing spine surgery.
This study revealed a pharmacological adverse reaction rate of 0.072% (95% CI 0% to 0.400%) among EOS patients. As these individuals are assumed to be at higher risk for such reactions because of their low weights, this finding supports empiric sentiments from several major centres that adverse pharmacological reactions to TVP are extremely rare. The single event recorded in our sample was a non-anaphylactoid sensitivity reaction without systemic effects found in a patient with neuromuscular scoliosis undergoing GR implantation. This reaction was attributed to TVP by the managing surgeon, although this cannot be fully proven retrospectively. This highlights new questions that this study was not designed to address. Among these is whether patients with different subtypes of EOS (i.e. neuromuscular) are predisposed to TVP reactions, how common are true anaphylactoid reactions and how do dose size and patient weight affect the probability of developing an adverse reaction.
This last question leads to one of our secondary findings: dosing practices greatly varied among the institutions studied here. While a recent study showed that larger doses of TVP result in higher systemic levels of vancomycin,16 the threshold for TVP dosing over which systemic drug levels may become toxic is not known and while the low reaction rate found here suggests that the institutions studied used safe doses, any objective conclusions we can make regarding the safety of TVP as used in this study are limited because the threshold for safe topical dosing is not well-defined. Further, as we did not simultaneously assess drug efficacy in preventing SSI, we could not directly compare the risk of pharmacological adverse reaction with drug effectiveness at any magnitude of dose.
Our study has several other limitations. While previous studies of outcomes with TVP in spine surgery have tracked non-pharmacological post-surgical complications such as SSIs and wound dehiscence,4 assessing associated rates of such complications was not within the scope of this project. We did not track such outcomes that focus on drug efficacy as this was similarly outside of project scope. Also, significant sample heterogeneity (different EOS aetiologies, patient ages, etc) was necessary in order to assemble a large enough patient cohort to assess the rare outcome of adverse pharmacological reaction.
With further regard to study limitations, it is important to note that failure to recognise an adverse reaction does not guarantee that one did not occur. Because our review methods sought only documented cases of reactions and did not include the direct review of reliable tests for the reactions of concern (i.e. liver function tests for hepatotoxicity), we cannot prove beyond reasonable doubt that the adverse reaction rate is as low as our data suggest. However, we can confidently note that the rate of clinically apparent reactions is quite low.
In summary, we found that TVP use is associated with an extremely low rate of recognised pharmacological adverse reaction. This finding should reassure those who care for young children requiring spinal surgery that using TVP for SSI prevention is very safe. Surgeons, nurses and anaesthesia personnel should, however, be aware of rare potential pharmacological adverse reactions and take care to communicate the timing of medication dosing, patient weight and dose quantity. Providers should always confirm drug allergies prior to medication administration. Future work regarding the use of TVP may establish evidence-based guidelines for drug dosing based upon patient characteristics like weight, age and underlying diagnosis (i.e. cerebral palsy) as well as type and magnitude of surgery.
Compliance with Ethical Standards
Funding Statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical Statement
Ethical Approval: This multicentre retrospective study qualified as human subjects research. It was approved by the Institutional Review Boards (IRBs).
Informed Consent: A waiver of informed consent and patient assent was approved by all proper IRBs.
References
- 1.Li Y, Glotzbecker M, Hedequist D.. Surgical site infection after pediatric spinal deformity surgery. Curr Rev Musculoskelet Med 2012;5:111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill KR, Smith JG, Abtahi AM, et al.. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J 2011;11:641-646. [DOI] [PubMed] [Google Scholar]
- 3.Mistovich RJ, Jacobs LJ, Campbell RM, et al.. Infection control in pediatric spinal deformity surgery: a systematic and critical analysis review. JBJS Rev 2017;5:e3. [DOI] [PubMed] [Google Scholar]
- 4.Armaghani SJ, Menge TJ, Lovejoy SA, Mencio GA, Martus JE.. Safety of topical vancomycin for pediatric spinal deformity: nontoxic serum levels with supratherapeutic drain levels. Spine (Phila Pa 1976) 2014;39:1683-1687. [DOI] [PubMed] [Google Scholar]
- 5.Gans I, Dormans JP, Spiegel DA, et al.. Adjunctive vancomycin powder in pediatric spine surgery is safe. Spine (Phila Pa 1976) 2013;38:1703-1707. [DOI] [PubMed] [Google Scholar]
- 6.Bakhsheshian J, Dahdaleh NS, Smith ZA.. Letter to the Editor concerning "Intrawound vancomycin to prevent infections after spine surgery: a systematic review and meta-analysis" by Evaniew N, Khan M, Drew B, Peterson D, Bhandari M, Ghert M (2014) Eur Spine J; DOI 10.1007/s00586-014-3357-0. Eur Spine J 2014;23:2014-2015. [DOI] [PubMed] [Google Scholar]
- 7.Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML.. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J 2014;14:397-407. [DOI] [PubMed] [Google Scholar]
- 8.Mariappan R, Manninen P, Massicotte EM, Bhatia A.. Circulatory collapse after topical application of vancomycin powder during spine surgery. J Neurosurg Spine 2013;19:381-383. [DOI] [PubMed] [Google Scholar]
- 9.Williams BA, Matsumoto H, McCalla DJ, et al.. Development and initial validation of the Classification of Early-Onset Scoliosis (C-EOS). J Bone Joint Surg [Am] 2014;96:1359-1367. [DOI] [PubMed] [Google Scholar]
- 10.Tukey JW. Exploratory Data Analysis. Reading, MA: Addison-Wesley, 1977. [Google Scholar]
- 11.Pull ter Gunne AF, van Laarhoven C, Cohen DB.. Incidence of surgical site infection following adult spinal deformity surgery: an analysis of patient risk. Eur Spine J 2010;19:982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedequist D, Haugen A, Hresko T, Emans J.. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976) 2009;34:60-64. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR 1995;44(No. RR-12). [PubMed] [Google Scholar]
- 14.Chotai S, Wright PW, Hale AT, et al.. Does intrawound vancomycin application during spine surgery create vancomycin-resistant organism? Neurosurgery 2017;80:746-753. [DOI] [PubMed] [Google Scholar]
- 15.Working ZM, Frederiksen H, Drew A, Loc-Carrillo C, Kubiak EN.. Bone penetrance of locally administered vancomycin powder in a rat femur fracture model. Injury 2017;48:1459-1465. [DOI] [PubMed] [Google Scholar]
- 16.Murphy EP, Curtin M, Shafqat A, et al.. A review of the application of vancomycin powder to posterior spinal fusion wounds with a focus on side effects and infection. A prospective study. Eur J Orthop Surg Traumatol 2017;27:187-191. [DOI] [PubMed] [Google Scholar]


