Abstract
The therapeutic effects of mesenchymal stromal/stem cells (MSCs) are mainly based on three characteristics: immunomodulation, tissue regeneration, and hematopoietic support. Cell therapy using culture-expanded MSCs is effective in some intractable bone and hemato-immune disorders; however, its efficacy is limited. In this article, we review the previous efforts to improve the clinical outcomes of cell therapy using MSCs for such disorders. We describe pharmacological targeting of endogenous bone marrow-derived MSCs as a crucial quality-based intervention to establish more effective MSC-based therapies.
Keywords: Mesenchymal stromal/stem cell, Hematopoiesis, Regeneration, Immunomodulation, Pharmacological modification, Cell therapy
Background
There are two types of multipotent cells in bone marrow (BM): hematopoietic stem/progenitor cells (HSCs) and mesenchymal stromal/stem cells (MSCs). HSCs produce all types of hematopoietic cells and are established as a central player in BM. MSCs support hematopoiesis in the BM microenvironment and have been considered to be a second-class player in BM, despite their ability to differentiate into a variety of mesenchymal cells [1–4]. Nevertheless, emerging evidence has revealed the active contribution of BM-derived MSCs (BM-MSCs) to the pathogenesis of hematological diseases. More importantly, culture-expanded MSCs are practically available in clinics as off-the-shelf stem cell products for the treatment of some intractable refractory diseases. This review describes the basic characteristics of human MSCs and their clinical applications in the past and present and looks ahead toward the new horizon of MSC-based therapy.
Main text
Characteristics of human MSCs
The International Society of Cellular Therapy (ISCT) has proposed the following minimal criteria of human MSCs to define their characteristics [5]: (1) the ability to adhere to plastic plates; (2) the ability to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro; and (3) the positive surface expression of CD105, CD73, and CD90 in the absence of surface human leukocyte antigen (HLA)-DR molecules and hematopoietic lineage markers of pan-leukocytes (CD45), endothelial/primitive cells (CD34), myeloid lineage cells (CD14 or CD11b), and B cell lineage cells (CD79α or CD19). MSCs are isolated from various tissues/organs via diverse methods in multiple institutions [6, 7]. Therefore, it is critical to determine the common characteristics of MSCs in order to discuss clinical and basic studies using these cells. The minimal criteria for MSCs proposed by the ISCT are appropriate for product identity but have no relevance to functions including hematopoietic support, immunomodulation, and tissue regeneration (Fig. 1).
Fig. 1.
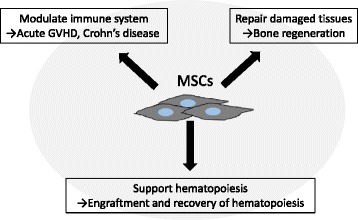
The main characteristics of MSCs. MSCs are multipotent stromal cells that have the ability to modulate the immune system, support hematopoiesis, and repair damaged tissues. These characteristics are applied to treat acute GVHD and Crohn’s disease, to regenerate bone, and to induce engraftment and recovery of hematopoiesis by infusing ex vivo expanded MSCs
There are two principal methods to isolate MSCs: classical isolation and prospective isolation. The classical isolation method selects cells that adhere to plastic dishes and form colonies. This method is simple and convenient; however, the isolated cells are heterogeneous. The prospective isolation method is based on cell sorting using surface markers that are expressed on MSCs [8, 9]. This method has the advantage of isolating a homogenous and high-quality cell population. According to the database provided by the National Institutes of Health (USA) at http://www.clinicaltrials.gov/, the conventional isolation method has been generally used in clinical trials using MSCs.
Clinical applications of human MSCs
Acute graft-versus-host disease (GVHD)
A substantial proportion of patients who undergo allogeneic hematopoietic stem/progenitor cell transplantation (HSCT) develop intractable acute graft-versus-host disease (GVHD). The European Group for Blood and Marrow Transplantation conducted a multi-institutional phase II study and showed that infusion of MSCs from multiple donor sources conferred an overall response rate of 71% (39 of 55 cases), with a complete response rate of 55% and a partial response rate of 16%, in cases with steroid-resistant acute GVHD [10]. The 2-year overall survival rate in cases with a complete response was 52%, which was better than that in historical controls (about 10%). These results suggested that intravenous infusion of MSCs is an effective therapy for patients with steroid-resistant acute GVHD.
In clinical trials using commercial off-the-shelf MSC products, their infusion was tolerable overall and they showed an efficacy to improve acute GVHD, especially in pediatric patients and gastrointestinal GVHD patients [11–15]. However, the preliminary results of a phase III study that was conducted outside of Japan showed that infusion of MSCs had an initial effect, but conferred no significant advantage in the longer term for acute GVHD patients [16]. A recent meta-analysis of 13 studies (336 patients) revealed that 241 (72%) patients achieved an overall response, with a 6-month overall survival rate of 63% in responders versus 16% in non-responders [17]. The overall response rate of individual organs was 49% for the gastrointestinal tract, 49% for the skin, and 28% for the liver. Although MSCs are certainly effective for the treatment of acute GVHD, the results of long-term follow-up are needed.
Skeletal disorders
Osteogenesis imperfecta (OI) is an inherited skeletal dysplasia characterized by osteopenia and frequent bone fractures. The molecular mechanism underlying this disease is a defect of type I collagen (COL1a1 and COL1a2) in progenies of MSCs, namely, osteoblasts. Allogeneic BM transplantation effectively improved the histological and clinical manifestations of OI in children [18, 19]. However, the engraftment of donor cells was not ensured via this strategy. In 2005, Le Blanc et al. performed in utero transplantation (IUT) of MSCs into a female fetus with severe OI [20]. A bone biopsy after delivery showed the engraftment of donor cells, suggesting that IUT is a promising strategy to solve the problem of engraftment and settlement of donor-derived MSCs.
Hypophosphatasia (HPP) is an inherited metabolic disorder characterized by low alkaline phosphatase activity and impaired bone formation. BM transplantation transiently improved the clinical features of HPP, but a boost of donor BM cells was required [21]. Tadokoro et al. reported successful BM and MSC transplantation into an 8-month-old patient with perinatal HPP [22]. Subsequently, the same group reported that transplantation of ex vivo expanded allogeneic MSCs following BM transplantation improved bone mineralization, muscle mass, respiratory function, and mental development in patients with HPP [23]. Combined BM and MSC transplantation may be effective to prevent the rejection of allogeneic donor-derived MSCs.
Cell therapy using MSCs has been applied for bone regeneration in adults. One important application is the repair of bone fractures or defects due to malignant bone tumors or external injuries. Quatro et al. reported three cases of successful autologous BM stromal cell transplantation to treat large bone defects in the tibia, ulna, and humerus [24]. They expanded osteoprogenitor cells isolated from BM cells and implanted them into the lesion sites with macroporous hydroxyapatite scaffolds. All three patients achieved improvement of bone function and radiographic examination findings. Following this report, many studies of local MSC transplantation for bone repair were conducted. However, the osteogenic differentiation potential of implanted MSCs in defected lesions was not certified in these reports.
Hematopoietic engraftment and recovery after HSCT
Attempts have been made to use MSCs to support hematopoiesis upon HSCT. For this purpose, two major interventions were applied: co-transplantation of HSCs and MSCs and transplantation of HSCs that were expanded ex vivo in the presence of MSCs.
In an early phase I/II trial of co-transplantation of autologous peripheral blood stem/progenitor cells (PBSCs) and culture-expanded autologous MSCs in advanced breast cancer patients that received high-dose chemotherapy, engraftment was effectively accelerated [25]. Following this report, clinical trials of co-transplantation of allogeneic BM or PBSCs and MSCs for patients with hematological malignant diseases were conducted (Table 1) [26–28]. Lazarus et al. co-administered HSCs and culture-expanded MSCs from the same donor (HLA-identical siblings) after myeloablative conditioning; however, acceleration of engraftment was not observed [26]. Le Blanc et al. conducted a pilot study of co-transplantation of MSCs and HSCs for patients with graft failure [27]. All patients achieved engraftment, indicating that such co-transplantation improves engraftment of cells from the second donor in salvage HSCT. MacMillan et al. reported that co-transplantation of MSCs supported rapid engraftment of unrelated cord blood cells in children with high-risk leukemia [28]. In summary, although co-transplantation of MSCs is not effective in a standard risk transplantation setting, it could be effective in cases of engraftment failure or delayed hematopoietic recovery, such as HSCT from HLA-haploidentical donors, cord blood transplantation, and retransplantation.
Table 1.
Clinical studies of co-infusion of MSCs with HSCs for hematopoietic recovery after hematopoietic stem/progenitor cell transplantation
| Number of patients | Median age of patients, years (range) | HSC donor | MSC donor | MSC dose (×106/kg) | Median time for Neut recovery (range) | Median time for Plt recovery (range) | Reference |
|---|---|---|---|---|---|---|---|
| 46 | 44.5 (19–61) | HLA-matched sibling | HSC donor | 1.0, 2.5, or 5.0 | Neut >500/μl at day 14 (11–26) | Plt >20,000/μl at day 20 (15–36) | [26] |
| 7 | 12 (1–44) | HLA-matched sibling in three cases Unrelated donor in three cases Cord blood in one case |
HLA-matched sibling or HLA-haploidentical donor | 1.0 | Neut >500/μl at day 12 (10–28) | Plt >30,000/μl at day 12 (8–36) | [27] |
| 8 | 7.5 (0.25–16) | Cord blood | HLA-haploidentical parent | 0.9–5.0 | Neut >500/μl at day 19 (9–28) | Plt >50,000/μl at day 53 (36–98) | [28] |
HLA human leukocyte antigen, HSC hematopoietic stem/progenitor cell, MSC mesenchymal stromal/stem cell, Neut neutrophil, Plt platelet
MSCs support the expansion of cord blood cells in vitro [29]. de Lima et al. studied whether cord blood cells culture-expanded in the presence of MSCs effectively induce hematopoietic recovery upon double cord blood cell transplantation [30]. Cord blood cells from one unit with a smaller cell number were expanded in co-culture with MSCs. These manipulated cells were co-transplanted with non-manipulated cord blood cells from another unit with a larger cell number. The time-to-engraftment of neutrophils and platelets was shorter in these patients than in the historical controls, indicating that ex vivo expansion of cord blood cells with MSCs is an effective strategy to improve engraftment.
Pharmacological targeting of endogenous BM-MSCs
In most clinical trials using allogeneic human MSCs, these cells were isolated from tissues/organs of volunteer donors, culture-expanded ex vivo, and intravenously infused into recipients. This intervention is a “quantity”-based approach to achieve therapeutic effects of MSCs. However, ex vivo expansion of MSCs might change their characteristics and reduce their quality. More importantly, a substantial proportion of intravenously infused donor MSCs become trapped within the lungs and are not distributed to the damaged tissues/organs of recipients [31]. There is obviously a limitation in the current strategy employed for cell therapy using MSCs because their effects are not dependent on the sustained settlement of infused cells or on proximate interactions with the target cells [32].
In a series of preclinical studies using model mice, we suggested that pharmacological treatment modifies the functions of endogenous BM-MSCs to achieve their therapeutic effects (Table 2) [33–37]. Acetylsalicylic acid (ASA), also known as aspirin, is a medication used to treat pain, fever, and inflammation. These therapeutic effects are mediated through inhibition or modification of cyclooxygenases [38, 39]. We showed that treatment with ASA ameliorates bone loss in osteoporotic mice due to the increased bone-forming capability of ASA-treated BM-MSCs [33]. Telomerase activity is enhanced in ASA-treated BM-MSCs [33]. This observation is consistent with a previous report that ASA contributes to the improvement of bone mineral density, although the contribution of MSCs is unknown [40]. These preclinical and clinical studies indicate the efficacy of ASA treatment for bone repair in patients with skeletal disorders through activation of endogenous BM-MSCs.
Table 2.
The effects of pharmacological treatment of MSCs
| Drug | Target cells | Clinical effect | MSC-mediated hematopoiesis | MSC-mediated bone regeneration | Mechanism(s) in MSCs | References |
|---|---|---|---|---|---|---|
| ASA | Broad cells | Anti-inflammation | N/T | ↑ | Telomerase activity↑ | [33] |
| EPO | Erythroid progenitors | Erythropoiesis | ↑ | ↑ | EPOR/Stat5 pathway↑ | [34] |
| PTH | Osteoblasts/Osteoclasts | Osteoporosis | ↑ | → | CDH11 expression↑ | [35] |
| VK2 | Osteoblasts | Osteoporosis | ↑ | ↑ | CXCL12 expression↓ | [37] |
| OICS | N/A | Osteoporosis | ↑ | → | CXCL12 and VCAM1 expression↓ | [36] |
Up arrows indicate up-regulation or activation. Down arrows indicate down-regulation or inactivation
ASA acetylsalicylic acid (aspirin), EPO erythropoietin, EPOR erythropoietin receptor, MSC mesenchymal stromal/stem cell, N/T not tested, OICS osteo-inductive cocktail (dexamethasone, phosphate, and vitamin C ), PTH parathyroid hormone, VCAM1 vascular cell adhesion protein 1, VK2 vitamin K2
Parathyroid hormone (PTH) is clinically used to treat osteoporosis because it has anabolic effects on bone formation though activating osteoblasts [41]. We demonstrated that short-term administration of PTH prolongs the survival of lethally irradiated mice that undergo BM transplantation, which is accompanied by enhanced hematopoietic marrow formation in BM [35]. PTH acts on human BM-MSCs to enhance their hematopoietic cell expansion capability through upregulation of the adhesion molecule cadherin-11 in BM-MSCs [35]. In another study, we showed that an erythropoiesis-stimulating agent, erythropoietin, acts on human BM-MSCs to enhance not only bone formation but also hematopoietic marrow formation in vivo, by using ectopically xeno-grafted mice [34]. The erythropoietin receptor/Stat5 pathway is enhanced in BM-MSCs as well as in erythroblast progenitor cells [34, 42]. Vitamin K2 (VK2) is clinically approved for the treatment of patients with osteoporosis. It is known that VK2 improves hematopoiesis in some patients with hematological diseases although the underlining mechanisms are not fully understood [43, 44]. In our study, the expression of CXCL12 in VK2-treated BM-MSCs was low, which suggested that CXCL12-CXCR4-mediated interaction between BM-MSCs and HSCs is released, thereby HSCs expand and differentiate into mature hematopoietic cells [37].
We have proposed that pharmacological targeting of endogenous MSCs is a quality-based intervention to achieve therapeutic effects in patients (Fig. 2). This strategy may enhance the therapeutic capability of MSCs to act closely on target cells through secretion of soluble factors and adherence in microenvironments, without requiring the redistribution of externally infused MSCs to damaged tissues/organs. However, attention needs to be paid to unexpected off-target effects of drugs in patients. To avoid this, we have sought drugs that act on MSCs and elicit therapeutic effects among compounds developed for medical purposes. We believe that this drug repositioning strategy shortens the drug development period, reduces medical costs, and provides patients with safe medications. In addition, there is a possibility that the characteristics of MSCs in patients might be affected [45]. Therefore, pharmacological stimulation of such affected MSCs may have unexpected effects on the pathogenesis of diseases. Thus, further investigations are needed to establish a quality-based, pharmacological, MSC-targeted strategy.
Fig. 2.
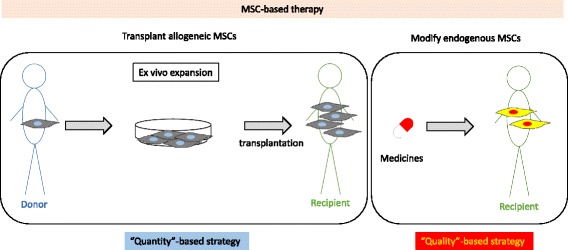
MSC-based therapy with pharmacological modification of endogenous MSCs. In a conventional approach, MSCs are isolated from donors, culture-expanded ex vivo, and then infused into recipients, mainly intravenously. This intervention is a “quantity”-based strategy to achieve the therapeutic effects of MSCs (left panel). We have proposed a strategy in which pharmacological treatment activates or modifies the functions of endogenous MSCs. This intervention is a “quality”-based strategy to achieve the therapeutic effects of MSCs (right panel)
Perspectives of MSC-based therapy
We recently reported that short-term treatment with ascorbic acid, inorganic phosphate, and dexamethasone (osteogenesis-inducing cocktails) accelerates hematopoietic recovery in mice that undergo BM transplantation, with altered chemotaxis- and adhesion-related gene expression profiles in BM-MSCs [36]. As well as treatment with a single pharmacological agent, combination treatment is also effective to achieve a therapeutic effect.
Recent studies reveal that MSCs are associated not only with normal hematopoiesis but also with the pathogenesis and progression of hematological malignant diseases. Our laboratory previously reported that defective MSCs are responsible for the impaired physiological early B cell lymphopoiesis in C/EBPβ-knockout mice [46]. Furthermore, MSC-mediated resistance to anti-cancer drugs in B cell precursor acute lymphoblastic leukemia cells can be ameliorated by pharmacological treatment of MSCs [47]. Raaijmakers et al. showed that deletion of Dicer1 in mouse osteoprogenitors causes myelodysplasia [48]. Balderman et al. suggested a novel therapeutic strategy to target the BM microenvironment for the treatment of myelodysplastic syndromes using model mice [49]. Collectively, the BM microenvironment is closely related to the pathogenesis and progression of hematological malignant diseases; therefore, targeting MSCs in this microenvironment is a crucial therapeutic strategy.
Conclusions
MSCs have a variety of biological characteristics. Cell therapy using MSCs is effective in a substantial proportion of intractable diseases; however, it is still in the process of development. Further investigations are needed to establish more effective MSC-based therapies.
Acknowledgements
We thank Dr. Masaki Iwasa, Dr. Aya Fujishiro, Dr. Sumie Fujii, Ms. Yoko Nakagawa, Dr. Satoshi Yoshioka, and Dr. Hisayuki Yao for their excellent work. The authors are grateful to Prof. Songtao Shi (University of Pennsylvania) for his mentorship on MSC studies.
Funding
This study was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (#26293277 and #15K09453, YM and TI; #16H00656, NS). This work was also supported in part by the Program of the network-type joint Usage/Research Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University (YM).
Availability of data and materials
Not applicable.
Authors’ contributions
NS and YM wrote the manuscript. TI, ATK, and TM provided the intellectual input to the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- ASA
Acetylsalicylic acid
- BM
Bone marrow
- BM-MSC
Bone marrow-derived mesenchymal stromal/stem cell
- GVHD
Graft-versus-host disease
- HLA
Human leukocyte antigen
- HPP
Hypophosphatasia
- HSC
Hematopoietic stem/progenitor cell
- HSCT
Hematopoietic stem/progenitor cell transplantation
- ISCT
International Society of Cellular Therapy
- IUT
In utero transplantation
- MSC
Mesenchymal stromal/stem cell
- OI
Osteogenesis imperfecta
- PBSC
Peripheral blood stem/progenitor cells
- PTH
Parathyroid hormone
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Gerson SL. Mesenchymal stem cells: no longer second class marrow citizens. Nat Med. 1999;5:262–264. doi: 10.1038/6470. [DOI] [PubMed] [Google Scholar]
- 3.Miura Y, Gao Z, Miura M, Seo BM, Sonoyama W, Chen W, Gronthos S, Zhang L, Shi S. Mesenchymal stem cell-organized bone marrow elements: an alternative hematopoietic progenitor resource. Stem Cells. 2006;24:2428–2436. doi: 10.1634/stemcells.2006-0089.. [DOI] [PubMed] [Google Scholar]
- 4.Miura Y. Human bone marrow mesenchymal stromal/stem cells: current clinical applications and potential for hematology. Int J Hematol. 2016;103:122–128. doi: 10.1007/s12185-015-1920-z. [DOI] [PubMed] [Google Scholar]
- 5.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka S, Miura Y, Iwasa M, Fujishiro A, Yao H, Miura M, Fukuoka M, Nakagawa Y, Yokota A, Hirai H, Ichinohe T, Takaori-Kondo A, Maekawa T. Isolation of mesenchymal stromal/stem cells from small-volume umbilical cord blood units that do not qualify for the banking system. Int J Hematol. 2015;102:218–229. doi: 10.1007/s12185-015-1828-7. [DOI] [PubMed] [Google Scholar]
- 7.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, Matsuzaki Y. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379:1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Mabuchi Y, Matsuzaki Y. Prospective isolation of resident adult human mesenchymal stem cell population from multiple organs. Int J Hematol. 2016;103:138–144. doi: 10.1007/s12185-015-1921-y. [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet (London, England) 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 11.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, Monroy R, Uberti J. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, Horn B, Yu L, Talano JA, Nemecek E, Mills CR, Chaudhury S. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20:229–235. doi: 10.1016/j.bbmt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Muroi K, Miyamura K, Ohashi K, Murata M, Eto T, Kobayashi N, Taniguchi S, Imamura M, Ando K, Kato S, Mori T, Teshima T, Mori M, Ozawa K. Unrelated allogeneic bone marrow-derived mesenchymal stem cells for steroid-refractory acute graft-versus-host disease: a phase I/II study. Int J Hematol. 2013;98:206–213. doi: 10.1007/s12185-013-1399-4. [DOI] [PubMed] [Google Scholar]
- 15.Muroi K, Miyamura K, Okada M, Yamashita T, Murata M, Ishikawa T, Uike N, Hidaka M, Kobayashi R, Imamura M, Tanaka J, Ohashi K, Taniguchi S, Ikeda T, Eto T, Mori M, Yamaoka M, Ozawa K. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol. 2016;103:243–250. doi: 10.1007/s12185-015-1915-9. [DOI] [PubMed] [Google Scholar]
- 16.Remberger M, Ringden O. Treatment of severe acute graft-versus-host disease with mesenchymal stromal cells: a comparison with non-MSC treated patients. Int J Hematol. 2012;96:822–824. doi: 10.1007/s12185-012-1218-3. [DOI] [PubMed] [Google Scholar]
- 17.Hashmi S, Ahmed M, Murad MH, Litzow MR, Adams RH, Ball LM, Prasad VK, Kebriaei P, Ringden O. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. The Lancet Haematology. 2016;3:e45–e52. doi: 10.1016/S2352-3026(15)00224-0. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.V97.5.1227. [DOI] [PubMed] [Google Scholar]
- 20.Le Blanc K, Gotherstrm C, Ringden O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U, Norden-Lindeberg S, Jansson M, Dalton A, Astrom E, Westgren M. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.TP.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 21.Whyte MP, Kurtzberg J, McAlister WH, Mumm S, Podgornik MN, Coburn SP, Ryan LM, Miller CR, Gottesman GS, Smith AK, Douville J, Waters-Pick B, Armstrong RD, Martin PL. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res. 2003;18:624–636. doi: 10.1359/jbmr.2003.18.4.624. [DOI] [PubMed] [Google Scholar]
- 22.Tadokoro M, Kanai R, Taketani T, Uchio Y, Yamaguchi S, Ohgushi H. New bone formation by allogeneic mesenchymal stem cell transplantation in a patient with perinatal hypophosphatasia. J Pediatr. 2009;154:924–930. doi: 10.1016/j.jpeds.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Taketani T, Oyama C, Mihara A, Tanabe Y, Abe M, Hirade T, Yamamoto S, Bo R, Kanai R, Tadenuma T, Michibata Y, Yamamoto S, Hattori M, Katsube Y, Ohnishi H, Sasao M, Oda Y, Hattori K, Yuba S, Ohgushi H, Yamaguchi S. Ex vivo expanded allogeneic mesenchymal stem cells with bone marrow transplantation improved osteogenesis in infants with severe hypophosphatasia. Cell Transplant. 2015;24:1931–1943. doi: 10.3727/096368914X685410. [DOI] [PubMed] [Google Scholar]
- 24.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 25.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 26.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Jr, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lonnies H, Nava S, Ringden O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 28.Macmillan ML, Blazar BR, DeFor TE, Wagner JE. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant. 2009;43:447–454. doi: 10.1038/bmt.2008.348. [DOI] [PubMed] [Google Scholar]
- 29.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, Kaur I, Fu P, Del Angel M, Messinger R, Flagge F, de Lima M, Decker W, Xing D, Champlin R, Shpall EJ. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, Alousi A, Saliba R, McMannis JD, Kaur I, Kebriaei P, Parmar S, Popat U, Hosing C, Champlin R, Bollard C, Molldrem JJ, Jones RB, Nieto Y, Andersson BS, Shah N, Oran B, Cooper LJ, Worth L, Qazilbash MH, Korbling M, Rondon G, Ciurea S, Bosque D, Maewal I, Simmons PJ, Shpall EJ. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Miura Y, Yoshioka S, Yao H, Takaori-Kondo A, Maekawa T, Ichinohe T. Chimerism of bone marrow mesenchymal stem/stromal cells in allogeneic hematopoietic cell transplantation. Chimerism. 2013;4:78–83. doi: 10.4161/chim.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, Le A, Wang CY, Chen W, Shi S. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One. 2008;3:e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaza T, Miura Y, Akiyama K, Bi Y, Sonoyama W, Gronthos S, Chen W, Le A, Shi S. Mesenchymal stem cell-mediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood. 2009;113:2595–2604. doi: 10.1182/blood-2008-10-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao H, Miura Y, Yoshioka S, Miura M, Hayashi Y, Tamura A, Iwasa M, Sato A, Hishita T, Higashi Y, Kaneko H, Ashihara E, Ichinohe T, Hirai H, Maekawa T. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells. 2014;32:2245–2255. doi: 10.1002/stem.1701. [DOI] [PubMed] [Google Scholar]
- 36.Sugino N, Miura Y, Yao H, Iwasa M, Fujishiro A, Fujii S, Hirai H, Takaori-Kondo A, Ichinohe T, Maekawa T. Early osteoinductive human bone marrow mesenchymal stromal/stem cells support an enhanced hematopoietic cell expansion with altered chemotaxis- and adhesion-related gene expression profiles. Biochem Biophys Res Commun. 2016;469:823–829. doi: 10.1016/j.bbrc.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 37.Fujishiro A, Miura Y, Iwasa M, Fujii S, Tamura A, Sato A, Yokota A, Sugino N, Hirai H, Ando A, Ichinohe T, Maekawa T. Vitamin K2 supports hematopoiesis through acting on bone marrow mesenchymal stromal/stem cells [Abstract] Blood. 2015;126:1192. [Google Scholar]
- 38.Cashman J, McAnulty G. Nonsteroidal anti-inflammatory drugs in perisurgical pain management. Mechanisms of action and rationale for optimum use. Drugs. 1995;49:51–70. doi: 10.2165/00003495-199549010-00005. [DOI] [PubMed] [Google Scholar]
- 39.Buttar NS, Wang KK. The “aspirin” of the new millennium: cyclooxygenase-2 inhibitors. Mayo Clin Proc. 2000;75:1027–1038. doi: 10.4065/75.10.1027. [DOI] [PubMed] [Google Scholar]
- 40.Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC, Barrow KD, Kritchevsky SB. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res. 2003;18:1795–1802. doi: 10.1359/jbmr.2003.18.10.1795. [DOI] [PubMed] [Google Scholar]
- 41.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 42.Kuhrt D, Wojchowski DM. Emerging EPO and EPO receptor regulators and signal transducers. Blood. 2015;125:3536–3541. doi: 10.1182/blood-2014-11-575357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takami A, Nakao S, Ontachi Y, Yamauchi H, Matsuda T. Successful therapy of myelodysplastic syndrome with menatetrenone, a vitamin K2 analog. Int J Hematol. 1999;69:24–26. [PubMed] [Google Scholar]
- 44.Nishimaki J, Miyazawa K, Yaguchi M, Katagiri T, Kawanishi Y, Toyama K, Ohyashiki K, Hashimoto S, Nakaya K, Takiguchi T. Vitamin K2 induces apoptosis of a novel cell line established from a patient with myelodysplastic syndrome in blastic transformation. Leukemia. 1999;13:1399–1405. doi: 10.1038/sj.leu.2401491. [DOI] [PubMed] [Google Scholar]
- 45.Ferrer RA, Wobus M, List C, Wehner R, Schonefeldt C, Brocard B, Mohr B, Rauner M, Schmitz M, Stiehler M, Ehninger G, Hofbauer LC, Bornhauser M, Platzbecker U. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica. 2013;98:1677–1685. doi: 10.3324/haematol.2013.083972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshioka S, Miura Y, Yao H, Satake S, Hayashi Y, Tamura A, Hishita T, Icinohe T, Hirai H, Takaor-Kondo A, Maekawa T. CCAAT/enhancer-binding protein beta expressed by bone marrow mesenchymal stromal cells regulates early B-cell lymphopoiesis. Stem Cells. 2014;32:730–740. doi: 10.1002/stem.1555. [DOI] [PubMed] [Google Scholar]
- 47.Iwasa M, Miura Y, Fujishiro A, Fujii S, Sugino N, Yoshioka S, Tamura A, Sato A, Yokota A, Kito K, Ando A, Hirai H, Takaori-Kondo A, Ichinohe T, Maekawa T. Bortezomib attenuates adhesion of B cell precursor acute lymphoblastic lleukemia cells to bone marrow mesenchymal stromal/stem cells via regulating SPARC expression [Abstract] Blood. 2015;126:786. [Google Scholar]
- 48.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balderman SR, Li AJ, Hoffman CM, Frisch BJ, Goodman AN, LaMere MW, Georger MA, Evans AG, Liesveld JL, Becker MW, Calvi LM. Targeting of the bone marrow microenvironment improves outcome in a murine model of myelodysplastic syndrome. Blood. 2016;127:616–625. doi: 10.1182/blood-2015-06-653113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


