Abstract
We herein report a case of hemichorea-hemiballism in an 85-year-old man diagnosed with diabetes at 76 years of age. After a one-year interruption in treatment, he was treated with a low-calorie diet, linagliptin, and nateglinide. Over 51 days, his HbA1c level decreased from 15.8% to 7.7%. After a prompt improvement in his hyperglycemia, he began experiencing involuntary movements in the right upper and lower extremities. T1-weighted magnetic resonance imaging showed a high signal intensity in the left lens nucleus. The patient was diagnosed with diabetic hemichorea-hemiballism and received haloperidol (1 mg/day) as treatment.
Keywords: diabetic hemichorea-hemiballism, prompt improvement of hyperglycemia, lens nucleus
Introduction
Glucose metabolism is considered to be clinically important. We recently encountered a patient who developed diabetic hemichorea-hemiballism (1-4) after a prompt improvement in hyperglycemia. The findings of this case may have important implications concerning blood glucose control in patients with diabetes.
Case Report
Case: An 85-year-old man with diabetes
Family history: Mother had diabetes
Past medical history: Hearing impairment (right > left) since approximately 75 years of age
Vocation history: Agriculture
Drinking history: 900 cc/day of sake from 20 to 50 years of age
Smoking history: 90 cigarettes/day from 20 to 50 years of age
The patient was diagnosed with diabetes at 76 years of age. He received treatment at our department, which included a diet of 1,600 kcal/day as well as oral antidiabetic drugs [linagliptin (5 mg, 1 tablet/day) and nateglinide (90 mg, 3 tablets/day)]. Subsequently, he was transferred to a nearby hospital. However, he did not continue to visit the hospital, and his treatment was interrupted for one year. In early May 2015, he began to experience difficulty walking owing to bilateral lower limb weakness. In mid-May 2015, he was admitted to our hospital for treatment. On admission, his blood glucose level was 563 mg/dL, and his HbA1c level was 17.0%. Treatment was initiated, which included an infusion of acetated Ringer's solution (500 mL/day intravenously) followed by the administration of linagliptin (5 mg, 1 tablet/day) and nateglinide (90 mg, 3 tablets/day). His blood glucose level stabilized at 120-220 mg/dL. His walking ability also recovered with physical rehabilitation. He was discharged from the hospital in early June 2015.
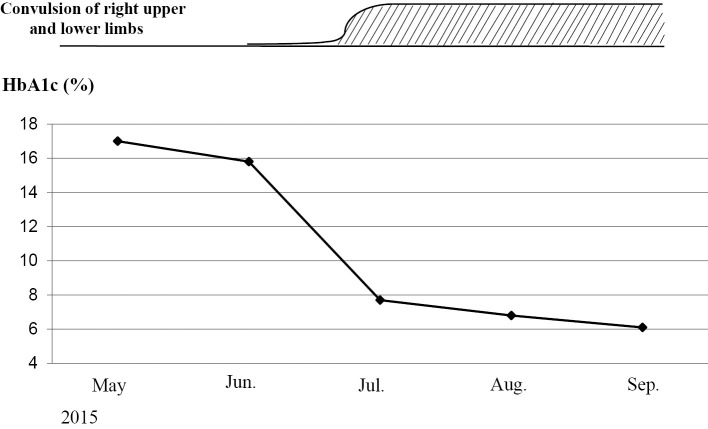
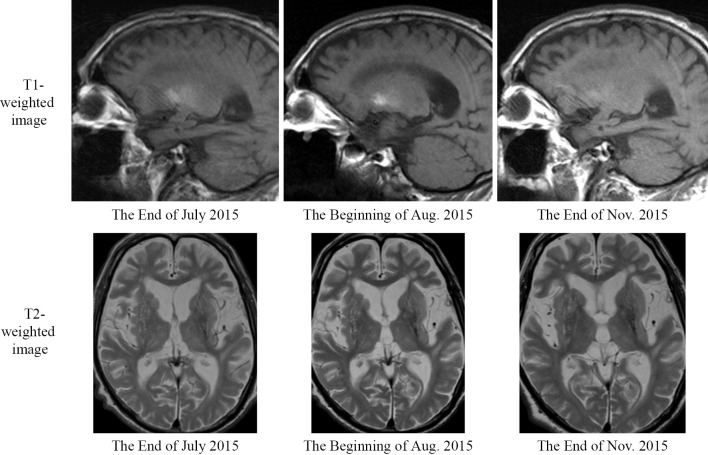
In late June 2015, he began experiencing involuntary movements in his right lower extremity. A few days later, he also began experiencing involuntary movements in his right upper extremity. His blood glucose level immediately before the appearance of involuntary movements was 102 mg/dL. At his first outpatient visit in July, the blood glucose level after the involuntary movements was recorded as 96 mg/dL. During the 51 days from the beginning of June 2015 to the end of July, his HbA1c level decreased by 8.1%, from 15.8% to 7.7% (Fig. 1). Coarse involuntary movements, such as jerks in the right upper and lower extremities, persisted. At the end of July 2015, he was readmitted to our department. T1-weighted magnetic resonance imaging (MRI) of his brain (sagittal section) showed a faint high signal intensity in the left lens nucleus (pallidus to putamen); however, the signal intensity was low on a T2-weighted image (horizontal section) (Fig. 2).
Figure 1.
Change in HbA1c levels. Over 51 days, from the beginning of June 2015 to the end of July 2015, the patient’s HbA1c level decreased by 8.1%, from 15.8% to 7.7%. At the end of June 2015, involuntary movements in his right lower extremity were observed. A few days later, the involuntary movements progressed to the right upper extremity.
Figure 2.
Brain magnetic resonance imaging findings. Brain magnetic resonance imaging was performed three times: at the end of July, at the beginning of August, and at the end of November, 2015. On the T1-weighted images, the high signal intensity observed in the lens nucleus peaked at the beginning of August and improved in November.
On admission, his vital signs were as follows: height, 167 cm; weight, 56.3 kg; blood pressure, 99/57 mmHg; pulse rate, 72 beats/min; and body temperature, 36.6℃. No abnormal chest or abdominal findings or edema in his lower extremities were identified on a physical examination. On a laboratory examination, his white blood cell count and lactate dehydrogenase, blood urea nitrogen, and creatinine levels were all elevated (Table). Chest radiographs of the lungs yielded unremarkable findings. Electrocardiograms revealed supraventricular premature contraction and first-degree atrioventricular block. After hospital admission, he was diagnosed with right-sided hemichorea-hemiballism (1-4) by a neurologist. He was administered haloperidol at 1 mg/day and discharged from the hospital at the end of July. After discharge, he continued to receive haloperidol therapy as an outpatient.
Table.
Laboratory Findings on Admission.
| Hematology and biochemistry | |||||
| WBC | 9,750 | /μL | BUN | 21.1 | mg/dL |
| RBC | 379×104 | /μL | Cr | 1.17 | mg/dL |
| Hb | 12.6 | g/dL | Na | 139 | mEq/L |
| Plt | 18.9×104 | /μL | K | 4.6 | mEq/L |
| TP | 8.1 | g/dL | CL | 104 | mEq/L |
| T-Bil | 0.7 | mg/dL | CK | 165 | IU/L |
| AST | 19 | IU/L | Amy | 98 | IU/L |
| ALT | 10 | IU/L | NT-pro BNP | 187 | pg/mL |
| LDH | 263 | IU/L | Glu | 96 | mg/dL |
| ALP | 198 | IU/L | HbA1c | 7.7 | % |
| γ-GTP | 12 | IU/L | eGFR | 46 | mL/min/1.732 |
| ChE | 247 | IU/L | D-dimer | 1.3 | µg/mL |
Urinalysis: pH 5.5 Glucose (-) Protein (-) Occult blood (-) Acetone body (-)
By the end of September 2015, the involuntary movements of his right upper extremity had improved. By January 2016, the involuntary movements of his right lower extremity had also improved, and walking became easier. MRI of his brain were obtained three times: at the end of July, at the beginning of August, and at the end of November 2015. On T1-weighted MRI, the high signal intensity of the lens nucleus peaked at the beginning of August and improved in November (Fig. 2).
Discussion
Diabetic hemichorea-hemiballism (1-4) often manifests as hyperglycemia. The underlying mechanism involves GABA, an inhibitory neurotransmitter, which is used as an energy resource under hyperglycemic conditions. Hyperglycemia results in the depletion of GABA, thereby reducing its inhibitory effects on neurons from the globus pallidus to the thalamus in the basal ganglia circuit. In the present case, hemichorea-hemiballism occurred after a rapid improvement in long-term, persistent hyperglycemia. In diabetic patients with poor blood glucose control, stress-induced ischemia may occur in the lens nucleus after rapid correction of blood glucose levels.
Striatal lesions may result from the weakening of the blood-brain barrier due to hyperglycemia (5). Alternatively, stress caused by rapid changes in the hyperglycemic status may result in ischemia-like changes in the striatum, consistent with the astrocyte proliferation observed in striatal biopsy specimens in such cases (2). Patients with diabetes who undergo rapid correction of blood glucose may need to be monitored for diabetic hemichorea-hemiballism. In addition, in these patients, retinopathy may worsen, or painful neuropathy may occur subsequent to the rapid improvement in blood glucose levels (6).
MRI of the patient's brain was performed three times: at the end of July, at the beginning of August, and at the end of November 2015. The high signal intensity peak observed in the lens nucleus on the T1-weighted images was slightly delayed relative to the onset of clinical symptoms, as was the high signal attenuation in conjunction with the improvement in clinical symptoms. In general, lesions due to diabetic hemichorea-hemiballism occur in the striatum (1-3,5). The development of lesions in the putamen is invariant, and similar lesions are observed in the caudate nucleus and the globus pallidus. Metabolic diseases that can cause lesions in the lens nucleus include Wilson's disease, which is a copper metabolism disorder (7), and ceruloplasmin deficiency, which is a hereditary iron metabolism abnormality (8-10). Wilson's disease is treated using a copper-chelating agent. In the present case, the symptoms along with the high signal intensity on the T1-weighted MRI improved without the administration of a copper-chelating agent; therefore, Wilson's disease was ruled out. In ceruloplasmin deficiency, lesions of the putamen and globus pallidus were detected at autopsy (8); patients tend to exhibit involuntary movements, such as chorea, and in many cases, diabetes mellitus also occurs (9,10). However, basal nucleus lesions appear as low signal intensities in both T1- and T2-weighted images, reflecting iron deposition.
Neurodegenerative diseases that cause lesions in the basal ganglia include Huntington's disease (11), spinocerebellar degeneration, such as dentatorubral pallidoluysian atrophy (12), and Creutzfeldt-Jakob, which involves elevated ubiquitin levels (13). Both neurological diseases are progressive in nature, and no treatment has been established; therefore, these diseases were also ruled out because the patient's symptoms improved. Pallidal lesions can also be caused by carbon monoxide poisoning (14); however, in such cases, the lesions appear as low signal intensities on T1-weighted images and high signal intensities on T2-weighted images.
Other diseases specifically associated with diabetes mellitus are mitochondrial diseases (15), Stiff-person syndrome due to decreased GABA activity (16), and myoclonus due to diabetic muscle atrophy (17). The patient in our case had a hearing impairment, and his mother had had diabetes. Therefore, it was necessary to include mitochondrial diseases in the differential diagnosis. A 3243-point mutation in the nucleotide sequence of mitochondrial DNA, which is associated with a family history of diabetes, is also observed in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS). Ataxia has been reported in MELAS occurring with diabetes mellitus. However, MELAS manifests as a cerebral infarct-like lesion in the occipital region. Stiff-person syndrome manifests as epileptic muscle spasms of the trunk and limb proximal muscles that spread throughout the body over a few months. In our patient, involuntary movements existed only in the right upper and lower extremities; therefore, stiff-person syndrome was also ruled out. Diabetic muscular atrophy is included in the concept of diabetic peripheral neuropathy. The findings in the present patient do not contradict the merger of diabetic peripheral neuropathy.
Given the patient's history of alcohol consumption, we also considered Marchiafava-Bignami disease (18), a disorder that occurs in heavy alcohol drinkers and presents with impaired gait as well as convulsions; however, hyperintensities are detectable at the corpus callosum on brain T2-weighted MRIs and fluid-attenuated inversion recovery images. Thus, Marchiafava-Bignami disease was also ruled out, and the patient was ultimately diagnosed with diabetic hemichorea-hemiballism.
Conclusion
Diabetic hemichorea-hemiballism often appears in conjunction with hyperglycemia. However, in diabetic patients with prolonged poor glycemic control, such as in the present case, hemichorea-hemiballism may become apparent after a prompt improvement in hyperglycemia. Therefore, clinicians should be attentive to the possibility of hemichorea-hemiballism after a prompt improvement in hyperglycemia among patients with prolonged poor glycemic control.
The gist of the present manuscript was presented at the 208th Japanese Society of Internal Medicine Tohoku district meeting in Sendai on July 9, 2016.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Nagai C, Kato T, Katagiri T, Sasaki H. Hyperintense putamen on T1-weighted MR images in a case of chorea with hyperglycemia. AJNR Am J Neuroradiol 16: 1243-1246, 1995. [PMC free article] [PubMed] [Google Scholar]
- 2. Shan DE, Ho DM, Chang C, Pan HC, Teng MM. Hemichorea-hemiballism: an explanation for MR signal changes. AJNR Am J Neuroradiol 19: 863-870, 1998. [PMC free article] [PubMed] [Google Scholar]
- 3. Nakajima N, Ueda M, Nagayama H, Katayama Y. Putaminal changes before the oncet of clinical symptoms in diabetic hemichorea-hemiballism. Intern Med 53: 489-491, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Lin JJ, Chang MK. Hemiballism-hemichorea and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry 57: 748-750, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwata A, Koike F, Arasaki K, Tamaki M. Blood brain barrier destruction in hyperglycemic chorea in a patient with poorly controlled diabetes. J Neurol Sci 163: 90-93, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Dahl-Jørgensen K, Brinchmann-Hansen O, Hanssen KF, Sandvik L, Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed) 290: 811-815, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss KH. Wilson Disease. GeneReviewsⓇ [Internet]. [cited 2016 Nov. 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1512/
- 8. Kawanami T, Kato T, Daimon M. Hereditary caeruloplasmin deficiency: clinicopathological study of a patient. J Neurol Neurosurg Psychiatry 61: 506-509, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasaki H, Yamatani K, Kato T, Kawanami T. Hereditary ceruloplasmin deficiency--a new type of diabetes mellitus. Intern Med 35: 596-597, 1996. [DOI] [PubMed] [Google Scholar]
- 10. Daimon M, Moriai S, Susa S. Hypocaeruloplasminaemia with heteroallelic caeruloplasmin gene mutation: MRI of the brain. Neuroradiology 41: 185-187, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Hashimoto K, Ito Y, Tanahashi H, Hayashi M, Yamakita N, Yasuda K. Hyperglycemic chorea-ballism or acute exacerbation of Huntington's chorea? Huntington's disease unmasked by diabetic ketoacidosis in type 1 diabetes mellitus. J Clin Endocrinol Metab 97: 3016-3020, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Nagaoka U, Suzuki Y, Kawanami T, et al. Regional differences in genetic subgroup frequency in hereditary cerebellar ataxia, and a morphometrical study of brain MR images in SCA1, MJD and SCA6. J Neurol Sci 164: 187-194, 1999. [DOI] [PubMed] [Google Scholar]
- 13. Manaka H, Kato T, Kurita K, et al. Marked increase in cerebrospinal fluid ubiquitin in Creutzfeldt-Jakob disease. Neurosci Lett 139: 47-49, 1992. [DOI] [PubMed] [Google Scholar]
- 14. Kawanami T, Kato T, Kurita K, Sasaki H. The pallidoreticular pattern of brain damage on MRI in a patient with carbon monoxide poisoning. J Neurol Neurosurg Psychiatry 64: 282, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura S, Yoshinari M, Wakisaka M, et al. Ketoacidosis accompanied by epileptic seizures in a patient with diabetes mellitus and mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Diabetes Metab 26: 407-410, 2000. [PubMed] [Google Scholar]
- 16. Chandra MS, Rajendra KP, Banshi LK, Dinesh K, Pankaj G. A unique combination of autoimmune limbic encephalitis, type 1 diabetes, and Stiff person syndrome associated with GAD-65 antibody. Ann Indian Acad Neurol 19: 146-149, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaidi SA, Chhetri SK, Lekwuwa G, Majeed T. An unusual presentation of diabetic amyotrophy: myoclonus. BMJ Case Rep bcr2012008245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pérez ÁA, Ramón CC, Morís TG, Pascual GJ. Marchiafava-Bignami disease triggered by poorly controlled diabetes mellitus. Neurologia 31: 498-500, 2015. [DOI] [PubMed] [Google Scholar]