Abstract
Neuroleptic malignant syndrome (NMS) with characteristic symptoms is a potentially lethal reaction to antipsychotic drugs. Atypical NMS usually lacks major symptoms and frequently occurs after treatment using atypical antipsychotics, such as aripiprazole. A 64-year-old man developed aripiprazole-induced NMS after surgery, and our early recognition of the NMS was based on high creatine kinase levels and low serum iron levels. His characteristic symptoms (a fever, rigidity, and altered mental status) were only present for a few hours and were resolved by aripiprazole discontinuation and supportive care. Aripiprazole-induced NMS can present with brief but major symptoms, and clinicians may overlook this “brief” appearance of NMS.
Keywords: antipsychotic agents, aripiprazole, early diagnosis, iron, neuroleptic malignant syndrome
Introduction
Patients with neuroleptic malignant syndrome (NMS) exhibit clinical features, such as a fever, rigidity, altered mental status, and dysautonomia, after treatment using neuroleptic agents or anti-Parkinson medication withdrawal (1). Atypical NMS appears without hyperthermia or muscle rigidity and frequently occurs after treatment using atypical antipsychotic drugs, such as aripiprazole (2,3). However, without careful observation, clinicians may overlook the signs and symptoms of NMS. We herein report a case of “brief” aripiprazole-induced NMS where a fever, rigidity, and altered mental status were present for only a few hours.
Case Report
A 64-year-old Japanese man with recent cholecystitis (treated using percutaneous transhepatic gallbladder drainage) was scheduled for laparoscopic cholecystectomy. A psychiatrist had previously diagnosed the patient with alcohol-related dementia, which was being treated using aripiprazole, pregabalin, and diazepam. The patient had no other significant medical history. On preoperative day 3, his axillary temperature was 36.6℃; heart rate, 73 beats/min; blood pressure, 126/68 mmHg; and oxygen saturation, 97% in ambient air. A physical examination and laboratory tests revealed normal findings, with a creatine kinase (CK) level of 217 U/L and serum iron level of 91 μg/dL (normal range: 54-181 μg/dL). The patient had been receiving aripiprazole (24 mg/day for 26 days), pregabalin (150 mg/day for 20 days), and diazepam (6 mg/day for 7 days) before laparoscopic cholecystectomy, although this treatment was stopped on the day of surgery.
In the operating room, the patient received general and epidural anesthesia using propofol, desflurane, fentanyl, remifentanil, levobupivacaine, and rocuronium. Laparoscopic cholecystectomy was completed in 155 minues, the intraoperative blood loss was 109 mL, and the anesthetic course and operation were uneventful. The patient was extubated and transported to the critical-care unit because of his dementia and subsequently received intravenous diazepam (5 mg) at bedtime. On postoperative day (POD) 1, his vital signs were normal, and laboratory tests revealed a leukocyte count of 11,000 cells/mm3, a C-reactive protein level of 2.73 mg/dL, a CK level of 97 U/L, and a serum iron level of 85 μg/dL. We therefore re-started his regular dementia treatment using the original drugs and doses.
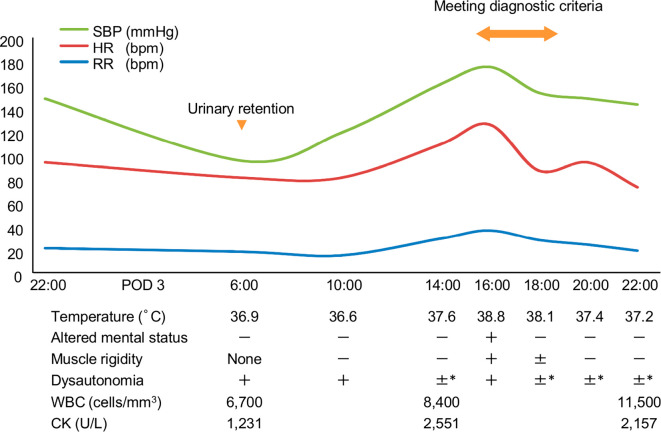
At 6 AM on POD 3, the patient had a temperature of 36.9℃, a heart rate of 81 beats/min, and a blood pressure of 95/68 mmHg (Figure). He was alert, and a physical examination revealed no remarkable findings, although he had acute urinary retention. We initially considered the hypotension and acute urinary retention to be complications of the epidural anesthesia, which prompted us to stop the continuous epidural anesthesia and place an indwelling urethral catheter. Routine laboratory tests revealed a leukocyte count of 6,700 cells/mm3, a creatinine level of 0.65 mg/dL, and a CK level of 1,231 U/L. Additional laboratory tests revealed a CK-MB level of 15 U/L, a troponin I level of 3.6 pg/mL, and a serum iron level of 47 μg/dL. Electrocardiography and cardiac ultrasonography showed normal findings, and we immediately stopped aripiprazole treatment on suspicion of NMS. Treatment for the elevated CK level was begun using fluid resuscitation. At 4 PM, he suddenly developed confusion and muscle rigidity with serious vital sign abnormalities, including a temperature of 38.8℃, a heart rate of 126 beats/min, a blood pressure of 175/100 mmHg, a respiratory rate of 36 breaths/min, and oxygen saturation of 90% in ambient air. His CK level peaked at 2,551 U/L despite adequate hydration maintenance with nasal oxygen at 2 L/min. We did not administer any antipyretic medication, and his fever, rigidity, and altered mental status resolved by 8 PM His CK level then decreased to 2,157 U/L, and his urine output reached >2 mL/kg/h. These results suggested that he had been successfully treated using only supportive care. On POD 4, his CK levels at 6 AM and 4 PM were 2,277 U/L and 1,835 U/L, respectively. On POD 7, the patient was transferred to the general ward, and he was subsequently discharged with prescriptions for clonazepam, pregabalin, and suvorexant.
Figure.
Patient’s clinical course. *Blood pressure approximately equal to the baseline value. Urinary retention was not considered to be present because of the urethral catheter. SBP: systolic blood pressure, HR: heart rate, RR: respiratory rate, POD: postoperative day, WBC: white blood cell count, CK: creatine kinase
Discussion
The present case highlights two important issues. First, the major symptoms of aripiprazole-induced NMS may only last a few hours. In normal cases, NMS is diagnosed according to the DSM-IV-TR and Levenson diagnostic criteria (3-5); however, atypical antipsychotic-induced NMS can be difficult to diagnose, as it presents without clear clinical features (2,3). A recent systematic review indicated that aripiprazole-induced neuroleptic malignant syndrome occurs after treatment with a mean dose of 18.9 mg and has a symptom duration of approximately 7.5 days. Furthermore, most NMS cases exhibit an altered mental status and rigidity; temperatures of ≥38℃ are less commonly observed (58.3% prevalence) (3). In the present case, our careful observation and physical examination detected a fever, rigidity, and an altered mental status that persisted for only a two-hour period, which met the DSM-IV-TR and Levenson diagnostic criteria. In addition, the patient was successfully treated using only supportive care. These findings suggest that atypical antipsychotic-induced NMS may be a self-limiting condition and can therefore be missed during treatment.
Second, sudden decreased serum iron levels may facilitate the early recognition of NMS, even in patients who have recently undergone surgery. In the present case, the first symptoms were hypotension and acute urinary retention, which mimicked the complications of epidural anesthesia. However, in the absence of elevated CK levels, the early diagnosis of NMS can be difficult. Serum iron levels can temporarily decrease during an NMS episode and subsequently return to normal with clinical improvement of the patient (6). Furthermore, serum iron levels are a negative acute-phase reactant (7) and are lowest on POD 1 in postoperative patients (8). In the present case, the serum iron levels of the patient were not markedly different on POD 1 (85 μg/dL) from before the operation (91 μg/dL) and decreased on POD 3 (47 μg/dL), prompting us to suspect NMS. In addition, the serum iron levels returned to 89 μg/dL in association with clinical improvement on POD 5. The serum iron levels are thought to be higher in the morning than in the afternoon or evening (7,9). Considering the importance of this diurnal variation of serum iron levels in the diagnosis of NMS, we measured the serum iron levels only in the morning.
Diagnosing NMS and determining its etiology is challenging, especially in postoperative patients. Postoperative NMS can have unusual causes, including anesthetics (10), anesthetic-related agents (11), neuroleptic discontinuation (12), and neuroleptic reinstitution (13). Furthermore, low serum iron is a potential risk factor for NMS (14,15). Therefore, patients experiencing surgical stress that results in low serum iron can potentially develop postoperative NMS. In the present case, surgical stress did not decrease the serum iron level. In our opinion, the present NMS case resulted from a high-dose administration for 28 days rather than neuroleptic discontinuation and reinstitution. This interpretation is consistent with the risk of NMS being associated with high-dose antipsychotic administration (16) and the median onset time being 27.5 days for atypical antipsychotic-induced NMS (17). Atypical antipsychotic drugs are commonly used for the management of behavioral and psychological symptoms of dementia (18). Our results suggest that atypical antipsychotic drug administration should be initiated at the lowest dose for the shortest time possible and in conjunction with other medical treatments for dementia (18).
Conclusion
Aripiprazole-induced NMS can present with major symptoms that last only a few hours. In these cases, the serum iron levels may facilitate the early diagnosis of NMS. Although many reports of atypical NMS can be found in the literature, this is the first report of a case of “brief” NMS, indicating that atypical antipsychotic-induced NMS may go unrecognized in the absence of careful observation and a physical examination. Further studies are required to determine whether or not atypical NMS is simply missed or indeed as rare as it seems, and whether or not an early diagnosis can prevent the worsening of symptoms.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry 72: 1222-1228, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Picard LS, Lindsay S, Strawn JR, Kaneria RM, Patel NC, Keck PE Jr. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy 28: 530-535, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Belvederi Murri M, Guaglianone A, Bugliani M, et al. Second-generation antipsychotics and neuroleptic malignant syndrome: systematic review and case report analysis. Drugs R D 15: 45-62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. American Psychiatric Association, Ed. Medication-induced movement disorders: neuroleptic malignant syndrome. American Psychiatric Association, Washington, D.C., 2000: 795-798. [Google Scholar]
- 5. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 142: 1137-1145, 1985. [DOI] [PubMed] [Google Scholar]
- 6. Rosebush PI, Mazurek MF. Serum iron and neuroleptic malignant syndrome. Lancet 338: 149-151, 1991. [DOI] [PubMed] [Google Scholar]
- 7. Burtis CA, Ashwood ER, Bruns DE. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed. Elsevier, Philadelphia, PA, 2012: 985-1030. [Google Scholar]
- 8. Mohammed R, McColl KE, Veitch D, Young R, Cumming RL, Parikh RK. Changes in iron metabolism following surgery. Br J Surg 70: 161-162, 1983. [DOI] [PubMed] [Google Scholar]
- 9. Dale JC, Burritt MF, Zinsmeister AR. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol 117: 802-808, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Tsuchiya N, Morimura E, Hanafusa T, Shinomura T. Postoperative neuroleptic malignant syndrome that occurred repeatedly in a patient with cerebral palsy. Paediatr Anaesth 17: 281-284, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Patel P, Bristow G. Postoperative neuroleptic malignant syndrome. A case report. Can J Anaesth 34: 515-518, 1987. [DOI] [PubMed] [Google Scholar]
- 12. Amore M, Zazzeri N. Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry 19: 1323-1334, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Zhao Z, Zhang H, Wang S, Chen X. Sudden discontinuation and reinstitution of olanzapine-associated atypical neuroleptic malignant syndrome in a patient undergoing lung surgery. Int J Clin Exp Med 8: 11639-11641, 2015. [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry 44: 499-507, 1998. [DOI] [PubMed] [Google Scholar]
- 15. Patil BS, Subramanyam AA, Singh SL, Kamath RM. Low serum iron as a possible risk factor for neuroleptic malignant syndrome. Int J Appl Basic Med Res 4: 117-118, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keck PE, Pope HG, Cohen BM, Mcelroy SL, Nierenberg AA. Risk factors for neuroleptic malignant syndrome. A case-control study. Arch Gen Psychiatry 46: 914-918, 1989. [DOI] [PubMed] [Google Scholar]
- 17. Trollor JN, Chen X, Chitty K, Sachdev PS. Comparison of neuroleptic malignant syndrome induced by first- and second-generation antipsychotics. Br J Psychiatry 201: 52-56, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Greenblatt HK, Greenblatt DJ. Use of Antipsychotics for the treatment of behavioral symptoms of dementia. J Clin Pharmacol 56: 1048-1057, 2016. [DOI] [PubMed] [Google Scholar]