Abstract
Human factor Va (hfVa) is the important regulatory subunit of prothrombinase. Recent modeling data have suggested a critical role for amino acid Arg701 of hfVa for human prothrombin (hPro) activation by prothrombinase. Furthermore, it has also been demonstrated that hfVa has a different effect than that of bovine fVa on prethrombin-1 activation by prothrombinase. The difference between the two cofactor molecules was also found within the Asn700–Arg701 dipeptide in the human factor V (hfV) molecule, which is replaced by the Asp–Glu sequence in bfV. As a consequence, we produced a recombinant hfV (rhfV) molecule with the substitution 700NR701→DE. rhfVNR→DE together with the wild-type molecule (rhfVWT) were expressed in COS7 cells, purified, and tested for their capability to function within prothrombinase. Kinetic studies showed that the Kd of rhfVaNR→DE for human fXa as well as the kcat and Km of prothrombinase made with rhfVaNR→DE for hPro activation were similar to the values obtained following hPro activation by prothrombinase made with rhfVaWT. Remarkably, sodium dodecyl sulfate polyacrylamide gel electrophoresis analyses of hPro activation time courses demonstrated that the rate of cleavage of hPro by prothrombinase reconstituted with rhfVaNR→DE was significantly delayed with substantial accumulation of meizothrombin, and delayed thrombin generation, when compared to activation of hPro by prothrombinase made with rhfVaWT. These unanticipated results provide significant insights on the role of the carboxyl-terminal end of the heavy chain of hfVa for hPro cleavage and activation by prothrombinase and show that residues 700NR701 regulate at least in part the enzyme–substrate/product interaction during fibrin clot formation.
Introduction
Human factor Va (hfVa) is the important regulatory subunit of prothrombinase that controls the rate of human prothrombin (hPro) activation by prothrombinase during hemostasis.1 This process is a highly regulated event, which involves various enzymatic entities that participate in a series of reactions. Prothrombinase activation of hPro is the penultimate step in the coagulation cascade implemented after any event that exposes a procoagulant membrane surface.2 In vivo, the procoagulant membrane surface is usually delivered by activated platelets and/or endothelial cells.3,4 Human factor Xa (hfXa), the enzymatic subunit of prothrombinase, can itself activate hPro after two consecutive cleavages at Arg271 and Arg320 to yield prethrombin-2 (Pre2). Association of the regulatory molecule hfVa with fXa will lead to the establishment of the prothrombinase complex, and a switch of the order of cleavages (Arg320 followed by Arg271) will yield the intermediate meizothrombin (MzT). This complex increases the enzymatic activity of hfXa by 300 000-fold.5−9 It is believed that the regulatory subunit of prothrombinase, hfVa, has a substantial effect on this reversal of cleavages, thus aiding in presenting Arg320 of the substrate to the catalytic subunit of prothrombinase.
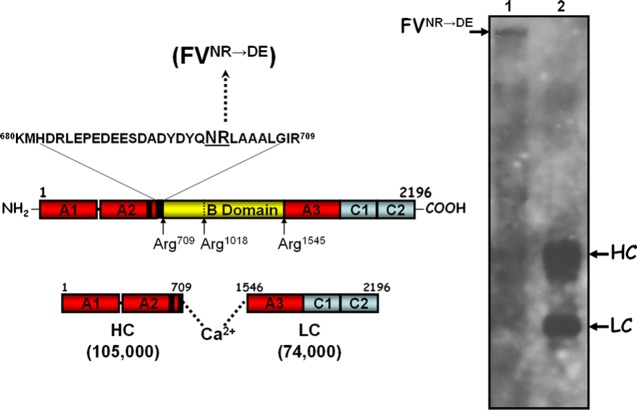
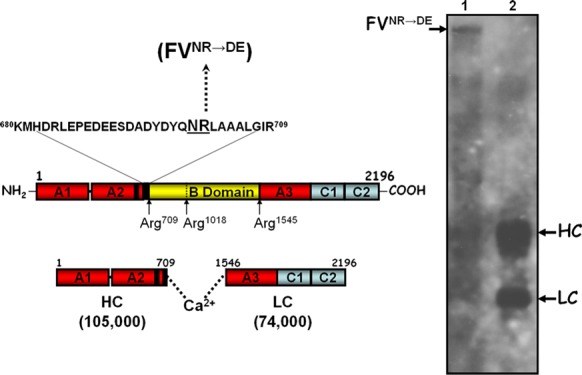
Human factor V (hfV) is present in blood at 20 nM as a single chain protein with a high molecular weight (Mr of 330 000). It is composed of three protein domains (A–C) that will produce a two-subunit protein made of light and heavy chains following cleavage and activation by thrombin at Arg709, Arg1018, and Arg1545 (Figure 1).10−13 The 105 000 heavy chain is bound to the 74 000 light chain through hydrophobic interactions. These hydrophobic interactions are only exposed on the hfV/hfVa molecule following its interaction with calcium ions ( Figure 1).14
Figure 1.
hfV. Left panel, hfV structure; hfV is composed of three A domains (red), two C domains (light blue), and a B region (yellow). hfV is activated following three sequential cleavages by α-thrombin at Arg709, Arg1018, and Arg1545. These cleavages are required to release the active cofactor composed of heavy (amino acids 1–709) and light (amino acids 1546–2196) chains noncovalently associated in the presence of divalent metal ions, and two activation fragments. The carboxyl-terminal portion of the heavy chain contains an acidic hirudin-like amino acid region that is important for cofactor function.26 Adjacent to this region are also the amino acids Asn700 and Arg701, which are important for cofactor activity.33 The amino acid substitutions within the heavy chain are indicated together with the designation for the recombinant mutant hfV molecule created and used throughout the article. Right panel; electrophoretic analyses of rhfVNR→DE and rhfVaNR→DE molecules. rhfVNR→DE was activated with thrombin as described in the Experimental Section and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Following transfer to a poly(vinylidene difluoride) (PVDF) membrane, immunoreactive fragments were detected with the monoclonal antibodies αHFVaHC17 (recognizing an epitope on the heavy chain of the cofactor between amino acid residues 307–506) and αHFVaHC9 (recognizing the light chain). The positions of the heavy/light chains of hfVa are shown on the right. Lane 1, rhfVNR→DE; lane 2, rhfVaNR→DE.
Mature hPro is an inactive zymogen with a Mr of 72 000 that has undergone substantial post-translational modifications before being secreted by the liver. Included in these modifications is the carboxylation of several glutamic acids at the NH2-terminus, a process that is vitamin K-dependent and is essential for proper binding of hPro to the procoagulant surface, which in turn is required for timely and efficient activation by prothrombinase. Thrombin has two charged regions (anion binding exosite I (ABE-I) and anion binding exosite II (ABE-II)), which are vital for its function. ABE-I is responsible for interacting with a plethora of proteins participating in the coagulation cascade like hfV, hfVa, and fibrinogen. ABE-II is the heparin-binding site.15−20
The Kd value of the hfVa–hPro interaction is 1 μM.21,22 hfVa provides binding sites for proexosite 1 and the Gla domain of hPro, explaining one of the possible mechanisms by which the cofactor functions to increase enzyme efficiency.20,23 It has been proven that several acidic amino acids at the very end of the heavy chain of hfVa are vital for hfVa cofactor activity24−30 and participate in the interaction of hPro with prothrombinase. It has been proposed that amino acids from the region 680–709 are required for proper interaction and catalysis of hPro by hfXa within prothrombinase.26,28,31 Further, we have hypothesized that these acidic residues may be involved in the direct binding of hfVa with hPro and/or thrombin through positively charged amino acids. This substantial increase in enzymatic activity resulting in rapid thrombin generation is credited to the precise and unique interactions of the cofactor with specific amino acids affiliated with both membrane-bound hfXa and membrane-bound hPro, as recently demonstrated.32 Accordingly, introduction of the nonenzymatic cofactor into prothrombinase equips the organism with the coagulation artillery necessary for the explosive arrest of vasculature bleeding.
A substantial difference was demonstrated when bovine factor Va (bfVa) or hfVa were used to activate prethrombin-1 (Pre1) by prothrombinase.33 The reason for the different effect of the cofactor on Pre1 was shown to be confined within the very last portion of the COOH-terminus of the hfVa heavy chain. A prominent difference in amino acid between the two cofactor molecules in that region is restricted to positions 700–701 where an Asn–Arg dipeptide in hfVa is replaced by the Asp–Glu sequence in bfVa,34−36 resulting in a total replacement of the net positive charge with two negative charges. Interestingly, very recently modeling data have shown that Arg701 of hfVa interacts with Glu345 of hPro.32 The work undertaken herein proposes to evaluate the significance of the 700Asn–Arg701 dipeptide of the hfVa heavy chain for enzyme–substrate recognition/interaction during hPro activation by prothrombinase. A substantial amino acid change, and a change in charge, in this portion of the cofactor will allow for an in-depth study of this very important contributor of prothrombinase. We directly tested the function of this portion of the heavy chain by using a recombinant molecule and several specific assays. The remarkable and unpredicted results presented herein solidify the notion that the acidic carboxyl-terminus of the heavy chain of hfVa is critical for proper prothrombinase function and dictates the timely interaction of hFVa with the substrate/product, as recently suggested by modeling studies of prothrombinase.32
Results and Discussion
Transient Expression and Analysis of rhfVNR→DE
To ascertain the significance of the amino acid region 700–701 of hfVa, we made a recombinant mutant factor V molecule (rhfV) with the substitution NR→DE (Figure 1, left panel). Figure 1, right panel, shows a typical quality control practice. The data show the makeup of rhfVNR→DE before (right panel, lane 1) and after (right panel, lane 2) activation by thrombin. After treatment with thrombin, no rhfVNR→DE was apparent on the gel, whereas fragments with the molecular weights of the two subunits were visible (a fragment of Mr 105 000 representing the heavy chain and a fragment of Mr 74 000 representing the light chain, both identified with monoclonal antibodies specifically made against each subunit). Clotting activity assays illustrated the fact that activation of rhfVWT gave rise to a molecule with similar activity to that of the plasma-derived molecule (Table 1).26,36 In contrast, using analogous experimental conditions, rhfVaNR→DE had ∼17-fold less clotting activity (Table 1). These results are unexpected, and they show that amino acids 700–701 are essential for expression of rhfVa clotting activity.
Table 1. Cofactor Properties of rhfVa Molecules.
| rhfVa species | clotting activity (U/mg) | decrease (-fold) | KDapp (nM) | Km (μM) | kcat (min–1)a | kcat/Km (M–1·s–1) × 108 | decrease (-fold) |
|---|---|---|---|---|---|---|---|
| rhfVaWT | 3631 ± 237 | 0.48 ± 0.10 | 0.207 ± 0.01 | 2688 ± 47 | 2.1 | ||
| rhfVaNR→DE | 210 ± 35 | 17.3 | 0.75 ± 0.30 | 0.214 ± 0.03 | 2641 ± 87 | 2.0 | 1.05 |
kcat = Vmax/[enzyme] (in the presence of fVa); the enzyme concentration of prothrombinase was 10 pM for all experiments.
Characterization of rhfVa Molecules through Kinetic Experiments
The kinetic effect that hfVa has on the prothrombinase-mediated activation of hPro has been well-studied over the past 50 years, but it is still not properly understood, and no specific molecular role has yet been assigned to the cofactor. Research with discontinuous assays using a chromogenic substrate for thrombin revealed that when hfVa is combined into the prothrombinase complex, the overall increase in activity of the enzymatic complex for the activation of hPro, compared to cleavage and activation of hPro by fXa alone, is substantially increased, making the two-subunit enzyme one of the most proficient catalysts known in the human body and similar to several other enzymes required for survival such as superoxide dismutase, catalase, and carbonic anhydrase. This significant increase in affinity of prothrombinase toward its substrate is attributed to tighter binding of the enzyme complex to hPro because of its localization on the membrane surface by hfVa. Thus, hfVa enhances thrombin generation by facilitating initial cleavage at Arg320 on hPro by hfXa that provides for the generation of an important enzymatic intermediate, MzT, with demonstrated anticoagulant activity.
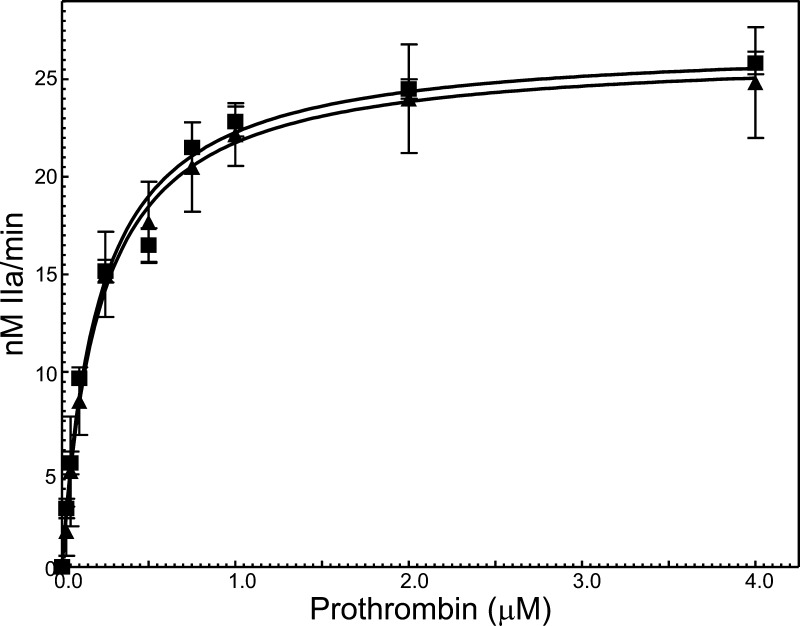
We next evaluated the ability of rhfVaNR→DE to bind rhfXa and form prothrombinase (Table 1). The data show that rhfVaWT has similar affinity for fXa as its plasma counterpart.26,36 Similarly, rhfVaNR→DE has comparable affinity for plasma-derived fXa, which is approximately the same as the affinity of rhfVaWT for fXa. These data demonstrate that residues 700–701 from the heavy chain of fVa, while being a major contributor for optimal expression of fVa clotting activity, do not contribute to the interaction between the cofactor and fXa. We subsequently assessed the consequence of the mutation on the Km and kcat of the enzyme. The resulting kinetic graphs are shown in Figure 2, and the constants of the enzyme made with either rhfVaWT or rhfVaNR→DE are provided in Table 1. The results surprisingly demonstrate that the amino acid substitutions had no substantial effect on either the Km of the reaction or on the catalytic efficiency of prothrombinase made with rhfVaNR→DE (Figure 2, Table 1). Compared to the data using the clotting assays, these unexpected results are somehow puzzling, and thus far demonstrate that the NR→DE substitution only affects the clotting activity of rhfVaNR→DE. The kinetic data alone do not provide an explanation for the impaired clotting activity of rhfVaNR→DE.
Figure 2.
Raw data used for the determination of the kinetic parameters of prothrombinase complex assembly and function. Initial rates of thrombin generation were determined as described in the Experimental Section. The data obtained were fit by nonlinear regression analysis to the Michaelis–Menten equation to obtain the Km and Vmax. Prothrombinase assembled with rhfVaWT is shown by filled squares (R2 = 0.98, three titrations with two different preparations of rhfVaWT) whereas prothrombinase assembled with rhfVaNR→DE is depicted by filled triangles (R2 = 0.96, three titrations with three different preparations of rhfVaNR→DE). The values of the Km and Vmax/ET (=kcat) extracted directly from these graphs are listed in Table 1.
Visualization of the Activation Pathway
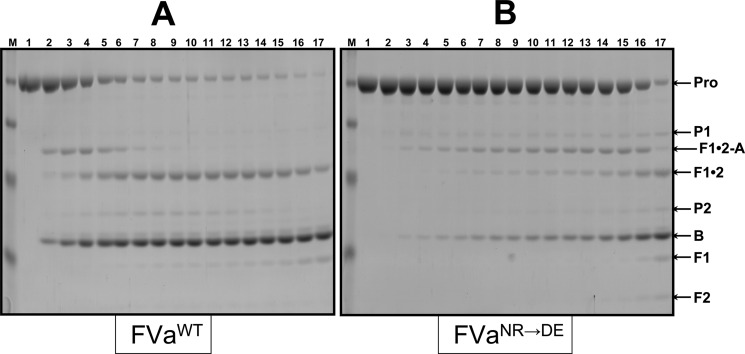
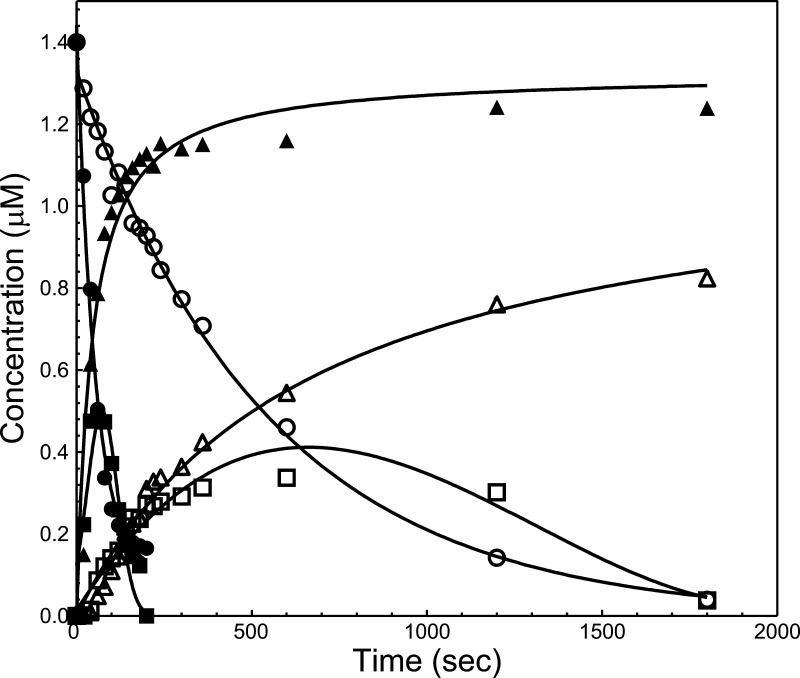
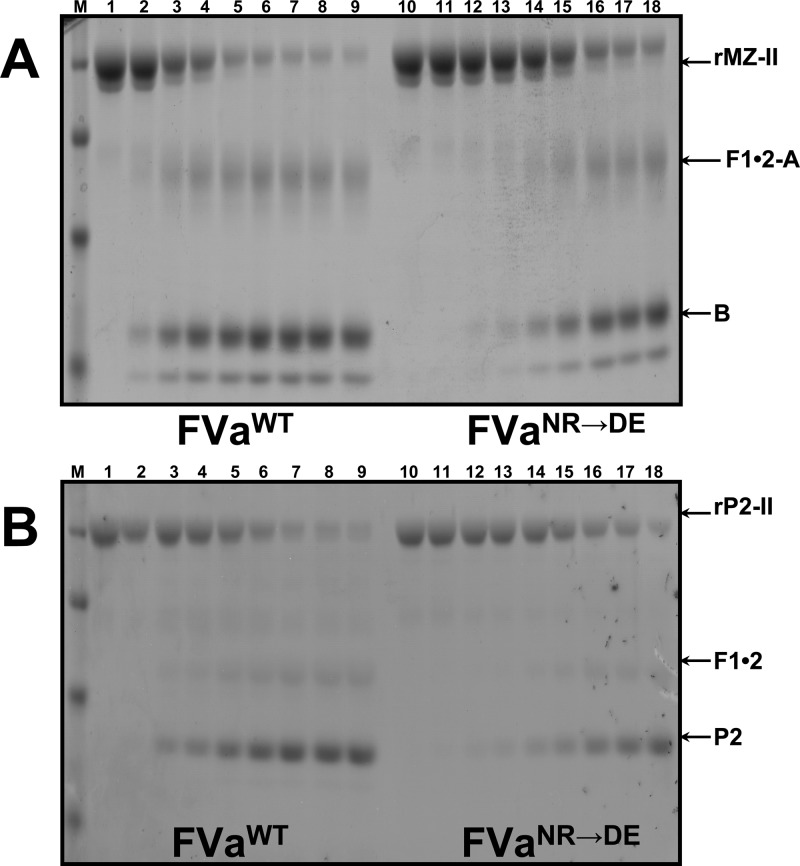
Because of the unexpected and contradictory results obtained in the activity assays (clotting vs kinetic assay), and to better appreciate the reason for the dearth in clotting activity of rhfVaNR→DE, we evaluated hPro activation by SDS-PAGE. The results display a considerable and significant delay in hPro activation by prothrombinase made with rhfVaNR→DE when related to cleavage of hPro by prothrombinase reconstituted with rhfVaWT with MzT lingering very late in the reaction and with little visible thrombin formation (Figure 3). Densitometry scanning of the SDS-PAGE results presented in Figure 3 established a 10-fold delay in hPro cleavage by prothrombinase made with rhfVaNR→DE when matched to the consumption of hPro with prothrombinase assembled with rhfVaWT (Figure 4, Table 2). Furthermore, it is clearly visible on the gels that when hPro is cleaved by prothrombinase reconstituted with rhfVaNR→DE, there is lingering of MzT during the activation time course. A peak for MzT is noticeable at 60 s when hPro is cleaved by prothrombinase made with rhfVaWT, and a peak for MzT is present at ∼600 s when hPro is processed by prothrombinase made with rhfVaNR→DE (Figure 4). These data can explain the paradoxical findings above and the apparent discrepancy between the clotting and kinetic assays, and they undeniably suggest that the slow accumulation of MzT is responsible for the poor clotting activity observed with rhfVaNR→DE. These data also verify our previous findings,26 and demonstrate that MzT can counterbalance the dearth of thrombin activity in the chromogenic test because its catalytic activity toward the chromogenic substrate used in our study is much higher than that of thrombin, as previously demonstrated.26,37,38
Figure 3.
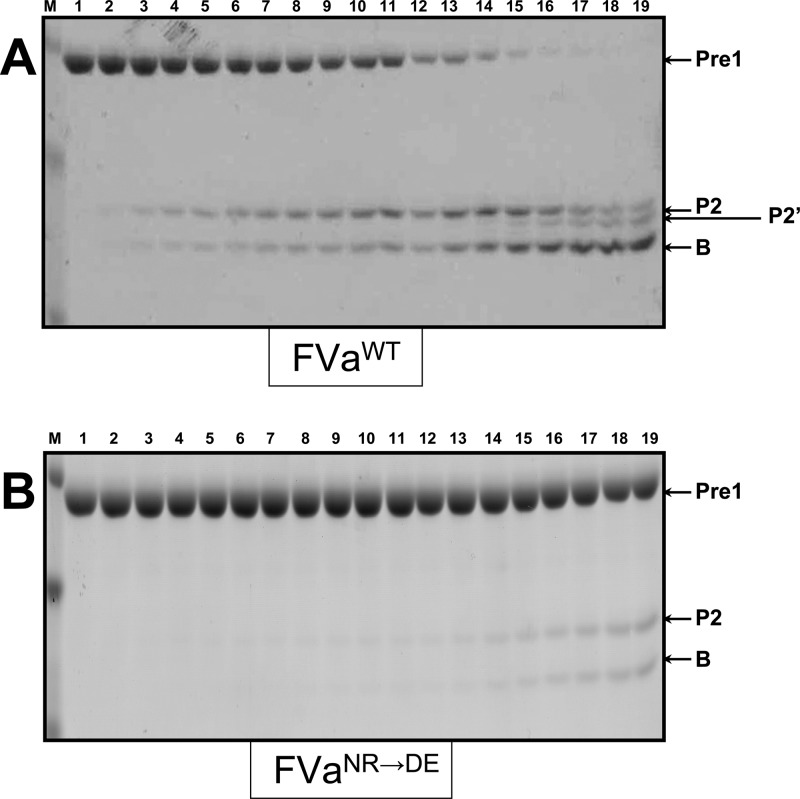
Analysis of the activation of plasma-derived hPro by prothrombinase. Plasma-derived hPro (1.4 μM) was incubated in different mixtures with PCPS vesicles (20 μM), and prothrombinase was assembled with either wild-type rhfVa (panel A, 20 nM) or rhfVaNR→DE (panel B, 20 nM), as described in the Experimental Section. At selected time intervals, aliquots of the reactions were withdrawn and treated as described in the Experimental Section. M represents the lane with the molecular weight markers (from top to bottom): Mr 98 000, Mr 64 000, Mr 50 000, Mr 36 000, Mr 22 000. Lanes 1–17 represent samples from the reaction mixture before (0 min) the addition of fXa and 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 240 s, 5, 6, 10, and 20 min, respectively, following the addition of fXa. The hPro-derived fragments are shown as follows: Pro (hPro, amino acid residues 1–579); P1 (prethrombin-1, amino acid residues 156–579); F1•2-A (fragment 1•2-A chain, amino acid residues 1–320); F1•2 (fragment 1•2, amino acid residues 1–271); P2 (prethrombin-2, amino acid residues 272–579); B (B chain of α-thrombin, amino acid residues 321–579). F1 (fragment 1, amino acid residues 1−155); F2 (fragment 2, amino acid residues 156−271).
Figure 4.
Reaction profiles for the activation of hPro by prothrombinase. Progress curves for products and reactants for the activation of hPro by prothrombinase assembled with rhfVaWT (filled symbols) or rhfVaNR→DE (open symbols) were obtained by quantitative densitometry of the gels shown in Figure 3A,B, as described in the Experimental Section. The graphs illustrate the disappearance of hPro (circles), the transient formation of MzT (squares), and the accumulation of the B chain of α-thrombin (triangles). The lines for the disappearance of hPro were drawn according to the equation of a one phase exponential decay (rhfVaWT, R2 = 0.99, and rhfVaNR→DE, R2 = 0.99). The lines depicting the formation of MzT and the accumulation of the B chain of α-thrombin were arbitrarily drawn. Additional data points extending to 1 h of incubation have been omitted for clarity.
Table 2. Rate of Various Substrate Cleavage by Prothrombinase.
| substrate | prothrombinase assembled with rhfVaWT (moles consumed·s–1·(mole factor Xa)−1) | prothrombinase assembled with rhfVaNR→DE (moles consumed·s–1·(mole factor Xa)−1) |
|---|---|---|
| plasma-derived prothrombin | 26.7 ± 2.5 (0.99)a | 2.6 ± 0.17 (0.99)a |
| rMZ-II | 11.8 ± 0.9 (0.99) | 1.7 ± 0.2 (0.99) |
| rP2-II | 3.0 ± 1.5 (0.86) | 1.5 ± 0.4 (0.97) |
| FPR-meizothrombin | 56.8 ± 0.8 (0.99) | 19.4 ± 1.7 (0.99) |
| prethrombin-1 | 46.7 ± 3.3 (0.99) | 12.1 ± 12 (0.77) |
The numbers in parentheses represent the goodness of fit for the fitting of the data to a first-order exponential decay.
Our data clearly show that although prothrombinase assembled with rhfVaNR→DE has similar Km and kcat as prothrombinase assembled with rhfVaWT, the clotting activity of rhfVNR→DE is severely impaired. In addition, visualization of the cleavage pattern of hPro activation by prothrombinase assembled with rhfVaNR→DE demonstrated a significantly different pattern of activation from the gels analyzing hPro activation by prothrombinase assembled with rhfVaWT. Thus, if we had limited our initial analysis of the mutant molecule to prothrombinase assays using purified proteins and a chromogenic substrate without using clotting assays and gel electrophoresis analysis, as is the case in the majority of the studies assessing mutations in the hfVa molecule, we would have missed and dismissed the particular critical regulatory function of this region of the molecule, as was the case on multiple occasions in the past.39
Activation of Recombinant Mutant hPro, FPR-MzT, and Pre1 by Prothrombinase Assembled with rhfVaNR→DE
The data obtained thus far show that both cleavages at Arg320 and Arg271 in hPro appeared to be impaired when using prothrombinase made with rhFVaNR→DE. To quantify the level of impairment of each of the cleavages separately in hPro that were specifically affected by the 700NR→DE701 substitution, we employed recombinant hPro molecules with only one site specific for prothrombinase cleavage (Arg320 for rMZ-II and Arg271 for rP2-II).40
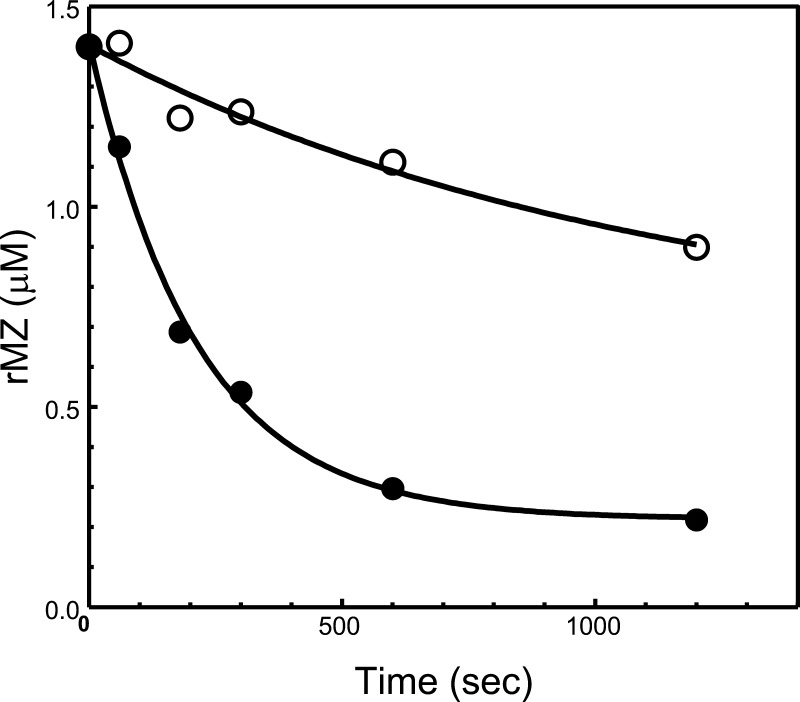
The results provided in Figure 5A illustrate a substantial delay in the rate of cleavage of rMZ-II by prothrombinase reconstituted with rhfVaNR→DE (lanes 10–18) when compared to the rate of activation of rMZ-II by prothrombinase composed with rhfVaWT (lanes 1–9). Scanning densitometry showed that activation of rMZ-II is slower by ∼10-fold when prothrombinase is made with rhfVaNR→DE compared to activation of rMZ-II by prothrombinase composed with rhVaWT (Table 2, Figure 6). A 2-fold slower hPro rate of activation was also detected when rP2-II was cleaved by prothrombinase reconstituted with rhfVaNR→DE when matched to the activation of rP2-II by prothrombinase made with rhfVaWT (Figure 5B, Table 2). These results support our data acquired with hPro. Altogether, the data suggest that prothrombinase-mediated cleavages at Arg320/Arg271 in hPro are significantly delayed when prothrombinase is made with rhfVaNR→DE. It is also clear, however, that cleavage at Arg320 is more affected by the substitution in hfVa than cleavage at Arg271.
Figure 5.
Analysis of the activation of rMZ-II and rP2-II. rMZ-II (1.4 μM, panel A) and rP2-II (1.4 μM, panel B) were incubated in different mixtures with PCPS vesicles (20 μM), DAPA (3 μM), and rhfVaWT (20 nM) or rhfVaNR→DE (20 nM). The reaction was started by the addition of fXa, and the samples were treated as detailed in the Experimental Section. Lanes 1–9 represent samples of the reaction mixture following incubation of prothrombinase assembled with rhfVaWT with rMZ-II or rP2-II before (lane 1) or following 1, 3, 5, 10, 20, 45, 60, and 120 min of incubation with fXa, respectively. Lanes 10–18 represent samples of the reaction mixture following incubation of prothrombinase assembled with rhfVaNR→DE with rMZ-II or rP2-II before (lane 10) or following 1, 3, 5, 10, 20, 45, 60, and 120 min of incubation with fXa, respectively. Positions of hPro-derived fragments are indicated to the right, as detailed in the legend of Figure 3. For easy reading of the article, the rhfVa species used for the reconstitution of prothrombinase are also shown.
Figure 6.
Analysis of rMZ-II consumption by prothrombinase assembled with rhfVa molecules. The gel shown in Figure 5 (A) was scanned and hPro consumption was recorded as described in the Experimental Section. Following scanning densitometry, the data representing recombinant mutant hPro consumption as a function of time (s) were plotted using nonlinear regression analysis according to the equation representing a first-order exponential decay using the software Prizm (GraphPad, San Diego, CA), as described in the Experimental Section. Prothrombinase was assembled with rhfVaWT (filled circles) or rhfVaNR→DE (open circles). The apparent first-order rate constant, k (s–1) was obtained directly from the fitted data, and the resulting numbers representing recombinant mutant hPro consumption are reported in Table 2.
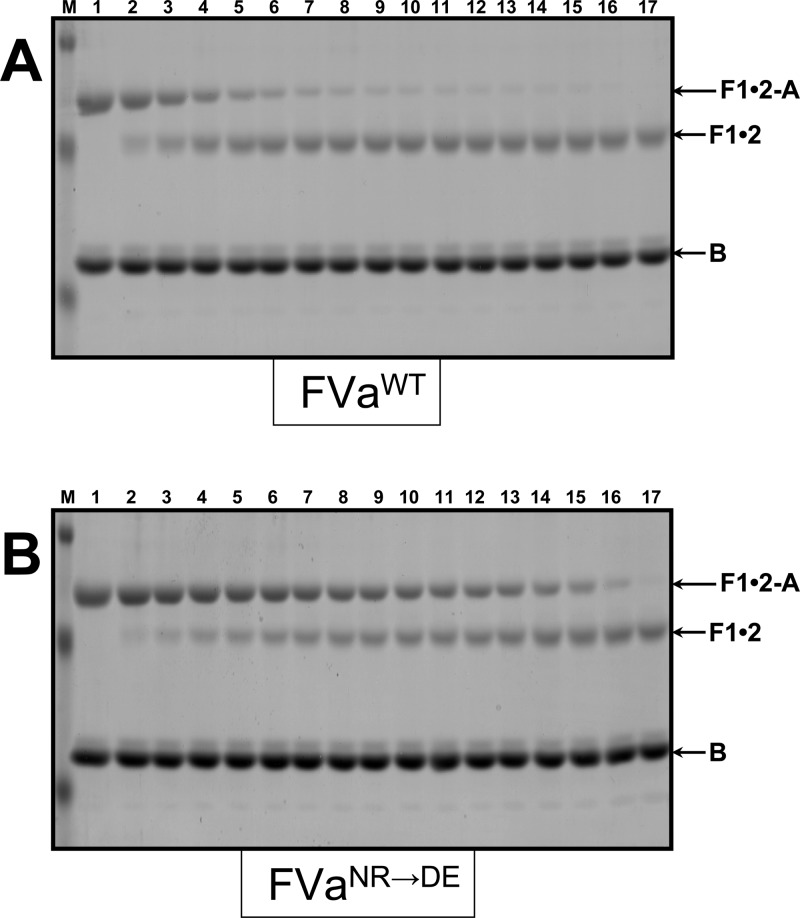
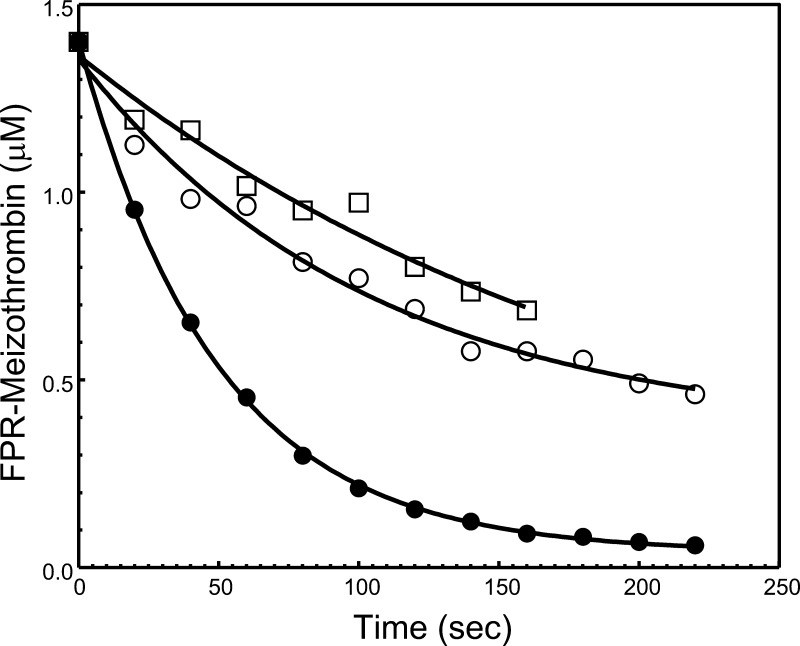
Examination of the results acquired up until now with plasma-derived and recombinant hPro demonstrates that the rate of activation of hPro or rMZ-II following cleavage at Arg320 is more sensitive to the mutation in rhfVa than the rate of activation of hPro or rP2-II following cleavage at Arg271. To understand the effect of the 700NR701→DE mutations on the cleavage of hPro at Arg271 alone after the conformational alteration occurring in hPro following cleavage at Arg320,41 we assessed the change in the rate of cleavage of FPR-meizothrombin (FPR-MzT) by prothrombinase reconstituted with rhfVaWT or rhfVaNR→DE (Figure 7). The results demonstrate a considerable delay for cleavage of FPR-MzT at Arg271 by prothrombinase made with rhfVaNR→DE (panel B) when related to the reaction that is catalyzed by prothrombinase reconstituted with rhfVaWT (panel A). Densitometry scanning analysis of the concentration of fragment 1•2-A confirmed an ∼3-fold delay in cleavage of FPR-MzT at Arg271 by prothrombinase made with rhfVaNR→DE (Figure 8, Table 2). However, it is worth noting that the maximum effect on the rate of cleavage at Arg271 of MzT credited to the binding of hfVa with hfXa on a cell/membrane surface is 4-fold.8,9 Thus, the 700NR701→DE mutations in the fVa heavy chain also considerably impede acceleration for cleavage at this site by prothrombinase.
Figure 7.
Gel electrophoresis analyses for cleavage of FPR-MzT. FPR-MzT (1.4 μM) was incubated in different mixtures with PCPS vesicles (20 μM) and rhfVa as described in the legend to Figure 3. The reactions were started by the addition of fXa, and the samples were further treated, scanned, and quantified as detailed in the Experimental Section. Panel A, prothrombinase assembled with rhfVaWT; panel B, prothrombinase assembled with rhfVaNR→DE; M represents the lane with the molecular weight markers (from top to bottom): Mr 50 000, Mr 36 000, Mr 22 000. Lanes 1–17 represent samples from the reaction mixture before (0 min) the addition of fXa and 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, and 240 s, 5, 6, 10, and 20 min, respectively, following the addition of fXa. The hPro-derived fragments are shown as detailed in the legend to Figure 3. The recombinant rhfVa species used for the reconstitution of prothrombinase is also shown under each panel.
Figure 8.
Analysis of FPR-MzT consumption by prothrombinase assembled with rhfVa molecules. The gels shown in Figure 7 were scanned and FPR-MzT consumption was recorded as described in the Experimental Section. Following scanning densitometry, the data representing FPR-MzT consumption as a function of time (s) were plotted using nonlinear regression analysis according to the equation representing a first-order exponential decay using the software Prizm (GraphPad, San Diego, CA), as described in the Experimental Section. The apparent first-order rate constant k (s–1) was obtained directly from the fitted data. Prothrombinase was assembled with rhfVaWT (filled circles) or rhfVaNR→DE (open circles); factor fXa alone cleavage of FPR-MzT is shown by the open squares. The resulting numbers representing FPR-MzT consumption are reported in Table 2.
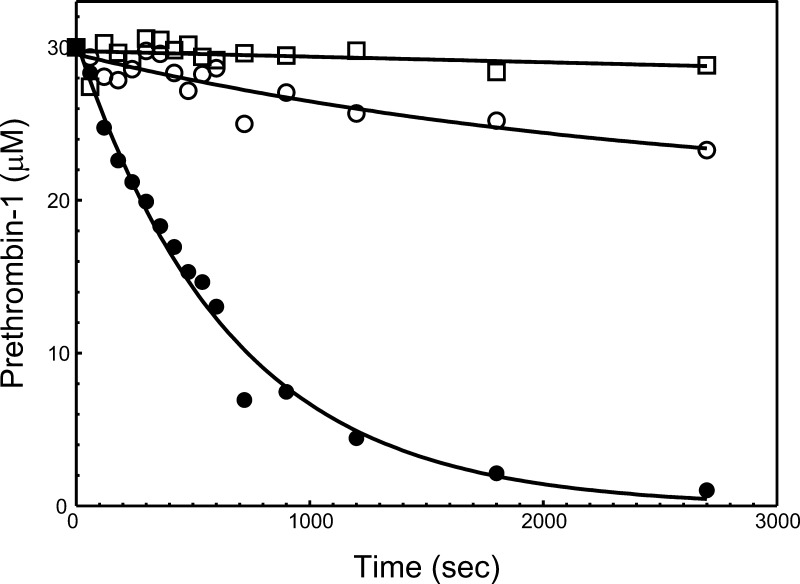
To determine the effect of the 700NR701→DE substitutions within the factor Va heavy chain on the cleavage of hPro at Arg320 alone when the substrate is not associated with the membrane surface, we compared the rates of activation of Pre1 by prothrombinase made with rhfVaWT and rhfVaNR→DE (Figure 9). Under the conditions used, the results show a substantial delay for activation of Pre1 following cleavage at Arg320 by prothrombinase made with rhfVaNR→DE (panel B) when related to the reaction catalyzed by prothrombinase reconstituted with hfVaWT (panel A). Scanning of Pre1 from the gels depicted in Figure 9 confirmed an ∼4-fold delay in activation of Pre1 at Arg320 by prothrombinase made with rhfVaNR→DE (Figure 10, Table 2). As a consequence, the 700NR701→DE mutations in the rhfVa heavy chain substantially impair activation of Pre1 by prothrombinase because of an impaired capability for cleavage at Arg320 even when the substrate (Pre1) is not associated with a membrane surface.
Figure 9.
Gel electrophoresis analyses for cleavage of Prethrombin-1. Prethrombin-1 was incubated in different mixtures with PCPS vesicles and rhfVa as described previously in detail.20 The reaction and the samples were further treated, scanned, and quantified as detailed in the Experimental Section. Panel A, control, rhfVaWT; panel B, rhfVaNR→DE. M represents the lane with the molecular weight markers (from top to bottom): Mr 50 000, Mr 36 000, Mr 22 000. Lanes 1–19 represent samples from the reaction mixture before and after the addition of fXa as previously described.20 The hPro-derived fragments are shown as detailed in the legend to Figure 3. The fragment denoted as P2′ depicts Pre2 cleaved at Arg284. For easy reading of the article, the rhfVa species used for the reconstitution of prothrombinase is also shown under each panel.
Figure 10.
Analysis of Prethrombin-1 consumption by prothrombinase assembled with rhfVa molecules. The gels shown in Figure 9 were scanned and Pre1 consumption was recorded as described in the Experimental Section. Following scanning densitometry, the data representing Pre1 consumption as a function of time (s) were plotted using nonlinear regression analysis according to the equation representing a first-order exponential decay using the software Prizm (GraphPad, San Diego, CA), as described in the Experimental Section. The apparent first-order rate constant k (s–1) was obtained directly from the fitted data. Prothrombinase was assembled with rhfVaWT (filled circles), rhfVaNR→DE (open circles); cleavage by fXa alone is shown by open squares. The resulting numbers representing Pre1 consumption are reported in Table 2.
Thus, because we have studied the consequence of the mutations on the clotting activity and on the rate of each cleavage individually, by analyzing activation of rMZ-II, rP2-II, FPR-MzT, and Pre1 by SDS-PAGE using prothrombinase made with rhfVaNR→DE, we can determine that both cleavages at Arg320 and Arg271 are impaired, resulting in MzT being present through the hPro activation process. In addition, a substantial increase in the concentration of MzT in our assays (as shown in Figure 4) can explain the initial inconsistent findings. The additional MzT molecules present throughout the time course, while lacking clotting activity, can compensate for the lack of thrombin activity in the assays using purified proteins because the molecule has augmented amidolytic activity toward chromogenic substrates that are, in general, used to evaluate thrombin activity, thus generating the wrong conclusion that the NR→DE substitution has negligible or no effect on prothrombinase activity.
The hypothesis that fVa confines and places hPro in an optimum position for efficient catalysis by fXa was confirmed by computational studies with prothrombinase by Shim et al.,32 who demonstrated that the acidic end of the heavy chain of hfVa is essential in its ability to capture the serine protease domain of hPro and reposition the Arg320 cleavage site at an optimal position for well-timed cleavage by fXa and hPro activation.42 These productive interactions between the acidic amino acids from the carboxyl-terminal portion of the hfVa heavy chain and hPro have been repeatedly suggested following experiments with synthetic peptides and recombinant hfVa molecules.26−28 In particular, Shim et al. proposed a direct interaction between Arg701 of fVa and Gln345 of hPro, the latter being only 24 amino acids away from the crucial activating hPro cleavage site at Arg320.32 They also proposed a salt bridge between Asp695 of hfVa and Lys340 of hPro, and between Tyr698 of fVa and Lys474 of hPro.32 We show that replacement of Arg701 in hfVa by a Glu, and the subsequent loss of the positive charge, results in a considerable delay in activating hPro following cleavage at Arg320. We have also shown repeatedly that Asp695 and Tyr698 are a part of a peptide portion of the hfVa heavy chain that represents a control switch for the activity of prothrombinase.27,28 Overall our experimental data together with the recent computational model of hfVa clearly establish a prolific interaction between the acidic carboxyl-terminus of the hfVa heavy chain and several residues adjacent or nearby to the crucial cleavage site at Arg320 of hPro for timely thrombin production.
Conclusions
The specific amino acid sequence 700–701 from hfVa is not strictly conserved among species36 (indicated by the vertical arrow in Figure 11). Although hfVa has the identical sequence as higher primates and fVa from horse, all other species shown in Figure 11 have different amino acids at these two positions, suggesting an important role of these two residues within fVa. Although there is no effect of the 700NR701→DE mutation in hfVa on the direct binding of hfVa to hfXa, there is a significant effect of the mutation on hPro activation by prothrombinase reconstituted with the mutated cofactor molecule, which is translated by hindered cleavage at both Arg320/Arg271. Consequently, our results strongly suggest that these amino acids are part of an important sequence that is accountable for the recognition and interaction of the substrate (Pro) with prothrombinase within different species. Furthermore, the data presented herein support the notion that Arg701 of the hfVa heavy chain makes a salt bridge with Glu345 of hPro, thus facilitating and promoting initial cleavage of hPro at Arg320, which was suggested by a recent computational model of prothrombinase.32 Collectively, the results strongly suggest that hfVa, the regulatory subunit of prothrombinase, undeniably controls the activity of hfXa within the enzymatic complex by directing the enzymatic subunit toward cleavage at Arg320, and thus actively participates in the cleavage and activation of hPro by prothrombinase. We must also conclude that all assays used herein to characterize rhfVaNR→DE are not redundant, but rather they are complimentary and a procedural requirement to understand the structural intricacies of the cofactor and its contributions to the activity of prothrombinase.
Figure 11.
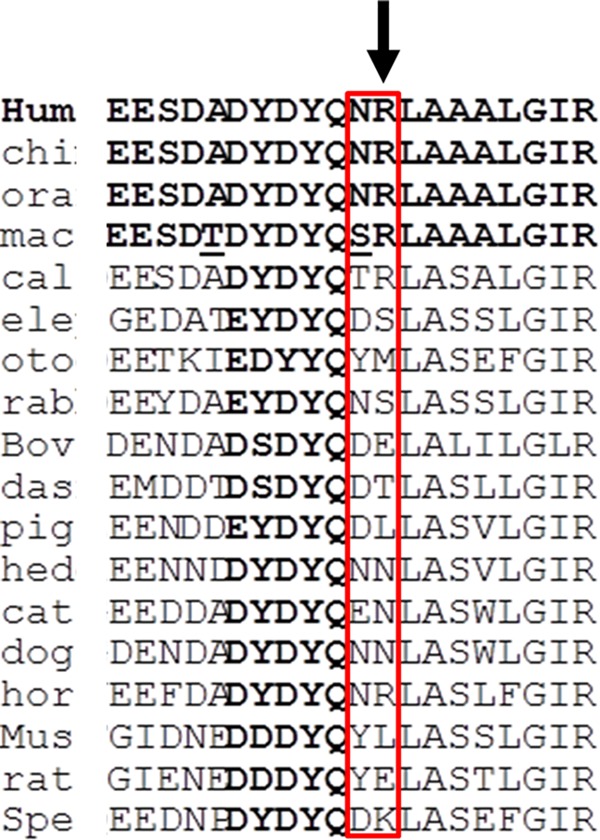
Comparison of the last 20 amino acids from the acidic carboxyl-terminal portion of the fVa heavy chain. The acidic sequence from 20 species is illustrated as adapted from ref (36). The special nomenclature of all species was previously described.36
Experimental Section
Materials and Reagents
Human fV cDNA was obtained from American Type Tissue Collection (ATCC# 40515 pMT2-V, Manassas, VA). The origin of all other chemicals, reagents, and proteins used by our laboratory is provided elsewhere.36 Recombinant hPro rMZ-II with only one site for hfXa (i.e., Arg320) and hPro rP2-II with only one site for hfXa (i.e., Arg271) were obtained as detailed previously.40
Mutagenesis and Transient Expression of Recombinant FV Molecules
A mutant hfV molecule mutated at the COOH-terminus of the heavy chain was produced using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), and was constructed as previously detailed26 with the mutagenic primers fVNR→DE mutant: 5′-GATGCTGACTATGATTACCAGGACGAACTGGCTGCAGCATT-3′ (sense) and 5′-GATTCCTAATGCTGCAGCCAGTTCGTCCTGGTAATCATAGT-3′ (antisense).
Expression of Recombinant Wild-Type and Mutant FV in Mammalian Cells
Expression in COS-7L cells was performed as previously detailed,26 and the concentration of recombinant proteins was obtained by enzyme-linked immunosorbent assay as previously shown.43 The activity of the recombinant molecules was verified by clotting assays using fV-deficient plasma.36
Gel Electrophoresis and Western Blotting
SDS-PAGE analyses were performed using the method of Laemmli.44 Western blotting using poly(vinylidene difluoride) (PVDF) membranes was performed according to Towbin et al.45 Following transfer to PVDF, fV heavy and light chain(s) were identified using the suitable monoclonal and polyclonal antibodies46−49 and chemiluminescence.
Analysis of hPro Activation by Gel Electrophoresis
hPro molecules (1.4 μM) were incubated with PCPS vesicles (20 μM), DAPA (50 μM), and fVa, and analysis was performed as detailed elsewhere.26,28,36
Measurement of Rates of hPro Activation
All rhfVa molecules were activated with human thrombin and tested as previously detailed using a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA).31,50
Scanning Densitometry of SDS-PAGE and Calculation of the Rate of hPro Consumption
Scanning densitometry of the gels was performed as described in detail elsewhere.50
Acknowledgments
This work was supported by funds from the Center for Gene Regulation in Health and Disease (GRHD) at Cleveland State University (to M.K.), and funds from the Division of Research and Graduate Studies at Cleveland State University (to M.K.). We thank Dr. Ken Mann and Dr. Tom Orfeo from the University of Vermont for the monoclonal antibodies to factor V.
Glossary
Abbreviations
- PS
l-α-phosphatidylserine
- PC
l-α-phosphatidylcholine
- PCPS
small unilamellar phospholipids vesicles composed of 75% PC and 25% PS (w/w)
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- rhfVWT
wild-type recombinant human fV
- rhfVaWT
wild-type recombinant human fV activated with thrombin
- rhfVNR→DE
recombinant human fV with the mutations 700NR701→DE
- rhfVaNR→DE
recombinant human fV with the mutations 700NR701→DE activated with thrombin
- hPro
human prothrombin
- hfXa
human factor Xa
- MzT
meizothrombin
- Pre1
prethrombin-1
- FPR
Phe-Pro-Arg-Chloromethylketone
Author Contributions
J.H. designed, performed, and analyzed the experiments and participated in the writing of the paper. M.K. conceived and coordinated the study, designed the experiments, and wrote the paper. Both authors reviewed the results and approved the final version of the manuscript.
The authors declare no competing financial interest.
References
- Kalafatis M.; Egan J. O.; van’t Veer C.; Cawthern K. M.; Mann K. G. The regulation of clotting factors. Crit. Rev. Eukaryotic Gene Expression 1997, 7, 241–280. 10.1615/critreveukargeneexpr.v7.i3.40. [DOI] [PubMed] [Google Scholar]
- Mann K. G.; Kalafatis M. Factor V: a combination of Dr Jekyll and Mr Hyde. Blood 2003, 101, 20–30. 10.1182/blood-2002-01-0290. [DOI] [PubMed] [Google Scholar]
- Tracy P. B.; Nesheim M. E.; Mann K. G. Coordinate binding of factor Va and factor Xa to the unstimulated platelet. J. Biol. Chem. 1981, 256, 743–751. [PubMed] [Google Scholar]
- Davie E. W.; Fujikawa K.; Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E.; Taswell J. B.; Mann K. G. The contribution of bovine factor V and factor Va to the activity of prothrombinase. J. Biol. Chem. 1979, 254, 10952–10962. [PubMed] [Google Scholar]
- Heldebrant C. M.; Mann K. G. The activation of prothrombin. I. Isolation and preliminary characterization of intermediates. J. Biol. Chem. 1973, 248, 3642–3652. [PubMed] [Google Scholar]
- Esmon C. T.; Jackson C. M. The conversion of prothrombin to thrombin. III. The factor Xa-catalyzed activation of prothrombin. J. Biol. Chem. 1974, 249, 7782–7790. [PubMed] [Google Scholar]
- Krishnaswamy S.; Church W. R.; Nesheim M. E.; Mann K. G. Activation of human prothrombin by human prothrombinase. Influence of factor Va on the reaction mechanism. J. Biol. Chem. 1987, 262, 3291–3299. [PubMed] [Google Scholar]
- Nesheim M. E.; Mann K. G. The kinetics and cofactor dependence of the two cleavages involved in prothrombin activation. J. Biol. Chem. 1983, 258, 5386–5391. [PubMed] [Google Scholar]
- Esmon C. T. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J. Biol. Chem. 1979, 254, 964–973. [PubMed] [Google Scholar]
- Nesheim M. E.; Foster W. B.; Hewick R.; Mann K. G. Characterization of Factor V activation intermediates. J. Biol. Chem. 1984, 259, 3187–3196. [PubMed] [Google Scholar]
- Kane W. H.; Majerus P. W. Purification and characterization of human coagulation factor V. J. Biol. Chem. 1981, 256, 1002–1007. [PubMed] [Google Scholar]
- Kalafatis M. Coagulation factor V: a plethora of anticoagulant molecules. Curr. Opin. Hematol. 2005, 12, 141–148. 10.1097/01.moh.0000155016.30296.90. [DOI] [PubMed] [Google Scholar]
- Adams T. E.; Hockin M. F.; Mann K. G.; Everse S. J. The crystal structure of Activated Protein C-inactivated bovine factor Va: Implications for cofactor function. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 8918–8923. 10.1073/pnas.0403072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. J.; Bock P. E. Role of prothrombin fragment 1 in the pathway of regulatory exosite I formation during conversion of human prothrombin to thrombin. J. Biol. Chem. 2003, 278, 44489–44495. 10.1074/jbc.M306916200. [DOI] [PubMed] [Google Scholar]
- Anderson P. J.; Nesset A.; Dharmawardana K. R.; Bock P. E. Role of proexosite I in factor Va-dependent substrate interactions of prothrombin activation. J. Biol. Chem. 2000, 275, 16435–16442. 10.1074/jbc.M001255200. [DOI] [PubMed] [Google Scholar]
- Hibbard L. S.; Nesheim M. E.; Mann K. G. Progressive development of a thrombin inhibitor binding site. Biochemistry 1982, 21, 2285–2292. 10.1021/bi00539a003. [DOI] [PubMed] [Google Scholar]
- Martin P. D.; Malkowski M. G.; Box J.; Esmon C. T.; Edwards B. F. New insights into the regulation of the blood clotting cascade derived from the X-ray crystal structure of bovine meizothrombin des F1 in complex with PPACK. Structure 1997, 5, 1681–1693. 10.1016/S0969-2126(97)00314-6. [DOI] [PubMed] [Google Scholar]
- Chen L.; Yang L.; Rezaie A. R. Proexosite-1 on prothrombin is a factor Va-dependent recognition site for the prothrombinase complex. J. Biol. Chem. 2003, 278, 27564–27569. 10.1074/jbc.M302707200. [DOI] [PubMed] [Google Scholar]
- Bukys M. A.; Orban T.; Kim P. Y.; Nesheim M. E.; Kalafatis M. The interaction of fragment 1 of prothrombin with the membrane surface is a prerequisite for optimum expression of factor Va cofactor activity within prothrombinase. Thromb. Haemostasis 2008, 99, 511–522. 10.1160/TH07-08-0532. [DOI] [PubMed] [Google Scholar]
- Esmon C. T.; Owen W. G.; Duiguid D. L.; Jackson C. M. The action of thrombin on blood clotting factor V: conversion of factor V to a prothrombin-binding protein. Biochim. Biophys. Acta, Protein Struct. 1973, 310, 289–294. 10.1016/0005-2795(73)90034-2. [DOI] [PubMed] [Google Scholar]
- Luckow E. A.; Lyons D. A.; Ridgeway T. M.; Esmon C. T.; Laue T. M. Interaction of clotting factor V heavy chain with prothrombin and prethrombin 1 and role of activated protein C in regulating this interaction: analysis by analytical ultracentrifugation. Biochemistry 1989, 28, 2348–2354. 10.1021/bi00431a055. [DOI] [PubMed] [Google Scholar]
- Anderson P. J.; Nesset A.; Dharmawardana K. R.; Bock P. E. Characterization of proexosite I on prothrombin. J. Biol. Chem. 2000, 275, 16428–16434. 10.1074/jbc.M001254200. [DOI] [PubMed] [Google Scholar]
- Camire R. M.; Kalafatis M.; Tracy P. B. Proteolysis of factor V by cathepsin G and elastase indicates that cleavage at Arg1545 optimizes cofactor function by facilitating factor Xa binding. Biochemistry 1998, 37, 11896–11906. 10.1021/bi980520v. [DOI] [PubMed] [Google Scholar]
- Gerads I.; Tans G.; Yukelson L. Y.; Zwaal R. F.; Rosing J. Activation of bovine factor V by an activator purified from the venom of Naja naja oxiana. Toxicon 1992, 30, 1065–1079. 10.1016/0041-0101(92)90052-7. [DOI] [PubMed] [Google Scholar]
- Hirbawi J.; Bukys M.; Barhoover M. A.; Erdogan E.; Kalafatis M. Role of the acidic hirudin-like COOH-terminal amino acid region of factor Va heavy chain in the enhanced function of prothrombinase. Biochemistry 2008, 47, 7963–7974. 10.1021/bi800593k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. O.; Bukys M. A.; Singh L. S.; Szabo K. A.; Kalafatis M. The contribution of amino acid region ASP695-TYR698 of factor V to procofactor activation and factor Va function. J. Biol. Chem. 2004, 279, 3084–3095. 10.1074/jbc.M306850200. [DOI] [PubMed] [Google Scholar]
- Bukys M. A.; Kim P. Y.; Nesheim M. E.; Kalafatis M. A control switch for prothrombinase: characterization of a hirudin-like pentapeptide from the COOH terminus of factor Va heavy chain that regulates the rate and pathway for prothrombin activation. J. Biol. Chem. 2006, 281, 39194–39204. 10.1074/jbc.M604482200. [DOI] [PubMed] [Google Scholar]
- Bakker H. M.; Tans G.; Thomassen M. C. L. G. D.; Yukelson L. Y.; Ebberink R.; Hemker H. C.; Rosing J. Functional properties of human factor Va lacking Asp683-Arg709 domain of the heavy chain. J. Biol. Chem. 1994, 269, 20662–20667. [PubMed] [Google Scholar]
- Kalafatis M.; Beck D. O.; Mann K. G. Structural requirements for expression of factor Va activity. J. Biol. Chem. 2003, 278, 33550–33561. 10.1074/jbc.M303153200. [DOI] [PubMed] [Google Scholar]
- Bukys M. A.; Blum M. A.; Kim P. Y.; Brufatto N.; Nesheim M. E.; Kalafatis M. Incorporation of factor Va into prothrombinase is required for coordinated cleavage of prothrombin by factor Xa. J. Biol. Chem. 2005, 280, 27393–27401. 10.1074/jbc.M503435200. [DOI] [PubMed] [Google Scholar]
- Shim J. Y.; Lee C. J.; Sangwook W.; Pedersen L. G. A model for the unique role of factor Va A2 domain extension in the human ternary thrombin-generating complex. Biophys. Chem. 2015, 199, 46–50. 10.1016/j.bpc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Kim P. Y.; Manuel R.; Nesheim M. E. Differences in prethrombin-I activation with human or bovine factor Va can be attributed to the heavy chain. Thromb. Haemostasis 2009, 102, 623–633. 10.1160/TH09-04-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny R. J.; Pittman D. D.; Toole J. J.; Kriz R. W.; Aldape R. A.; Hewick R. M.; Kaufman R. J.; Mann K. G. Complete cDNA and derived amino acid sequence of human factor V. Proc. Natl. Acad. Sci. U.S.A. 1987, 84, 4846–4850. 10.1073/pnas.84.14.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinto E. R.; Esmon C. T.; Mann K. G.; MacGillivray R. T. The complete cDNA sequence of bovine coagulation factor V. J. Biol. Chem. 1992, 267, 2971–2978. [PubMed] [Google Scholar]
- Hirbawi J.; Vaughn J. L.; Bukys M. A.; Vos H. L.; Kalafatis M. Contribution of amino acid region 659–663 of factor Va heavy chain to the activity of factor Xa within prothrombinase. Biochemistry 2010, 49, 8520–8534. 10.1021/bi101097t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. F.; Haley P. E. Meizothrombin: active intemediate formed during prothrombin-catalyzed activation of prothrombin. Methods Enzymol. 1993, 222, 299–313. 10.1016/0076-6879(93)22020-g. [DOI] [PubMed] [Google Scholar]
- Doyle M. F.; Mann K. G. Multiple active forms of thrombin. Relative activities of meizothrombins. J. Biol. Chem. 1990, 265, 10693–10701. [PubMed] [Google Scholar]
- Toso R.; Camire R. M. Role of Hirudin-like factor Va heavy chain sequences in prothrombinase function. J. Biol. Chem. 2006, 281, 8773–8779. 10.1074/jbc.M511419200. [DOI] [PubMed] [Google Scholar]
- Côté H. C. F.; Stevens W. K.; Bajzar L.; Banfield D. K.; Nesheim M. E.; MacGillivray T. A. Characterization of a stable form of human meizothrombin derived from recombinant prothrombin (R155A, R271A, and R284A). J. Biol. Chem. 1994, 269, 11374–11380. [PubMed] [Google Scholar]
- Bianchini E. P.; Orcutt S. J.; Panizzi P.; Bock P. E.; Krishnaswamy S. Racheting of the substrate from the zymogen to proteinase conformations directs the sequential cleavage of prothrombin by prothrombinase. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 10099–10104. 10.1073/pnas.0504704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinto E. R.; Esmon C. T. Loss of prothrombin and of factor Xa-factor Va interactions upon inactivation of factor Va by Activated Protein C. J. Biol. Chem. 1984, 259, 13986–13992. [PubMed] [Google Scholar]
- Singh L. S.; Bukys M. A.; Beck D. O.; Kalafatis M. Amino acids Glu323, Tyr324, Glu330, and Val331 of factor Va heavy chain are essential for expression of cofactor activity. J. Biol. Chem. 2003, 278, 28335–28345. 10.1074/jbc.M300233200. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Nature 1970, 227, 680–685. 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H.; Staehlin T.; Gordon J. Proc. Natl. Acad. Sci. U.S.A. 1979, 76, 4350–4354. 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalafatis M.; Bertina R. M.; Rand M. D.; Mann K. G. Characterization of the molecular defect in factor VR506Q. J. Biol. Chem. 1995, 270, 4053–4057. 10.1074/jbc.270.8.4053. [DOI] [PubMed] [Google Scholar]
- Kalafatis M.; Haley P. E.; Lu D.; Bertina R. M.; Long G. L.; Mann K. G. Proteolytic events that regulate factor V activity in whole plasma from normal and activated protein C (APC)-resistant individuals during clotting: an insight into the APC-resistance assay. Blood 1996, 87, 4695–4707. [PubMed] [Google Scholar]
- van’t Veer C.; Kalafatis M.; Bertina R. M.; Simioni P.; Mann K. G. Increased tissue factor-initiated prothrombin activation as a result of the Arg506 --> Gln mutation in factor VLEIDEN. J. Biol. Chem. 1997, 272, 20721–20729. 10.1074/jbc.272.33.20721. [DOI] [PubMed] [Google Scholar]
- van’t Veer C.; Golden N. J.; Kalafatis M.; Mann K. G. Inhibitory mechanism of the protein C pathway on tissue factor-induced thrombin generation. Synergistic effect in combination with tissue factor pathway inhibitor. J. Biol. Chem. 1997, 272, 7983–7994. 10.1074/jbc.272.12.7983. [DOI] [PubMed] [Google Scholar]
- Bukys M. A.; Orban T.; Kim P. Y.; Beck D. O.; Nesheim M. E.; Kalafatis M. The structural integrity of anion binding exosite-I of thrombin is required and sufficient for timely cleavage and activation of factor V and factor VIII. J. Biol. Chem. 2006, 281, 18569–18580. 10.1074/jbc.M600752200. [DOI] [PubMed] [Google Scholar]