Abstract
Background
Neuroinflammation has been implicated in the pathophysiology of post-hemorrhagic hydrocephalus (PHH) of prematurity, but no comprehensive analysis of signaling molecules has been performed using human cerebrospinal fluid (CSF).
Methods
Lumbar CSF levels of key cytokines (IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, TGF-β1, IFN-γ) and chemokines (XCL-1, CCL-2, CCL-3, CCL-19, CXCL-10, CXCL-11, CXCL-12) were measured using conventional and multiplexed Enzyme-linked Immunosorbent Assays and compared between preterm infants with PHH and those with no known neurological injury. The relationships between individual biomarker levels and specific CSF cell counts were examined.
Results
Total protein (TP) CSF levels were elevated in the PHH subjects compared to controls. CSF levels of IL-1α, IL-4, IL-6, IL-12, TNF-α, CCL-3, CCL-19, and CXCL-10 were significantly increased in PHH whereas XCL-1 was significantly decreased in PHH. When normalizing by TP, IL-1α, IL-1β, IL-10, IL-12, CCL-3, and CCL-19 levels were significantly elevated compared to controls, while XCL-1 levels remained significantly decreased. Among those with significantly different levels in both absolute and normalized levels, only absolute CCL-19 levels showed a significant correlation with CSF nucleated cells, neutrophils, and lymphocytes. IL-1β and CXCL-10 also were correlated with total cell count, nucleated cells, red blood cells, and neutrophils.
Conclusions
Neuroinflammation is likely to be an important process in the pathophysiology of PHH. To our knowledge, this is the first study to investigate CSF levels of chemokines in PHH as well as the only one to show XCL-1 selectively decreased in a diseased state. Additionally, CCL-19 was the only analyte studied that showed significant differences between groups and had significant correlation with cell count analysis. The selectivity of CCL-19 and XCL-1 should be further investigated. Future studies will further delineate the role of these cytokines and chemokines in PHH.
Keywords: Cytokines, Chemokines, Post-hemorrhagic, Hydrocephalus, CSF, Cerebrospinal fluid, Preterm, Prematurity
Background
Post-hemorrhagic hydrocephalus (PHH) develops in up to 25% of preterm infants with intraventricular hemorrhage (IVH) and is a leading cause of infant hydrocephalus in North America [1, 2]. While the association between IVH and PHH is well established [3], the pathophysiological mechanisms linking these two conditions remain unclear. Data from experimental studies and limited clinical series have implicated neuroinflammation in the pathogenesis of PHH [4–7]. IVH-related blood or blood breakdown products may trigger inflammatory fibrosis or arachnoiditis with gliosis that may contribute to an imbalance in cerebrospinal fluid (CSF) production, absorption, or transit [4, 8].
A number of reports have detailed changes in the CSF levels of IL-1β, IL-6, IL-8, TNF-α, IFN-γ, TGF-β1, and TGF-β2 in the setting of experimental or human PHH [1, 9–13]. Indeed, there is experimental evidence that inhibition of TGF-β or lysophosphatidic acid may prevent the development of PHH [9, 14]. To date, human studies into the neuroinflammatory basis of PHH have largely targeted select proteins and, to our knowledge, have not considered the role of chemokines. Informed by our previous work in CSF proteomics [15], we decided to take a broad approach to survey neuro-inflammatory processes at play in the CSF of human infants with PHH. Commercially available multiplex assays offer the advantage of simultaneously measuring proteins from multiple pathways involved in inflammatory modulation while using very little CSF volume. In the current study, we used multiplex analyses investigate the CSF levels of key inflammatory cytokines (IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, TGF-β1, IFN-γ) and chemokines (XCL-1, CCL-2, CCL-3, CCL-19, CXCL-10, CXCL-11, CXCL-12) in the setting of human infant PHH.
These and related proteins have been evaluated in various clinical contexts previously and have been reviewed in detail by Turner et al. 2014 [16]. IL-1α, IL-1β, IL-6, IL-8, TNF-α, and IFN-γ, among other functions, provide pro-inflammatory signaling for bone marrow cell proliferation, IgG production, chemotaxis, phagocyte cell activation, and anti-viral, macrophage activation. IL-10 and IL-12 are anti-inflammatory signaling molecules and inhibit cytokine production and activate natural killer cells. TGF-β1 has been shown to inhibit T and B cell proliferation. Chemokines also function in inflammatory and immunological responses. XCL-1 provides chemotactic activity specific for lymphocytes while contributing to regulatory T cell development. CCL-2, CCL-3, and CCL-19 among other functions serve to recruit monocytes to inflammation sites and regulate proliferation of progenitor cells. CXCL-10, -11, and -12 are chemotactic for monocytes and T-lymphocytes and with the exception of CXCL-11, have been upregulated in post-traumatic brain injury studies [16–23].
As these studies suggest, neuroinflammation is likely to be an important process in the pathophysiology of PHH and its associated neurological injury. In the current study, we used multiplex analyses to broadly investigate proteins involved in inflammatory modulation and their relationship to CSF cell counts. Based on the results presented herein, future studies will delineate the role of specific cytokines and chemokines in the pathophysiology of PHH.
Methods
Research subjects
Washington University Human Research Protection Office (WU-HRPO) approval was obtained prior to beginning this study (WU-HRPO #201101887). Research subjects comprised two study groups: control and PHH. Control CSF samples were acquired from 31 infants born ≤ 35 weeks post-menstrual age (PMA) without known neurological injury via lumbar puncture (LP) performed as part of routine sepsis/meningitis evaluation. Final microbiological cultures were verified as sterile in all controls. PHH CSF samples were acquired from infants born ≤ 30 weeks PMA with PHH as described previously via clinically-indicated LP [24]. Prior to LP, all PHH subjects demonstrated progressive increase in occipital-frontal circumference, full fontanel, splaying of the sagittal suture ≥ 2 mm [25] and a frontal-occipital horn ratio (FOR) ≥ 0.55 [26]. Thirteen of the 14 PHH infants included in this report required ventriculo-peritoneal (VP) shunts between 34 and 59 weeks PMA (Table 1).
Table 1.
Characteristics of study subjects with post-hemorrhagic hydrocephalus
| Subject ID |
Sex | PMA at birth (weeks) | TP (μg/ml) | PMA at CSF sample (weeks) | Temporizing neurosurgical procedure | PMA at temporizing procedure (weeks) | VP shunt surgery PMA (weeks) |
|---|---|---|---|---|---|---|---|
| 1 | M | 24.00 | 2369 | 26.86 | RES | 27.00 | 37.57 |
| 2 | F | 29.57 | 1745 | 30.29 | RES | 31.29 | 36.57 |
| 3 | F | 24.00 | 6072 | 26.86 | RES | 27.86 | 34.71 |
| 4 | M | 29.00 | 4564 | 31.14 | RES | 31.57 | 40.86 |
| 5 | M | 26.00 | 9596 | 27.43 | RES | 28.14 | 38.14 |
| 6 | M | 28.14 | 2606 | 28.71 | RES | 30.14 | 37.14 |
| 7 | F | 24.57 | 1270 | 28.00 | RES | 30.57 | 39.29 |
| 8 | M | 24.71 | 1869 | 30.57 | RES | 34.14 | 59.29 |
| 9 | M | 25.43 | 1420 | 27.57 | NAa | NA | NA |
| 10 | M | 25.43 | 2620 | 28.71 | RES | 31.00 | 53.57 |
| 11 | M | 25.86 | 1902 | 27.71 | RES | 28.14 | NAb |
| 12 | M | 25.29 | 3735 | 27.71 | RES | 28.00 | 40.00 |
| 13 | F | 29.57 | 2331 | 30.00 | RES | 31.29 | 36.57 |
| 14 | F | 24.00 | 4300 | 26.43 | RES | 27.00 | 37.57 |
PMA post-menstrual age, TP total protein, CSF cerebrospinal fluid, VP ventriculo-peritoneal, RES ventricular reservoir, NA not available
aSubject 9 expired after withdrawal of care by family
bSubject 11 developed a S. capitis RES infection 12 weeks after RES implantation and device tapping for cerebrospinal fluid removal (CSF). CSF samples prior to the 12-week sample were sterile on culture. After infection, the RES was removed and replaced with an external ventricular drain, which was later successfully weaned, and no shunt was implanted. Subject 10 underwent endoscopic third ventriculostomy with choroid plexus cauterization prior to VP shunt placement. Subject 1 had a shunt malfunction within 6 months of VP shunt implantation
Cerebrospinal fluid processing
CSF samples were acquired via LP under sterile conditions for clinical purposes and transferred to the St. Louis Children’s Hospital clinical laboratory. The clinical laboratory performed cell counts of the PHH CSF including total cell count, nucleated cells, and red blood cells (all measured as cells/µL). They also performed a differential analysis including neutrophils, lymphocytes, and monocytes (measured as percentages). The laboratory then stored the samples at – 80 °C. At the time of experimental analysis, samples were thawed and centrifuged at 2500 rpm for 6 min. The supernatant was aliquoted and used for biomarker assays.
Total protein measurements
The Pierce Bicinchoninic Acid protein assay kit (Thermo Scientific; Waltham, Massachusetts) was used to estimate total protein (TP) concentration in each CSF sample. Serum albumin standards as well as CSF samples were placed in microplate wells in duplicate. After addition of the working reagent, the plate was incubated at 37 °C for 30 min. The plate was then cooled to room temperature and absorbance at 562 nm was measured on a plate reader. Total protein concentrations were determined with a 4-parameter logistic standard curve.
Chemokine and cytokine analysis
Enzyme-linked Immunosorbent Assays (ELISAs) were used to measure concentrations of TGF-β1 and the chemokine CXCL-12 in both control and PHH CSF samples (R&D systems, catalog # DY240 and DY350 respectively, Minneapolis, MN). Sigma-Aldrich ELISA kits (Sigma, catalog #RAB0073 and RAB0515) were used for the measurement of CCL-3 and XCL-1 concentrations. All ELISA samples were run in duplicate and the absorbance at 450 nm was measured on a Versamax plate reader (Molecular Devices, Sunnyvale, CA). Chemokine and cytokine concentrations were determined using a 4 parameter logistic standard curve. Aushon (Billerica, MA) human cytokine array #2 and human chemokine array #2 multiplexes were used to measure concentrations of IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ, CCL-2, CCL-19, CXCL-10, and CXCL-11. Prefabricated assays for these analytes were run according to the manufacturer’s instructions. Multiplex samples were also run in duplicate and analyzed on the Aushon Ciraplex® Assays system.
Due to differences in TP between samples, we normalized analyte measurements by dividing absolute levels by their corresponding TP. Where specified, statistical comparisons were conducted using these normalized measurements.
Statistical analysis
CSF analyte levels were reported as mean ± standard deviation (SD), and mean difference between groups with a 95% confidence interval. Comparisons between control and PHH groups were conducted using two-tailed independent samples t tests assuming unequal variances in Prism 5.0 (GraphPad Software, La Jolla, CA). Linear regressions between CSF cell count parameters and absolute levels of PHH CSF cytokines and chemokines were performed using Spearman correlation coefficients in SAS 9.3 (SAS Institute, Cary NC). A predetermined significance level of 0.05 was used for all statistical tests.
Results
Subject characteristics
Fourteen preterm infants with PHH (Table 1) were included in this study. Ventricular reservoirs (RES) were placed in all infants except one, where the family opted for withdrawal of care. Of the 13 remaining subjects, all except one required a VP shunt. The subject not requiring a VP shunt developed a S. capitis infection 12 weeks after RES implantation (after weeks of sterile cultures) and underwent device removal and replacement with an external ventricular drain, which was later weaned and removed. Subject 10 had endoscopic third ventriculostomy with choroid plexus cauterization prior to VP shunt placement. Subject 1 underwent VP shunt revision within 6 months of VP shunt implantation. The control group presented with a broad array of clinical reasons for LP, but the primary reason was for sepsis diagnosis. For both groups, the CSF sampled cultures were monitored for 3.68 ± 0.13 days and remained negative.
Seven out of the 31 control subjects were female, while 5 out of the 15 PHH subjects were female. The mean birth PMA for the PHH group was 25.75 ± 2.19 weeks and for the control group was 30.12 ± 3.23 weeks (p < 0.0001). The CSF sample PMA was also significantly different between the groups (31.86 ± 2.83 for control, 28.88 ± 2.97 for PHH; p = 0.003). Ventricular size measurements were only available for 7 out of the 31 control subjects due to a lack of cranial images. Of the 7, 3 of them had ventricular sizes too small to be accurately measured and the other 4 had an FOR of 0.40 ± 0.05. PHH subjects demonstrated much higher FOR measurements (0.62 ± 0.07) for imaging performed within 24 h of CSF sample collection (p < 0.0001).
Cerebrospinal fluid total protein, cytokines and chemokines
CSF TP, cytokine, and chemokine concentrations were measured and compared between control and PHH subjects (Fig. 1a; Tables 2, 3). TP was significantly elevated in PHH versus control (3285 ± 2266 µg/ml vs 1540 ± 985.6 µg/ml, respectively, p = 0.0008). CSF levels of IL-1α, IL-4, IL-6, IL-12, TNF-α, CCL-3, CCL-19, and CXCL-10 were significantly elevated in PHH versus control while IL-1β, IL-8, IL-10, TGFβ1, IFN-γ, CCL-2, CXCL-11, and CXCL-12 were not (Tables 2 and 3). After normalizing by total protein, IL-1α, IL-1β, IL-10, IL-12, CCL-3, and CCL-19 were significantly elevated compared with control, while IL-4, IL-6, IL-8, TNF-α, TGFβ1, IFN-γ, CCL-2, CXCL-10, CXCL-11, and CXCL-12 were not significantly different (Tables 2 and 3). XCL-1 was the only analyte that was significantly decreased in PHH, even when normalized by TP (Table 3). The most robust candidate neuroinflammatory CSF biomarkers—those that retained statistical significance irrespective of normalization by TP levels—included IL-1α (increased), IL-12 (increased), CCL-3 (increased), CCL-19 (increased), and XCL-1 (decreased) (Fig. 1b–d).
Fig. 1.
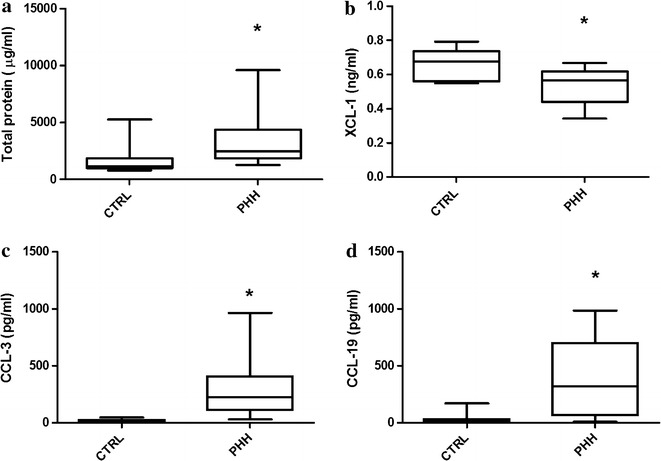
Lumbar cerebrospinal fluid levels of total protein (a), XCL-1 (b), CCL-3 (c), and CCL-19 (d) in human preterm infants without (control) or with post-hemorrhagic hydrocephalus. Boxes represent the median with 25th and 75th percentiles and the whiskers show interquartile range multiplied by 1. Levels of Total protein, CCL-3 and CCL-19 were significantly increased in PHH. The levels of XCL-1 were decreased in PHH subjects. *Denotes significance at p ≤ 0.05. CTRL control, PHH post-hemorrhagic hydrocephalus
Table 2.
Comparison of cerebrospinal fluid absolute and normalized concentrations of common cytokines in control and post-hemorrhagic hydrocephalus subjects
| Control mean (SD) | PHH mean (SD) | Mean difference | 95% confidence interval | p value | |
|---|---|---|---|---|---|
| Total protein (μg/ml) | 1540 (985.6) | 3285 (2265.9) | 1745.0 | 2714–775.3 | 0.0008 |
| IL-1α (pg/ml) | 2.385 (3.758) | 13.51 (11.03) | 11.13 | 17.67–4.577 | 0.0019 |
| Normalized IL-1α | 1.58E−7 (2.72E−7) | 4.89E−7 (4.56E−7) | 3.31E−7 | 6.40E−7 to 2.31E−8 | 0.0363 |
| IL-1β (pg/ml) | 4.600 (15.05) | 79.89 (146.3) | 75.29 | 156.2 to − 5.664 | n.s. |
| Normalized IL-1β | 1.27E−7 (2.93E−7) | 1.90E−6 (2.2E−6) | 1.78E−6 | 3.02E−6 to 5.31E−7 | 0.0072 |
| IL-4 (pg/ml) | 0.266 (0.4465) | 1.010 (0.5653) | 0.7444 | − 1.173 to − 0.3164 | 0.0016 |
| Normalized IL-4 | 1.64E−9 (2.53E−9) | 4.20E-9 (3.55E−9) | 2.56E−9 | 5.13E−9 to − 1.11E−11 | n.s. |
| IL-6 (pg/ml) | 268.2 (667.8) | 981.3 (932.6) | 713.1 | 1389–37.32 | 0.0395 |
| Normalized IL-6 | 1.8E−5 (5.6E−5) | 4.27E−5 (5.82E−5) | 2.49E−5 | 7.38E−5 to − 2.40E−5 | n.s. |
| IL-8 (pg/ml) | 522.5 (528) | 1958 (3158) | 1436 | 3205 to − 333.3 | n.s. |
| Normalized IL-8 | 4.4E−5 (5.3E−5) | 1.04E−4 (1.95E−4) | 6.0E−5 | 1.9E−4 to − 6.7E−5 | n.s. |
| IL-10 (pg/ml) | 5.154 (14.59) | 128 (274.8) | 122.8 | 274.1 to − 28.38 | n.s. |
| Normalized IL-10 | 1.81E−7 (3.13E−7) | 2.53E−6 (3.75E−6) | 2.35E−6 | 4.42E−6 to 2.84E−7 | 0.0277 |
| IL-12 (pg/ml) | 1.763 (2.446) | 11.58 (9.234) | 9.819 | 15.14 to 4.497 | 0.0009 |
| Normalized IL-12 | 1.3E−7 (2.2E−7) | 3.75E−7 (3.12E−7) | 2.45E−7 | 4.70E−7 to 2.043E−8 | 0.0339 |
| TNF-α (pg/ml) | 2.147 (3.337) | 9.71 (7.384) | 7.566 | 12.18–2.951 | 0.0026 |
| Normalized TNF-α | 1.61E−7 (3.0E−7) | 3.42E−7 (2.97E−7) | 1.81E−7 | 4.38E−7 to − 7.55E−8 | n.s. |
| TGF-β1 (ng/ml) | 0.4504 (0.8607) | 1.161 (2.270) | 0.7103 | 2.278 to − 0.857 | n.s. |
| Normalized TGF-β1 | 1.9E−5 (2.0E−5) | 2.4E−5 (2.1E−5) | 4.55E−6 | 2.02E−5 to − 1.5E−5 | n.s. |
| IFN-γ (pg/ml) | 0.6463 (1.552) | 1.191 (1.357) | 0.5446 | 1.812 to − 0.7226 | n.s. |
| Normalized IFN-γ | 2.93E−8 (5.65E−8) | 3.42E−8 (2.35E−8) | 4.91E−9 | 4.44E−8 to − 3.45E−8 | n.s. |
SD standard deviation, PHH: post-hemorrhagic hydrocephalus
Table 3.
Comparison of cerebrospinal fluid absolute and normalized concentrations of common chemokines in control and post-hemorrhagic hydrocephalus subjects
| Control mean (SD) |
PHH mean (SD) |
Mean difference | 95% confidence interval | p value | |
|---|---|---|---|---|---|
| XCL-1 (ng/ml) | 0.6680 (0.0833) | 0.5528 (0.1121) | − 0.1152 | − 0.0224 to − 0.2079 | 0.0176 |
| Normalized XCL-1 | 5.4E−5 (1.6E−5) | 2.19E−5 (1.1E−5) | − 3.3E−5 | − 1.76E−5 to − 4.8E−5 | 0.0002 |
| CCL-2 (pg/ml) | 9436 (5117) | 9535 (5089) | 99.45 | − 4622 to 4821 | n.s. |
| Normalized CCL-2 | 7.79E−4 (5.76E−4) | 4.16E−4 (4.08E−4) | − 3.62E−4 | − 8.47E−4 to 1.21E−4 | n.s. |
| CCL-3 (pg/ml) | 16.50 (11.02) | 360.0 (327.7) | 343.5 | 537.9–149.1 | 0.0018 |
| Normalized CCL-3 | 0.015 (0.0092) | 1.2E−5 (9.9E−6) | − 0.0146 | − 0.00658 to − 0.0227 | 0.0014 |
| CCL-19 (pg/ml) | 28.46 (45.15) | 410.8 (377.9) | 382.4 | 591.8–173.0 | 0.0011 |
| Normalized CCL-19 | 1.73E−6 (2.14E−6) | 1.41E−5(2.1E−5) | 1.23E−5 | 2.36E−5 to 9.96E−7 | 0.0345 |
| CXCL-10 (pg/ml) | 809.6 (776.6) | 1480 (742.6) | 670 | 1325–15.09 | 0.0454 |
| Normalized CXCL-10 | 5.9E−5 (5.8E−5) | 5.72E−5 (4.42E−5) | − 2.14E−6 | 4.32E-5 to − 4.75E-5 | n.s. |
| CXCL-11 (pg/ml) | 5.162 (10.68) | 5.15 (4.58) | − 8.7E−3 | 7.869 to − 7.886 | n.s. |
| Normalized CXCL-11 | 4.08E−7 (1.01E−6) | 1.38E−7 (8.01E−8) | − 2.71E−7 | 4.39E−7 to − 9.80E−7 | n.s. |
| CXCL-12 (ng/ml) | 0.5187 (0.5528) | 0.9930 (1.0002) | 0.4743 | 1.052 to − 0.1033 | n.s. |
| Normalized CXCL-12 | 4.5E−5 (5.3E−5) | 1.4E−5 (9.6E−6) | − 3.01E−5 | 2.01E-5 to − 7.5E−5 | n.s. |
SD standard deviation, PHH post-hemorrhagic hydrocephalus
Cerebrospinal fluid cytokines and chemokines correlation with cell counts
Total cell count measurements for PHH CSF were 72964.86 ± 114192 cells/µL, nucleated cells were 4057.2 ± 13319 cells/µL, and red blood cells were 68907.64 ± 103612 cells/µL. Neutrophils, lymphocytes, and monocytes within the 14 PHH CSF samples were 48.15 ± 33.60, 19.54 ± 21.96, and 21.79 ± 17.32%, respectively. The individual data points for these cell counts were analyzed for a correlation with absolute cytokine and chemokine levels within PHH CSF (Tables 4 and 5). IL-1α correlated with neutrophils with a Spearman r value of 0.73 and a p value of 0.0246. IL-1β correlated with total cell count (r: 0.64; p = 0.0479), red blood cells (r: 0.64; p = 0.0479), neutrophils (r: 0.88; p = 0.0016), and lymphocytes (r: − 0.75; p = 0.0199). IL-6 and IL-10 were significantly correlated with neutrophils (r: 0.67; p = 0.0499 and r: 0.78; p = 0.0075 respectively). TGF-β1 was significantly correlated with nucleated cells (r: 0.83; p = 0.0102). CCL-19 showed significant and strong correlation with nucleated cells (r: 0.71; p = 0.0465), neutrophils (r: 0.78; p = 0.0208), and lymphocytes (r: − 0.74; p = 0.0366). CXCL-10 was significantly correlated with total cell counts (r: 0.63; p = 0.0498), red blood cells (r: 0.63; p = 0.0498), neutrophils (r: 0.88; p = 0.0018), and lymphocytes (r: 0.78; p = 0.0125). CXCL-11 showed correlation with neutrophils (r: 0.67; p = 0.0499), while CXCL-12 showed correlation with lymphocytes (r: − 0.73; p = 0.0396) and monocytes (r: − 0.80; p = 0.0165). XCL-1 and CCL-3 levels did not correlate significantly with any cell counts.
Table 4.
Post-hemorrhagic hydrocephalus cerebrospinal fluid absolute cytokine Spearman correlations with cell counts
| IL-1α r p value |
IL-1β r p value |
IL-4 r p value |
IL-6 r p value |
IL-8 r p value |
IL-10 r p value |
IL-12 r p value |
TNF-α r p value |
TGF-β1 r p value |
IFN-γ r p value |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Total cell count | 0.52727 n.s. |
0.63636 0.0479 |
0.29697 n.s. |
0.26061 n.s. |
0.16387 n.s. |
0.49091 n.s. |
0.26061 n.s. |
0.06667 n.s. |
0.69048 n.s. |
0.23636 n.s. |
| Nucleated cells | 0.49091 n.s. |
0.57576 n.s. |
0.15152 n.s. |
0.20000 n.s. |
0.16387 n.s. |
0.50303 n.s. |
0.24848 n.s. |
0.06667 n.s. |
0.83333 0.0102 |
0.21212 n.s. |
| Red blood cells | 0.52727 n.s. |
0.63636 0.0479 |
0.29697 n.s. |
0.26061 n.s. |
0.16387 n.s. |
0.49091 n.s. |
0.26061 n.s. |
0.06667 n.s. |
0.69048 n.s. |
0.23636 n.s. |
| Neutrophils | 0.73333 0.0246 |
0.88333 0.0016 |
0.61667 n.s. |
0.66667 0.0499 |
0.12780 n.s. |
0.78333 0.0125 |
0.31667 n.s. |
0.05000 n.s. |
0.45238 n.s. |
0.41667 n.s. |
| Lymphocytes | − 0.61667 n.s. |
− 0.75000 0.0199 |
− 0.28333 n.s. |
− 0.16667 n.s. |
− 0.20083 n.s. |
− 0.56667 n.s. |
− 0.30000 n.s. |
− 0.13333 n.s. |
− 0.38095 n.s. |
− 0.26667 n.s. |
| Monocytes | − 0.40122 n.s. |
− 0.46201 n.s. |
− 0.14590 n.s. |
− 0.16413 n.s. |
0.03424 n.s. |
− 0.46201 n.s. |
− 0.14590 n.s. |
0.09726 n.s. |
− 0.35714 n.s. |
− 0.19453 n.s. |
r Spearman correlation coefficient
Table 5.
Post-hemorrhagic hydrocephalus cerebrospinal fluid absolute chemokine Spearman correlations with cell counts
| XCL-1 r p value |
CCL-2 r p value |
CCL-3 r p value |
CCL-19 r p value |
CXCL-10 r p value |
CXCL-11 r p value |
CXCL-12 r p value |
|
|---|---|---|---|---|---|---|---|
| Total cell count | − 0.37143 n.s. |
0.09524 n.s. |
0.48571 n.s. |
0.64286 n.s. |
0.63222 0.0498 |
0.48333 n.s. |
0.37126 n.s. |
| Nucleated cells | − 0.20000 n.s. |
0.30952 n.s. |
0.08571 n.s. |
0.71429 0.0465 |
0.58967 n.s. |
0.63333 n.s. |
0.46707 n.s. |
| Red blood cells | − 0.37143 n.s. |
0.09524 n.s. |
0.48571 n.s. |
0.64286 n.s. |
0.63222 0.0498 |
0.48333 n.s. |
0.37126 n.s. |
| Neutrophils | − 0.25714 n.s. |
0.21429 n.s. |
0.70000 n.s. |
0.78571 0.0208 |
0.87867 0.0018 |
0.66667 0.0499 |
0.70660 n.s. |
| Lymphocytes | 0.31429 n.s. |
− 0.09524 n.s. |
− 0.54286 n.s. |
− 0.73810 0.0366 |
− 0.78333 0.0125 |
− 0.58333 n.s. |
− 0.73055 0.0396 |
| Monocytes | − 0.25714 n.s. |
− 0.30952 n.s. |
− 0.55078 n.s. |
− 0.54762 n.s. |
− 0.46951 n.s. |
− 0.53333 n.s. |
− 0.80241 0.0165 |
r Spearman correlation coefficient
Discussion
This study aimed to advance the current understanding of neuroinflammation in PHH by measuring key inflammatory cytokines and chemokines in the CSF. Among 17 CSF biomarkers, 8 were significantly increased (IL-1α, IL-4, IL-6, IL-12, TNF-α, CCL-3, CCL-19, and CXCL-10) and one was significantly decreased (XCL-1) in PHH. Of note, CSF in the clinical setting of PHH contains high levels of protein related to the IVH itself (e.g. albumin); in order to account for any potential effect of CSF protein on CSF biomarker levels, we also report the level of each cytokine and chemokine after normalization by total CSF protein (TP). When normalized by TP, IL-1α, IL-1β, IL-10, IL-12, CCL-3, CCL-19 were elevated, while XCL-1 remained decreased. The most robust candidate CSF biomarkers were significantly altered in PHH irrespective of normalization by TP, which included IL-1α, IL-12, CCL-3, and CCL-19 as increased, and XCL1 as decreased. Of those 5, only absolute levels of CCL-19 correlated with CSF nucleated cells, neutrophils, and lymphocytes, strongly implicating this chemokine in the neuroinflammatory processes of PHH pathophysiology. Neuroinflammatory profile specificity may have important implications in the pathophysiology of PHH and could shape future pharmacological studies in treating PHH.
Neuroinflammation accompanying IVH and PHH is complex and hypothesized to be initiated by blood and its breakdown products in the ventricular system prompting ventriculitis, gliosis and arachnoiditis. Past studies demonstrate that infants with PHH and periventricular white matter injury have increased CSF concentrations of IL-1β, IL-6, IL-8, and TGF-β1 [10, 12, 13, 27, 28]. Although increased levels of TGF-β1 have been implicated in white matter injury in the setting of IVH and PHH, Heep et al. [11] contrarily found no significant difference. A recent review by Szpecht et al. [29] on the role of cytokines in the pathogenesis of IVH suggested an association between IL-1β, IL-6, IL-8, and TNF-α with increased risk of PHH [12, 13, 30].
The current report demonstrates that CSF levels of the cytokines TNF-α, IL-1α, IL-4, IL-6, and IL-12 are significantly increased in PHH. TNF-α and IL-1α findings are consistent with previous studies of their association with acute phase reactions and pro-inflammatory responses to pathogen or tissue injury [31, 32]. Astrocytes, microglia, and neurons typically produce basal levels of TNF-α and maintain homeostasis in normal central nervous system (CNS) physiology; however, just microglia and astrocytes are assumed to be responsible for elevated levels during neuroinflammation [33–37]. IL-1α is constitutively secreted by many cell types, but its expression surges in response to pathogens or brain tissue injury [38]. IL-1α behaves as an upstream signal for multiple proinflammatory cytokines, chemokines, and prostaglandins [31]. Di Paolo et al. demonstrated that IL-1α was the only cytokine to show absolute differences between the two groups with a significant Spearman correlation in the cell count analysis. IL-1α and TNF-α may be associated with acute phase reactions as well as pro-inflammatory states that occur in PHH pathophysiology. Increased levels of TNF-α and IL-6 are also consistent with Savman et al. [12]. Mainly secreted by T-cells, macrophages, and endothelial cells, IL-6 also increases in PHH, which is consistent with previous studies which showed its involvement in neuronal and glial function as well as neuroinflammation pathways in the CNS [39, 40].
IL-4 is a regulatory cytokine that assumes a myriad of immune and non-immune functions. In extravascular tissues, it is involved in alternative activation of macrophages into M2 cells (repair macrophages) and inhibits activation of M1 cells (inflammatory macrophages) resulting in decreased pathological inflammation [41]. It is plausible that increased CSF levels of IL-4 in PHH could be associated with decreased M1 macrophage activity to contain secondary injury from inflammatory cells. Zundler and Neurath [42] describe the main sources of IL-12 as macrophages, monocytes, dendritic cells, granulocytes and B cells; it induces production of IFN-γ to foster both innate and adaptive cell-mediated immune responses. Increased levels of IL-12 in PHH could be caused by microglial activation during neuroinflammation to enhance the innate immune response for phagocytosis of extravascular blood, blood-breakdown products, and cell debris resulting from IVH.
Normalization to TP significantly altered the results such that we found selective and significant increases in CSF IL-1α, IL-1β, IL-10, and IL-12 levels in PHH. Furthermore, absolute IL-1β levels significantly correlated with total cell count, red blood cells, neutrophils, and lymphocytes. IL-1β is a potent pro-inflammatory cytokine that initiates and amplifies innate immunity and host responses to microbial and tissue injury [43]. A previous study by Savman et al. [12] showed that IL-1β is significantly elevated in the CSF of premature infants with PHH. Innate responses from phagocytic immune cells, such as macrophages and neutrophils, may be associated with increased IL-1β levels in PHH. We also found a significant correlation between IL-6 and IL-10 levels with neutrophil counts. IL-10 functions in the activation, inhibition, growth, and migration of hematopoietic cells. In the context of tissue injury, whether by infectious or sterile inflammation, IL-10 downregulates and terminates inflammation [44]. Increased IL-10 levels may be associated with down-regulation of neuroinflammation in the setting of IVH or PHH. Contrary to past studies, we did not observe increased TGF-β1 levels in the CSF of PHH subjects [10, 11, 28]. This may have been due to differences between studies such as location of CSF (LP versus ventricular), methods and timing of CSF sample acquisition, or methods of detection. However, there was a significant correlation between absolute TGF-β1 levels and nucleated cells.
To our knowledge, this is the first study investigating CSF chemokine levels in PHH. CSF levels of seven chemokines (XCL-1, CCL-2, CCL-3, CCL-19, CXCL-10, CXCL-11, and CXCL-12) were measured and compared between PHH and control groups. Absolute levels of CCL-3, CCL-19, and CXCL-10 were significantly elevated in the CSF of PHH subjects; CCL-3 and CCL-19 levels remained elevated after normalization with TP. CCL-3, also known as Macrophage Inflammatory Protein 1-α, is primarily secreted by astrocytes, microglia, endothelial cells, and neurons [45–49] and has been found to be upregulated in the CSF of patients after traumatic brain injury [50–53]. CCL-3 is believed to be a potent chemo-attractant of polymorphonuclear leukocytes (PMNLs; neutrophils) in humans and mice [54] and induces peroxide production in PMNLs [55, 56]. In IVH and PHH, reactive oxygen species produced by activated PMNLs may cause local tissue injury, ventriculitis, ependymal layer scarring, and denudation. Chui et al. [48] reported that CCL-3 is a critical early inflammatory chemokine that is greatly upregulated in active Schwann cells and infiltrating macrophages in areas of brain tissue injury. In our CSF samples, CCL-3 is several-fold higher in PHH than controls, thus suggesting that it may have an important role in neuroinflammation.
CCL-19 and CXCL-10 correlated with cell counts, either total, nucleated cells, red blood cells, neutrophils, and lymphocytes. CCL-19, is also known as Macrophage Inflammatory Protein-3β, is involved in immune surveillance of the CNS by lymphocytes. Its ectopic expression may also trigger activation and/or recruitment of infiltrating leukocytes in response to specific offending stimuli [57]. Since CCL-19 is primarily expressed in cerebrovascular endothelium and choroid plexus [57–61], it is readily available to potentially promote neuroinflammation in IVH and PHH. Absolute concentration of CXCL-10 was significantly increased in PHH but there was no difference after normalization by CSF TP. We also found correlations between CXCL10 and total cell count, red blood cells, neutrophils, and lymphocytes, suggesting a possible role in PHH neuroinflammation. CXCL-10 has been reported to be upregulated in post-traumatic brain injury in some studies, but others found absent mRNA expression of CXCL-10 after head injury [21, 50, 62, 63]. Interestingly, both absolute and normalized levels of XCL-1 were decreased in PHH, but absolute levels did not correlate significantly with any cell counts. XCL-1, also known as Lymphotactin, is a chemokine of the –C– class that is expressed by T Cells (Natural Killer Cells, Natural Killer T Cells) in response to pathogenic or injurious stimuli [64]. It is expressed in various infectious and autoimmune diseases, suggesting its predominant role in protective and pathological immune responses [65].
There are a number of limitations in this study. Small sample sizes and inherent clinical heterogeneity were present within both our control and PHH groups. Indeed, non-neurological challenges or conditions could affect CSF levels of neuro-inflammatory markers in both groups. Further, there were also differences between groups in terms of birth and sample PMA (4 and 3 weeks, respectively), raising the possibility of age-dependent variability in CSF flow rate and maturity of arachnoid villi, though in theory, the effect of arachnoid villi maturity would be expected to be minor, since their development is limited until term equivalent age [66–68]. In the PHH group, heterogeneity was inevitably compounded by the complex and dynamic neuro-inflammatory response to IVH and the timing of acquisition of CSF samples, particularly since small molecules, such as those measured in this study, may be metabolized or undergo reuptake into cells in the interstitial fluids and along the CSF pathways. Any combination of these factors could impact cytokine and chemokine levels to such a degree that changes in levels can occur simultaneous with, prior to, or after CSF sampling. Finally, the data for CSF cell types and cell counts reported here were all taken from clinical laboratory reports. Thus, we were reliant on existing institutional clinical laboratory methods and were unable to measure or subtype many cell types (e.g. Th2 cells, CD8 cells) that could provide insight into the immune response itself (protein elaboration, cellular recruitment). These analyses and additional validation studies must be conducted through multi-institutional collaboration and experimental models.
Conclusion
In summary, the neuroinflammatory processes associated with PHH pathophysiology are complex and remain incompletely understood. In our current study, we measured the levels of CSF cytokines and chemokines in PHH relative to control. CSF levels of IL-1α, IL-4, IL-6, IL-12, TNF-α, CCL-3, CCL-19, CXCL-10, and TP were significantly increased in PHH, whereas XCL-1 was significantly decreased. However, only the cytokines IL-1α and IL-12, and the chemokines CCL-3, CCL-19, and XCL-1 retained their statistical significance for PHH when normalized to CSF TP levels. Furthermore, CCL-19 was the only analyte studied that also had a significant correlation with CSF cell counts. To our knowledge, this is the first study to investigate CSF levels of chemokines in PHH as well as the only one to show that XCL-1 selectively decreased in a diseased state, whether absolute or normalized levels are considered. The selectivity of CCL-19 and XCL-1 should be further investigated. These findings provide novel insights into the neuro-inflammatory processes at play in IVH and PHH, and may help inform future studies of pharmacological treatments for PHH.
Authors’ contributions
GH made substantial contributions to conception and design, analysis and interpretation of data, drafting the manuscript. DMM made substantial contribution to acquisition of data, analysis and interpretation of data, and revising the manuscript. CDM made substantial contribution to analysis and interpretation of data, and revising the manuscript. JPM made substantial contribution to analysis and interpretation of data, and revising the manuscript. TSC made substantial contribution to cell count analysis and re-interpretation of data, and revising the manuscript. RHH made substantial contribution to statistical analysis and revising the manuscript. MG made substantial contribution to interpretation of data, and revising the manuscript. BB made substantial contribution to data acquisition and maintenance. DM made substantial contribution to data acquisition and maintenance. DDL made substantial contributions to general supervision of the research group, acquisition of funding, conception and design, acquisition of data, analysis and interpretation of data, drafting and revising the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Dr. David Gibson and his staff at the Immunoassay Core at Washington University in St. Louis for their technical assistance in running the multiplex analysis. We would also like to thank Dr. Yan Yan at Washington University in St. Louis, for his statistical assistance throughout the manuscript.
Competing interests
Drs. DDL and JPM have received research, educational, and philanthropic support for unrelated research through Medtronic, Inc. and Karl Storz, Inc.
Availability of data and materials
The datasets used during the current study is available from Diego M. Morales (d.morales@wustl.edu) upon reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Washington University Human Research Protection Office (WU-HRPO) approval was obtained prior to beginning this study (WU-HRPO#201101887).
Funding
This project was supported by NIH/NINDS K23 NS075151, ICTS UL1 TR000448, CTSA UL1 RR024992, TL1 TR000449, and a grant from the Children’s Surgical Sciences Institute, St. Louis Children’s Hospital (D.L.). JPM received support from a Hydrocephalus Association Innovator Award, a Seed Grant from the Washington University Hope Center for Neurological Disorders, the Rudi Schulte Research Institute, and STARS-kids.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CCL
CC chemokine ligands
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CXCL
CXC chemokine ligands
- ELISA
enzyme-linked immunosorbent assay
- FOR
frontal-occipital horn ratio
- IFN
interferon gamma
- IL
interleukins
- IVH
intraventricular hemorrhage
- LP
lumbar puncture
- PHH
post-hemorrhagic hydrocephalus
- PMA
post-menstrual age
- PMNL
polymorphonuclear leukocytes
- RES
ventricular reservoir
- SD
standard deviation
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TP
total protein
- VP
ventriculo-peritoneal
- HRPO
Human Research Protection Office
- XCL
XC chemokine ligand
Footnotes
Gakwaya Habiyaremye and Diego M. Morales contributed equally to this work
Contributor Information
Gakwaya Habiyaremye, Email: habiyaremyeg@wudosis.wustl.edu.
Diego M. Morales, Phone: (314) 454-4688, Email: d.morales@wustl.edu
Clinton D. Morgan, Email: clinton.morgan@barrowbrainandspine.com
James P. McAllister, Email: mcallisterp@wudosis.wustl.edu
Travis S. CreveCoeur, Email: crevecoeur.travis@wustl.edu
Rowland H. Han, Email: rowland.han@wustl.edu
Mohamed Gabir, Email: mgabir@wustl.edu.
Brandon Baksh, Email: brandonbaksh@wustl.edu.
Deanna Mercer, Email: mercerd@wudosis.wustl.edu.
David D. Limbrick, Jr., Email: limbrickd@wustl.edu
References
- 1.Merhar S. Biomarkers in neonatal posthemorrhagic hydrocephalus. Neonatology. 2012;101:1–7. doi: 10.1159/000323498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–F41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsitouras V, Sgouros S. Infantile posthemorrhagic hydrocephalus. Child’s Nerv Syst. 2011;27:1595–1608. doi: 10.1007/s00381-011-1521-y. [DOI] [PubMed] [Google Scholar]
- 4.Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14:305–311. doi: 10.1111/j.1750-3639.2004.tb00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, Altay O, Tang J, Zhang JH. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol. 2012;236:69–78. doi: 10.1016/j.expneurol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012;3:25–38. doi: 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takizawa T, Tada T, Kitazawa K, Tanaka Y, Hongo K, Kameko M, Uemura KI. Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol Res. 2001;23:724–730. doi: 10.1179/016164101101199243. [DOI] [PubMed] [Google Scholar]
- 8.Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 2012;9:242–258. doi: 10.3171/2011.12.PEDS11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botfield H, Gonzalez AM, Abdullah O, Skjolding AD, Berry M, McAllister JP, 2nd, Logan A. Decorin prevents the development of juvenile communicating hydrocephalus. Brain. 2013;136:2842–2858. doi: 10.1093/brain/awt203. [DOI] [PubMed] [Google Scholar]
- 10.Chow LC, Soliman A, Zandian M, Danielpour M, Krueger RC., Jr Accumulation of transforming growth factor-beta2 and nitrated chondroitin sulfate proteoglycans in cerebrospinal fluid correlates with poor neurologic outcome in preterm hydrocephalus. Biol Neonate. 2005;88:1–11. doi: 10.1159/000083945. [DOI] [PubMed] [Google Scholar]
- 11.Heep A, Stoffel-Wagner B, Bartmann P, Benseler S, Schaller C, Groneck P, Obladen M, Felderhoff-Mueser U. Vascular endothelial growth factor and transforming growth factor-beta1 are highly expressed in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus. Pediatr Res. 2004;56:768–774. doi: 10.1203/01.PDR.0000141524.32142.53. [DOI] [PubMed] [Google Scholar]
- 12.Savman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A. Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr. 2002;91:1357–1363. doi: 10.1111/j.1651-2227.2002.tb02834.x. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz T, Heep A, Groenendaal F, Huseman D, Kie S, Bartmann P, Obladen M, Felderhoff-Muser U. Interleukin-1beta, interleukin-18, and interferon-gamma expression in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus—markers of white matter damage? Pediatr Res. 2007;61:722–726. doi: 10.1203/pdr.0b013e31805341f1. [DOI] [PubMed] [Google Scholar]
- 14.Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med. 2011;3:99ra87. doi: 10.1126/scitranslmed.3002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales DM, Townsend RR, Malone JP, Ewersmann CA, Macy EM, Inder TE, Limbrick DD., Jr Alterations in protein regulators of neurodevelopment in the cerebrospinal fluid of infants with posthemorrhagic hydrocephalus of prematurity. Mol Cell Proteom. 2012;11(M111):011973. doi: 10.1074/mcp.M111.011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Trifilo MJ, Lane TE. Adenovirus-mediated expression of CXCL10 in the central nervous system results in T-cell recruitment and limited neuropathology. J Neurovirol. 2003;9:315–324. doi: 10.1080/13550280390201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trifilo MJ, Lane TE. The CC chemokine ligand 3 regulates CD11c+ CD11b+ CD8alpha− dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327:8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein RS. Regulation of neuroinflammation: the role of CXCL10 in lymphocyte infiltration during autoimmune encephalomyelitis. J Cell Biochem. 2004;92:213–222. doi: 10.1002/jcb.20052. [DOI] [PubMed] [Google Scholar]
- 20.Karman J, Chu HH, Co DO, Seroogy CM, Sandor M, Fabry Z. Dendritic cells amplify T cell-mediated immune responses in the central nervous system. J Immunol. 2006;177:7750–7760. doi: 10.4049/jimmunol.177.11.7750. [DOI] [PubMed] [Google Scholar]
- 21.Israelsson C, Bengtsson H, Kylberg A, Kullander K, Lewen A, Hillered L, Ebendal T. Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J Neurotrauma. 2008;25:959–974. doi: 10.1089/neu.2008.0562. [DOI] [PubMed] [Google Scholar]
- 22.Itoh T, Satou T, Ishida H, Nishida S, Tsubaki M, Hashimoto S, Ito H. The relationship between SDF-1alpha/CXCR4 and neural stem cells appearing in damaged area after traumatic brain injury in rats. Neurol Res. 2009;31:90–102. doi: 10.1179/174313208X332995. [DOI] [PubMed] [Google Scholar]
- 23.Schutt RC, Burdick MD, Strieter RM, Mehrad B, Keeley EC. Plasma CXCL12 levels as a predictor of future stroke. Stroke. 2012;43:3382–3386. doi: 10.1161/STROKEAHA.112.660878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales DM, Silver SA, Morgan CD, Mercer D, Inder TE, Holtzman DM, Wallendorf MJ, Rao R, McAllister JP, Limbrick DD., Jr Lumbar cerebrospinal fluid biomarkers of posthemorrhagic hydrocephalus of prematurity: amyloid precursor protein, soluble amyloid precursor protein alpha, and L1 cell adhesion molecule. Neurosurgery. 2016;80(1):82–90. doi: 10.1227/NEU.0000000000001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellons JC, 3rd, Holubkov R, Browd SR, Riva-Cambrin J, Whitehead W, Kestle J, Kulkarni AV. Hydrocephalus Clinical Research N: the assessment of bulging fontanel and splitting of sutures in premature infants: an interrater reliability study by the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 2013;11:12–14. doi: 10.3171/2012.10.PEDS12329. [DOI] [PubMed] [Google Scholar]
- 26.O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245–249. doi: 10.1159/000028730. [DOI] [PubMed] [Google Scholar]
- 27.Baumeister FA, Pohl-Koppe A, Hofer M, Kim JO, Weiss M. IL-6 in CSF during ventriculitis in preterm infants with posthemorrhagic hydrocephalus. Infection. 2000;28:234–236. doi: 10.1007/s150100070043. [DOI] [PubMed] [Google Scholar]
- 28.Whitelaw A, Christie S, Pople I. Transforming growth factor-beta1: a possible signal molecule for posthemorrhagic hydrocephalus? Pediatr Res. 1999;46:576–580. doi: 10.1203/00006450-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Szpecht D, Wiak K, Braszak A, Szymankiewicz M, Gadzinowski J. Role of selected cytokines in the etiopathogenesis of intraventricular hemorrhage in preterm newborns. Childs Nerv Syst. 2016;32:2097–2103. doi: 10.1007/s00381-016-3217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 31.Di Paolo NC, Shayakhmetov DM. Interleukin 1alpha and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rochfort KD, Collins LE, McLoughlin A, Cummins PM. Tumour necrosis factor-alpha-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6. J Neurochem. 2016;136:564–572. doi: 10.1111/jnc.13408. [DOI] [PubMed] [Google Scholar]
- 33.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 34.Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB. Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. J Comp Neurol. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- 35.Welser-Alves JV, Milner R. Microglia are the major source of TNF-alpha and TGF-beta1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem Int. 2013;63:47–53. doi: 10.1016/j.neuint.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gahring LC, Carlson NG, Kulmar RA, Rogers SW. Neuronal expression of tumor necrosis factor alpha in the murine brain. Neuroimmunomodulation. 1996;3:289–303. doi: 10.1159/000097283. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 38.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 39.Rochfort KD, Cummins PM. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans. 2015;43:702–706. doi: 10.1042/BST20140319. [DOI] [PubMed] [Google Scholar]
- 40.Rothaug M, Becker-Pauly C, Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochem Biophys Acta. 2016;1863:1218–1227. doi: 10.1016/j.bbamcr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Seder RA, Paul WE, Davis MM. Fazekas de St Groth B: the presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zundler S, Neurath MF. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 2015;26:559–568. doi: 10.1016/j.cytogfr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Eder C. Mechanisms of interleukin-1beta release. Immunobiology. 2009;214:543–553. doi: 10.1016/j.imbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 45.Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME. Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. J Neuroimmunol. 1995;60:143–150. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 47.Okamura T, Katayama T, Obinata C, Iso Y, Chiba Y, Kobayashi H, Yamada Y, Harashima H, Minami M. Neuronal injury induces microglial production of macrophage inflammatory protein-1alpha in rat corticostriatal slice cultures. J Neurosci Res. 2012;90:2127–2133. doi: 10.1002/jnr.23105. [DOI] [PubMed] [Google Scholar]
- 48.Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010;7:1. doi: 10.1186/1742-2094-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am J Pathol. 1998;153:31–37. doi: 10.1016/S0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stefini R, Catenacci E, Piva S, Sozzani S, Valerio A, Bergomi R, Cenzato M, Mortini P, Latronico N. Chemokine detection in the cerebral tissue of patients with posttraumatic brain contusions. J Neurosurg. 2008;108:958–962. doi: 10.3171/JNS/2008/108/5/0958. [DOI] [PubMed] [Google Scholar]
- 51.Helmy A, De Simoni MG, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol. 2011;95:352–372. doi: 10.1016/j.pneurobio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmin S, Hojeberg B. In situ detection of intracerebral cytokine expression after human brain contusion. Neurosci Lett. 2004;369:108–114. doi: 10.1016/j.neulet.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 54.Jaerve A, Muller HW. Chemokines in CNS injury and repair. Cell Tissue Res. 2012;349:229–248. doi: 10.1007/s00441-012-1427-3. [DOI] [PubMed] [Google Scholar]
- 55.Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe SD, Masiarz F, Coit D, Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J Exp Med. 1988;168:2251–2259. doi: 10.1084/jem.168.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolpe SD, Davatelis G, Sherry B, Beutler B, Hesse DG, Nguyen HT, Moldawer LL, Nathan CF, Lowry SF, Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lalor SJ, Segal BM. Lymphoid chemokines in the CNS. J Neuroimmunol. 2010;224:56–61. doi: 10.1016/j.jneuroim.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32:2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 59.Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis SM, Lassmann H, Ransohoff RM. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- 60.Williams JL, Holman DW, Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. 2014;8:154. doi: 10.3389/fncel.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krumbholz M, Theil D, Steinmeyer F, Cepok S, Hemmer B, Hofbauer M, Farina C, Derfuss T, Junker A, Arzberger T, Sinicina I, Hartle C, Newcombe J, Hohlfeld R, Meinl E. CCL19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J Neuroimmunol. 2007;190:72–79. doi: 10.1016/j.jneuroim.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 62.Israelsson C, Bengtsson H, Lobell A, Nilsson LN, Kylberg A, Isaksson M, Wootz H, Lannfelt L, Kullander K, Hillered L, Ebendal T. Appearance of Cxcl10-expressing cell clusters is common for traumatic brain injury and neurodegenerative disorders. Eur J Neurosci. 2010;31:852–863. doi: 10.1111/j.1460-9568.2010.07105.x. [DOI] [PubMed] [Google Scholar]
- 63.Helmy A, Antoniades CA, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Principal component analysis of the cytokine and chemokine response to human traumatic brain injury. PLoS ONE. 2012;7:e39677. doi: 10.1371/journal.pone.0039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Guttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, Jack RS, Henn V, Kroczek RA. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 65.Lei Y, Takahama Y. XCL1 and XCR1 in the immune system. Microbes Infect. 2012;14:262–267. doi: 10.1016/j.micinf.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Gomez DG, DiBenedetto AT, Pavese AM, Firpo A, Hershan DB, Potts DG. Development of arachnoid villi and granulations in man. Acta Anat (Basel) 1982;111:247–258. doi: 10.1159/000145473. [DOI] [PubMed] [Google Scholar]
- 67.Chazal J, Tanguy A, Irthum B, Janny P, Vanneuville G. Dilatation of the subarachnoid pericerebral space and absorption of cerebrospinal fluid in the infant. Anat Clin. 1985;7:61–66. doi: 10.1007/BF01654631. [DOI] [PubMed] [Google Scholar]
- 68.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–316. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study is available from Diego M. Morales (d.morales@wustl.edu) upon reasonable request.


