Abstract
Macrophages are essential for protection against influenza A virus infection, but are also implicated in the morbidity and mortality associated with severe influenza disease, particularly during infection with highly pathogenic avian influenza (HPAI) H5N1 virus. While influenza virus infection of macrophages was once thought to be abortive, it is now clear that certain virus strains can replicate productively in macrophages. This may have important consequences for the antiviral functions of macrophages, the course of disease and the outcome of infection for the host. In this article, we review findings related to influenza virus replication in macrophages and the impact of productive replication on macrophage antiviral functions. A clear understanding of the interactions between influenza viruses and macrophages may lead to new antiviral therapies to relieve the burden of severe disease associated with influenza viruses.
Keywords: Influenza A virus, macrophage, replication
Abbreviations
AM, alveolar macrophage; BAL, bronchoalveolar lavagep; BMDM, bone marrow-derived macrophage; pH1N1, pandemic H1N1; HA, hemagglutinin; HPAI, highly pathogenic avian influenza; IAV, influenza A virus; LPS, lipopolysaccharide; MDM, monocyte-derived macrophage; MSR1, macrophage scavenger receptor 1; NA, neuraminidase; NP, nucleoprotein; PBMC, peripheral blood mononuclear cell; PEC, peritoneal cavity; PI3K, phosphoinositide 3-kinase.
Introduction
Influenza A viruses (IAVs) have a worldwide distribution and cause annual epidemics of acute respiratory illness. Additionally, over the past 100 years global influenza pandemics have occurred approximately every 10–30 years and are associated with higher mortality rates than seasonal influenza. The threat of future pandemics, an incomplete understanding of the mechanisms of enhanced disease severity in certain patient populations and with particular virus strains, and the use of a sub-optimal, though effective, vaccine, combine to highlight the need to better understand influenza virus pathogenesis in humans.
Influenza viruses have a segmented RNA genome coding for at least 10 viral proteins. Two of these proteins, the haemagglutinin (HA) and neuraminidase (NA), are responsible for virus entry and release, respectively, and determine the virus subtype. Sixteen HA and nine NA subtypes have been discovered in wild waterfowl, which are the natural host reservoir for all known IAVs. While only H1N1 and H3N2 IAV are currently established in humans, novel strains emerge periodically, often from wild or domestic birds, to cause human disease [1–3]. While these outbreaks are not always widespread, they often come with a higher mortality rate than seasonal influenza virus. For example, highly pathogenic avian influenza (HPAI) H5N1 first emerged to infect humans in 1997 in Hong Kong and has caused nearly 900 confirmed cases as of March 2017, with a reported mortality rate of ~50 %. The mechanism of exacerbated disease severity following H5N1 infection is not entirely understood.
Epithelial cells lining the respiratory mucosa are the major cell type supporting IAV replication in humans. Given their location, other cell types, such as macrophages, may be among the first cells to respond to IAV infection. Virus antigen can be detected in macrophages in vivo [4–6], suggesting that these cells may become directly infected by IAV. Macrophages exist in the lung and various fluid compartments as a heterogeneous population of cells that act as sentinels to detect and respond to infection. As professional phagocytic cells, macrophages clear infectious particles by internalization and lysosomal degradation, remove cellular debris through the uptake of apoptotic cells, and aid in the adaptive immune response via antigen presentation to T cells. Macrophages produce a wide range of cytokines that balance immune-mediated tissue damage with repair and homeostatic maintenance. The manner in which macrophages respond to IAV infection is complex, with >1000 cellular genes being up- or down-regulated following infection [7]. Thus, the response of these cells to IAV infection is likely to be of critical importance to disease outcome. The importance of macrophages for protection against IAV infection is well established, as ablation of macrophages enhances IAV-associated immunopathology and mortality [8–12]. The importance of macrophages during IAV infection goes beyond protection from virus-induced pneumonia. Experimental IAV infection in mice causes a transient depletion of alveolar macrophages, resulting in enhanced susceptibility to secondary bacterial infection and pneumonia [13]. On the other hand, macrophages have been implicated in the pathogenesis of severe IAV disease. Excessive pulmonary infiltration of macrophages secreting enhanced levels of pro-inflammatory cytokines is a hallmark of IAV-induced severe pneumonia [14–17]. Recently, efforts to understand the role of macrophages in IAV pathogenesis have focused on whether influenza viruses have the ability to replicate productively in macrophages. It is now recognized that IAV replication in macrophages is both strain- and macrophage type-dependent, with the most compelling and consistent evidence for productive replication coming from studies performed with HPAI H5N1 viruses. In this review, we discuss the current literature related to IAV replication in macrophages and the impact of virus replication on macrophage antiviral functions. To maintain consistency with the literature summarized herein, we make a distinction between productive replication (defined as the successful replication and release of new infectious virions) and abortive replication (defined as an infection event that fails to result in the production and release of infectious virus particles). We feel that this is a useful distinction due to the impact that productive replication may have on disease progression. Despite presumably being an infrequent occurrence, extrapulmonary detection of H5N1 IAV in infected humans has been reported in the literature [18, 19]. Productive virus replication in macrophages may be the mechanism by which systemic infection occurs in these rare cases, as has already been demonstrated for other viruses [20]. Thus, an understanding of the mechanisms and impact of H5N1 IAV replication, per se, in macrophages may be important for the development of clinical interventions to prevent these rare but serious cases of disease. Regardless, the distinction that we make between productive and abortive infection does not indicate that non-productive replication comes without potentially important changes to macrophage function and infection outcome. Indeed, as we point out below, certain measures of macrophage dysregulation during IAV infection require virus entry and protein/nucleic acid accumulation independent of the release of new infectious virions, suggesting that productive replication may not always be required for changes in macrophage function to occur that will negatively impact on the host’s ability to clear virus infection.
Along the way, we will point to areas of investigation that future studies should focus on to better understand the interaction between IAV and macrophages. A clear understanding of the involvement of macrophages in both protection and pathogenesis of IAV infection may lead to targeted therapies that have the potential to reduce the burden of disease associated with these viruses.
Macrophage populations and experimental models
Macrophages exist in vivo as a heterogeneous population of cells that are tissue-resident and/or derived from bone marrow and blood monocytes. Alveolar macrophages (AMs) are tissue-resident cells found in the lumen of the airways and are involved in homeostatic regulation of airway function and early responses to pulmonary infection. Peripheral blood mononuclear cells (PBMC) from mice or humans can be cultured to generate monocyte-derived macrophages (MDMs) and are typically used experimentally to represent tissue macrophages and/or macrophages that are recruited to a site of infection or inflammation. AMs and MDMs share certain features, but also have important phenotypic and functional distinctions that should be kept in mind when interpreting data related to macrophage functions and responses to infectious threats. RAW264.7 cells are a transformed monocyte/macrophage cell line derived from ascites of a mouse tumour and offer a convenient and abundant source of cells that are commonly used to study the macrophage response to virus infection [21–23].
Given the heterogeneity of macrophage populations in vivo, a similarly diverse response to IAV infection is expected. Further, the various in vitro macrophage models have been seen to respond differently to IAV infection. For this review, rather than separating the discussion by specific populations of cells (i.e. IAV interactions with AMs versus MDMs), we will discuss related responses and experiments together, being careful to point out which specific macrophage population was used to obtain the data.
Replication of IAV in macrophages
Historically, IAV infection of macrophages was considered to be abortive, with neither peritoneal cavity (PEC) macrophages nor AMs from mice supporting productive replication of seasonal IAV [24, 25]. Likewise, human AMs were shown to be abortively infected with seasonal IAV [26]. In this case, virus nucleoprotein (NP) accumulated in 20 % of infected cells, but virus release was undetected [26]. Other studies support the findings of early experiments [11, 27–29]. However, certain seasonal H1N1 and H3N2 strains are capable of limited productive replication in mouse bone marrow-derived macrophages (BMDMs) or human MDMs, respectively [30–33]. Thus, the situation is more complex than originally thought and it is now evident that productive virus replication can occur in macrophages in a virus strain- and macrophage subtype-dependent manner. IAV replication in human macrophages is reviewed well in [34], but a brief discussion is included here and is summarized in Table 1.
Table 1. Summary of IAV replication in mouse/human macrophages.
| Author | Species | Macrophage type | Virus | Productive replication? |
|---|---|---|---|---|
| Chan et al. [30] | Mouse | BMDM | Various H1/H5 | WSN only (limited) |
| Cline et al. [39] | AM/RAW264.7 | H1–H16 | HPAI H5 only | |
| Londrigan et al. [27] | PEC/RAW264.7 | Various seasonal H1/H3 | Moderate rep. only of A/Brazil/11/78 in RAW264.7 | |
| Marvin et al. [32] | RAW264.7 | Human/avian; various HA types | HPAI H5 and WSN only | |
| Perrone et al. [17] | Lung digest | Various H1/H5 | HPAI H5 only | |
| Rodgers and Mims [24] | AM/PEC | Seasonal H1 | No | |
| Tate et al. [11] | BAL/PEC | Seasonal H1/H3 | No | |
| Tate et al. [28] | BAL | Seasonal H1/H3 | No | |
| Wells et al. [25] | PEC | seasonal H3 | No | |
| Friesenhagen et al. [38] | Human | MDM | Various H1/H5/H7 | No |
| Hoeve et al. [31] | MDM | Seasonal H3 | Yes | |
| Marvin et al. [32] | MDM | Human/avian; various HA types | HPAI H5 and WSN only | |
| Mok et al. [35] | MDM | HPAI H5 Seasonal H1 | Yes; all viruses | |
| Perrone et al. [17] | MDM | Various H1/H5 | Yes; all viruses | |
| Rodgers and Mims [26] | AM | Seasonal H1/H3 | No | |
| Sakabe et al. [36] | MDM | Various H1/H3/H5 | Variable | |
| Van Riel et al. [37] | AM/MDM | HPAI H5 Seasonal H1/H3 | Yes – H3/H5 in MDM None in AM | |
| Wang et al. [29] | AM | H1 | No | |
| Yu et al. [33] | AM/MDM | HPAI H5 Seasonal H1 | Yes – H5 only in AM; Both viruses in MDM |
Early studies on IAV replication in macrophages were performed prior to the emergence of HPAI H5N1 viruses in humans and the implication of macrophages in disease severity. The majority of studies addressing the replication of HPAI H5N1 viruses in human MDMs demonstrate productive virus replication [32, 33, 35–37]. Moreover, a direct comparison of human MDMs and AMs suggested that, while AMs did not support productive replication of IAV, an HPAI H5N1 virus was more efficient at infecting AMs than seasonal viruses were [37]. In contrast to these studies, Friesenhagen et al. showed that infection of human MDMs with either a HPAI H5 or H7 virus is abortive [38]. The use of different H5N1 strains or different cell culture methods may explain these discordant results. A comparison of the replicative capacity of seasonal low-pathogenicity H1N1, reverse genetics-derived 1918 H1N1, and HPAI H5N1 IAV demonstrated that each of the viruses was capable of productive replication in human MDMs [17]. Interestingly, however, the release of new infectious virions into the cell culture supernatant was delayed for the two H1N1 strains relative to the H5N1 viruses. This suggests that, similar to studies performed in epithelial cell culture, H5N1 IAV may possess the ability to replicate with faster kinetics compared to non-H5 strains in macrophages. The same study shows that mouse lung macrophages support productive replication of an H5N1 virus, but not of either of the two H1N1 viruses [17]. Varying results between studies may be explained by the use of different virus strains. In some cases, particularly for those instances where primary cells are used, differences may also be explained by host genetic factors. Regardless of these differences, most of the published data converge on the notion that human MDMs, but not AMs, support productive replication of certain IAV subtypes.
Most studies on IAV replication in macrophages have been performed with HPAI H5N1 or seasonal H1N1 and H3N2 viruses. Using a RAW264.7 murine macrophage cell line model, Cline et al. carried out the first systematic comparison of the replicative capacity of all 16 HA subtypes in macrophages. The results demonstrated that HPAI H5N1 IAV is unique in its ability to replicate productively in macrophages. In contrast to what has been shown for human AMs, primary mouse AMs were also shown to support productive replication of HPAI H5N1, but not an H1N1 virus [39]. A comparison of the replication of 23 different IAV strains of different HA subtypes in RAW264.7 cells also showed that HPAI H5N1 viruses and the H1N1 WSN strain were the only viruses that could productively replicate. In summary, the data on IAV replication collected in RAW264.7 cells appear to be consistent with most studies that have been performed in human MDMs and mouse lung macrophages in demonstrating that H5N1 IAVs have a unique ability to overcome blocks to virus replication in these cells.
Mechanism of IAV restriction in macrophages
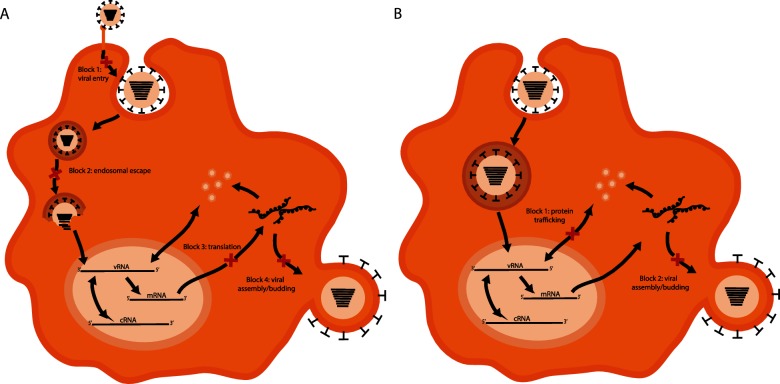
Understanding how macrophages restrict IAV replication may be important for the development of targeted therapies to mitigate severe disease caused by avian influenza viruses. Additionally, there may be applications to other pathogens that infect and replicate in macrophages to cause disease. The mechanism by which macrophages restrict IAV replication has recently begun to be elucidated. Blocks at several stages of the virus replication cycle have been described. These are described below and are summarized in Fig. 1.
Fig. 1.
IAV replication blocks in mouse and human macrophages. Various experimental models have been used to describe multiple blocks to productive IAV replication in mouse (a) and human (b) macrophages. The IAV replication cycle is depicted with the points at which macrophages restrict virus replication indicated by a red ‘X’. (a) In mouse macrophages, four blocks have been shown to exist. (1) Inefficient attachment and internalization. (2) An inability to successfully escape from the cellular endosome mediated by the acid stability of the viral HA protein. (3) Translation of late viral proteins. (4) Defective assembly and budding of infectious virions following protein translation. (b) In human macrophages only the late blocks have been described. (1) Dysfunctional nucleocytoplasmic trafficking of viral proteins. (2) Defective assembly and budding of infectious virions.
Virus preference for α2,6-linked or α2,3-linked sialic acid receptors does not predict whether productive replication will occur, as viruses with specificity for either sialic acid linkage can replicate productively in RAW264.7 cells, which express both receptors [32, 40]. In contrast, mouse PEC macrophages and bronchoalveolar lavage (BAL) macrophages that predominantly express α2,6-linked sialic acid receptors show poor susceptibility to infection with a PR8 IAV strain that binds preferentially to α2,3-linked sialic acids [11, 28, 41]. Variability in the receptor-binding preference of PR8 viruses of different origins has been reported, with the PR8 Warwick strain binding equally well to α2,3-linked and α2,6-linked receptors [42]. Thus, the inability of a PR8 to infect PEC and BAL macrophages may reflect variations in different PR8 viruses rather than a general restriction of α2,3 sialic acid-binding IAVs. PR8 also failed to productively replicate in RAW264.7 cells. The restriction of PR8 in RAW264.7 cells has been linked to inefficient attachment and entry [27] and to a block downstream of virus internalization but upstream of nuclear entry [32, 39]. The differences in the results presented by these investigators may be attributed to differences in the preparation or source of the exact PR8 virus used in the studies. The pandemic influenza A/California/04/2009 (CA/09) and PR8 H1N1 viruses (incapable of productive replication), and the HPAI H5N1 virus strain HK/483 (capable of productive replication), were internalized by RAW264.7 macrophages with equal efficiency, with >95 % of cells staining positive for the viral NP protein 30 min post-infection. However, by 4 h post-infection, only 13 % of CA/09-infected macrophages were positive for viral NP, in contrast to 60 % of HK/483-infected cells retaining virus antigen [39]. Consistent with this, minimal synthesis of viral RNA and proteins was observed in CA/09-infected macrophages. The ability of the HK/483 virus to replicate productively in RAW264.7 cells was HA-dependent, as expression of the HK/483 HA gene was sufficient to rescue replication of CA/09 in macrophages. Thus, the HA protein of HPAI H5N1 viruses mediates productive replication of IAV in macrophages by overcoming an early block in the replication cycle.
In addition to a block prior to nuclear entry, two post-nuclear entry replication blocks exist for viruses that do not replicate productively in RAW264.7 cells. The H3N2 virus A/HK/68 is representative of the first phenotype, wherein virus antigen accumulates in the nucleus and late virus protein translation occurs without the release of infectious virus into the culture supernatant. The second phenotype is represented by the H5N6 A/Duck/Potsdam/2216-4/84 virus. In this case, virus antigen accumulated in the nucleus, but late protein expression did not occur [32]. In contrast to mouse macrophages, the pre-nuclear entry block does not occur in human MDMs. The CA/09 virus was not restricted from nuclear entry in human MDMs, and nor was the production of viral RNA, mRNA or proteins inhibited. Despite this, infectious virus particles were not detected in the culture supernatant due to an observed defect in virus protein trafficking [32]. These data suggest that the utility of RAW264.7 cells as a model for studying blocks to IAV replication in human macrophages depends on the virus strain being used. Relevance to human infection is more likely if the experiments are performed with viruses that successfully enter the nucleus and express viral proteins without productive release of infectious virions.
Two blocks to productive replication of IAV in macrophages were also reported in a separate study using two virus strains that only differ in the HA gene. The infectivity of reverse genetics-derived PR8 was poor in RAW264.7 cells at 2 h post-infection, while a reassortant PR8 virus expressing the HA gene from influenza A/Brazil/11/78 H1N1 (RG-Braz-HA) was able to efficiently infect RAW264.7 cells [27]. The differences in infection efficiency were unrelated to sialic acid binding, as both viruses were found to bind equally well to cell surface receptors. The infectivity of PR8 was enhanced in the presence of the neuraminidase inhibitor zanamivir [27]. This suggests a role for the viral NA protein in entry into macrophages. Consistent with this notion, Marvin et al. found that a recombinant WSN virus expressing the HA protein of CA/09 retains the productive replication phenotype of WSN in macrophages, but co-expressing the CA/09 HA and NA genes on the WSN genetic background abrogates replication. The particular mechanism by which the NA protein contributes to virus entry has not been described. While RAW264.7 cells support a modest amount of productive replication of RG-Braz-HA over the first 24 h post-infection, the PR8 virus, despite enhanced entry efficiency in the presence of zanamivir, does not productively replicate, confirming the presence of a replication block downstream of nuclear entry. In contrast to RAW264.7 cells, mouse PEC macrophages did not support productive replication of either virus strain, with the block being downstream of RNA and protein synthesis and upstream of virus budding [27]. This result appears to be consistent with that of Marvin et al. and suggests that mouse PEC macrophages may also serve as a good model for the restriction of IAV replication in human macrophages.
The PR8 and Brazil/78 HA proteins differ in their glycosylation status, with the globular head of the PR8 HA being poorly glycosylated, while the Brazil/78 virus contains four glycosylation sites in the HA head [27]. Glycosylation of the HA protein may impact on the ability of the virus to interact efficiently with C-type lectins, such as the mannose receptor and the galactose-type lectin, which, in addition to sialic acid receptors, act as attachment and entry receptors for IAVs [41, 43–45]. In support of this hypothesis, IAV infection of macrophages with RG-Braz-HA in the presence of mannan or asialofetuin, which have been shown to block C-type lectin interactions, is reduced to levels similar to that of PR8 [27]. Since 2009, descendant variants of the 2009 pandemic H1N1 (pH1N1) virus have been isolated that have acquired multiple additional glycosylation sites [46, 47]. Given that the initial isolates of pH1N1 (i.e. CA/09) do not replicate in macrophages, it will be interesting to determine whether the addition of glycosylation sites on this virus enhances virus infectivity and replication in macrophages.
Upon the internalization of IAVs, the endosomal pH gradually decreases until a threshold is reached at which point the viral HA protein undergoes a conformational change facilitating fusion of the viral and endosomal membranes. This threshold, called the pH of HA activation, is virus strain-specific and has been described as a virulence determinant for IAVs in ducks and mice [48–50]. The ability of IAVs to productively replicate in RAW264.7 cells correlated positively with the expression of an acid-labile HA protein (pH of activation, 5.9), while the HA of viruses causing abortive infection was acid-stable (pH of activation, 5.4). Perhaps the CA/09 HA is not exposed to a pH in the endosome of RAW264.7 cells that is sufficiently acidic to trigger HA-mediated fusion, leading to virus retention in the endosome until fusion with the lysosome exposes the virus to its degradative enzymes. In support of this hypothesis, while there was no difference between the CA/09 (abortive in macrophages) and WSN (productive replication in macrophages) viruses in the extent or duration of virus co-localization with lysosomal markers in permissive epithelial cells, co-localization of CA/09 with the lysosome in macrophages steadily increased over the first 4 h post-infection [32]. Interestingly, differences in the pH of activation between virus strains do not affect replication in epithelial cells because endosomal acidification occurs more rapidly in these cells relative to macrophages [32]. It is intriguing to speculate that the slower pace of endosomal acidification in macrophages may have evolved, in part, to restrict infection by pathogens that enter through pH-dependent pathways. Conversely, the poorly acid-stable nature of HPAI H5N1 viruses may have evolved as a means of subverting this block to productive replication. The kinetics of endosomal acidification in macrophages of other mouse or human sources has not been described. It will be interesting to determine if the phenomenon described by Marvin et al. is only a feature of RAW264.7 cells, or if it applies to more physiologically relevant macrophages as well.
A single amino acid substitution (K58I) in the HA of influenza A/Vietnam/1203 (VN/1203) H5N1 virus lowers the pH of HA activation to resemble that of CA/09. Nuclear accumulation of NP in RAW264.7 cells infected with a mutant VN/1203 containing this HA substitution (VN-HA2-K58I) was reduced relative to that of WT VN/1203. However, the reduction was modest, with ~60 % of VN-HA2-K58I-infected cells still staining NP-positive, while only 8 % of CA/09-infected RAW264.7 cells contain NP-positive nuclei at the same time point [32]. Further, the release of infectious virus into the cell culture supernatant was equivalent to that of cells infected with wild-type VN/1203, suggesting that HA acid stability alone is not sufficient to explain the ability of H5N1 IAV to replicate productively in macrophages. It is also important to note that the pH of HA activation has not been determined for all of the viruses that were shown by Marvin et al. to be restricted at the pre-nuclear entry stage. Thus, the existence of a pre-nuclear entry block that is independent of both the pH of HA activation and HA glycosylation status cannot be ruled out.
Impact of productive replication in macrophages on IAV pathogenesis
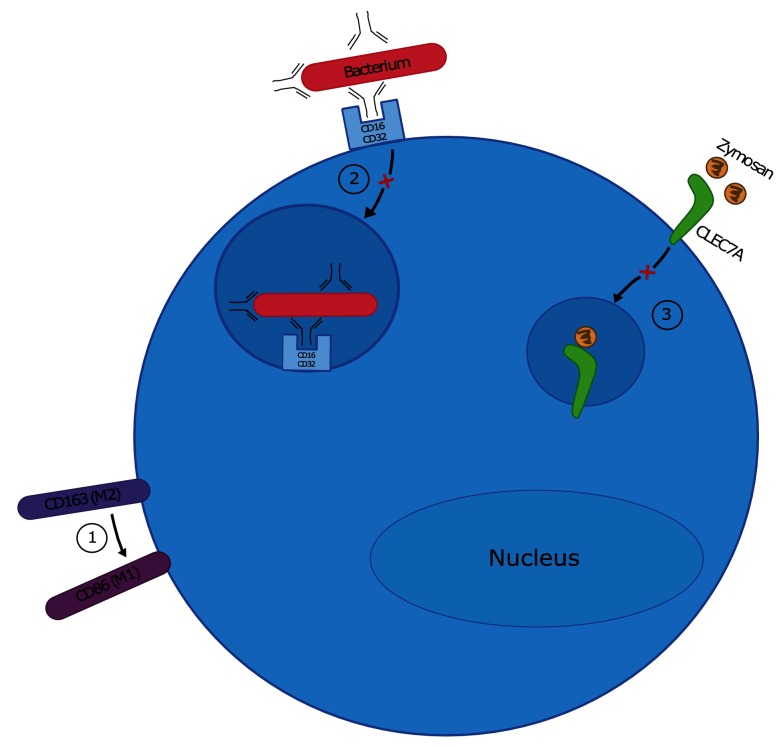
Macrophages are required for protection against IAV infection, but also contribute to severe disease associated with HPAI H5N1 infection. When the replicative capacity of a panel of eight H5N1 viruses was tested in RAW264.7 cells, only those associated with high virulence in mammals were capable of productive replication, demonstrating a correlation between replication in macrophages and disease severity [39]. Further, a reassortant CA/09 virus expressing the HA protein from a HPAI H5 virus was capable of productive replication in macrophages and was associated with higher morbidity and mortality in mice relative to the WT CA/09 virus, which does not replicate in macrophages [51]. The increased disease severity was not linked directly to the ability of the virus to replicate in macrophages in this study, but it is intriguing to speculate that productive replication of H5N1 viruses in macrophages may alter antiviral macrophage functions in ways that lead to enhanced disease severity. This is discussed below and is summarized in Fig. 2.
Fig. 2.
IAV alters the antiviral functions of macrophages. Various experimental models of IAV infection have been used to describe the impact of IAV infection on macrophage function. (1) Infection with IAV strains that replicate productively in macrophages can override the programming of M2 macrophages, causing them to phenotypically resemble pro-inflammatory M1 macrophages. (2) Infection with IAV strains that replicate productively in macrophages inhibits phagocytic uptake of opsonized bacteria by decreasing cellular expression of CD16 and CD32. (3) IAV infection decreases macrophage phagocytic capacity for zymosan by decreasing transcription of CLEC7A.
IAV replication and hypercytokinemia
Inflammatory cytokines produced by macrophages during IAV infection are important for virus clearance [8, 14, 17]. However, an exaggerated inflammatory response is believed to contribute to H5N1-associated morbidity and mortality [52–55]. For example, the resistance of pigs to severe H5N1-mediated disease correlated with the lack of a strong pro-inflammatory response following infection [56–58]. Combined with evidence that macrophages support productive replication of some IAV strains, excessive cytokine production following H5N1 infection may be a result of productive virus replication in macrophages. Of particular interest because of its role in immunopathology, the cytokine TNF-α is induced to high levels in human MDMs infected with HPAI H5N1 viruses or with a seasonal H3N2 virus [16, 52, 59]. While these studies did not directly correlate TNF-α production with virus replication, in another report, HPAI H5N1 and seasonal H3N2 IAV were shown to replicate in human MDMs [37]. Further evidence that productive replication of IAV in macrophages results in excessive cytokine expression is provided by experiments demonstrating that an HPAI H5N1, but not a seasonal H1N1 virus, induces enhanced expression of several pro-inflammatory cytokines [33, 59]. Elevated levels of TNF-α or TGF-β did not correlate with replication in MDMs, as the H3N2 virus induced significantly less of each cytokine compared to the HPAI H5N1 virus [37]. Additionally, an H1N1 virus did not productively replicate in human MDM despite inducing high expression of TGF-β [37]. The H5N1 viruses HK/483 and HK/486 both induce high levels of TNF-α in human MDMs relative to seasonal viruses, despite the fact that HK/486 does not replicate in human MDMs [32, 52]. Moreover, the induction of TNF-α in WSN-infected human MDMs is not elevated when compared to infection with seasonal H1N1 or H3N2 viruses [60]. Taken together, these findings indicate that excessive cytokine production does not always correlate with productive virus replication in macrophages.
None of these studies directly address the hypothesis that elevated cytokine production in IAV-infected macrophages requires productive virus replication. The induction of pro-inflammatory cytokines in response to HPAI H5 infection of human MDMs was inhibited by β-propiolactone-inactivation of the virus [36]. Although this result suggests that replication is required for the induction of pro-inflammatory cytokines following H5N1 IAV infection of macrophages, β-propiolactone was shown to cause a 16-fold reduction in viral HA activity [61]. Thus, decreased cytokine expression may have resulted from decreased virus attachment/entry rather than from decreased productive replication per se. When the virus was inactivated by UV irradiation, PR8-induced cytokine expression in human AMs was shown to be replication-dependent [29]. Consistent with other studies on PR8 [11, 27, 28, 32], this study reported very little, if any, productive release of PR8 into macrophage culture supernatants, even in the absence of UV inactivation. However, virus antigen in cells infected with live virus increased over the first 48 h post-infection, a response that was abrogated by UV inactivation of the virus. These results suggest that virus entry and synthesis of viral RNA/proteins is sufficient, in some cases, for the induction of pro-inflammatory cytokines in macrophages by seasonal IAV, independent of the productive assembly and release of new virus particles. To avoid the difficulties in interpretation that arise from the use of different virus strains, macrophage types and virus inactivation techniques, future studies should focus on UV inactivation of virus strains and the infection of a macrophage model for which productive replication has previously been demonstrated. A direct comparison of pro-inflammatory cytokine expression following the infection of live, or UV-inactivated, H5 and seasonal IAV strains in different sources of both human and mouse macrophages is required to adequately address the role of H5N1 virus replication in macrophage cytokine overexpression. Even so, the results from such an experiment may not be entirely conclusive, as it is likely that some variation will exist in human macrophages from different donors.
IAV replication and macrophage phagocytosis
As professional phagocytes, macrophages assist in clearing infectious organisms through the internalization and degradation of pathogens and by phagocytosis of apoptotic cells. Phagocytosis can be mediated directly through macrophage surface receptors binding to structural moieties on the phagocytic target, or indirectly through binding of macrophage Fcγ receptors (i.e. CD16, CD32) to opsonized infectious organisms.
Phagocytosis plays an important role in protection against IAV infection. The phagocytic capacity of mouse PEC macrophages is enhanced when they are cultured with IAV-infected epithelial cells [62]. Similarly, BAL macrophages from IAV-challenged mice contain engulfed apoptotic cells and AMs from IAV-infected mice show greater phagocytic capacity towards apoptotic thymocytes than AMs from uninfected mice [63, 64]. Together, these data suggest that macrophages play a role in clearing dead infected epithelial cells during IAV infection. Phagocytosis of apoptotic epithelial cells is a protective host response, as increased phagocytic capacity correlates with lower IAV lung titres in mice and inhibition of phagocytosis during IAV infection in vivo enhances virus lethality [63, 64].
Direct infection of macrophages by IAV could contribute to disease outcome by altering the phagocytic capacity of macrophages. The infection of human MDMs with a seasonal H3N2 virus strain enhanced the macrophage phagocytic capacity for apoptotic epithelial cells in a replication-dependent manner [31]. Phagocytosis of apoptotic cells is mediated by the recognition of phosphatidylserine on the surface of apoptotic cells by macrophage cell surface receptors [63, 65–67]. Thus, one mechanism for the enhancement of phagocytosis by IAV is upregulated expression of phosphatidylserine receptors on infected macrophages. Future studies should investigate this, as well as the question of whether HPAI H5N1 IAV similarly enhances macrophage phagocytosis of apoptotic cells.
While the expression of phosphatidylserine receptors on macrophages infected with IAV has not been investigated, changes in the expression of other phagocytosis receptors have been studied. In contrast to infection of human MDMs with an H3N2 IAV, PR8 infection of human AMs decreased phagocytic uptake of the fungal cell wall component zymosan. This was accompanied by a corresponding decrease in the transcription of CLEC7A, a receptor for fungal cell wall glucans [29]. PR8 infection is abortive in RAW264.7, PEC macrophages and BAL macrophages [11, 28, 32], and was, at best, shown to be only moderately productive in human AMs by Wang et al. Thus, it is unlikely that the decreased phagocytic capacity and CLEC7A expression following IAV infection are due to productive virus replication. A direct comparison of the effects on macrophage phagocytosis of live and UV-inactivated PR8 infection is necessary to conclude what role virus replication plays in decreasing phagocytic capacity.
Consistent with the work of Wang et al., RAW264.7 cells and human MDMs showed decreased phagocytic capacity towards IgG-opsonized, fluorescently tagged Staphylococcus aureus. Interestingly, this effect was only seen when the macrophages were infected with virus strains that productively replicate in these cells [32]. Decreased phagocytic uptake of opsonized bacteria correlated with a decrease in cell surface expression of CD16 and CD32. While these data suggest that IAV inhibits macrophage phagocytic capacity in a replication-dependent manner, macrophage phagocytic capacity following infection with UV-inactivated virus was not reported. Considering that the CA/09 virus used by Marvin et al. in this study is not restricted from accumulating viral protein in human MDMs, it is possible that, in this case, productive replication is required for the inhibition of phagocytosis. Alternatively, a specific interaction between a viral protein and host phagocytosis machinery that is unique to WSN and H5N1 IAV may mediate the inhibitory activity in a replication-dependent manner.
Further details on the mechanism by which IAV decreases macrophage phagocytic capacity need to be elucidated. Fc receptor-mediated phagocytosis and phagocytic uptake of apoptotic cells is dependent on the activation of phosphoinositide 3-kinase (PI3K) [68]. A PI3K inhibitor decreased expression of CD16 on microglial cells (central nervous system-resident macrophages), suggesting a link between PI3K signalling and Fc receptor expression [69]. Although seasonal H1N1 and H3N2 IAV activate the PI3K signalling pathway in mouse AMs, an H5N1 IAV fails to do the same in infected epithelial cells [70, 71]. While the status of PI3K signalling in H5-infected macrophages has not been investigated, perhaps a failure to activate the PI3K pathway in macrophages is one explanation for the observed decrease in CD16/32 expression that leads to the decreased phagocytic capacity of H5-infected macrophages.
Apart from inhibiting the uptake and clearance of apoptotic epithelial cells in the lung, how might the direct inhibition of macrophage phagocytosis impact on IAV pathogenesis? IAV infection is often made more severe by secondary bacterial infection of the upper respiratory tract [72]. While AM should serve as a protection against bacterial co-infection, bacterial clearance is impaired in a model of IAV/S. pneumoniae co-infection, and the phagocytic capacity of AM from IAV-infected mice is decreased relative to macrophages from uninfected mice [13, 73]. The macrophage scavenger receptor-1 (MSR1) and CD36 have been implicated for their role in protection against bacterial pneumonia [74, 75]. IAV infection of human AMs led to a decrease in MSR1 and CD36 mRNA, suggesting a mechanism for bacterial colonization of IAV-infected hosts [29]. However, despite decreased MSR1 and CD36 transcription, phagocytosis of bacteria by human AMs from IAV-infected mice was not impaired. This result is likely due to the fact that IAV infection decreased MSR1 and CD36 mRNA levels, but not surface expression of MSR1 and CD36 protein [29]. In contrast, decreased surface expression of CD16 and CD32 has been reported for macrophages infected with viruses that replicate productively in macrophages [32]. It is tempting to hypothesize that decreased CD16/CD32 expression on macrophages contributes to IAV pathogenesis by enhancing secondary bacterial infection. Future studies are required to determine whether CD16/CD32 expression is lowered on macrophages during IAV infection in vivo, whether this requires productive virus replication in macrophages, and whether it impacts on the ability of the host to control bacterial colonization. Moreover, the expression of other macrophage phagocytosis receptors (e.g. the LPS receptor) should be investigated to determine whether IAV infection induces a global inhibition of phagocytosis, or whether specific pathways are inhibited.
Macrophage differentiation state and susceptibility to IAV infection
Macrophages do not exist as a homogeneous population of cells in vivo. Rather, they exist on a spectrum of differentiation states with accompanying variations in function. While it is unlikely to reflect the full diversity of macrophage heterogeneity that exists in vivo, traditionally, macrophages have been characterized as either classically activated (M1) or alternatively activated (M2) macrophages. This classification is defined by the specific cytokines that drive macrophage activation and by the gene expression profile of the activated macrophage. M1 macrophages develop in response to stimulation with IFN-γ and/or lipopolysaccharide (LPS) and are considered to be pro-inflammatory, producing TNF-α and IL-1β, and the surface markers CD80 and CD86. In contrast, differentiation to the M2 phenotype occurs in the presence of IL-4 or IL-10. M2 macrophages are generally considered to be involved in tissue repair and wound healing processes and produce the cytokines IL-10 and TGF-β. Markers of M2 differentiation include arginase-1 and the surface marker CD163 [76–80].
The differentiation status of macrophages affects their susceptibility and response to IAV infection. Human MDMs differentiated to an M2 phenotype by culturing in the presence of IL-4 are more susceptible to infection with the CA/09 and HPAI H5N1 viruses relative to M1 macrophages. However, M1 versus M2 polarization had no effect on the release of infectious virions into the cell culture supernatant in this model [81]. Changes in sialic acid receptor expression could not explain differences in susceptibility to IAV infection, as both α2,3-linked and α2,6-linked receptors were present on the surface of macrophages following both IL-4 and IFN-γ treatment. Expression of the macrophage mannose receptor is upregulated in M2-polarized macrophages [78, 82]. Having been shown to be involved in IAV infection of macrophages [45], upregulation of the mannose receptor might explain the increased susceptibility of M2 macrophages to IAV infection, although this has not been tested directly.
Mouse BMDMs cultured in the presence of IL-4 were also more susceptible to the WSN IAV strain than cells polarized to an M1 phenotype by IFN-γ. However, despite being more susceptible to IAV infection and supporting greater production of viral RNA, M2 macrophages did not support productive IAV replication to a greater extent than M1 macrophages [83].
M2 macrophages were more susceptible to apoptotic cell death than classically activated M1 macrophages following IAV infection [83]. While IAV-infected M1 macrophages expressed the highest levels of pro-inflammatory cytokines such as TNF-α, IAV infection of M2 macrophages was able to override the anti-inflammatory cytokine profile of these cells and cause them to secret TNF-α and other M1-like cytokines. Importantly, this programming override to a more pro-inflammatory phenotype was only seen during the infection of macrophages with WSN and HPAI H5N1, strains that have been shown to replicate productively in macrophages [32, 39]. However, a requirement for productive replication for the M2 to M1 phenotype switch has not been investigated directly. The model that appears to be forming, then, is that IAV can infect both M1 and M2 macrophages. M1 macrophages are protected from IAV-induced apoptosis relative to M2 macrophages, but, prior to cell death, M2 macrophages experience a shift to an M1-like cytokine expression profile in what may be a virus replication-dependent manner. Together, this may contribute to the hypercytokinemia and enhanced inflammatory response following severe H5N1 infection.
Concluding remarks
Influenza viruses continue to burden even the most highly developed healthcare systems, and the continued incidence of human infection with novel influenza viruses highlights the deficiencies in our understanding of severe influenza disease and our ability to control zoonotic infections of humans. Of particular interest is the continued global spread of HPAI H5N1 viruses and their derivatives in wild waterfowl and domestic poultry, with the threat of spillover into human populations [84–87]. A multi-faceted approach to influenza pandemic preparedness is necessary and one aspect of this is to develop improved therapeutic treatments. This requires a better understanding of the mechanisms of virus pathogenesis. In addition to respiratory epithelium, IAV can also infect macrophages [4, 88, 89]. A consensus on the fate of IAV infection of macrophages and its impact on virus pathogenesis has not been reached. This is due, in part, to the use of varied virus strains and macrophage types, which have given rise to contrasting results. Compounding the problem, variations in the cell culture and infection protocols employed by different laboratories are likely to result in different conclusions. Further, a better understanding of the complex interactions between lung-resident and infiltrating macrophages with the inflammatory milieu present in the lungs before and during virus infection is necessary to piece together an accurate picture of what role macrophages play during IAV infection. As these processes are highly dynamic, an organized and systematic approach to these questions must be undertaken in order to fully understand the impact of IAV infection of macrophages on virus pathogenesis. Regardless, it is evident that some IAV strains have the capacity to replicate productively in macrophages and impact on their antiviral functions. A determination of whether productive virus replication in macrophages occurs in vivo, and which specific macrophage subsets support replication in vivo, will be important for developing a complete picture of how the infection of macrophages impacts on disease outcome.
Most studies have focused on IAV infection and replication in murine and human macrophages, with few studies investigating the potential for IAV to replicate in avian macrophages, or macrophages from other mammalian hosts [90–92]. Lymphoid necrosis associated with virulent H5N9 IAV infection of chickens correlated with the ability of the virus to infect macrophages [93]. The infection of macrophages with several viruses contributes to increased virus dissemination [20]. Because HPAI H5N1 viruses replicate in multiple tissues of an infected chicken, it will be interesting to determine whether infected macrophages act to disseminate the virus to various tissues. Given the zoonotic potential of influenza viruses and the economic burden of IAV infection in commercially important animals, it will be important to determine the extent to which IAV replication occurs in these macrophages, and whether this contributes to disease severity.
Funding information
T. D. C is supported by R15 award #AI111307-01A1 from the National Institute of Allergy and Infectious Diseases.
Acknowledgements
We thank Analucia Barragan Trejo and Renee Margolin for critical reading of the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
No person employed by the funder played a role in the preparation of this manuscript or in the decision to submit to this journal.
References
- 1.Dugan VG, Chen R, Spiro DJ, Sengamalay N, Zaborsky J, et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 2008;4:e1000076. doi: 10.1371/journal.ppat.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen Hadisoedarsuno W, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355:2186–2194. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- 3.Peiris JS, de Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007;20:243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, et al. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci USA. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogiwara H, Yasui F, Munekata K, Takagi-Kamiya A, Munakata T, et al. Histopathological evaluation of the diversity of cells susceptible to H5N1 virulent avian influenza virus. Am J Pathol. 2014;184:171–183. doi: 10.1016/j.ajpath.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wonderlich ER, Swan ZD, Bissel SJ, Hartman AL, Carney JP, et al. Widespread virus replication in alveoli drives acute respiratory distress syndrome in aerosolized H5N1 influenza infection of macaques. J Immunol. 2017;198:1616–1626. doi: 10.4049/jimmunol.1601770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lietzén N, Ohman T, Rintahaka J, Julkunen I, Aittokallio T, et al. Quantitative subcellular proteome and secretome profiling of influenza A virus-infected human primary macrophages. PLoS Pathog. 2011;7:e1001340. doi: 10.1371/journal.ppat.1001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HM, Lee YW, Lee KJ, Kim HS, Cho SW, et al. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol. 2008;82:4265–4274. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purnama C, Ng SL, Tetlak P, Setiagani YA, Kandasamy M, et al. Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur J Immunol. 2014;44:2003–2012. doi: 10.1002/eji.201344359. [DOI] [PubMed] [Google Scholar]
- 10.Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10:e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84:7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumpey TM, García-Sastre A, Taubenberger JK, Palese P, Swayne DE, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoneim HE, Thomas PG, Mccullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol. 2013;191:1250–1259. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Högner K, Wolff T, Pleschka S, Plog S, Gruber AD, et al. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 2013;9:e1003188. doi: 10.1371/journal.ppat.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 16.Lee SM, Gardy JL, Cheung CY, Cheung TK, Hui KP, et al. Systems-level comparison of host-responses elicited by avian H5N1 and seasonal H1N1 influenza viruses in primary human macrophages. PLoS One. 2009;4:e8072. doi: 10.1371/journal.pone.0008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrone LA, Plowden JK, García-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 19.Zhou JJ, Fang DY, Fu J, Tian J, Zhou JM, et al. Infection and replication of avian influenza H5N1 virus in an infected human. Virus Genes. 2009;39:76–80. doi: 10.1007/s11262-009-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klepper A, Branch AD. Macrophages and the viral dissemination super highway. EC Microbiol. 2015;2:328–336. [PMC free article] [PubMed] [Google Scholar]
- 21.Brana C, Biggs TE, Barton CH, Sundstrom LE, Mann DA. A soluble factor produced by macrophages mediates the neurotoxic effects of HIV-1 Tat in vitro. AIDS. 1999;13:1443–1452. doi: 10.1097/00002030-199908200-00002. [DOI] [PubMed] [Google Scholar]
- 22.Steurbaut S, Merckx E, Rombaut B, Vrijsen R. Modulation of viral replication in macrophages persistently infected with the DA strain of Theiler's murine encephalomyelitis virus. Virol J. 2008;5:89. doi: 10.1186/1743-422X-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Bloch N, Nguyen LA, Kim B, Landau NR. SAMHD1 restricts HIV-1 replication and regulates interferon production in mouse myeloid cells. PLoS One. 2014;9:e89558. doi: 10.1371/journal.pone.0089558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers B, Mims CA. Interaction of influenza virus with mouse macrophages. Infect Immun. 1981;31:751–757. doi: 10.1128/iai.31.2.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells MA, Albrecht P, Daniel S, Ennis FA. Host defense mechanisms against influenza virus: interaction of influenza virus with murine macrophages in vitro. Infect Immun. 1978;22:758–762. doi: 10.1128/iai.22.3.758-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers BC, Mims CA. Influenza virus replication in human alveolar macrophages. J Med Virol. 1982;9:177–184. doi: 10.1002/jmv.1890090304. [DOI] [PubMed] [Google Scholar]
- 27.Londrigan SL, Short KR, Ma J, Gillespie L, Rockman SP, et al. Infection of mouse macrophages by seasonal influenza viruses can be restricted at the level of virus entry and at a late stage in the virus life cycle. J Virol. 2015;89:12319–12329. doi: 10.1128/JVI.01455-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011;24:77–88. doi: 10.1089/vim.2010.0118. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Nikrad MP, Travanty EA, Zhou B, Phang T, et al. Innate immune response of human alveolar macrophages during influenza A infection. PLoS One. 2012;7:e29879. doi: 10.1371/journal.pone.0029879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan RW, Leung CY, Nicholls JM, Peiris JS, Chan MC. Proinflammatory cytokine response and viral replication in mouse bone marrow derived macrophages infected with influenza H1N1 and H5N1 viruses. PLoS One. 2012;7:e51057. doi: 10.1371/journal.pone.0051057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeve MA, Nash AA, Jackson D, Randall RE, Dransfield I. Influenza virus A infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS One. 2012;7:e29443. doi: 10.1371/journal.pone.0029443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marvin SA, Russier M, Huerta CT, Russell CJ, Schultz-Cherry S. Influenza virus overcomes cellular blocks to productively replicate, impacting macrophage function. J Virol. 2017;91:e01417-16. doi: 10.1128/JVI.01417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu WC, Chan RW, Wang J, Travanty EA, Nicholls JM, et al. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J Virol. 2011;85:6844–6855. doi: 10.1128/JVI.02200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Short KR, Brooks AG, Reading PC, Londrigan SL. The fate of influenza A virus after infection of human macrophages and dendritic cells. J Gen Virol. 2012;93:2315–2325. doi: 10.1099/vir.0.045021-0. [DOI] [PubMed] [Google Scholar]
- 35.Mok CK, Lee DC, Cheung CY, Peiris M, Lau AS. Differential onset of apoptosis in influenza A virus H5N1- and H1N1-infected human blood macrophages. J Gen Virol. 2007;88:1275–1280. doi: 10.1099/vir.0.82423-0. [DOI] [PubMed] [Google Scholar]
- 36.Sakabe S, Iwatsuki-Horimoto K, Takano R, Nidom CA, Le M, et al. Cytokine production by primary human macrophages infected with highly pathogenic H5N1 or pandemic H1N1 2009 influenza viruses. J Gen Virol. 2011;92:1428–1434. doi: 10.1099/vir.0.030346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Riel D, Leijten LM, van der Eerden M, Hoogsteden HC, Boven LA, et al. Highly pathogenic avian influenza virus H5N1 infects alveolar macrophages without virus production or excessive TNF-alpha induction. PLoS Pathog. 2011;7:e1002099. doi: 10.1371/journal.ppat.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friesenhagen J, Boergeling Y, Hrincius E, Ludwig S, Roth J, et al. Highly pathogenic avian influenza viruses inhibit effective immune responses of human blood-derived macrophages. J Leukoc Biol. 2012;92:11–20. doi: 10.1189/jlb.0911479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cline TD, Karlsson EA, Seufzer BJ, Schultz-Cherry S. The hemagglutinin protein of highly pathogenic H5N1 influenza viruses overcomes an early block in the replication cycle to promote productive replication in macrophages. J Virol. 2013;87:1411–1419. doi: 10.1128/JVI.02682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahata M, Iwasaki N, Nakagawa H, Abe Y, Watanabe T, et al. Sialylation of cell surface glycoconjugates is essential for osteoclastogenesis. Bone. 2007;41:77–86. doi: 10.1016/j.bone.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Ng WC, Liong S, Tate MD, Irimura T, Denda-Nagai K, et al. The macrophage galactose-type lectin can function as an attachment and entry receptor for influenza virus. J Virol. 2014;88:1659–1672. doi: 10.1128/JVI.02014-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng B, Marriott AC, Dimmock NJ. The receptor preference of influenza viruses. Influenza Other Respir Viruses. 2010;4:147–153. doi: 10.1111/j.1750-2659.2010.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang WC, White MR, Moyo P, McClear S, Thiel S, et al. Lack of the pattern recognition molecule mannose-binding lectin increases susceptibility to influenza A virus infection. BMC Immunol. 2010;11:64. doi: 10.1186/1471-2172-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson B, Zhou X, White M, Hartshorn K, Takahashi K, et al. Recombinant human mannose-binding lectin dampens human alveolar macrophage inflammatory responses to influenza A virus in vitro. J Leukoc Biol. 2014;95:715–722. doi: 10.1189/jlb.0313161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reading PC, Miller JL, Anders EM. Involvement of the mannose receptor in infection of macrophages by influenza virus. J Virol. 2000;74:5190–5197. doi: 10.1128/JVI.74.11.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Job ER, Deng YM, Barfod KK, Tate MD, Caldwell N, et al. Addition of glycosylation to influenza A virus hemagglutinin modulates antibody-mediated recognition of H1N1 2009 pandemic viruses. J Immunol. 2013;190:2169–2177. doi: 10.4049/jimmunol.1202433. [DOI] [PubMed] [Google Scholar]
- 47.Sun S, Wang Q, Zhao F, Chen W, Li Z. Glycosylation site alteration in the evolution of influenza A (H1N1) viruses. PLoS One. 2011;6:e22844. doi: 10.1371/journal.pone.0022844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed ML, Bridges OA, Seiler P, Kim JK, Yen HL, et al. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity and transmissibility in ducks. J Virol. 2010;84:1527–1535. doi: 10.1128/JVI.02069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholtissek C. Stability of infectious influenza A viruses at low pH and at elevated temperature. Vaccine. 1985;3:215–218. doi: 10.1016/0264-410X(85)90109-4. [DOI] [PubMed] [Google Scholar]
- 50.Zaraket H, Bridges OA, Russell CJ. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus replication and pathogenesis in mice. J Virol. 2013;87:4826–4834. doi: 10.1128/JVI.03110-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cline TD, Karlsson EA, Freiden P, Seufzer BJ, Rehg JE, et al. Increased pathogenicity of a reassortant 2009 pandemic H1N1 influenza virus containing an H5N1 hemagglutinin. J Virol. 2011;85:12262–12270. doi: 10.1128/JVI.05582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/S0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 53.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maines TR, Szretter KJ, Perrone L, Belser JA, Bright RA, et al. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev. 2008;225:68–84. doi: 10.1111/j.1600-065X.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 55.Hui KP, Lee SM, Cheung CY, Ng IH, Poon LL, et al. Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J Immunol. 2009;182:1088–1098. doi: 10.4049/jimmunol.182.2.1088. [DOI] [PubMed] [Google Scholar]
- 56.Choi YK, Nguyen TD, Ozaki H, Webby RJ, Puthavathana P, et al. Studies of H5N1 influenza virus infection of pigs by using viruses isolated in Vietnam and Thailand in 2004. J Virol. 2005;79:10821–10825. doi: 10.1128/JVI.79.16.10821-10825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipatov AS, Kwon YK, Sarmento LV, Lager KM, Spackman E, et al. Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathog. 2008;4:e1000102. doi: 10.1371/journal.ppat.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelli RK, Dunham SP, Kuchipudi SV, White GA, Baquero-Perez B, et al. Mammalian innate resistance to highly pathogenic avian influenza H5N1 virus infection is mediated through reduced proinflammation and infectious virus release. J Virol. 2012;86:9201–9210. doi: 10.1128/JVI.00244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geiler J, Michaelis M, Sithisarn P, Cinatl J. Comparison of pro-inflammatory cytokine expression and cellular signal transduction in human macrophages infected with different influenza A viruses. Med Microbiol Immunol. 2011;200:53–60. doi: 10.1007/s00430-010-0173-y. [DOI] [PubMed] [Google Scholar]
- 60.Monteerarat Y, Sakabe S, Ngamurulert S, Srichatraphimuk S, Jiamtom W, et al. Induction of TNF-alpha in human macrophages by avian and human influenza viruses. Arch Virol. 2010;155:1273–1279. doi: 10.1007/s00705-010-0716-y. [DOI] [PubMed] [Google Scholar]
- 61.Jonges M, Liu WM, van der Vries E, Jacobi R, Pronk I, et al. Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling. J Clin Microbiol. 2010;48:928–940. doi: 10.1128/JCM.02045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J Virol. 2000;74:3399–3403. doi: 10.1128/JVI.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe Y, Hashimoto Y, Shiratsuchi A, Takizawa T, Nakanishi Y. Augmentation of fatality of influenza in mice by inhibition of phagocytosis. Biochem Biophys Res Commun. 2005;337:881–886. doi: 10.1016/j.bbrc.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 65.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 66.Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, et al. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci. 2009;122:3365–3373. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi N, Karisola P, Peña-Cruz V, Dorfman DM, Jinushi M, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leverrier Y, Okkenhaug K, Sawyer C, Bilancio A, Vanhaesebroeck B, et al. Class I phosphoinositide 3-kinase p110β is required for apoptotic cell and Fcgamma receptor-mediated phagocytosis by macrophages. J Biol Chem. 2003;278:38437–38442. doi: 10.1074/jbc.M306649200. [DOI] [PubMed] [Google Scholar]
- 69.Wang P, He Y, Li D, Han R, Liu G, et al. Class I PI3K inhibitor ZSTK474 mediates a shift in microglial/macrophage phenotype and inhibits inflammatory response in mice with cerebral ischemia/reperfusion injury. J Neuroinflammation. 2016;13:192. doi: 10.1186/s12974-016-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W, Wang G, Zhang H, Shen Y, Dai J, et al. Inability of NS1 protein from an H5N1 influenza virus to activate PI3K/Akt signaling pathway correlates to the enhanced virus replication upon PI3K inhibition. Vet Res. 2012;43:36. doi: 10.1186/1297-9716-43-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X, Dai J, Xiao X, Wu L, Zeng J, et al. PI3K/Akt signaling pathway modulates influenza virus induced mouse alveolar macrophage polarization to M1/M2b. PLoS One. 2014;9:e104506. doi: 10.1371/journal.pone.0104506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith AM, McCullers JA. Secondary bacterial infections in influenza virus infection pathogenesis. Curr Top Microbiol Immunol. 2014;385:327–356. doi: 10.1007/82_2014_394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jakab GJ. Immune impairment of alveolar macrophage phagocytosis during influenza virus pneumonia. Am Rev Respir Dis. 1982;126:778–782. doi: 10.1164/arrd.1982.126.5.778. [DOI] [PubMed] [Google Scholar]
- 74.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, et al. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol. 2006;35:474–478. doi: 10.1165/rcmb.2006-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, et al. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med. 2000;191:147–156. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 77.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado JD, Popovich PG, et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sang Y, Miller LC, Blecha F. Macrophage polarization in virus-host interactions. J Clin Cell Immunol. 2015;6 doi: 10.4172/2155-9899.1000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isabelle Dutry JL, Li PH, Bruzzone R, Malik Peiris JS, Jamue M. The effects of macrophage polarity on influenza virus replication and innate immune responses. J Clin Cell Immunol. 2015;6:297. [Google Scholar]
- 82.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell GM, Nicol MQ, Dransfield I, Shaw DJ, Nash AA, et al. Susceptibility of bone marrow-derived macrophages to influenza virus infection is dependent on macrophage phenotype. J Gen Virol. 2015;96:2951–2960. doi: 10.1099/jgv.0.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arriola CS, Nelson DI, Deliberto TJ, Blanton L, Kniss K, et al. Infection risk for persons exposed to highly pathogenic avian influenza A H5 virus-infected birds, United States, December 2014-March 2015. Emerg Infect Dis. 2015;21:2135–2140. doi: 10.3201/eid2112.150904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Briand FX, Schmitz A, Ogor K, Le Prioux A, Guillou-Cloarec C, et al. Emerging highly pathogenic H5 avian influenza viruses in France during winter 2015/16: phylogenetic analyses and markers for zoonotic potential. Euro Surveill. 2017;22:pii: 30473. doi: 10.2807/1560-7917.ES.2017.22.9.30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee DH, Bahl J, Torchetti MK, Killian ML, Ip HS, et al. Highly pathogenic avian influenza viruses and generation of novel reassortants, United States, 2014–2015. Emerg Infect Dis. 2016;22:1283–1285. doi: 10.3201/eid2207.160048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Global Consortium for H5N8 and Related Influenza Viruses Role for migratory wild birds in the global spread of avian influenza H5N8. Science. 2016;354:213–217. doi: 10.1126/science.aaf8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuyama S, Katsura H, Zhao D, Ozawa M, Ando T, et al. Multi-spectral fluorescent reporter influenza viruses (Color-flu) as powerful tools for in vivo studies. Nat Commun. 2015;6:6600. doi: 10.1038/ncomms7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lakdawala SS, Shih AR, Jayaraman A, Lamirande EW, Moore I, et al. Receptor specificity does not affect replication or virulence of the 2009 pandemic H1N1 influenza virus in mice and ferrets. Virology. 2013;446:349–356. doi: 10.1016/j.virol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lyon JA, Hinshaw VS. Replication of influenza A viruses in an avian macrophage cell line. J Gen Virol. 1991;72:2011–2013. doi: 10.1099/0022-1317-72-8-2011. [DOI] [PubMed] [Google Scholar]
- 91.Kasloff SB, Weingartl HM. Swine alveolar macrophage cell model allows optimal replication of influenza A viruses regardless of their origin. Virology. 2016;490:91–98. doi: 10.1016/j.virol.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 92.Powe JR, Castleman WL. Canine influenza virus replicates in alveolar macrophages and induces TNF-α. Vet Pathol. 2009;46:1187–1196. doi: 10.1354/vp.08-VP-0229-P-FL. [DOI] [PubMed] [Google Scholar]
- 93.van Campen H, Easterday BC, Hinshaw VS. Virulent avian influenza A viruses: their effect on avian lymphocytes and macrophages in vivo and in vitro. J Gen Virol. 1989;70:2887–2895. doi: 10.1099/0022-1317-70-11-2887. [DOI] [PubMed] [Google Scholar]