Abstract
Background
Several observational studies and meta-analyses have suggested that treating hyperuricemia in patients with gout and moderate or severe chronic kidney disease (CKD) may improve renal and cardiovascular (CV) outcomes.
Objective
To evaluate the impact of initiating allopurinol or febuxostat treatment on major CV events in patients with gout, preexisting CV disease (CVD) or heart failure (HF), and stage 3 or 4 CKD in a real-world setting.
Methods
Patients with gout (aged >18 years) who initiated allopurinol or febuxostat treatment between 2009 and 2013 after a diagnosis of stage 3 or 4 CKD and CVD—including coronary artery disease (CAD), cerebrovascular disease, and peripheral vascular disease (PVD)—or HF were selected from the MarketScan databases. The major CV events included CAD-specific, cerebrovascular disease–specific, and PVD-specific events. Cox proportional hazards modeling identified the predictors of major CV events in aggregate, and of CAD, cerebrovascular disease, and PVD events, individually.
Results
During follow-up, 2426 patients (370 receiving febuxostat and 2056 receiving allopurinol; 63% male; mean age, 73 years) had 162 major CV events (3.8% in those receiving febuxostat vs 7.2% in those receiving allopurinol; P = .015). The rates of major CV events per 1000 person-years were 51.8 (95% confidence interval [CI], 28–87) in patients initiating febuxostat and 99.3 (95% CI, 84–117) among those initiating allopurinol. Overall, 49.4% of patients had a CAD event, 32.5% had a PVD event, and 23.5% had a cerebrovascular disease–specific event. Febuxostat initiation was associated with a significantly lower risk for a major CV event versus patients who initiated allopurinol (hazard ratio, 0.52; P = .02), driven in large part by lower PVD-specific events (P = .026).
Conclusion
Patients with moderate-to-severe CKD and CVD or HF who initiated febuxostat treatment had a significantly lower rate of major CV events than patients who initiated allopurinol.
Keywords: allopurinol, cardiovascular disease, chronic kidney disease, febuxostat, gout, hyperuricemia, major CV events, urate-lowering therapies, xanthine oxidase inhibitors
Gout is a metabolic disorder that causes flares of arthritis in the joints and occurs with the onset of inflammation as a result of excess serum uric acid in the blood (ie, hyperuricemia) and the deposition of crystals in tissue. Gout affects 3.9% of the US adult population,1 and its prevalence is rising as a result of the increasing rates of comorbidities that promote hyperuricemia and extensive use of thiazide and loop diuretics for the treatment of cardiovascular (CV) diseases (CVD).2–4
Patients with gout have substantial rates of renal disease and CVD.5 Among patients with gout in the 2007–2008 National Health and Nutrition Examination Survey study, 71% had stage ≥2 chronic kidney disease (CKD), 14% had a history of myocardial infarction (MI), 11% had a history of heart failure (HF), and 10% had a history of stroke.5 Conversely, the presence of CKD was associated with a significantly higher incidence and prevalence of gout, with some studies suggesting that the prevalence of gout increases 2- to 3-fold for each 30-mL/min/1.73 m2 decrease in glomerular filtration rate (GFR).6,7 Roughley and colleagues recently estimated the pooled prevalence of moderate to severe (stage ≥3) CKD in patients with gout to be 24%.8 Patients with CKD are at increased risk for CVD, and studies have suggested that they are more likely to die from CVD than to progress to end-stage renal disease (ESRD).9
KEY POINTS
-
▸
Emerging evidence suggests a link between hyperuricemia and CVD, although this continues to be the subject of some debate.
-
▸
This retrospective, real-world cohort study compares major cardiovascular events in patients with gout and concurrent CVD and chronic kidney disease who receive febuxostat or allopurinol.
-
▸
Major cardiac events were 48% lower among patients initiating febuxostat therapy versus those initiating allopurinol therapy (P = .021), driven largely by lower rates of peripheral vascular disease.
-
▸
More real-world clinical trials are needed to address challenges faced by clinicians who manage gout in renally compromised patients with multiple chronic diseases.
A number of researchers have suggested an independent link between hyperuricemia and an increased risk for metabolic syndrome, diabetes, hypertension, kidney disease, and CVD, including HF,10–18 with the relationship between hyperuricemia and renal function itself the subject of several recent systematic reviews and meta-analyses.9,19,20 Kanji and colleagues used trial data to conduct a meta-analysis that evaluated whether treating hyperuricemia in patients with stages 3 to 5 CKD might improve renal and CV outcomes.9 The investigators observed a small but significant improvement in estimated GFR (eGFR) and serum creatinine; however, they also noted, as did 2 other systematic reviews on this topic, that the analyses were limited by the overall paucity of data, which was insufficient to support an evaluation of CVD and almost exclusively focused on a single urate-lowering agent.9,19,20
Urate-lowering therapies, such as allopurinol and febuxostat, are the primary therapies for the management of patients with chronic gout and for the prevention of gout flares.21 The goal of treatment is to achieve a serum urate level of <6 mg/dL.2 However, the management of gout in patients with concomitant CVD and CKD is complex, with several factors influencing treatment choice, most notably in patients with advanced disease (ie, stage ≥3).22 A reduced starting dose of allopurinol is recommended in patients with renal impairment because of the potential for hypersensitivity reactions.23 These patients may fail to reach target serum urate, leading to an increased risk for gout flares for which nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated, and corticosteroids and colchicine are used with restriction.24
Several studies have suggested that febuxostat may be more effective in reducing serum urate levels than allopurinol,22,25–28 but concerns about the higher drug acquisition cost of febuxostat29,30 and the potential for serious CV events may also influence treatment choice.22,31,32 Conversely, some studies suggest that febuxostat may be less costly when evaluating the overall patient expenditure,33–36 particularly when evaluating CVD-specific cost,34 although theoretical models have suggested that dose escalation with allopurinol may affect its overall cost-effectiveness.37
The goal of this study was to evaluate the impact of initiating allopurinol or febuxostat treatment on major CV events in patients with gout, preexisting CVD or HF, and stage 3 or 4 CKD in a real-world setting.
Methods
This retrospective cohort study used data from the Truven MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits databases. Eligible patients (aged ≥18 years) had to have gout, stage 3 or 4 CKD, and a history of CVD or HF and received first-line treatment with a xanthine oxidase inhibitor (ie, allopurinol or febuxostat) for ≥31 days of continuous therapy between January 1, 2009, and June 30, 2013. Gout was defined as ≥1 diagnoses of gout (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 274.xx) and CKD was defined as stage 3 or 4 disease (ICD-9-CM 585.3 or 585.4) on a nondiagnostic claim during the 12-month baseline period after the study index date (ie, the date of initiating allopurinol or febuxostat).
A history of CVD was defined as an ICD-9-CM diagnosis (see Appendix Table 1, at www.AHDBonline.com) of coronary artery disease (CAD), cerebrovascular disease, or peripheral vascular disease (PVD) on ≥1 inpatient claims or ≥2 nondiagnostic outpatient claims, or evidence of a previous revascularization procedure or nontraumatic lower-extremity amputation (see Appendix Table 2, at www.AHDBonline.com). Patients were included if they were continuously enrolled in the MarketScan databases for ≥12 months before (baseline) and ≥31 days after the index date, with complete data availability.
Patients were excluded if they received pegloticase ≤2 months before the index date, or if they had evidence of organ transplant at baseline, ESRD (ie, dialysis, a diagnosis of stage 5 CKD), a nonskin malignancy, or HIV/AIDS. Patients whose baseline CV or HF status could not be determined were also excluded from the study. Enrolled patients were followed until their disenrollment from the MarketScan databases, discontinuation of the qualifying study agent, use of the alternate study agent, onset of any of the exclusion disease states, or postindex exposure to pegloticase. Treatment discontinuation was defined as no additional evidence of a prescription for the qualifying agent or a gap of >45 days in the available supply of medication.
The primary outcome was the occurrence of major CV events during follow-up. The 3 types of major CV events included CAD-specific, cerebrovascular disease–specific, and PVD-specific events. CAD-specific major CV events required evidence of MI or coronary revascularization. Acute MI was captured using a validated definition38 (sensitivity, 79%; specificity, 99.5%; positive predictive value, 86.1%), which required a discharge diagnosis of MI (ICD-9-CM 410.x0 or 410.x1) in the primary or secondary diagnosis position on an inpatient claim. CAD revascularization was defined as coronary artery bypass graft and/or percutaneous revascularization procedures.
Cerebrovascular disease–specific major CV events included ischemic or hemorrhagic stroke, transient ischemic attack, or cerebrovascular disease revascularization. Patients were classified as having an ischemic or hemorrhagic stroke if they had ≥1 inpatient claims with a discharge ICD-9-CM diagnosis code (primary or secondary) of 433.x1, 434.xx-436.xx (ischemic), or 430.xx-431.xx (hemorrhagic). CVD-specific revascularization was defined as embolectomy, thrombectomy, thromboendarterectomy, percutaneous revascularization, and/or bypass graft.
PVD-specific major CV events included lower-limb amputation (ie, toe, metatarsal, foot, ankle, thigh, leg, or abdominopelvic) classified as nontraumatic (ie, no evidence of ICD-9-CM diagnosis codes 800–999) and lower-limb revascularization, which was defined as resection, atherectomy, endarterectomy, or angioplasty.
The secondary outcome was the incidence of gout flares, identified based on an algorithm that defined an acute gout attack using 1 of 2 main criteria of (1) an office visit for gout (ICD-9-CM 274.xx), with the diagnosis of gout in any position on the claim and a new dispensing of colchicine, selective or nonselective NSAIDs, or oral or injectable glucocorticoids within 14 days of the office visit, or (2) an emergency department or inpatient visit coded (diagnosis in any position on the claim) for gout.39 A clean period or “gap” of 21 days between attacks was required.
Cox proportional hazards models assessed the predictors of any major CV event in aggregate, and of CAD, cerebrovascular disease, and PVD events individually. The Cox proportional hazards model was tested to ensure that proportionality assumptions were met. Appropriate fit was assessed using the likelihood ratio, score, and Wald tests. Explanatory variables tested for inclusion in the model included basic demographics, baseline CVD type, baseline history of other comorbid conditions, baseline medication exposure, and CKD stage nearest the index date.
Measures of gout severity in the model included baseline evidence of tophi (ICD-9-CM diagnosis 274.03, 274.81, or 274.82), baseline gout flare frequency, and acute gout flare medications. For the secondary outcome of gout flares, an additional extended Cox model (Anderson-Gill) was developed to assess the relative hazard of the repeated measure of gout flare.
Results
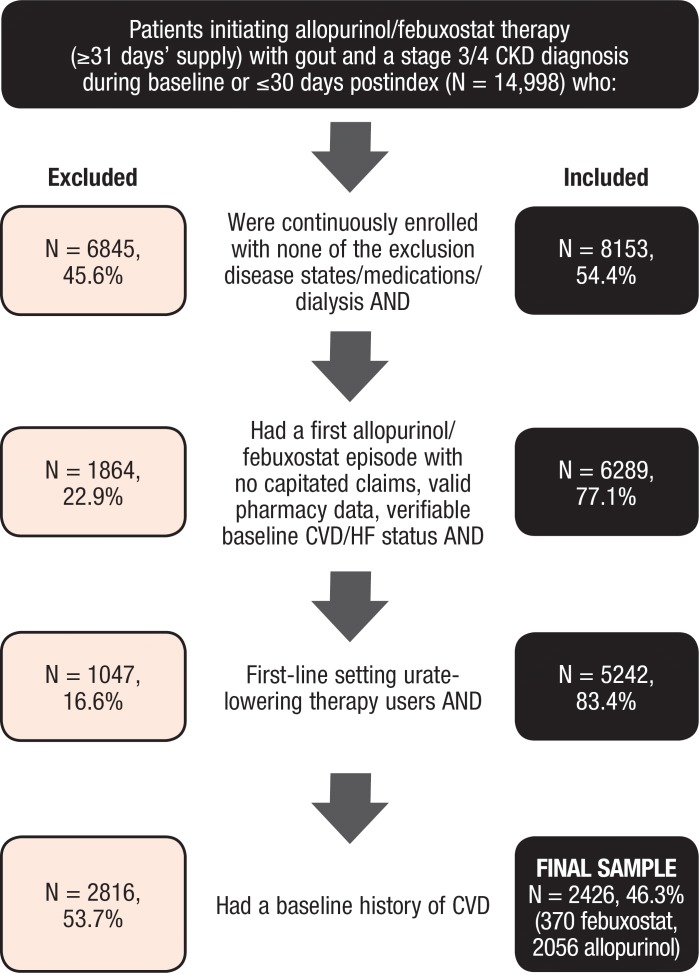
Among the 1.2 million adults with a diagnosis of gout in the MarketScan databases between January 1, 2009, and June 30, 2013, a total of 14,998 patients who initiated treatment with allopurinol or with febuxostat had at least a 31-day supply of the drug and a diagnosis of stage 3 or 4 CKD. Figure 1 depicts the impact of the population selection criteria.
Figure 1. Study Eligibility and Attrition.
CKD indicates chronic kidney disease; CVD, cardiovascular disease; HF, heart failure.
The final study population included 2426 patients (370 receiving febuxostat and 2056 receiving allopurinol). The mean preperiod enrollment was fixed at 12 months, and the mean duration during follow-up was 9 months (standard deviation [SD], 7.5 months) in the febuxostat cohort and 9.2 months (SD, 8.2 months) in the allopurinol cohort. The primary reason for the end of observation window was the discontinuation of urate-lowering therapies or a gap in receiving urate-lowering therapies. The median daily dose was 150 mg (interquartile range [IQR], 100–250 mg) in the allopurinol cohort and 40 mg (IQR, 40–60 mg) in the febuxostat cohort.
Overall, 63.1% of the study population were male, with a similar mean age in the cohorts (73.4 years with febuxostat vs 73.1 years with allopurinol; P = .733). Both cohorts had many comorbidities, with a mean Deyo-adapted Charlson Comorbidity Index40 of 4.9 (SD, 1.8) for febuxostat and 4.8 (SD, 1.9) for allopurinol. Overall, 50% of the patients had diabetes, 39.1% had dyslipidemia, and 50.6% had evidence of HF. The majority of patients had CAD (63.5% and 67.0% with febuxostat vs allopurinol, respectively; P = .194), followed by cerebrovascular disease (17.6% vs 18.2%, respectively; P = .774) and PVD (26.5% vs 22.9%, respectively; P = .129). In all, 24% of patients had a baseline hospitalization for CVD or HF (21.4% with febuxostat vs 24.0% with allopurinol; P = .265); 70.5% and 73.1% (P = .318) had moderate (stage 3) CKD, and 24.9% and 22.3% (P = .274) had severe (stage 4) CKD. (These percentages do not add up to 100% because CKD status can change over time, and these data reflect the first 30 days after the index date.) There were no significant differences between the cohorts in baseline CKD stage. See Table 1 (at www.AHDBonline.com) for baseline comorbidities.
Table 1.
Baseline Clinical Characteristicsa
| Baseline comorbid conditions, N (%) | Allopurinol (N = 2056) | Febuxostat (N = 370) | P value |
|---|---|---|---|
| Asthma | 91 (4.4) | 10 (2.7) | .127 |
| Cerebrovascular disease (any) | 374 (18.2) | 65 (17.6) | .774 |
| Hemorrhagic stroke | 12 (0.6) | 1 (0.3) | .447 |
| Ischemic stroke | 144 (7.0) | 25 (6.8) | .864 |
| Transient ischemic attack | 77 (3.7) | 17 (4.6) | .436 |
| Occlusion/stenosis precerebral arteries | 180 (8.8) | 29 (7.8) | .563 |
| Other/unspecified hemorrhage | 6 (0.3) | 1 (0.3) | .943 |
| Other cerebrovascular disease | 107 (5.2) | 18 (4.9) | .786 |
| Cerebral/carotid revascularization | 17 (0.8) | 5 (1.4) | .327 |
| CAD/CHD (any) | 1377 (67.0) | 235 (63.5) | .194 |
| Myocardial infarction | 201 (9.8) | 29 (7.8) | .241 |
| Unstable angina | 98 (4.8) | 20 (5.4) | .599 |
| Other CHD | 1317 (64.1) | 226 (61.1) | .274 |
| Unknown CAD/CHD | 23 (1.1) | 4 (1.1) | .949 |
| Coronary revascularization | 333 (16.2) | 53 (14.3) | .365 |
| PVD (any) | 470 (22.9) | 98 (26.5) | .129 |
| Lower-extremity PAD | 411 (20.0) | 85 (23.0) | .190 |
| Abdominal aortic aneurysm | 62 (3.0) | 12 (3.2) | .815 |
| Amputation | 8 (0.4) | 3 (0.8) | .266 |
| Unknown PVD | 4 (0.2) | 2 (0.5) | .217 |
| PVD revascularization | 48 (2.3) | 11 (3.0) | .463 |
| Other circulatory system disorders | 1845 (89.7) | 331 (89.5) | .871 |
| Aneurysms (not abdominal aortic) | 19 (0.9) | 7 (1.9) | .096 |
| Cardiomyopathy | 282 (13.7) | 41 (11.1) | .170 |
| Dysrhythmias and conduction disorders | 851 (41.4) | 160 (43.2) | .506 |
| Embolism and thrombosis | 88 (4.3) | 13 (3.5) | .497 |
| Hypertension | 1588 (77.2) | 288 (77.8) | .800 |
| Infectious/inflammatory conditions | 19 (0.9) | 3 (0.8) | .832 |
| Valvular disorders | 49 (2.4) | 8 (2.2) | .796 |
| Heart failure | 1064 (51.8) | 164 (44.3) | .009 |
| Chronic liver disease | 16 (0.8) | 5 (1.4) | .273 |
| Chronic obstructive pulmonary disease | 447 (21.7) | 60 (16.2) | .016 |
| Diabetes mellitus | 1027 (50.0) | 197 (53.2) | .244 |
| Dyslipidemia | 813 (39.5) | 136 (36.8) | .312 |
| Osteoarthritis | 378 (18.4) | 72 (19.5) | .625 |
| Psoriasis | 8 (0.4) | 3 (0.8) | .266 |
| Rheumatoid arthritis | 38 (1.8) | 9 (2.4) | .453 |
| Index CKD stageb, N (%) | |||
| Stage 1 | 18 (0.9) | 4 (1.1) | .701 |
| Stage 2 | 78 (3.8) | 13 (3.5) | .794 |
| Stage 3 | 1502 (73.1) | 261 (70.5) | .318 |
| Stage 4 | 458 (22.3) | 92 (24.9) | .274 |
| Polychronic diseases, mean (SD) | 7.3 (2.4) | 7.2 (2.3) | .379 |
| CCI, mean (SD) | 4.8 (1.9) | 4.9 (1.8) | .484 |
| CVD/HF-specific hospitalizations,c N (%) | 494 (24.0) | 79 (21.4) | .265 |
| Baseline cost per month,d $, mean (SD) | 2394 (2900) | 2565 (2940) | .297 |
| Baseline medications, N (%) | |||
| Antibioticse | 1460 (71.0) | 286 (77.3) | .013 |
| Antidiabetes agents | 921 (44.8) | 183 (49.5) | .097 |
| Antigout agents | |||
| Colchicine | 732 (35.6) | 182 (49.2) | <.001 |
| Glucocorticoids | 1184 (57.6) | 235 (63.5) | .033 |
| Prescription NSAIDs | 643 (31.3) | 115 (31.1) | .941 |
| Probenecid | 26 (1.3) | 16 (4.3) | <.001 |
| Opiate analgesics and agonists | 1299 (63.2) | 233 (63.0) | .939 |
| Antihyperlipidemics | 1572 (76.5) | 284 (76.8) | .901 |
| Cardiac agents | |||
| ACE inhibitors | 883 (42.9) | 139 (37.6) | .054 |
| Angiotensin II receptor blockers | 688 (33.5) | 143 (38.6) | .053 |
| Antiarrhythmic agents | 237 (11.5) | 45 (12.2) | .726 |
| Anticoagulants | 674 (32.8) | 129 (34.9) | .433 |
| Antiplatelets | 659 (32.1) | 125 (33.8) | .512 |
| Beta blockers | 1565 (76.1) | 290 (78.4) | .346 |
| Calcium channel blockers | 893 (43.4) | 192 (51.9) | .003 |
| Digitalis preparations | 229 (11.1) | 34 (9.2) | .267 |
| Diuretics | 1647 (80.1) | 298 (80.5) | .847 |
| Hemorrheologic agents | 20 (1.0) | 4 (1.1) | .846 |
| Vasodilators | 943 (45.9) | 162 (43.8) | .456 |
| Medication classes, mean (SD) | 7.9 (2.4) | 8.3 (2.2) | .001 |
| Baseline gout severity measures, N (%) | |||
| Presence of tophi | 52 (2.5) | 17 (4.6) | .028 |
| Patients with ≥1 gout attacks | 975 (47.4) | 207 (55.9) | .003 |
| Mean (SD) number of attacks | 0.6 (0.7) | 0.8 (0.8) | <.001 |
As measured during baseline (ie, 12 months before the index date), unless otherwise noted.
CKD stage based on baseline data plus the first 30 days postindex. Each patient is represented once, by the CKD stage reported closest to index. If multiple stages were reported on the same date, we report the highest stage.
Hospitalization with a primary discharge diagnosis of CAD/CHD, cerebrovascular disease, or PVD.
Baseline monthly cost included the actual health plan paid amounts, coordination of benefits, and patient copayment, deductible, and coinsurance amount, standardized to 2013 US dollars using the medical component of the Consumer Price Index.
Antibiotic classes captured include beta-lactams, glycopeptides, aminoglycosides, quinolones, sulfonamides, and macrolides.
ACE indicates angiotensin-converting enzyme; CAD, coronary artery disease; CCI, Charlson Comorbidity Index; CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; HF, heart failure; NSAID, nonsteroidal anti-inflammatory drug; PAD, peripheral arterial disease; PVD, peripheral vascular disease; SD, standard deviation.
Although the 2 cohorts were generally well-balanced, several important and significant differences exist in baseline characteristics. The patients who initiated allopurinol were significantly more likely than patients initiating febuxostat to have a baseline diagnosis of HF (51.8% vs 44.3%, respectively; P = .009) and chronic obstructive pulmonary disease (21.7% vs 16.2%, respectively; P = .016), as well as significantly greater use of an angiotensin-converting enzyme inhibitor at baseline (42.9% vs 37.6%, respectively; P = .054). The patients who initiated febuxostat had significantly higher baseline use of calcium channel blockers compared with those who initiated allopurinol (51.9% vs 43.4%, respectively; P = .003) and antibiotics (77.3% vs 71.0%, respectively; P = .013), as well as marginally higher use of angiotensin II receptor blockers (38.6% vs 33.5%, respectively; P = .053) and antidiabetes agents (49.5% vs 44.8%, respectively; P = .097).
Patients who initiated febuxostat also had more aggressive gout during baseline than those initiating allopurinol. The patients who initiated febuxostat were significantly more likely than those initiating allopurinol to fill prescriptions for colchicine (49.2% vs 35.6%, respectively; P <.0001), glucocorticoids (63.5% vs 57.6%, respectively; P <.03), and probenecid (4.3% vs 1.3%, respectively; P <.0001). Those who initiated febuxostat were also more likely to have tophi documented at baseline (4.6% vs 2.5%, respectively; P <.03), have had at least 1 baseline gout flare (55.9% vs 47.4%, respectively; P <.003), and higher mean baseline monthly flares (SD, 0.8 vs 0.6, respectively; P <.001).
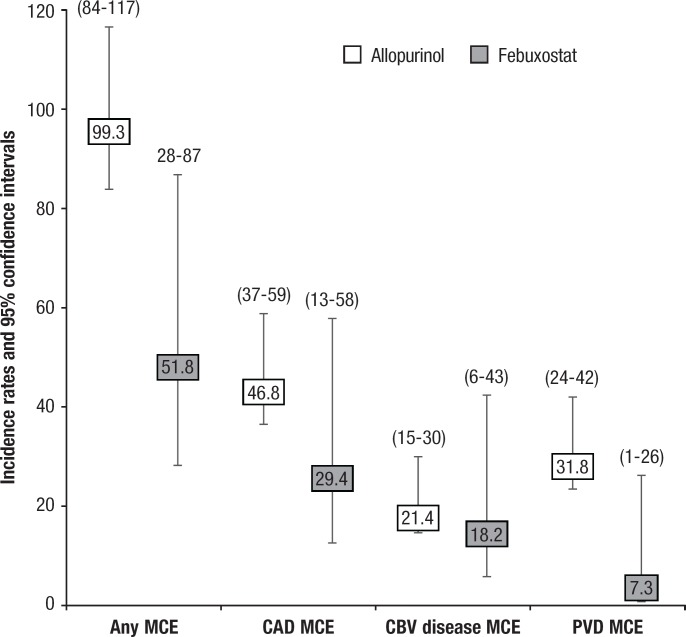
Overall, 162 (6.7%) patients had at least 1 of the major CV events during follow-up (3.8% with febuxostat vs 7.2% with allopurinol; P = .015) with an unadjusted incidence rate per 1000 person-years of 51.8 (95% confidence interval [CI], 28–87) in the febuxostat cohort and 99.3 (95% CI, 84–117) in the allopurinol cohort (Figure 2).
Figure 2. Major CV Event Rates per 1000 Person Years of Observation, Unadjusted, by Urate-Lowering Therapy Cohort.
CAD indicates coronary artery disease; CBV, cerebrovascular; CV, cardiovascular; MCE, major CV event; PVD, peripheral vascular disease.
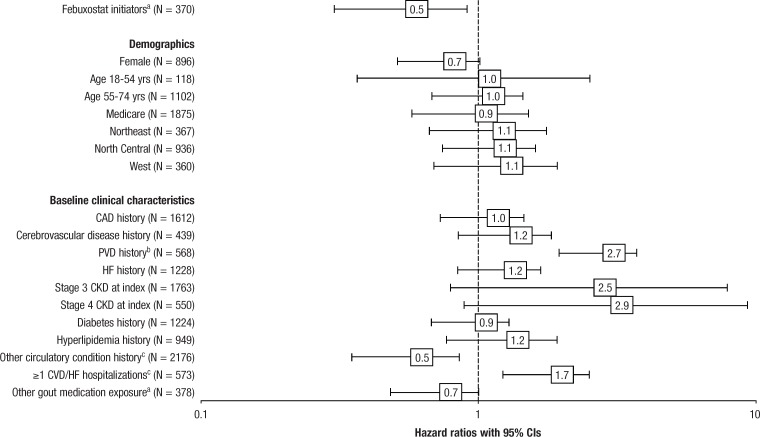
Cox model results showed a significantly increased likelihood of any major CV event among patients with PVD (hazard ratio [HR], 2.69; 95% CI, 1.95–3.71; P <.001) or a baseline CVD or HF hospitalization (HR, 1.75; 95% CI, 1.22–2.50; P = .002), and a significantly decreased likelihood of any major CV event among febuxostat initiators (HR, 0.52; 95% CI, 0.30–0.91; P = .021), patients with a baseline history of an “other” circulatory disorder (primarily hypertension, dysrhythmia, and conduction disorders; HR, 0.54; 95% CI, 0.35–0.85; P = .008), and those receiving baseline antigout medications, such as colchicine, glucocorticoids, or NSAIDs (HR, 0.69; 95% CI, 0.48–1.00; P = .049; Figure 3).
Figure 3. Multivariable Adjusted Hazard Ratios for Any Major CV Event.
aP <.05.
bP <.001.
cP <.01.
CAD indicates coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; HF, heart failure; PVD, peripheral vascular disease.
A total of 80 (3.3%) patients had a CAD-specific major CV event (2.2% with febuxostat vs 3.5% with allopurinol; P = .184), with incidence rates per 1000 person-years of 29.4 (95% CI, 13–58) and 46.8 (95% CI, 37–59) in the febuxostat and allopurinol cohorts, respectively. There was a significantly increased likelihood of CAD major CV events among patients with baseline PVD (HR, 2.04; 95% CI, 1.27–3.26; P = .003) and those with a baseline CVD or HF hospitalization (HR, 1.83; 95% CI, 1.11–3.02; P = .018).
A total of 38 (1.6%) patients had a cerebrovascular disease–specific major CV event (1.4% with febuxostat vs 1.6% with allopurinol; P = .718), with an incidence rate per 1000 person-years of 18.2 (95% CI, 6–43) for febuxostat and 21.4 (95% CI, 15–30) for allopurinol. The likelihood of a cerebrovascular-specfiic major CV event was increased among patients with baseline cerebrovascular disease (HR, 2.55; 95% CI, 1.25–5.20; P = .010) and those with a baseline CVD or HF hospitalization (HR, 2.20; 95% CI, 1.03–4.71; P = .042). In addition, a significantly decreased likelihood of cerebrovascular disease–related major CV events was seen in female patients (HR, 0.41; 95% CI, 0.19–0.91; P = .029) and those with a history of a non-CVD circulatory system disorder (HR, 0.32; 95% CI, 0.15–0.71; P = .005).
A total of 51 (2.1%) patients had a PVD-specific major CV event (0.5% with febuxostat vs 2.4% with allopurinol; P = .023), with incidence rates per 1000 person-years of 7.3 (95% CI, 1–26) and 31.8 (95% CI, 24–42) in the febuxostat and allopurinol cohorts, respectively. The likelihood of a PVD-related major CV event was significantly increased among patients with a baseline PVD event (HR, 6.02; 95% CI, 3.36–10.79; P <.001) and among those aged 55 to 74 years at the time of treatment initiation compared with being aged ≥75 years (HR, 2.05; 95% CI, 1.09–3.87; P = .027). Febuxostat initiation was associated with a significant reduction in PVD-related major CV events (HR, 0.20; 95% CI, 0.05–0.82; P = .026).
The frequency of acute gout flare (patients with ≥1 flares) was 23% overall, 27.8% for febuxostat initiators, and 22.6% for allopurinol initiators (P = .028). Unadjusted, the febuxostat cohort had a significantly higher incidence (488.0; 95% CI, 398–592) of acute gout flare per 1000 person-years than the allopurinol cohort (359.6; 95% CI, 328–394). The adjusted analysis demonstrated a significantly increased likelihood of gout flare among patients with baseline hypertension (HR, 1.29; 95% CI, 1.04–1.60; P = .019), colchicine prescription (HR, 1.26; 95% CI, 1.06–1.50; P = .010), steroid prescription (HR, 1.56; 95% CI, 1.29–1.88; P <.001), and those with ≥1 baseline gout flares (HR, 1.19; 95% CI, 1.07–1.32; P = .002). The likelihood of a gout flare was reduced significantly in patients with a diuretic prescription (HR, 0.80; 95% CI, 0.65–1.00; P = .046) and in those with stage 3 or 4 CKD (HR, 0.70; 95% CI, 0.50–0.98; P = .036).
Discussion
This is one of the first real-world, retrospective cohort studies to compare major CV event outcomes in patients with gout and concurrent CVD and CKD who initiated allopurinol or febuxostat therapy. This study follows a recent study that showed that increased gout-specific cost (P <.001) in patients initiating febuxostat treatment was offset by significantly reduced cardiac- and renal-related expenses (P <.001).34 The significantly lower major CV event rate in febuxostat initiators in the current study, which was driven in large part by lower PVD events, may help to elucidate the clinical drivers of the lower cost observed in other studies. A number of studies have assessed the impact of urate-lowering therapies on CVD-specific outcomes in the general population. MacIsaac and colleagues (2016) reported that treatment at different dosing levels of allopurinol significantly improved CVD outcomes in 2032 patients with hypertension who were aged ≥65 years using data from the United Kingdom Clinical Research Practice Datalink.41 These patients were propensity matched and compared with a control group without exposure. Active treatment with allopurinol reduced the risk for stroke by 50% and for a CV event (MI, acute coronary syndrome) by 39%; the reduction was significantly larger in patients who received higher dosages of allopurinol.
Dubreuil and colleagues also used the UK general practitioners database to assess the impact of allopurinol on mortality in patients aged ≥40 years who had at least 1 episode of hyperuricemia.42 Dubreuil and colleagues observed modest reductions in the risk for death in patients with hyperuricemia and patients with gout.42 Similarly, in a retrospective case-matched cohort study in Taiwan, Chen and colleagues assessed the impact of urate-lowering therapies (ie, allopurinol, benzbromarone) on CV mortality.43 The authors observed significantly lower all-cause mortality in the cohort receiving urate-lowering therapies, but they were constrained by the small number of mortality events in their evaluation of CVD-specific mortality.
Wei and colleagues also conducted a cohort study using a record-linked UK-based database to evaluate the impact of allopurinol on the combined outcome of nonfatal MI, nonfatal stroke, and CVD mortality.44 The authors found no significant impact of allopurinol on CVD risk; however, fewer than 50% of patients reached their target urate concentration. In addition, compared with low-dose allopurinol use, high-dose allopurinol use was associated with reduced CVD events (adjusted HR, 0.69; 95% CI, 0.50–0.94) and all-cause mortality (adjusted HR, 0.75; 95% CI, 0.59–0.94).
Finally, Kim and colleagues conducted a cohort study among patients with gout using US insurance claims data to compare incidence ratios of a composite CVD outcome (MI, coronary revascularization, stroke, or HF) for initiators of xanthine oxidase inhibitors and nonusers.45 The investigators concluded that xanthine oxidase inhibitor initiators were not at an increased or decreased risk for a CVD event, although they did note that low adherence to xanthine oxidase inhibitor therapies may explain the lack of association.
Although notable, the 5 studies discussed above did not restrict study entry based on renal function and, differences in outcome definition aside, may not be adequate benchmarks for the existing study results. In addition, all of these studies were retrospective and observational in nature. Data from clinical trials are limited. Kanji and colleagues used data from randomized controlled trials in a meta-analysis designed to evaluate whether treating hyperuricemia in patients with stages 3 to 5 CKD might improve renal and CV outcomes.9 The researchers observed a small but significant improvement in eGFR and serum creatinine, but they were unable to evaluate CVD-specific outcomes, because of the paucity of data.
The most notable and relevant trial cited, Goicoechea and colleagues conducted a 2-year, single-blind, randomized controlled trial of allopurinol treatment in patients with CKD to assess renal and CVD outcomes (ie, MI, coronary revascularization or angina pectoris, congestive HF, cerebrovascular disease, and PVD).46 Patients who received allopurinol in that study had fewer CVD events compared with the control group (HR, 0.43; 95% CI, 0.21–0.88; P = .02).46 Although the findings from this study are similar to our own, the population was not restricted to patients with CKD, concurrent gout, and preexisting CVD. Moreover, because it was a controlled trial, the population might have been less heterogeneous and more adherent to treatment than patients in the real world. Given the lack of specificity about allopurinol dosing protocols in renally compromised patients, we expect that there was less variability in the dosing levels of allopurinol.
In a subgroup analysis by Sezai and colleagues of 109 patients with CKD from the NU-FLASH trial, patients with hyperuricemia who had undergone cardiac surgery were randomized to febuxostat or to allopurinol therapy.27 The end points included serum urate levels and a variety of antioxidant and inflammatory markers. Serum creatinine, urinary albumin, cystatin-C, oxidized low-density lipoprotein, eicosapentaenoic acid/arachidonic acid ratio, high-sensitivity C-reactive protein, and serum urate values were significantly lower in the febuxostat group than in the allopurinol group.27
The results of our study may have important implications for the management of patients with gout and polychronic disease. Although the mechanisms linking elevated serum urate levels with CVD are likely multifactorial, studies have implicated low-grade systemic inflammation, xanthine oxidase activity, and the harmful effects of hyperuricemia itself on CVD.15,28 In a recent editorial, Borghi and Desideri have suggested that a decrease in serum urate levels may moderate hypertension in patients with CVD and, hence, reduce all-cause and CVD-specific mortality.15 Study results have also suggested that the alkaloid colchicine, which is indicated for the treatment of acute gout, may also reduce the risk for CV events, most particularly in patients with cardiac disease.47
Limitations
This study has several limitations. The study database was a nonrandom sample of patients, primarily with employer-sponsored coverage, and may therefore not be generalizable to other populations. The sample selection was dependent on claims-based diagnostic data, which are limited in their clinical detail.
In addition, event counts were likely constrained by the short duration of follow-up observed in this study in both cohorts. Cerebrovascular disease and PVD models were most affected by low event counts, with the number of events per predictor model lower than the customary threshold of 10. Although simulation models have suggested that type II errors are of greatest concern under these circumstances,48 it is possible, given the low prevalence of some of the predictor variables, that bias away from the null might have been exacerbated.
To maximize event counts per predictor, baseline covariates, which were marginally significant, were not included in the Cox models unless they improved the model fit and/or altered the study findings. For example, including baseline exposure to angiotensin II receptor blockers in MV models did not improve model fit or alter the study findings, because the HR (0.52) and P value (= .02) for febuxostat were unchanged.
Furthermore, because of the lack of sufficient laboratory data, it was not possible to account for differences in serum urate levels between the cohorts.
Finally, because the study design was observational, there is potential unmeasured confounding as a result of channeling bias, specifically regarding socioeconomic factors that cannot be measured in this data source, especially given the label warnings for the 2 agents considered in this study. For example, it is conceivable that clinicians are cautious in using febuxostat in patients with CVD, given the label warning about potential CV thromboembolic events; they may therefore self-select patients with comparatively stable CVD. The CARES trial is a phase 3B, multicenter, randomized, double-blind study comparing the CV safety of febuxostat and allopurinol in patients with gout and a history of major CVD or cerebrovascular disease. Initiated in 2010, the study planned to enroll and follow approximately 7500 patients until 624 major CV events occurred (approximately 9 years).49,50 It is also conceivable that clinicians are cautious in using allopurinol in patients with renal disease, given the label warning of the risk for hypersensitivity reaction. Accordingly, they may self-select patients with comparatively stable renal function.
Conclusion
The study results suggest that patients with gout and moderate-to-severe CKD and CVD or HF who initiate febuxostat treatment may have lower rates of major CV events than patients who initiate allopurinol therapy. It is unclear if this is because of channeling, allopurinol underdosing in renally impaired patients, or greater clinical effectiveness of febuxostat, either directly through lower serum urate levels or indirectly by reduced oxidative stress or other pleiotropic effects on the endothelium. This finding adds to a growing body of observational studies suggesting that lower uric acid levels are associated with fewer CV events in renally compromised patients. Clinical trials that mitigate the selection bias associated with observational studies are needed to reflect real-world challenges facing clinicians in treating renally compromised patients with gout and polychronic disease.
Acknowledgments
The authors wish to thank Amelito Torres and Laurie Costa for their SAS programming expertise and Jennifer Banovic for her editorial guidance and research support.
Source of Funding
This study was funded by Takeda Pharmaceuticals USA, Inc.
Author Disclosure Statement
Dr Foody is an employee of Janssen Pharmaceuticals; Dr Turpin and Dr Lawrence are employees of Takeda Pharmaceuticals USA; Ms Tidwell and Ms Schulman are consultants to Takeda Pharmaceuticals USA.
Contributor Information
JoAnne Foody, Associate Professor, Harvard Medical School, Boston, MA, and Executive Director–Cardiovascular, Janssen Pharmaceuticals, New Brunswick, NJ.
Robin S. Turpin, Director and Head, Health Economics and Outcomes Research, Medical Affairs, Takeda Pharmaceuticals USA, Deerfield, IL.
Beni A. Tidwell, Research Associate, Outcomes Research Solutions, Shrewsbury, MA.
Debra Lawrence, Director, Health Economics and Outcomes Research; Medical Affairs, Takeda Pharmaceuticals USA.
Kathy L. Schulman, Research Scientist and Principal, Outcomes Research Solutions, Shrewsbury, MA.
References
- 1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. [DOI] [PubMed] [Google Scholar]
- 2. Khanna D, Fitzgerald JD, Khanna PP, et al; for the American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawrence RC, Felson DT, Helmick CG, et al; for the National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suresh E, Das P. Recent advances in management of gout. QJM. 2012;105:407–417. [DOI] [PubMed] [Google Scholar]
- 5. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–687.e1. [DOI] [PubMed] [Google Scholar]
- 6. Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS One. 2012;7:e50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang W, Bhole VM, Krishnan E. Chronic kidney disease as a risk factor for incident gout among men and women: retrospective cohort study using data from the Framingham Heart Study. BMJ Open. 2015;5:e006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanji T, Gandhi M, Clase CM, Yang R. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2015;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanbay M, Segal M, Afsar B, et al. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–766. [DOI] [PubMed] [Google Scholar]
- 11. Perez-Ruiz F, Dalbeth N, Bardin T. A review of uric acid, crystal deposition disease, and gout. Adv Ther. 2015;32:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richette P, Perez-Ruiz F, Doherty M, et al. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. 2014;10:654–661. [DOI] [PubMed] [Google Scholar]
- 13. Stamp LK, Chapman PT. Gout and its comorbidities: implications for therapy. Rheumatology (Oxford). 2013;52:34–44. [DOI] [PubMed] [Google Scholar]
- 14. Grassi D, Desideri G, Di Giacomantonio AV, et al. Hyperuricemia and cardiovascular risk. High Blood Press Cardiovasc Prev. 2014;21:235–242. [DOI] [PubMed] [Google Scholar]
- 15. Borghi C, Desideri G. Urate-lowering drugs and prevention of cardiovascular disease: the emerging role of xanthine oxidase inhibition. Hypertension. 2016;67:496–498. [DOI] [PubMed] [Google Scholar]
- 16. Qin T, Zhou X, Wang J, et al. Hyperuricemia and the prognosis of hypertensive patients: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2016;18:1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braga F, Pasqualetti S, Ferraro S, Panteghini M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. 2016;54:7–15. [DOI] [PubMed] [Google Scholar]
- 18. Mallat SG, Al Kattar S, Tanios BY, Jurjus A. Hyperuricemia, hypertension, and chronic kidney disease: an emerging association. Curr Hypertens Rep. 2016;18:74. [DOI] [PubMed] [Google Scholar]
- 19. Bose B, Badve SV, Hiremath SS, et al. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2014;29:406–413. [DOI] [PubMed] [Google Scholar]
- 20. Wang H, Wei Y, Kong X, Xu D. Effects of urate-lowering therapy in hyperuricemia on slowing the progression of renal function: a meta-analysis. J Ren Nutr. 2013;23:389–396. [DOI] [PubMed] [Google Scholar]
- 21. Ye P, Yang S, Zhang W, et al. Efficacy and tolerability of febuxostat in hyperuricemic patients with or without gout: a systematic review and meta-analysis. Clin Ther. 2013;35:180–189. [DOI] [PubMed] [Google Scholar]
- 22. Abdellatif AA, Elkhalili N. Management of gouty arthritis in patients with chronic kidney disease. Am J Ther. 2014;21:523–534. [DOI] [PubMed] [Google Scholar]
- 23. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529–2536. [DOI] [PubMed] [Google Scholar]
- 24. Sarawate CA, Patel PA, Schumacher HR, et al. Serum urate levels and gout flares: analysis from managed care data. J Clin Rheumatol. 2006;12:61–65. [DOI] [PubMed] [Google Scholar]
- 25. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–2461. Erratum in: N Engl J Med. 2006;354: 1532–1533. [DOI] [PubMed] [Google Scholar]
- 27. Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU-FLASH trial for CKD). J Cardiol. 2015;66:298–303. [DOI] [PubMed] [Google Scholar]
- 28. Borghi C, Perez-Ruiz F. Urate lowering therapies in the treatment of gout: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20:983–992. [PubMed] [Google Scholar]
- 29. Beard SM, von Scheele BG, Nuki G, Pearson IV. Cost-effectiveness of febuxostat in chronic gout. Eur J Health Econ. 2014;15:453–463. Erratum in: Eur J Health Econ. 2014;15: 897. [DOI] [PubMed] [Google Scholar]
- 30. Gray CL, Walters-Smith NE. Febuxostat for treatment of chronic gout. Am J Health Syst Pharm. 2011;68:389–398. [DOI] [PubMed] [Google Scholar]
- 31. National Institute for Health and Care Excellence. Febuxostat for the management of hyperuricaemia in people with gout. NICE technology appraisal guidance [TA164]. December 17, 2008. www.nice.org.uk/Guidance/ta164. Accessed October 11, 2016.
- 32. Uloric (febuxostat) tablet [prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America; August 2017. [Google Scholar]
- 33. Gandhi PK, Gentry WM, Ma Q, Bottorff MB. Cost-effectiveness analysis of allopurinol versus febuxostat in chronic gout patients: a US payer perspective. J Manag Care Spec Pharm. 2015;21:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitri G, Wittbrodt ET, Turpin RS, et al. Cost comparison of urate-lowering therapies in patients with gout and moderate-to-severe chronic kidney disease. J Manag Care Spec Pharm. 2016;22:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smolen LJ, Gahn JC, Mitri G, Shiozawa A. Febuxostat in the management of gout: a cost-effectiveness analysis. J Med Econ. 2016;19:265–276. [DOI] [PubMed] [Google Scholar]
- 36. Smolen LJ, Gahn JC, Mitri G, Shiozawa A. The budget impact of increased use of febuxostat in the management of gout: a US health plan managed care pharmacy and medical costs perspective. Clin Ther. 2016;38:1710–1725. [DOI] [PubMed] [Google Scholar]
- 37. Jutkowitz E, Choi HK, Pizzi LT, Kuntz KM. Cost-effectiveness of allopurinol and febuxostat for the management of gout. Ann Intern Med. 2014;161:617–626. [DOI] [PubMed] [Google Scholar]
- 38. Hlatky MA, Ray RM, Burwen DR, et al. Use of Medicare data to identify coronary heart disease outcomes in the Women's Health Initiative. Circ Cardiovasc Qual Outcomes. 2014;7:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim SC, Schmidt BM, Franklin JM, et al. Clinical and health care use characteristics of patients newly starting allopurinol, febuxostat, and colchicine for the treatment of gout. Arthritis Care Res (Hoboken). 2013;65:2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 41. MacIsaac RL, Salatzki J, Higgins P, et al. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension. 2016;67:535–540. [DOI] [PubMed] [Google Scholar]
- 42. Dubreuil M, Zhu Y, Zhang Y, et al. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis. 2015;74:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen JH, Lan JL, Cheng CF, et al. Effect of urate-lowering therapy on all-cause and cardiovascular mortality in hyperuricemic patients without gout: a case-matched cohort study. PLoS One. 2015;10:e0145193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei L, Mackenzie IS, Chen Y, et al. Impact of allopurinol use on urate concentration and cardiovascular outcome. Br J Clin Pharmacol. 2011;71:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim SC, Schneeweiss S, Choudhry N, et al. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am J Med. 2015;128:653.e7–653.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65:543–549. [DOI] [PubMed] [Google Scholar]
- 47. Verma S, Eikelboom JW, Nidorf SM, et al. Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2015;15:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. [DOI] [PubMed] [Google Scholar]
- 49. White WB, Chohan S, Dabholkar A, et al. Cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular comorbidities. Am Heart J. 2012;164:14–20. [DOI] [PubMed] [Google Scholar]
- 50. ClinicalTrials.gov. Cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular comorbidities (CARES). Updated August 11, 2017. https://clinicaltrials.gov/show/NCT01101035. Accessed November 1, 2017.