Abstract
Background
Ovarian cancer is the eighth most common cancer among women, but ranks fifth in cancer-related causes of death, the majority of which are detected in late stages, after the cancer has metastasized. The CA125 test is the standard of care for assessing suspicious pelvic masses. However, the primary use of CA125 is to monitor treatment progress rather than to screen for disease, and its sensitivity is exceedingly low, unlike the multivariate assay OVA1. A cost-effective treatment of ovarian cancer requires early and accurate diagnosis of pelvic masses and reduced referrals of patients with benign tumors to a gynecologic oncologist.
Objective
To analyze the economic impact of increased utilization of a multivariate assay, such as OVA1, to guide the treatment of ovarian cancer.
Methods
The study population was drawn from Medicare and commercial health plan claims data. A budget impact model was constructed to estimate the economic consequences of substituting the multivariate assay OVA1 to replace the single biomarker assay CA125 to assess the likelihood of pelvic mass malignancy in premenopausal and/or postmenopausal women. All patients selected for the analysis had CA125 testing before surgical intervention.
Results
A total of 92,843 health plan members were included for analysis, comprising 48,113 commercially insured members and 44,730 Medicare beneficiaries. Estimates of future health plan expenditures, which were calculated from base-case assumptions, projected overall savings of $0.05 per-member per-month (PMPM) for commercially insured members and $0.01 PMPM for Medicare beneficiaries as a result of increased utilization of OVA1. Sensitivity analysis revealed potential savings of up to $0.17 PMPM for commercially insured patients and up to $0.05 for Medicare beneficiaries.
Conclusion
The results of the budget impact model support the use of OVA1 instead of CA125 by indicating that modest cost-savings can be achieved, while reaping the clinical benefits of improved diagnostic accuracy, early disease detection, and reductions in multiple, and possibly unnecessary, referrals to gynecologic oncologists.
Keywords: budget impact analysis, CA125, gynecologic oncologist, Medicare, multivariate assay, OVA1, ovarian cancer, pelvic mass, postmenopausal women, premenopausal women
KEY POINTS
-
▸
Early detection and appropriate care improve outcomes of women with ovarian cancer, but the CA125 diagnostic test is poor at identifying early-stage cancer.
-
▸
This budget impact model compared the economic impact of OVA1 and CA125 tests in guiding ovarian cancer treatment.
-
▸
The use of OVA1 could result in a 3.27% shift in cancer detection from late to early stage among premenopausal women and of 0.38% among postmenopausal women.
-
▸
This test may result in a $0.05 PMPM savings by enabling physicians to better manage patients in the appropriate care setting.
-
▸
Sensitivity analysis revealed potential savings of up to $0.17 PMPM for commercially insured women and up to $0.05 for Medicare beneficiaries.
-
▸
The OVA1 test results in modest cost-savings and improved outcomes.
Ovarian cancer is the eighth most common cancer among women, but the disease ranks fifth in cancer-related causes of death among women.1 This translates to 22,440 new ovarian cancer cases diagnosed annually in the United States.2 Of these cases, 60% are detected in late stages of disease, after the cancer has metastasized. The 5-year survival rate for late-stage ovarian cancer is only 28.9%, but when detected in an early stage, the survival rate increases to 92.2%.3 The current standard of care for assessing the risk for malignancy when evaluating a complex ovarian mass relies heavily on the single-protein biomarker CA125, which is exceedingly poor at identifying early stages of ovarian cancer.4,5
An additional concern with the existing standard of care is the unnecessary referral of low-risk patients to the limited supply of gynecologic oncologists. Although referral to a gynecologic oncologist is common practice, the majority of pelvic masses are benign and can safely and cost-effectively be removed by a general gynecologic surgeon. Moreover, it is estimated that approximately 20% of women will develop a pelvic mass or a benign ovarian cyst during their lifetime6; therefore, referring all such women to the limited number of gynecologic oncologists is not a feasible practice.
The American College of Obstetricians and Gynecologists (ACOG) released updated guidelines in November 2016 of its recommended criteria for referring patients with a pelvic mass to a gynecologic oncologist.7 Previous ACOG guidelines from 2007 recommended referral to a gynecologic oncologist based on the results of ultrasound imaging studies, the presence of ascites, the existence of a nodular or fixed pelvic mass, a family history of breast or ovarian cancer, evidence of abdominal or distant metastasis, and elevated CA125 levels.8 An elevated CA125 level indicates an increased risk for malignancy; however, a woman's CA125 level may be elevated for reasons other than ovarian cancer.9
The 2007 ACOG guidelines provided a recommended CA125 level for premenopausal women informed by expert opinion, not by clinical evidence.8 However, this recommendation was removed in the 2016 guidelines and was replaced with the statement, “no evidence-based threshold is currently available.”7 The updated guidelines now include the use of a multivariate index assay, such as OVA1, Risk of Malignancy Index, Risk of Ovarian Malignancy Algorithm, or International Ovarian Tumor Analysis, as alternatives to CA125.7 Specifically, the guidelines state that CA125 or a multivariate index assay should only be performed when level A recommendations (ultrasound and physical examination) are inconclusive.
When used as a stand-alone diagnostic to determine the risk for malignancy, the overall sensitivity of CA125 is between 68.4% and 77.0%, and declines to 61.0% to 65.7% in patients with early-stage ovarian cancer.4,5,10–12 When CA125 is used to determine the risk for malignancy in conjunction with results from ultrasound imaging and physical examination, the sensitivity rises only to 77.0% to 79.3% across all women11,13 and to 76.7% in women with early-stage cancer (47.1% for premenopausal women vs 88.2% for postmenopausal women).4,13
Early detection and appropriate intervention improve member clinical outcomes, and evidence shows that treatment costs for late-stage ovarian cancer are considerably more expensive.14 The cost of treating advanced ovarian cancer, especially in older individuals, is substantial, averaging $65,908 for the initial treatment of a Medicare beneficiary.15 Accordingly, the current dependence on a single-protein biomarker for assessing the risk for malignancy is arguably inadequate.
OVA1 combines the results of 5 single-protein biomarkers (ie, CA125, apolipoprotein A1, beta 2 microglobulin, prealbumin, and transferrin) to determine the risk for malignancy, and has an overall sensitivity of 92.2% as a stand-alone test and rises to 98.1% when accompanied by ultrasound imaging and physical examination.4,13 This represents a 23.8% increase in sensitivity versus CA125. Moreover, the sensitivity and negative predictive value of OVA1 are considerably higher for patients with early-stage ovarian cancer.13 The negative predictive value (ie, the proportion of true negative test results) for OVA1 ranges from 92.0% to 96.9%,4,5,10–13 allowing physicians to maintain clinical responsibility more confidently for patients who are at low risk for ovarian cancer rather than referring to a gynecologic oncologist for evaluation of pelvic masses that are predominately benign.
The sensitivity of OVA1 in premenopausal women is 88.2% when combined with ultrasound imaging and physical evaluation, whereas CA125 only reaches 47.1%.13 Therefore, premenopausal women are at an increased risk for failure to be diagnosed at early-stage disease, resulting in an increased mortality risk associated with advanced disease.3
Two studies have examined the economic impact of using OVA1 to determine which health plan members should be referred to a gynecologic oncologist for removal of a pelvic mass. One study found a “refer all” strategy to be least expensive, but did not account for quality-adjusted life-years (QALYs) or for patient survival rates.16 The second study, which did account for member-perceived quality of life as well as survival rates, showed that OVA1 had an incremental cost-effectiveness ratio (ICER) of $35,094 per QALY gained compared with the 2007 modified ACOG guidelines and an ICER of $12,189 per QALY gained compared with CA125 alone.17 In the United States, an ICER of <$62,000 is thought to illustrate an efficient intervention, supporting the cost-effectiveness of OVA1.18 Moreover, in the second study the “refer all” strategy was determined to be less expensive, but was associated with reduced QALYs.17
Although there is a robust body of literature to support the improved test sensitivity of OVA1 compared with the standard of care, few studies have examined the economic impact of OVA1 from the perspective of payers. The cost of cancer care is projected to increase by 27% from 2010 to 2020,19 partially because of the introduction of new treatments, and payers need to better understand the economic impact on a per-member per-month (PMPM) basis. The present study is intended to help inform health plan decision makers about the true cost of incorporating the use of OVA1 to aid the diagnosis of female members who currently rely on CA125 to determine their risk for pelvic mass malignancy.
Methods
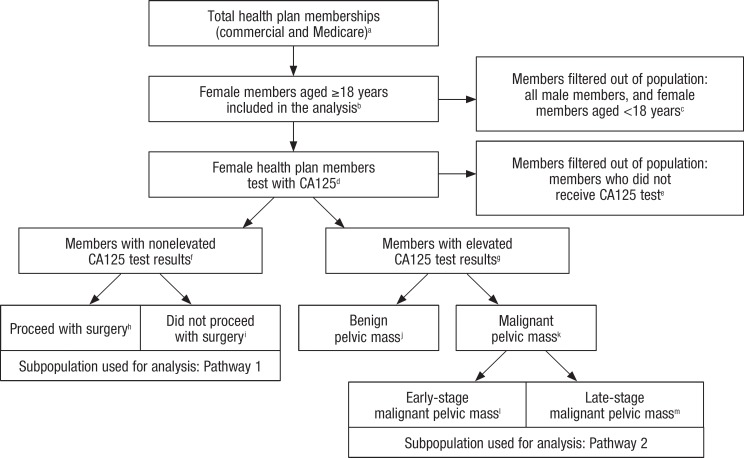
The budget impact model in this study was constructed from the perspective of health plan policymakers and comprises commercially insured and Medicare members. The model starts with a current-state scenario, in which CA125 is used along with the current clinical guidelines to determine the risk for pelvic mass malignancy.7 In this scenario, the model applies a sequence of filters to identify the relevant subset of plan members for inclusion. The Figure (see www.AHDBonline.com) provides a flow diagram of the filtering process for this study. The filtering process distributes plan members who receive a CA125 into 2 pathways: the first pathway analyzes the course of treatment for health plan members whose CA125 results were nonelevated, and the second pathway analyzes the course of treatment for those with elevated CA125 results whose pelvic mass was determined to be in the early versus late stages of malignancy.
Figure.
Flow Diagram of Study Members
aCommercial members, 25,243,5771; Medicare members, 37,345,7122
bCommercial, premenopausal %, 25.91; Commercial, postmenopausal %, 9.61; Medicare %, 54.03
cCommercial members, 16,275,3001; Medicare members, 17,179,028
dCommercial %, 0.571; Medicare %, 0.704,5
eCommercial members, 16,224,560; Medicare members, 17,036,896
fCommercial %, 70.606; Medicare %, 66.807
gCommercial %, 29.406; Medicare %, 33.207
hCommercial %, 39.208; Medicare %, 39.208
iCommercial %, 60.808; Medicare %, 60.808
jCommercial %, 49.007; Medicare %, 30.307
kCommercial %, 51.007; Medicare %, 69.707
lCommercial, premenopausal %, 37.509; Commercial, postmenopausal %, 26.609; Medicare %, 26.609
mCommercial, premenopausal %, 62.509; Commercial, postmenopausal %, 73.409; Medicare %, 73.409
1OptumInsight. Single payer database. 2014. Data on file at Baker Tilly, LLP.
2Centers for Medicare & Medicaid Services. On its 50th anniversary, more than 55 million Americans covered by Medicare. Press release. July 28, 2015. www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2015-Press-releases-items/2015-07-28.html.
3America's Health Insurance Plans Center for Policy and Research. Medicare Advantage demographics report. February 2015. https://ahip.org/wp-content/uploads/2015/02/MADemo_Report2015.pdf.
4OptumInsight. Medicare Outpatient Standard Analytic File. 2014. Data on file at Baker Tilly, LLP.
5Centers for Medicare & Medicaid Services. Physician and other supplier data CY 2014. www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Physician-and-Other-Supplier2014.html.
6Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. J Clin Pathol. 2005;58:308–312.
7Bristow RE, Hodeib M, Smith A, et al. Impact of a multivariate index assay on referral patterns for surgical management of an adnexal mass. Am J ObstetGynecol. 2013;209:581.e1–581.e8.
8Partridge EE, Greenlee RT, Riley TL, et al. Assessing the risk of ovarian malignancy in asymptomatic women with abnormal CA 125 and transvaginal ultrasound scans in the Prostate, Lung, Colorectal, and Ovarian Screening Trial. Obstet Gynecol. 2013;121:25–31.
9Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–46.
The model then devises a future-state scenario, in which OVA1 is substituted for CA125 in a proportion of members to determine their risk for pelvic mass malignancy. Relevant plan members are split into the 2 pathways using the same filtering logic that was applied in the current-state scenario; however, several assumptions are incorporated, including (1) the OVA1 market penetration rate, (2) the improved accuracy of OVA1 compared with CA125, and (3) assumed variation in key parameters pertaining to clinical resource utilization.
The first parameter variation captures a shift in procedures that were performed from the inpatient setting to the outpatient setting, which was enabled by an increase in diagnostic accuracy, allowing physicians to manage the patient in a less intense setting because of a low risk for malignancy based on OVA1. Second, increased physician confidence in OVA1 is assumed to also drive a shift in the number of procedures performed by physician type—moving away from gynecologic oncologists to general gynecologists. Finally, the model incorporates the possibility that a proportion of patients with a very low risk for malignancy may avoid surgery, based on OVA1 results combined with clinical assessment and ultrasound imaging instead of surgery.
The economic outcomes of the budget impact model (ie, estimated total costs and PMPM computations) for each pathway of the model are calculated separately for commercially insured and Medicare members. First, we estimate the total procedure costs accumulated in the current-state and future-state scenarios for members filtered into the nonelevated CA125/OVA1 pathway. The PMPM economic impact is then calculated for each scenario. Future-state PMPMs are subtracted from respective current-state PMPMs to determine the overall budget impact of OVA1 at the health plan population level.
Outcomes were calculated using the same method for the elevated CA125/OVA1 pathway. However, instead of total procedure costs, this analysis used the 24-month total episode-of-care costs to examine the impact of OVA1 on the total cost of early- and late-stage cancer, because the total cost of managing these groups of patients is very different.
The model culminates in a sensitivity analysis in which we permit uncertainty around the assumptions made in the future-state scenario to vary across a range of values to examine the resulting impact on PMPM expenditures.
Data Sources
The metrics used to conduct the filtering processes in operationalizing the current- and future-state scenarios originated from real-world claims data and literature sources. Claims data were used to filter the total payer population to include only females tested with CA125. Because claims compendia do not contain the entire set of data elements required (particularly the results of diagnostic tests) to complete the filtering process, literature sources were utilized to obtain values for the missing elements, including the percent of CA125 results that are elevated versus nonelevated, the percent of cases with nonelevated CA125 results who did and did not proceed with surgery, the percent of cases with elevated CA125 results whose pelvic mass is determined to be benign versus malignant, and the percent of malignant cases detected in an early versus late stage of cancer. In addition, CA125 and OVA1 sensitivities were taken from the literature. A complete list of values and their respective data sources are available in the Figure.
Data Sources for Procedure Mix and Costs
The breakdown of the procedure mix and the associated costs utilized in the first pathway of the analyses (ie, nonelevated CA125/OVA1 analysis) were derived from insurance claims. Commercial claims data were extracted from the OptumInsight, Inc (Eden Prairie, MN) database comprising approximately 25 million members. Claims used to derive the procedure costs were required to meet certain criteria, including (1) female members who had CA125 testing, as identified by Current Procedural Terminology (CPT) code 86304 from April 1, 2013, through March 31, 2015, and (2) the absence of a cancer diagnosis at the time of the procedure or within 6 months after the procedure (to ensure that members receiving CA125 testing to monitor ovarian cancer treatment response were not included in the analysis). Appendix A (see www.AHDBonline.com) provides a list of the diagnosis codes used to make this distinction.
Surgical cases were classified as a hysterectomy, salpingectomy and/or oophorectomy, cystectomy, or no surgery based on the procedure codes identified during the 6 months after the initial CA125 test. Because it is possible for members to receive multiple procedures, a hierarchy was established (in the same order as the procedures listed above) to restrict consideration to the most invasive procedure for each member (eg, a claim with hysterectomy and oophorectomy procedure codes would be classified as a member who received a hysterectomy). A complete list of CPT procedure codes used in the classification scheme is reported in Appendix B (see www.AHDBonline.com).
Claims for these procedures were further categorized by place of service (ie, inpatient and outpatient), physician type (ie, gynecologist and gynecologic oncologist), and menopausal status. Because claims data do not specify menopausal status, member age was used as a proxy. Women aged 18 to 51 years were classified as premenopausal, and women aged 52 to 64 years were classified as postmenopausal. Women aged >64 years were excluded from the commercial data to differentiate from Medicare. The paid claims amounts were used to represent the actual reimbursement levels by payers.
The commercial cost data utilized for the elevated CA125/OVA1 pathway were gathered through a 24-month retrospective claims analysis of female members with ovarian cancer (International Classification of Diseases, Ninth Revision [ICD-9] code 138.0) who received CA125 testing between July 1, 2013, and June 30, 2014. ICD-9 diagnosis codes for ovarian cancer do not distinguish between early- and late-stage cancers; therefore, clinician experts were consulted on the appropriate method for classifying members.
Late-stage ovarian cancer was defined as the metastasis of cancer outside of the pelvic region or to lymph nodes. Claims meeting these criteria between July 1, 2013, and June 30, 2014, were classified as late-stage ovarian cancer. Conversely, early-stage cancer was defined as the absence of these diagnosis codes suggesting spread of the disease. A list of CPT codes used in the classification scheme is shown in Appendix B. The episode-of-care costs comprised reimbursement for the healthcare expenditures, including inpatient care, outpatient care, physician services, and pharmacy.
The claims used to derive the costs for Medicare calculations were identified from the Medicare Inpatient Standard Analytic File and the Medicare Outpatient Standard Analytic File between January 1, 2014, and December 31, 2015, for the nonelevated CA125/OVA1 analysis and between January 1, 2013, and December 31, 2013, for the elevated CA125/OVA1 analysis.
The Medicare files contain data for approximately 37 million fee-for-service members. The claims data for women aged ≥65 years were extracted from Medicare data sets using the same criteria for the commercially insured members, and these women were deemed to be postmenopausal. However, because the cost data for physician, pharmacy, and outpatient services were not available in the Medicare data sets, the cost for these categories was estimated to be 80% of the commercial payment rate.
Finally, the total cost estimates in the nonelevated and elevated CA125/OVA1 analyses are inclusive of the CA125 and OVA1 costs. Reimbursement for CA125 testing was derived from claims data ($36, commercial; $31, Medicare). OVA1 pricing was provided by the manufacturer—$552 for commercial, and $225 for Medicare.
Results
A total of 92,843 plan members were included in the analysis, which comprised 48,113 commercially insured members and 44,730 Medicare beneficiaries. In the nonelevated CA125/OVA1 pathway of the model, 44,620 commercially insured members and 38,214 Medicare beneficiaries were retained for analysis. The elevated CA125/OVA1 pathway comprised 3493 commercial insurance members (ie, 2646 with early-stage cancer and 847 with late-stage cancer) and 6516 Medicare beneficiaries (ie, 2747 with early-stage cancer and 3769 with late-stage cancer).
Nonelevated CA125/OVA1 Pathway
The economic impact of OVA1 use on the subset of members currently tested with CA125 was calculated subject to the following conservative assumptions:
OVA1 market penetration of 20%
5% shift of procedures from inpatient to outpatient setting
5% of procedures currently performed by gynecologic oncologists shift to general gynecologists
5% of patients whose procedures are currently performed by a gynecologist will avoid surgery.
Our model assumptions were developed in consultation with the lead author (who is a clinician who actively manages patients with ovarian cancer and a former medical director of a large health plan) who urged conservatism in our estimate; the model assumptions were further informed by expert opinions and market intelligence offered by the manufacturer.
As a result of these assumptions, the future-state scenario estimates that commercially insured patients will experience a 0.11% decrease in the total procedures performed by gynecologic oncologists, a 0.20% decrease in the total procedures performed by gynecologists, and a 0.30% increase in surgery avoidance. The Medicare population experienced a 0.22% decrease in the total procedures performed by gynecologic oncologists, a 0.04% increase in the total procedures performed by gynecologists, and a 0.18% increase in surgery avoidance.
Table 1 provides full details on the procedure incidence rates by setting and by physician type for the commercially insured and Medicare populations. Average payer reimbursements per procedure type and by care setting are shown in Table 2.
Table 1.
Surgical Procedure Incidence Rates for Current- and Future-State Scenarios (Nonelevated CA125 Test Results)a
| Commercially insured incidence rates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | Total | |||||||
| Surgical procedure | Current state, % | Future state, % | Change, % | Current state, % | Future state, % | Change, % | Current state, % | Future state, % | Change, % |
| Gynecologist | |||||||||
| Hysterectomy | 5.40 | 5.31 | −0.09 | 6.72 | 6.70 | −0.02 | 12.12 | 12.01 | −0.11 |
| Salpingectomy and/or oophorectomy | 2.17 | 2.13 | −0.04 | 10.50 | 10.47 | −0.03 | 12.67 | 12.60 | −0.07 |
| Cystectomy | 0.59 | 0.58 | −0.01 | 4.35 | 4.34 | −0.01 | 4.94 | 4.92 | −0.02 |
| Total | 8.16 | 8.02 | −0.14 | 21.57 | 21.51 | −0.06 | 29.73 | 29.53 | −0.20 |
| Gynecologic oncologist | |||||||||
| Hysterectomy | 2.31 | 2.26 | −0.05 | 3.06 | 3.04 | −0.02 | 5.37 | 5.30 | −0.07 |
| Salpingectomy and/or oophorectomy | 0.77 | 0.76 | −0.01 | 2.50 | 2.48 | −0.02 | 3.27 | 3.24 | −0.03 |
| Cystectomy | 0.18 | 0.17 | −0.01 | 0.66 | 0.66 | 0.00 | 0.84 | 0.83 | −0.01 |
| Total | 3.26 | 3.19 | −0.07 | 6.22 | 6.18 | −0.04 | 9.48 | 9.37 | −0.11 |
| Avoided surgeries | N/A | N/A | N/A | N/A | N/A | N/A | 60.80 | 61.10 | 0.30 |
| Medicare incidence rates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | Total | |||||||
| Surgical procedure | Current state, % | Future state, % | Change, % | Current state, % | Future state, % | Change, % | Current state, % | Future state, % | Change, % |
| Gynecologist | |||||||||
| Hysterectomy | 3.28 | 3.25 | −0.03 | 4.08 | 4.11 | 0.03 | 7.36 | 7.36 | 0.00 |
| Salpingectomy and/or oophorectomy | 1.09 | 1.08 | −0.01 | 9.25 | 9.30 | 0.05 | 10.34 | 10.38 | 0.04 |
| Cystectomy | 0.07 | 0.07 | 0.00 | 0.18 | 0.18 | 0.00 | 0.25 | 0.25 | 0.00 |
| Total | 4.44 | 4.40 | −0.04 | 13.51 | 13.59 | 0.08 | 17.95 | 17.99 | 0.04 |
| Gynecologic oncologist | |||||||||
| Hysterectomy | 4.32 | 4.23 | −0.09 | 9.68 | 9.62 | −0.06 | 14.00 | 13.85 | −0.15 |
| Salpingectomy and/or oophorectomy | 1.22 | 1.19 | −0.03 | 5.85 | 5.82 | −0.03 | 7.07 | 7.01 | −0.06 |
| Cystectomy | 0.03 | 0.03 | 0.00 | 0.16 | 0.15 | −0.01 | 0.19 | 0.18 | −0.01 |
| Total | 5.57 | 5.45 | −0.12 | 15.69 | 15.59 | −0.10 | 21.26 | 21.04 | −0.22 |
| Avoided surgeries | N/A | N/A | N/A | N/A | N/A | N/A | 60.80 | 60.98 | 0.18 |
Model assumes 20% OVA1 penetration, 5% shift from inpatient to outpatient, 5% shift from gynecologic oncologist to gynecologist to avoidance of surgery.
N/A indicates not available.
Table 2.
Average Reimbursement by Surgical Procedures (Nonelevated CA125 Test Results)a
| Commercial insurance reimbursement | Medicare reimbursement | |||
|---|---|---|---|---|
| Surgical procedure | Inpatient, $ | Outpatient, $ | Inpatient, $ | Outpatient, $ |
| Gynecologist | ||||
| Hysterectomy | 14,932 | 7421 | 8743 | 3342 |
| Salpingectomy and/or oophorectomy | 13,505 | 6019 | 8436 | 3370 |
| Cystectomy | 13,866 | 5471 | 8483 | 3262 |
| Gynecologic oncologist | ||||
| Hysterectomy | 16,689 | 7669 | 9947 | 3128 |
| Salpingectomy and/or oophorectomy | 16,088 | 6229 | 9617 | 3380 |
| Cystectomy | 15,303 | 6598 | 9650 | 3181 |
Reimbursement rates are static between current and future states.
As expected, the costs for inpatient procedures were substantially higher than for outpatient procedures. In the commercially insured group, the total cost of a hysterectomy performed by a gynecologist as an inpatient procedure averaged $14,932 compared with $7421 when performed as an outpatient procedure. The cost difference remained consistent among the Medicare population, in whom the total payments for an inpatient hysterectomy performed by a gynecologist averaged $8743 compared with $3342 for the equivalent outpatient procedure.
Furthermore, procedures performed by gynecologic oncologists were more expensive than when performed by a gynecologist. An inpatient hysterectomy procedure averaged $14,932 when completed by a gynecologist and $16,689 when performed by a gynecologic oncologist. This pattern persisted in the Medicare population, where an inpatient hysterectomy cost averaged $8743 for a gynecologist and $9947 for a gynecologic oncologist.
Elevated CA125/OVA1 Pathway
Early- and late-stage cancer incidence rates for the current- and future-state scenarios by payer population are presented in Table 3. In the commercially insured population, the future adoption of OVA1 at the 20% level produced a 3.27% shift in cancer detection from late stage to early stage among premenopausal women and a shift of 0.38% among postmenopausal women. In the Medicare population, future utilization of OVA1 at the 20% adoption rate produced a 0.38% shift of cancer detection from late stage to early stage, where all women are presumed to be postmenopausal.
Table 3.
Early- and Late-Stage Cancer Rates for Current- and Future-State Scenariosa
| Commercially insured incidence rates | ||||||
|---|---|---|---|---|---|---|
| Premenopausal women | Postmenopausal women | |||||
| Cancer stage | Current state, % | Future state, %b | Change, % | Current state, % | Future state, %b | Change, % |
| Early-stage | 37.50 | 40.77 | 3.27 | 26.60 | 26.98 | 0.38 |
| Late-stage | 62.50 | 59.23 | −3.27 | 73.40 | 73.02 | −0.38 |
| Medicare incidence rates | ||||||
|---|---|---|---|---|---|---|
| Premenopausal womenc | Postmenopausal women | |||||
| Cancer stage | Current state, % | Future state, % | Change, % | Current state, % | Future state, %b | Change, % |
| Early-stage | N/A | N/A | N/A | 26.60 | 26.98 | 0.38 |
| Late-stage | N/A | N/A | N/A | 73.40 | 73.02 | −0.38 |
Model assumes 20% OVA1 penetration rate.
Model assumes that 50% of early-stage cancers are misdiagnosed by CA125 test.10
Model assumes all Medicare cases are postmenopausal.
N/A indicates not applicable.
Table 4 presents the 24-month episode-of-care costs for early-stage and late-stage cancers, by payer. The costs were substantially higher for late-stage cancer compared with early-stage cancer in the commercially insured and Medicare populations. Commercially insured premenopausal women with early-stage cancer had 24-month episode costs that averaged $35,754, whereas premenopausal women with late-stage cancer had an average 24-month episode cost of $224,922.
Table 4.
A 24-Month Average Reimbursement, by Payera
| Commercial insurance reimbursement | Medicare reimbursement | |||
|---|---|---|---|---|
| Cancer stage | Premenopausal women, $ | Postmenopausal women, $ | Premenopausal women, $b | Postmenopausal women, $ |
| Early-stage cancer | 35,754 | 37,195 | N/A | 53,681 |
| Late-stage cancer | 224,922 | 197,757 | N/A | 161,369 |
Reimbursement rates are static between the current and future states.
All Medicare cases are assumed to be postmenopausal.
N/A indicates not applicable.
PMPM Calculations and Sensitivity Analysis
PMPM calculations were completed for the commercially insured and Medicare populations, combining the economic impact of OVA1 on both pathways that were analyzed in the model. In the future-state scenario (using our base-case assumptions), the commercial population experiences a $0.05 PMPM savings and the Medicare population experiences a $0.01 PMPM savings.
Table 5 presents a sensitivity analysis of PMPM savings that was conducted to determine the range of the economic impact that OVA1 may have on a payer, given variations in the key model inputs. The model assumptions that were allowed to vary in the sensitivity analysis included (1) an OVA1 market penetration rate ranging from 10% to 50%, (2) a shift of procedure location ranging from 2.5% to 20%, and (3) a shift of physician type (which includes surgery avoidance) from 2.5% to 20%.
Table 5.
Sensitivity Analysis of Economic Impact on Total PMPM Expenditures for the Use of OVA1 as a Substitute for CA125a
| Model assumptions | Commercially insured | Medicare | ||||||
|---|---|---|---|---|---|---|---|---|
| Penetration rate, % | Inpatient to outpatient, % | Gynecologic oncologist to gynecologist to avoidance of surgery, % | Current state, $ | Future state, $ | PMPM change, $ | Current state, $ | Future state, $ | PMPM change, $ |
| 10.0 | 2.5 | 2.5 | 2.83 | 2.81 | −0.02 | 6.34 | 6.33 | −0.01 |
| 20.0 | 5.0 | 5.0 | 2.83 | 2.78 | −0.05 | 6.34 | 6.33 | −0.01 |
| 25.0 | 7.5 | 7.5 | 2.83 | 2.76 | −0.07 | 6.34 | 6.33 | −0.01 |
| 30.0 | 10.0 | 10.0 | 2.83 | 2.74 | −0.09 | 6.34 | 6.32 | −0.02 |
| 35.0 | 12.5 | 12.5 | 2.83 | 2.72 | −0.11 | 6.34 | 6.32 | −0.02 |
| 40.0 | 15.0 | 15.0 | 2.83 | 2.70 | −0.13 | 6.34 | 6.31 | −0.03 |
| 45.0 | 17.5 | 17.5 | 2.83 | 2.68 | −0.15 | 6.34 | 6.30 | −0.04 |
| 50.0 | 20.0 | 20.0 | 2.83 | 2.66 | −0.17 | 6.34 | 6.29 | −0.05 |
Calculations are based on 10 member months annually as reported by OptumInsight.20
PMPM indicates per-member per-month.
The results of the sensitivity analyses revealed that increased adoption of OVA1 to 50% market penetration could yield payer savings up to $0.17 PMPM in the commercial population and $0.05 PMPM in the Medicare population. It is best to calculate the total dollar savings at the plan-specific level.
Discussion
More than 22,000 new cases of ovarian cancer are diagnosed annually in the United States, with the majority of them being late-stage disease at the time of diagnosis. The results of our budget impact model estimate that average treatment costs for late-stage cancer could be as high as $224,922 over a 24-month episode. This finding, coupled with extremely high mortality rates for this disease, poses a formidable challenge for physicians and payers.
Our study findings suggest that, because of its increased sensitivity and negative predictive value, the use of OVA1 may result in savings for payers of $0.05 PMPM by enabling physicians to better manage patients in the appropriate setting of care. These savings can be attributed to a reduction in the total procedures performed by gynecologic oncologists (up to 0.22%), decreases in total procedures performed by gynecologists (up to 0.20%), a 0.30% increase in surgery avoidance for patients with nonelevated test results, as well as a shift in cancer detection from late-stage to early-stage cancer reaching 3.27%.
The results of the budget impact model support the use of OVA1 by indicating that modest cost-savings can be achieved by health plans while reaping the clinical benefits of improved diagnostic accuracy, early disease detection, and reductions in multiple, and possibly unnecessary, referrals to gynecologic oncologists. The observed favorable differential of savings in the commercial population is likely attributable to the increased sensitivity of OVA1 in premenopausal women compared with the current standard of care. Overall, the addition of a multivariate index assay is cost-neutral, and possibly cost-sparing, across the commercially insured and Medicare populations.
Limitations
This study has its limitations. First, reliance on administrative claims data makes it difficult to categorize precisely the clinical conditions and the chronologic occurrence of events.
Second, using 80% of commercial reimbursements as a proxy for Medicare physician, pharmacy, and outpatient payments might have underestimated or overestimated Medicare costs, depending on the region of the country.
Third, our assumption that a portion of low-risk cases may be managed by gynecologists in the outpatient setting may be imprecise, especially in the Medicare population, because the model is not able to accurately account for clinical modifiers such as comorbidities and surgical risks, which could drive these patients to inpatient settings if present.
Fourth, our assumptions regarding adoption rates, changes in referral patterns, and changes in setting of care are subject to challenge; however, such uncertainties are addressed by the sensitivity analysis. Our analyses of direct medical costs did not include coinsurance or deductible amounts paid by Medicare patients, but patient liability is included in commercial data.
Finally, the analysis did not consider the indirect costs associated with time off from work, travel time to visit the offices of thinly distributed gynecologic oncologists, and the psychological stress associated with seeing a cancer specialist.
Conclusion
The budget impact model presented in this study is offered as a first step toward assisting health plan policymakers in weighing the financial consequences—that is, differences between inpatient and outpatient procedure reimbursements and early- versus late-stage cancer reimbursements in premenopausal versus postmenopausal women—of expanded utilization of multivariate assay testing in relation to its clinical advantages in treating this silent killer, which often presents at a late stage. Future investigations of this type should further refine our model, by extending the analyses longitudinally and by more closely coupling economic data with clinical measures. We also encourage testing of the model using regional and plan-specific data to assess its robustness.
Acknowledgments
We would like to thank Brittany Blau for her assistance with literature research and drafting of the manuscript, and David Gregory for conceptualization of the study.
Source of Funding
This study was funded by Vermillion Inc, Austin, TX, which developed the OVA1 test.
Author Disclosure Statement
Dr Brodsky is on the Speaker's Bureau for Myriad Genetics. Dr Owens is a consultant to Eli Lilly, Genentech, Inspire Medical Systems, Johnson & Johnson, Novartis, Roche Diagnostics, and Sun Pharmaceuticals. Dr Scotti, Mr Needham, and Ms Cool are consultants to Vermillion.
Contributor Information
Burton S. Brodsky, Assistant Professor of Obstetrics and Gynecology, The University of Toledo Medical Center, Toledo, OH.
Gary M. Owens, President, Gary Owens Associates, Ocean View, DE.
Dennis J. Scotti, Alfred E. Driscoll Professor of Healthcare & Life Sciences Management, Fairleigh Dickinson University, Teaneck, NJ.
Keith A. Needham, Senior Consultant, Baker Tilly LLP, New York, NY.
Christina L. Cool, Manager, Baker Tilly LLP..
References
- 1. Ovarian Cancer Research Fund Alliance. Statistics. www.ocrfa.org/members/about-ovarian-cancer/statistics/. Accessed November 22, 2016.
- 2. American Cancer Society. What are the key statistics about ovarian cancer? Revised January 6, 2017. www.cancer.org/cancer/ovariancancer/detailedguide/ovarian-cancer-key-statistics. Accessed February 16, 2017.
- 3. National Cancer Institute. SEER cancer stat facts: ovarian cancer. www.seer.cancer.gov/statfacts/html/ovary.html. Accessed September 7, 2017.
- 4. Longoria TC, Ueland FR, Zhang Z, et al. Clinical performance of a multivariate index assay for detecting early-stage ovarian cancer. Am J Obstet Gynecol. 2014;210:78.e1–78.e9. [DOI] [PubMed] [Google Scholar]
- 5. Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117:1289–1297. [DOI] [PubMed] [Google Scholar]
- 6. Moore RG, Bast RC., Jr. How do you distinguish a malignant pelvic mass from a benign pelvic mass? Imaging, biomarkers, or none of the above. J Clin Oncol. 2007;25:4159–4161. [DOI] [PubMed] [Google Scholar]
- 7. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210–e226. [DOI] [PubMed] [Google Scholar]
- 8. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol. 2007;110:201–214. [DOI] [PubMed] [Google Scholar]
- 9. Mayo Clinic. Tests and procedures: CA 125 test: definition. April 14, 2014. www.mayoclinic.org/tests-procedures/ca-125-test/basics/definition/prc-20009524. Accessed February 7, 2017.
- 10. Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128:252–259. [DOI] [PubMed] [Google Scholar]
- 11. Bristow RE, Hodeib M, Smith A, et al. Impact of a multivariate index assay on referral patterns for surgical management of an adnexal mass. Am J Obstet Gynecol. 2013;209:581.e1–581.e8. [DOI] [PubMed] [Google Scholar]
- 12. Goodrich ST, Bristow RE, Santoso JT, et al. The effect of ovarian imaging on the clinical interpretation of a multivariate index assay. Am J Obstet Gynecol. 2014;211:65e1–65.e11. [DOI] [PubMed] [Google Scholar]
- 13. Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists' ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol. 2011;117:1298–1306. [DOI] [PubMed] [Google Scholar]
- 14. Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. [DOI] [PubMed] [Google Scholar]
- 15. Urban RR, He H, Alfonso-Cristancho R, et al. The cost of initial care for Medicare patients with advanced ovarian cancer. J Natl Compr Canc Netw. 2016;14:429–437. [DOI] [PubMed] [Google Scholar]
- 16. Kim KH, Zsebik GN, Straughn JM, Jr, Landen CN., Jr. Management of complex pelvic masses using a multivariate index assay: a decision analysis. Gynecol Oncol. 2012;126:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forde GK, Hornberger J, Michalopoulos S, Bristow RE. Cost-effectiveness analysis of a multivariate index assay compared to modified American College of Obstetricians and Gynecologists criteria and CA-125 in the triage of women with adnexal masses. Curr Med Res Opin. 2016;32:321–329. [DOI] [PubMed] [Google Scholar]
- 18. Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–437. [DOI] [PubMed] [Google Scholar]
- 19. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. Erratum in: J Natl Cancer Inst 2011;103: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. OptumInsight. Single payer database. 2014. Data on file at Baker Tilly, LLP.