Abstract
Background
Hematopoietic stem-cell transplantation (HSCT) requires highly specialized, resource-intensive care. Myeloablative conditioning regimens used before HSCT generally require inpatient stays and are more intensive than other preparative regimens, and may therefore be more costly.
Objective
To estimate the costs associated with inpatient HSCT according to the type of the conditioning regimen used and other potential contributors to the overall cost of the procedure.
Method
We used data from the Truven Health MarketScan insurance claims database to analyze healthcare costs for pediatric (age <18 years) and adult (age ≥18 years) patients who had autologous or allogeneic inpatient HSCT between January 1, 2010, and September 23, 2013. We developed an algorithm to determine whether conditioning regimens were myeloablative or nonmyeloablative/reduced intensity.
Results
We identified a sample of 1562 patients who had inpatient HSCT during the study period for whom the transplant type and the conditioning regimen were determinable: 398 patients had myeloablative allogeneic HSCT; 195 patients had nonmyeloablative/reduced-intensity allogeneic HSCT; and 969 patients had myeloablative autologous HSCT. The median total healthcare cost at 100 days was $289,283 for the myeloablative allogeneic regimen cohort compared with $253,467 for the nonmyeloablative/reduced-intensity allogeneic regimen cohort, and $140,792 for the myeloablative autologous regimen cohort. The mean hospital length of stay for the index (first claim of) HSCT was 35.6 days in the myeloablative allogeneic regimen cohort, 26.6 days in the nonmyeloablative/reduced-intensity allogeneic cohort, and 21.8 days in the myeloablative autologous regimen cohort.
Conclusion
Allogeneic HSCT was more expensive than autologous HSCT, regardless of the regimen used. Myeloablative conditioning regimens led to higher overall costs than nonmyeloablative/reduced-intensity regimens in the allogeneic HSCT cohort, indicating a greater cost burden associated with inpatient services for higher-intensity preparative conditioning regimens. Pediatric patients had higher costs than adult patients. Future research should involve validating the algorithm for identifying conditioning regimens using clinical data.
Keywords: allogeneic HSCT, autologous HSCT, healthcare costs, hospitalization, inpatient, myeloablative conditioning regimen, nonmyeloablative/reduced-intensity conditioning regimen
KEY POINTS
-
▸
Hematopoietic stem-cell transplantation (HSCT) is costly, and its use is steadily rising in the treatment of cancer.
-
▸
This retrospective study of claims data compared the costs of inpatient autologous or allogeneic HSCT, based on the use of a myeloablative or a nonmyeloablative/reduced-intensity conditioning regimen.
-
▸
The median total healthcare cost at 100 days was twice as high with a myeloablative regimen before allogeneic HSCT ($289,283) than before autologous HSCT ($140,792).
-
▸
The algorithm developed for this study showed that transplant type, conditioning regimen, and patient age affect the cost of HSCT.
-
▸
Overall, allogeneic HSCT was more expensive than autologous HSCT based on this algorithm.
-
▸
Myeloablative conditioning is costlier than nonmyeloablative/higher-intensity conditioning in patients with allogeneic transplant, likely because of added complications.
-
▸
Future research is needed to validate the algorithm for identifying conditioning regimens used with HSCT based on clinical data.
Cancer is costly. As new cancer therapies become available that extend survival, and as the US population ages and continues to grow, the cost of cancer care is estimated to reach almost $158 billion in 2020, according to the National Cancer Institute.1 The costs of cancer care vary considerably by cancer type and stage of treatment. In the year after a cancer diagnosis, treatment costs can exceed $110,000 for cancers of the brain or pancreas, for example, whereas end-of-life costs are much higher for all cancers, approaching $200,000 in the last year of life for patients with leukemia or with brain cancer.1
The growing cost burden of cancer care to our healthcare system compels society to seek value in cancer treatments by maximizing cost benefits, and by finding ways to reduce costs. By exploring several significant drivers of cost, we want to identify ways in which to optimize these costs, particularly for addressable factors, such as long hospital stays that lack a clinical basis. Using the example of hematopoietic stem-cell transplantation (HSCT), a lifesaving, increasingly common, and expensive procedure for hematologic or bone marrow disorders, we evaluated the total cost of care and specific cost drivers for patients undergoing HSCT.
Stem-cell transplantation in the United States has risen steadily for more than 2 decades, with 340,000 cumulative HSCTs having been performed by 2014; the annual number of HSCTs performed in 2014 surpassed 8000 allogeneic and 10,000 autologous transplants.2 The hospital costs associated with HSCT have also grown by approximately 85% to nearly $1.3 billion between 2004 and 2007, making it one of the hospital procedures with the largest increase over that period.3
Several previous studies have analyzed the costs of HSCT (which range from approximately $87,000 to $300,000), but few have examined the conditioning regimen as a determinant of the cost, and none used a population sample derived from all geographic regions of the United States.4–11 For this study, we used health insurance claims covering 50 million individuals in the United States12 to investigate potential drivers of HSCT costs in the oncology setting, including conditioning regimens, transplant type, and patient age.
Methods
In this retrospective cohort study design we used commercial insurance data from the Truven Health MarketScan claims database to analyze the 100-day and 1-year costs for patients who received inpatient autologous or allogeneic HSCT between January 1, 2010, and September 23, 2013 (ie, the identification period), and stratified patients by the type of the conditioning regimen and age-group, including pediatric (age <18 years) and adult (age ≥18 years) patients.
Patients were required to have at least 1 claim with an appropriate International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure code indicating the HSCT type. Allogeneic and autologous transplants were distinguished using ICD-9-CM and Current Procedural Terminology (CPT) 4 codes (see Appendix Table 1 at www.AHDBonline.com). The MarketScan database of employer-sponsored health plans in the United States contained information on enrollment and demographics, in addition to the data reported on administrative claims, including inpatient and outpatient services and costs, diagnoses and procedures (CPT and ICD-9-CM procedure codes), and outpatient pharmacy medication dispensing information (eg, days of drug supply, fill dates, and National Drug Codes).
The study population comprised patients who underwent allogeneic or autologous HSCT and received myeloablative or nonmyeloablative/reduced-intensity conditioning regimens before transplantation. The first date of an HSCT claim (eg, with one of the codes listed in Appendix Table 1) during the identification period was defined as the index date. To qualify for study inclusion, patients were required to be continuously enrolled in a health plan for 1 year before the index date (ie, the baseline period) and for 100 days after this date. This 1-year baseline period was used to ensure that the inpatient HSCT was the first such transplant.
Allogeneic and autologous HSCT are billed using different ICD-9-CM codes, making it straightforward to distinguish between them. Distinguishing between different conditioning regimens is far more complicated, because the regimen types do not have their own codes and are billed by the individual chemotherapy or radiation components used. Furthermore, although chemotherapeutic agents administered in the outpatient setting are generally identifiable in claims, inpatient claims do not provide sufficient detail to distinguish one agent from another.
Finally, although differing doses of radiation are an important determinant of whether a conditioning regimen is myeloablative, radiation dosing is not easily determined in claims. Therefore, we relied on published literature and expert clinician input to develop an algorithm that distinguished myeloablative from nonmyeloablative/reduced-intensity conditioning regimens.13,14 The expert clinical input came from a variety of sources, including experienced hematologist/oncologists who treat adults and children, coding specialists at the American Society for Radiation Oncology, and the Center for International Blood and Marrow Transplant Research. The algorithm included data on the underlying malignancy, type of treatment (ie, chemotherapy type, radiation, and total body irradiation procedures), location of service, and therapy timing (see Appendix Table 2 at www.AHDBonline.com) to identify a subset of patients for whom we determined we could reliably define their conditioning regimen type.
We made several assumptions about capturing the costs of HSCT through claims data. First, we assumed that all HSCT-related care occurring in the oncology setting could be observed through claims. Second, we assumed that all conditioning regimen–related costs could be captured, which we believe was reasonable for outpatient regimen procedures, because outpatient claims provide sufficient detail about such services. However, any inpatient conditioning regimen costs were not isolated, because inpatient claims lack specific regimen information. Finally, based on expert clinical input, we used a 10-day look-back period from the date of the HSCT to fully capture any billing associated with the conditioning regimen, which is typically given 6 to 8 days before HSCT, but also to avoid including unrelated costs associated with earlier care.
We computed healthcare costs, our primary outcome, using the fee-for-service equivalent or amount paid field in the claims. The costs were calculated for the HSCT hospital admission and for services in the 10 days before transplantation; the latter was done to account for expenses related to the conditioning regimens administered before HSCT. We estimated the mean and median costs for total, inpatient (all services, including intensive care unit care), outpatient, and pharmacy services at 100 days and at 1 year (all calculated by summing the costs, regardless of diagnosis on the claims). The 100-day and 1-year costs included relevant services in the 10 days before transplantation.
Outpatient HSCT did not qualify patients for inclusion in the study; however, a small fraction (0.8%) of patients had outpatient HSCT before their qualifying inpatient HSCT. These outpatient costs had a negligible effect on the overall HSCT cost estimates. Hospitalization was measured by the mean length of stay (LOS) during the index HSCT admission (by definition, a single hospital stay). In addition, we examined subsequent hospitalization at 100 days and at 1 year of follow-up, which was defined by the proportion of patients hospitalized after HSCT and by mean LOS across all subsequent hospitalizations.
Patients were divided into 3 groups, based on the conditioning regimen and the type of transplant: (1) patients receiving a myeloablative regimen before an allogeneic transplant (henceforth labeled “allogeneic MA”); (2) patients receiving a nonmyeloablative/reduced-intensity regimen before an allogeneic transplant (“allogeneic NMA”); and (3) those receiving a myeloablative regimen before an autologous transplant (“autologous MA”). We did not include patients (N = 2) who received a nonmyeloablative/reduced-intensity regimen and underwent an autologous transplant because of their infrequency.
To compare the study groups, we measured several baseline variables available in the claims data, including age, sex, and geographic region. We also considered the cancer diagnosis(es) reported at the index HSCT hospitalization, which was based on the presence of ICD-9-CM codes in any diagnosis field for acute myeloid leukemia (ICD-9-CM: 205.0x, 205.3x, 206.0x, 207.0x, 207.2x), acute lymphocytic leukemia (ICD-9-CM: 204.0x), chronic myeloid leukemia (ICD-9-CM: 205.10), myelodysplastic syndrome (ICD-9-CM: 238.72, 238.73, 238.75), lymphoma (ICD-9-CM: 196.xx, 200.xx, 201.xx, 202.xx), multiple myeloma and plasma-cell neoplasms (ICD-9-CM: 203.xx, 277.3x), chronic lymphocytic leukemia (ICD-9-CM: 204.1x), aplastic anemia (ICD-9-CM: 284.xx), and sarcoma (ICD-9-CM: 171.xx).
All analyses were stratified according to transplant type, conditioning regimen, and age-group. Descriptive statistics, including means, medians, standard deviations, and percentages, were reported for all study measures as appropriate. The costs were updated to 2013 US dollars using the healthcare component of the Consumer Price Index.15 All data transformations and analyses were performed using SAS version 9.4 (SAS Institute, Inc; Cary, NC).
Results
We identified 6671 patients who had an HSCT during the identification period, 4474 of whom were continuously enrolled in a health plan during the 1 year before and 100 days after the index date. From this population, we derived our final study cohort of 1562 patients who had inpatient HSCT and for whom the transplant type and the conditioning regimen were determinable and were divided into the 3 cohorts—allogeneic MA cohort (N = 398); allogeneic NMA cohort (N = 195); and autologous MA cohort (N = 969).
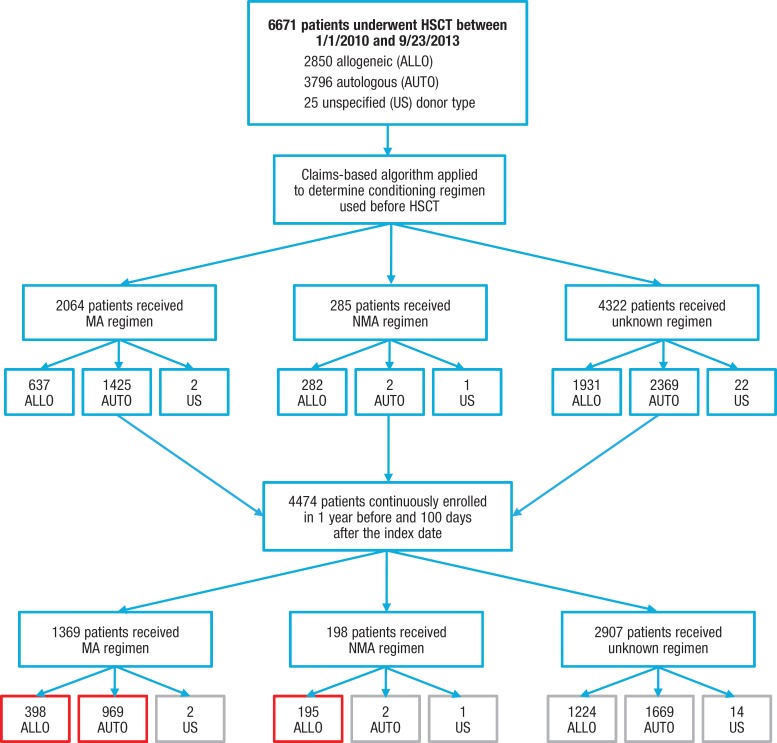
Figure 1 (see AHDBonline.com) describes the patient identification process for the study. Table 1 outlines the patients' baseline characteristics. The majority (87.5%) of patients had myeloablative conditioning. Our sample included 61% males, and 92.9% of the patients were aged ≥18 years (mean age, 48.6 years; standard deviation, 16.4 years). Overall, the most common malignancy diagnoses reported during admission for HSCT were lymphoma (62.5%), aplastic anemia (46.3%), acute lymphocytic leukemia (21.5%), and acute myeloid leukemia (11.2%; not mutually exclusive; Table 1).
Figure 1.
The figure shows the number of patients who were initially identified in the study database, the number of patients meeting the continuous enrollment requirement, and the number identified by the algorithm as having a myeloablative (MA), nonmyeloablative/reduced-intensity conditioning (NMA), or unknown conditioning regimen.
Table 1.
Characteristics of Patients with Allogeneic and Autologous Transplants, by Conditioning Regimen
| Allogeneic transplant | Autologous transplant | |||
|---|---|---|---|---|
| Characteristic | Myeloablative regimen (N = 398) | Nonmyeloablative/reduced-intensity regimen (N = 195) | Myeloablative regimen (N = 969) | Total (N = 1562)a |
| Age, yrs, mean (SD) | 37.5 (18.4) | 54.8 (11.6) | 51.9 (14.0) | 48.6 (16.4) |
| <18 yrs, N (%) | 80 (20.1) | 4 (2.1) | 27 (2.8) | 111 (7.1) |
| 18–40 yrs, N (%) | 125 (31.4) | 12 (6.2) | 150 (15.5) | 287 (18.4) |
| 41–60 yrs, N (%) | 157 (39.4) | 116 (59.5) | 513 (52.9) | 786 (50.3) |
| ≥61 yrs, N (%) | 36 (9.0) | 63 (32.3) | 279 (28.8) | 378 (24.2) |
| Female, N (%) | 166 (41.7) | 82 (42.1) | 361 (37.3) | 609 (39.0) |
| Male, N (%) | 232 (58.3) | 113 (58.0) | 608 (62.8) | 953 (61.0) |
| Region, N (%) | ||||
| Midwest | 84 (21.1) | 58 (29.7) | 285 (29.4) | 427 (27.3) |
| Northeast | 102 (25.6) | 56 (28.7) | 195 (20.1) | 353 (22.6) |
| South | 129 (32.4) | 54 (27.7) | 313 (32.3) | 496 (31.8) |
| West | 83 (20.9) | 27 (13.8) | 176 (18.2) | 286 (18.3) |
| Diagnosis of cancer type at index HSCT, N (%)b | 398 (100.0) | 192 (98.5) | 968 (99.9) | 1558 (99.7) |
| Acute myeloid leukemia | 114 (28.6) | 54 (27.7) | 7 (0.7) | 175 (11.2) |
| Acute lymphocytic leukemia | 312 (78.4) | 18 (9.2) | 6 (0.6) | 336 (21.5) |
| Chronic myeloid leukemia | 12 (3.0) | 8 (4.1) | 0 (0.0) | 20 (1.3) |
| Myelodysplastic syndrome | 21 (5.3) | 23 (11.8) | 2 (0.2) | 46 (2.9) |
| Lymphoma | 47 (11.8) | 38 (19.5) | 891 (92.0) | 976 (62.5) |
| Multiple myeloma and plasma-cell neoplasms | 8 (2.0) | 4 (2.1) | 99 (10.2) | 111 (7.1) |
| Chronic lymphocytic leukemia | 7 (1.8) | 96 (49.2) | 9 (0.9) | 112 (7.2) |
| Aplastic anemia | 184 (46.2) | 87 (44.6) | 452 (46.6) | 723 (46.3) |
| Sarcoma | 0 (0.0) | 0 (0.0) | 2 (0.2) | 2 (0.1) |
Patients (N = 2) who received autologous nonmyeloablative/reduced-intensity HSCT were not included in the study because of the low frequency.
Including 4 patients who underwent transplant but did not have evidence of a selected malignancy at the index HSCT.
HSCT indicates hematopoietic stem-cell transplantation; SD, standard deviation.
100-Day Costs and Hospitalization
The descriptive findings on the first 100 days after transplantation are presented in Table 2. The median total healthcare cost was $289,283 for patients in the allogeneic MA cohort versus $253,467 for the allogeneic NMA cohort and $140,792 for the autologous MA cohort. The median inpatient cost, which made up the largest share of the total costs, was $239,959 for the allogeneic MA cohort compared with $182,256 for the allogeneic NMA cohort and $113,272 for the autologous MA cohort.
Table 2.
Healthcare Costs and Hospitalization at 100-Day and 1-Year Follow-Up for Patients Receiving Myeloablative or Nonmyeloablative/Reduced-Intensity Conditioning Regimen
| 100-day follow-up | 1-year follow-up | ||||||
|---|---|---|---|---|---|---|---|
| Allogeneic transplant | Autologous transplant | Allogeneic transplant | Autologous transplant | ||||
| Cost parameter | Myeloablative regimen (N = 398) | Nonmyeloablative/reduced-intensity regimen (N = 195) | Myeloablative regimen (N = 969) | Myeloablative regimen (N = 398) | Nonmyeloablative/reduced-intensity regimen (N = 195) | Myeloablative regimen (N = 969) | |
| Total healthcare costs, $a | Mean | 401,566 | 300,871 | 164,049 | 549,208 | 432,157 | 231,259 |
| SD | 397,479 | 238,034 | 137,214 | 508,350 | 297,707 | 191,665 | |
| Median | 289,283 | 253,467 | 140,792 | 408,876 | 374,065 | 181,933 | |
| Inpatient costs, $a | Mean | 343,352 | 231,463 | 134,268 | 422,973 | 295,749 | 160,581 |
| SD | 396,217 | 238,505 | 129,394 | 480,513 | 272,290 | 163,290 | |
| Median | 239,959 | 182,256 | 113,272 | 276,620 | 235,620 | 121,277 | |
| Outpatient costs, $a | Mean | 50,235 | 60,805 | 27,698 | 104,923 | 117,248 | 63,081 |
| SD | 42,093 | 59,245 | 28,331 | 93,951 | 104,741 | 68,919 | |
| Median | 40,655 | 41,349 | 18,400 | 81,575 | 83,435 | 42,294 | |
| Pharmacy costs, $a | Mean | 7979 | 8603 | 2083 | 21,312 | 19,159 | 7597 |
| SD | 7217 | 7629 | 3847 | 21,124 | 16,814 | 15,462 | |
| Median | 6451 | 6551 | 673 | 14,429 | 15,487 | 2043 | |
| Costs of index HSCT hospitalization, $b | Mean | 306,959 | 198,676 | 119,678 | N/A | N/A | N/A |
| SD | 366,665 | 223,887 | 68,462 | N/A | N/A | N/A | |
| Median | 208,857 | 161,241 | 110,209 | N/A | N/A | N/A | |
| Length of stay for HSCT admission, days | Mean (SD) | 35.6 (26.4) | 26.6 (22.1) | 21.8 (12.8) | N/A | N/A | N/A |
| Any subsequent hospitalizationc | N (%) | 169 (42.5) | 85 (43.6) | 202 (20.8) | 268 (67.3) | 135 (69.2) | 367 (37.9) |
| Total length of stay, daysd | Mean (SD) | 9.0 (15.0) | 11.0 (16.8) | 6.5 (12.8) | 26.6 (32.5) | 30.3 (31.9) | 18.0 (21.4) |
All costs include the claims from 10 days before through 100 days (or 1 year) after the transplant.
Represents the costs of index transplant, including conditioning regimen; inpatient transplants include the costs from 10 days before admission through discharge from index admission; outpatient transplants includes the costs from 10 days before day of first outpatient ICD-9 diagnosis code for HSCT.
Within 100 days (or 1 year) of follow-up.
Among patients with hospitalization subsequent to the HSCT admission and within 100 days (or 1 year) of follow-up. Total represents hospital days across all admissions subsequent to HSCT admission.
HSCT indicates hematopoietic stem-cell transplantation; ICD-9, International Classification of Diseases, Ninth Revision; N/A, not applicable; SD, standard deviation.
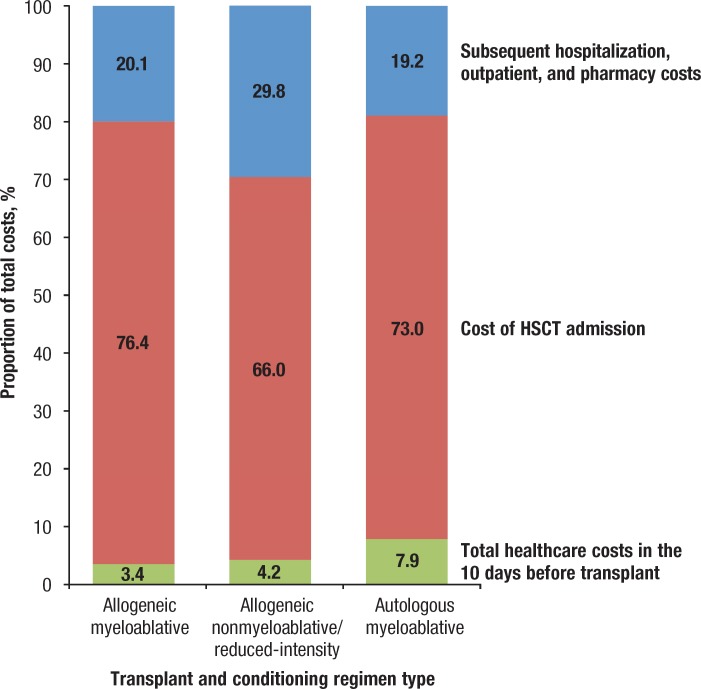
The cost of HSCT hospitalization made up 73% to 76% of the 100-day costs for those receiving a myeloablative conditioning regimen and 66% for patients receiving a nonmyeloablative/reduced-intensity conditioning regimen (Figure 2). The highest median costs for HSCT hospitalization were for the allogeneic MA cohort, at $208,857, followed by $161,241 and $110,209 for the allogeneic NMA and autologous MA cohorts, respectively.
Figure 2.
Relative Contribution of Cost of Total Healthcare in the 10 Days Leading Up to Transplantation, the Cost of HSCT Admission, and Subsequent Hospitalization, Outpatient, and Pharmacy Costs in the First 100 Days After Transplantation
NOTE: For each of the 3 categories of patients shown (allogenic transplant with myeloablative conditioning, allogeneic transplant with nonmyeloablative/reduced-intensity conditioning, and autologous transplant with myeloablative conditioning), the bar segments indicate the proportion of the total cost accounted for in each of these 3 time periods: the 10 days before transplant, the transplant admission (<1% of patients in our sample had a transplant in the outpatient setting), and the remainder of the 100-day follow-up.
HSCT indicates hematopoietic stem-cell transplantation.
The median outpatient costs and pharmacy costs were similar between the allogeneic NMA cohort ($41,349 and $6551, respectively) and the allogeneic MA cohort ($40,655 and $6451); these costs were higher than the costs for the autologous MA cohort ($18,400 and $673; Table 2). The mean LOS for the index HSCT hospitalization was 35.6 days for the allogeneic MA cohort, 26.6 days for the allogeneic NMA cohort, and 21.8 days for the autologous MA cohort. Subsequent hospitalization occurred for 42.5% of the allogeneic MA cohort, with a mean LOS of 9 days, compared with 43.6% for the allogeneic NMA cohort, with a mean LOS of 11 days, and 20.8% for the autologous MA cohort, with a mean LOS of 6.5 days (Table 2).
1-Year Costs and Hospitalization
The 100-day costs were more than 66% of the total median costs at 1 year, which were $408,876, $374,065, and $181,933 for the allogeneic MA, allogeneic NMA, and autologous MA groups, respectively (Table 2). The median inpatient costs at 1 year were $276,620 for the allogeneic MA cohort compared with $235,620 for the allogeneic NMA cohort and $121,277 for the autologous MA cohort (Table 2).
The 1-year median outpatient and pharmacy costs were similar among the allogeneic NMA cohort ($83,435 and $15,487, respectively) and the allogeneic MA cohort ($81,575 and $14,429), but these were higher than the costs for the autologous MA cohort ($42,294 and $2043).
Subsequent hospitalization at 1 year occurred at a similar rate for the allogeneic transplant groups (allogeneic MA, 67.3% with a mean LOS of 26.6 days vs allogeneic NMA, 69.2% with 30.3 days) but occurred less frequently for the autologous MA cohort (37.9% with 18 days; Table 2).
Pediatric Patients versus Adults
Table 3 shows the cost and hospitalization results according to age. The costs for the index HSCT hospitalization and for 100-day inpatient and outpatient healthcare services were greater for pediatric patients than for adult patients for the 2 transplant types. The median costs of the index HSCT hospitalization for myeloablative allogeneic and autologous transplants (we had insufficient numbers of pediatric patients receiving a nonmyeloablative/reduced-intensity regimen before transplant for comparison) were $363,379 and $154,266, respectively, in pediatric patients versus $191,541 and $109,113, respectively, in adults. The median inpatient costs for myeloablative allogeneic and autologous transplants were $406,195 and $194,125, respectively, for pediatric patients versus $212,332 and $111,419, respectively, for adults.
Table 3.
Healthcare Costs and Hospitalization at 100-Day Follow-Up for Those Receiving a Myeloablative Conditioning Regimen: Adults versus Pediatric Patients
| Pediatric patients | Adults | ||||
|---|---|---|---|---|---|
| Parameter | Allogeneic transplant (N = 80) | Autologous transplant (N = 27) | Allogeneic transplant (N = 318) | Autologous transplant (N = 942) | |
| Total healthcare costs, $a | Mean | 585,300 | 244,337 | 355,344 | 161,747 |
| SD | 441,427 | 74,986 | 372,341 | 137,921 | |
| Median | 445,916 | 243,257 | 264,632 | 138,966 | |
| Inpatient costs, $a | Mean | 529,994 | 191,320 | 296,398 | 132,633 |
| SD | 445,287 | 60,464 | 369,038 | 130,484 | |
| Median | 406,195 | 194,125 | 212,332 | 111,419 | |
| Outpatient costs, $a | Mean | 50,552 | 51,883 | 50,156 | 27,005 |
| SD | 32,916 | 40,436 | 44,147 | 27,627 | |
| Median | 43,814 | 44,929 | 40,424 | 17,893 | |
| Pharmacy costs, $a | Mean | 4754 | 1134 | 8790 | 2110 |
| SD | 5121 | 1305 | 7443 | 3893 | |
| Median | 2865 | 916 | 7174 | 662 | |
| Cost of index HSCT hospitalization, $b | Mean | 494,621 | 162,439 | 259,749 | 118,453 |
| SD | 432,533 | 65,081 | 332,603 | 68,195 | |
| Median | 363,379 | 154,266 | 191,541 | 109,113 | |
| Length of stay of HSCT hospitalization, days | Mean (SD) | 54.1 (37.2) | 25.6 (14.5) | 30.9 (20.6) | 21.6 (12.7) |
| Any subsequent hospitalizationc | Days, N (%) | 31 (38.8) | 11 (40.7) | 138 (43.4) | 191 (20.3) |
| Total length of stay, daysd | Mean (SD) | 7.1 (9.7) | 10.4 (11.7) | 9.4 (16.0) | 6.3 (12.8) |
All costs include the claims from 10 days before through 100 days (or 1 year) after the transplant.
Represents the costs of the index transplant, including conditioning regimen; inpatient transplants include the costs from 10 days before admission through discharge from index admission; outpatient transplants include the costs from 10 days before the day of first outpatient ICD-9 diagnosis code for HSCT.
Within 100 days (or 1 year) of follow-up.
Among patients with hospitalization subsequent to the HSCT admission and within 100 days (or 1 year) of follow-up. Total represents hospital days across all admissions subsequent to HSCT admission.
HSCT indicates hematopoietic stem-cell transplantation; ICD-9, International Classification of Diseases, Ninth Revision; SD, standard deviation.
The median outpatient costs for myeloablative allogeneic and autologous transplants were $43,814 and $44,929, respectively, for pediatric patients versus $40,424 and $17,893, respectively, for adults. However, the median 100-day pharmacy costs were higher for adults than for pediatric patients in the allogeneic transplant groups, but not in the autologous groups. The median pharmacy costs for myeloablative allogeneic and autologous transplants were $2865 and $916, respectively, for pediatric patients versus $7174 and $662, respectively, for adults (Table 3).
The mean LOS for the index HSCT hospitalization was higher for pediatric patients than for adults in the allogeneic MA and autologous MA cohorts—54.1 days and 25.6 days for pediatric patients versus 30.9 and 21.6 days for adults. Subsequent hospitalization and LOS were not substantially different across the pediatric and adult allogeneic MA and autologous MA groups—38.8% (mean LOS, 7.1 days) and 40.7% (mean LOS, 10.4 days) versus 43.4% (mean LOS, 9.4 days) and 20.3% (mean LOS, 6.3 days; Table 3).
Discussion
This real-world study of administrative claims data provides evidence that in a selected sample of transplant recipients, conditioning regimens given before HSCT, transplant type, and age are all important determinants of the associated costs. In the sample of predominantly adults undergoing inpatient HSCT between 2010 and 2013, the highest overall 100-day and 1-year costs were for patients in the allogeneic MA cohort, followed by the allogeneic NMA and autologous MA cohorts. The majority of healthcare spending associated with HSCT occurred in the first 100 days, mainly as a result of the inpatient costs associated with the index HSCT hospitalization and the subsequent hospitalization. After 100 days, the inpatient costs declined, whereas the outpatient and pharmacy costs grew as a proportion of the total cost.
This study included a selected group of patients. Data limitations, in particular the lack of detailed information on inpatient chemotherapy regimens and total body irradiation, made it impossible to identify the conditioning regimens for a representative sample of patients undergoing transplant.2 Therefore, we developed an algorithm that could identify the conditioning regimen in a select group of patients. Despite the use of this nonrepresentative sample, our findings are consistent with previous studies.4,16,17
For example, in our sample, allogeneic HSCT was nearly twice as costly as autologous HSCT for total, inpatient, outpatient, and pharmacy costs; this difference is similar to the findings by Majhail and colleagues who studied a larger group.4 This cost difference, which we observed at 100 days and at 1 year, may be primarily a result of the greater complexity of allogeneic HSCT than of autologous HSCT, in addition to allogeneic transplant–associated graft-versus-host disease and other complications linked to prolonged hospital admission and rates of readmission.16,17 In fact, we found that patients receiving myeloablative conditioning before allogeneic HSCT had longer index HSCT hospitalization and higher rates of subsequent hospitalization than patients undergoing autologous HSCT. Of course, the cost differences between patients undergoing allogeneic and autologous transplants are also undoubtedly influenced by the underlying clinical differences that guide the selection of HSCT grafting approach.
Our study also highlights the importance of the conditioning regimen with regard to 100-day and 1-year costs. The inpatient and total costs were higher for patients undergoing the allogeneic MA transplant who received myeloablative conditioning than those receiving nonmyeloablative/reduced-intensity conditioning, which is consistent with findings from single-institution studies.8,9 In our sample, patients in the allogeneic MA cohort had longer LOS in the index HSCT hospitalization than patients in the allogeneic NMA group. Despite the difference in cost according to the conditioning regimen, the inpatient costs for the allogeneic NMA cohort (the lower-cost group) were still substantial.
We observed considerable cost differences between the pediatric patients and adults who had HSCT, which is consistent with past research.18 Pediatric patients receiving myeloablative allogeneic or autologous HSCT had much higher 100-day inpatient, outpatient, and total costs than adults. These findings, particularly related to inpatient costs, likely reflect the different approaches to caring for pediatric patients and adults who have had HSCT. Children and adolescents tend to stay in the hospital or intensive care unit for longer periods after HSCT than adults, despite comparable rates of major complications18; this difference may reflect the special clinical needs of pediatric patients or the additional time needed to prepare parents for home-based caregiving.
Our analysis shows that pediatric HSCT hospital stays were nearly twice as long as adult hospitalizations. Such prolonged hospital stays can account for large increases in HSCT costs. For 100-day pharmacy costs, adults who had myeloablative allogeneic transplant, but not autologous transplant, had higher costs than pediatric patients, which could be caused by the greater use of age-related chronic medications among adults.
To our knowledge, this methodologic approach of identifying conditioning regimens to calculate their contribution to HSCT costs has not been used previously and therefore adds to the existing research.
Limitations
The approach we took has significant limitations and should be considered only a first step in a process that will, if validated, allow the use of large, administrative data sets to examine more detailed questions than was previously possible.
Although our algorithm for identifying conditioning regimens was developed through multiple rounds of clinical input from a variety of sources, as noted before, it has not been validated. The algorithm could have misclassified regimens in ways that would have biased our findings, and validation would therefore be crucial to strengthening our findings. Specifically, we excluded many patients whose conditioning regimens were undeterminable, making our results sample-specific and thus not generalizable to all patients undergoing HSCT.
Our analysis includes a very small number (ie, 54) of patients who had an outpatient HSCT before their inpatient transplant (their index event); therefore our conclusions may not be generalizable to the broader group of such patients. In addition, few patients in the autologous NMA group were identified, because such patients are typically managed in the outpatient setting. We therefore limited the analysis of adults to the 3 remaining groups.
Our examination of costs by age-group focused only on myeloablative conditioning, because we identified few pediatric patients who received nonmyeloablative/reduced-intensity conditioning, reflecting real-world patterns.19 Still, the costs for pediatric patients in our study may not be generalizable, because we focused on HSCT performed in the oncology setting, whereas the indications for pediatric transplants vary and include oncologic conditions, immunodeficiencies, and other genetic conditions.
We calculated the costs in the 10 days before HSCT as part of pretransplant conditioning. Some true conditioning-related costs might have occurred more than 10 days before HSCT, and some costs not related to conditioning might have occurred within the 10-day window, which may have led to overestimating or underestimating the conditioning regimen–related costs.
Because of small sample sizes and a lack of clinical detail in claims, our analysis did not adjust for other possible confounders that might have contributed to the HSCT costs, such as disease severity. For example, more fit patients are generally considered better candidates for the myeloablative conditioning regimen. However, we accounted for the factors of the transplant type, conditioning regimen, and age using a stratified analysis. We did not conduct multivariate analyses, because the goal of the study was to compare the actual costs for different types of conditioning regimens. The groups differed significantly, and these differences were related to or were drivers of the choice of the conditioning regimen.
We analyzed the costs at 1 year, but not all patients were alive or enrolled in a health plan at the end of the year. The costs generally increase in the months just before death, and because our intent was to examine the actual costs, limiting the analysis to patients who were still alive would have biased the results.
Finally, our analysis excluded donor-related costs, which, if added, would raise our estimates of the total costs associated with HSCT. Furthermore, our findings may not be representative of HSCT costs for patients with public or noncommercial insurance.
Conclusion
Our findings indicate that among patients who receive myeloablative conditioning regimens before a transplant, allogeneic HSCT is more expensive than autologous HSCT. Among patients undergoing allogeneic transplant, a myeloablative conditioning regimen is costlier than a nonmyeloablative/higher-intensity conditioning regimen, likely because of additional complications associated with more complex grafting procedures and regimens. Overall, pediatric HSCT is more expensive than HSCT in adults, which may be attributable to precautions used for pediatric patients, such as longer hospital stays. A crucial step for future research is to validate the accuracy of the algorithm in this study through clinical records, such as patient charts.
Funding Source
This research was funded by Jazz Pharmaceuticals.
Author Disclosure Statement
Dr Broder, Dr Chang, and Dr Reddy are employees of Partnership for Health Analytic Research, which received funding from Jazz Pharmaceuticals for this research; Dr Quock is an employee of Prothena Biosciences; Dr Agarwal-Hashmi and Dr Arai reported no conflicts of interest; Ms Villa is an employee and shareholder of Jazz Pharmaceuticals.
Contributor Information
Michael S. Broder, President and CEO, Partnership for Health Analytic Research, Beverly Hills, CA.
Tiffany P. Quock, Associate Director, Health Economics & Outcomes Research, Jazz Pharmaceuticals, Palo Alto, CA, during this study.
Eunice Chang, Chief Statistician, Partnership for Health Analytic Research.
Sheila R. Reddy, Director, Health Services Research, Partnership for Health Analytic Research.
Rajni Agarwal-Hashmi, Associate Professor, Pediatrics (Stem Cell Transplantation), Stanford University School of Medicine, Palo Alto, CA.
Sally Arai, Associate Professor, Medicine (Blood and Marrow Transplantation), Stanford University School of Medicine.
Kathleen F. Villa, Executive Director, Health Economics & Outcomes Research, Jazz Pharmaceuticals..
References
- 1. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. Erratum in: J Natl Cancer Inst 2011;103: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2015. www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/Pages/index.aspx. Accessed September 15, 2015.
- 3. Stranges E, Russo CA, Friedman B. Procedures with the most rapidly increasing hospital costs, 2004–2007. Healthcare Cost and Utilization Project statistical brief #82. December 2009. Agency for Healthcare Research and Quality; Rockville, MD. www.hcup-us.ahrq.gov/reports/statbriefs/sb82.jsp. Accessed March 16, 2016.
- 4. Majhail NS, Mau LW, Denzen EM, Arneson TJ. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large National Private Claims Database. Bone Marrow Transplant. 2013;48:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khera N, Storer B, Sandmaier BM, et al. Costs of second allogeneic hematopoietic cell transplantation. Transplantation. 2013;96:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khera N, Emmert A, Storer BE, et al. Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. Oncologist. 2014;19:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. [DOI] [PubMed] [Google Scholar]
- 9. Saito AM, Zahrieh D, Cutler C, et al. Lower costs associated with hematopoietic cell transplantation using reduced intensity vs high-dose regimens for hematological malignancy. Bone Marrow Transplant. 2007;40:209–217. [DOI] [PubMed] [Google Scholar]
- 10. Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cordonnier C, Maury S, Esperou H, et al. Do minitransplants have minicosts? A cost comparison between myeloablative and nonmyeloablative allogeneic stem cell transplant in patients with acute myeloid leukemia. Bone Marrow Transplant. 2005;36:649–654. [DOI] [PubMed] [Google Scholar]
- 12. Hansen LG, Chang S. Health research data for the real world: the MarketScan databases. White paper. July 2011. http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf. Accessed April 21, 2016.
- 13. Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bureau of Labor Statistics; US Department of Labor. CPI inflation calculator. June 2013. www.bls.gov/data/inflation_calculator.htm. Accessed April 25, 2016.
- 16. Dignan FL, Potter MN, Ethell ME, et al. High readmission rates are associated with a significant economic burden and poor outcome in patients with grade III/IV acute GvHD. Clin Transplant. 2013;27:E56–E63. [DOI] [PubMed] [Google Scholar]
- 17. Spring L, Li S, Soiffer R, et al. Readmissions following allogeneic hematopoietic stem cell transplantation. J Clin Oncol. 2013;31(31 suppl):265. [Google Scholar]
- 18. Majhail NS, Mothukuri JM, Macmillan ML, et al. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatr Blood Cancer. 2010;54:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verneris MR, Eapen M, Duerst R, et al. Reduced intensity conditioning regimens for allogeneic transplantation in children with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2010;16:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]