Abstract
Introduction
The intentional strategy (aggressive side branch (SB) protection strategy: elective two-stent strategy or jailed balloon technique) is thought to be associated with lower SB occlusion rate than conventional strategy (provisional two-stent strategy or jailed wire technique). However, most previous studies showed comparable outcomes between the two strategies, probably due to no risk classification of SB occlusion when enrolling patients. There is still no randomised trial compared the intentional and conventional strategy when treating bifurcation lesions with high risk of SB occlusion. We aim to investigate if intentional strategy is associated with significant reduction of SB occlusion rate compared with conventional strategy in high-risk patients.
Methods and analysis
The Conventional versus Intentional straTegy in patients with high Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion (CIT-RESOLVE) is a prospective, randomised, single-blind, multicentre clinical trial comparing the rate of SB occlusion between the intentional strategy group and the conventional strategy group (positive control group) in a consecutive cohort of patients with high risk of side branch occlusion defined by V-RESOLVE score, which is a validated angiographic scoring system to evaluate the risk of SB occlusion in bifurcation intervention and used as one of the inclusion criteria to select patients with high SB occlusion risk (V-RESOLVE score ≥12). A total of 21 hospitals from 10 provinces in China participated in the present study. 566 patients meeting all inclusion/exclusion criteria are randomised to either intentional strategy group or conventional strategy group. The primary endpoint is SB occlusion (defined as any decrease in thrombolysis in myocardial infarction flow grade or absence of flow in SB after main vessel stenting). All patients are followed up for 12-month postdischarge.
Ethics and dissemination
The protocol has been approved by all local ethics committee. The ethics committee have approved the study protocol, evaluated the risk to benefit ratio, allowed operators with a minimum annual volume of 200 cases to participate in the percutaneous coronary intervention procedure and permitted them to perform both conventional and intentional strategies. Written informed consent would be acquired from all participants. The findings of the trial will be shared by the participant hospitals and disseminated through peer-reviewed journals.
Trial registration number
NCT02644434; Pre-results.
Keywords: coronary bifurcation intervention, randomized comparison, conventional strategy, intentional strategy, side branch occlusion
Strengths and limitations of this study.
Conventional versus Intentional straTegy in patients with high Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion (CIT-RESOLVE) is the leading trial that intends to investigate if intentional strategy could decrease the rate of side branch (SB) occlusion in patients with high risk of SB occlusion.
This study enrols high-risk patients by using an inclusion criteria of SB occlusion risk (V-RESOLVE score ≥12 points).
This study would provide evidence for interventionalists in strategy selection when treating bifurcation with high risk of SB occlusion.
Not all bifurcation lesions are included in the present study; left main diseases are excluded.
Introduction
Approximately 15% to 20% of percutaneous coronary interventions (PCI) are performed to treat coronary bifurcation lesions.1–3 Previous studies have shown similar short-term and long-term clinical outcomes between the conventional strategy (eg, provisional two-stent strategy or jailed wire technique4–6) and the intentional strategy (eg, elective two-stent strategy or jailed balloon technique);7 8 thus, the conventional strategy is generally preferred for its easy use and reduced procedure time. However, the optimal interventional strategy selection for complex coronary bifurcation lesions remains somewhat controversial because of the variability in side branch (SB) disease and the desire to preserve patency of large diseased SBs. SB occlusion after main vessel (MV) stenting is one of the most serious complications during the procedure and may be the major reason why operators prefer more aggressive strategy in the complex bifurcation lesions. Our study has shown that the rate of SB occlusion was 7.37% in patients underwent conventional strategy,9 which was in accordance with previous studies (SB occlusion rate: 8.4%–19%).10–12 SB occlusion can result in vessel closure and ischaemia, with clinically significant myocardial infarction (MI) and even death depending on the size of the SB (and the myocardial territory subtended by it).12 13
The risk and incidence of SB occlusion are important factors impacting the interventional strategy selection and clinical outcome.9 However, since the lack of useful tool for risk prediction of SB occlusion, no previous studies have considered the risk of SB occlusion as one of the inclusion criteria during patient enrolment. Previous randomised clinical trials performed randomisation of all categories of bifurcation lesions by using computer-generated random sequence totally ignored the individual lesion anatomical characteristics and the risk of SB occlusion. Now, we have developed an angiographic tool for risk prediction of SB occlusion, the Visual estimation for Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion (V-RESOLVE) score, which can help risk stratification of SB occlusion and could also be used as a tool to select high-risk patients in randomised study. The SB occlusion rate was significantly higher in the high-risk group (V-RESOLVE score ≥12, rate of SB occlusion: 16.7%) than the non-high-risk group (V-RESOLVE score <12, rate of SB occlusion: 4.3%) as assessed by the V-RESOVLE score.14
Bifurcation lesions with high risk of SB occlusion may need intentional interventional strategy, which is more aggressive in SB protection than conventional strategy and considered to be associated with lower SB occlusion rate. However, no randomised trials were performed to compare the rate of SB occlusion between intentional strategy and conventional strategy in high-risk patients.
Accordingly, the present study is designed to enrol patients with high risk of SB occlusion (V-RESOLVE score ≥12) and investigate if intentional strategy is associated with significant reduction of SB occlusion rate compared with conventional strategy in patients with high risk of SB occlusion.
Methods and analysis
Hypothesis to be tested
We hypothesised that for patients at high risk of SB occlusion (V-RESOLVE score ≥12), intentional strategy (a more aggressive SB protection strategy: elective two-stent strategy or jailed balloon technique) is associated with significant reduction of SB occlusion rate compared with conventional strategy (provisional two-stent strategy or jailed wire technique). Thus, the hypothesis to be decided on are as follows: H0, for patients with high risk prediction of SB occlusion, there is no difference in the rate of SB occlusion between intentional strategy group and the conventional strategy group, versus H1, the rate of SB occlusion in intentional strategy group would be significantly lower than that of conventional strategy group.
Study design
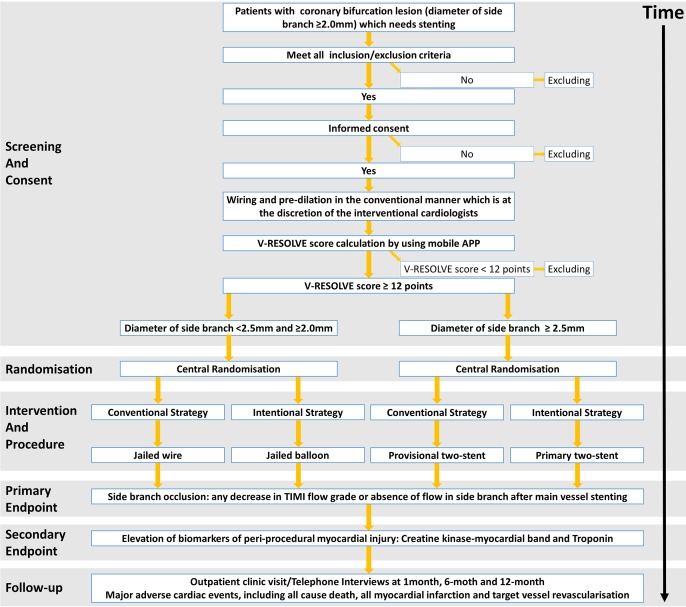
The CIT-RESOLVE is a prospective, randomised (1:1), single-blind, multicentre clinical trial comparing the rate of SB occlusion between the conventional strategy group and the intentional strategy group in a consecutive cohort of high-risk coronary bifurcation patients. Although operators are not blinded, all individuals analysing the data are masked to treatment assignment. A total of 21 centres in China will enrol patients. This study is registered on www.clinicaltrials.gov, and the registration number is NCT 02644434. The study flowchart is shown in figure 1 and its legend.
Figure 1.
Study flowchart screening, randomisation, intervention, procedure, study endpoint and follow-up of CIT-RESOLVE trial. CIT-RESOLVE, Conventional versus Intentional straTegy in patients with high Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion.
This trial is conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The conduct of the trial has been approved by the ethics committee. Written informed consent would be acquired from all participants. Patient data in the Data Management System are protected by password and only available to users designated by the study with appropriate authorisation levels. Deidentified data will be used for data analysis.
Risk prediction of SB occlusion
V-RESOLVE score would be used for risk prediction of SB occlusion. The RESOLVE (Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion) score, which is developed on the basis of quantitative coronary angiography (QCA), is a validated angiographic scoring system to evaluate the risk of SB occlusion in bifurcation intervention.9 The QCA-based RESOLVE score system contains six independent risk factors of SB occlusion: including two visual estimation predictors (plaque distribution and MV thrombolysis in MI (TIMI) flow grade before stenting) and four QCA analysis predictors (preprocedural diameter stenosis of bifurcation core, bifurcation angle, diameter ratio between MV/SB and diameter stenosis of SB before MV stenting).
Although QCA provides a more objective determination of the extent and severity of coronary artery disease, it may be more time-consuming and/or not immediately available in real-time. As a result, the inclusion of QCA data within the QCA-based RESOLVE score limits its ability to be used at the time of bifurcation intervention.15 Therefore, we evaluated the ability of a visually estimated RESOLVE (V-RESOLVE) score to predict the risk of SB occlusion during bifurcation intervention. We found that the V-RESOLVE score, an easy-to-use score system based on visual estimation, can help risk stratification of SB occlusion during coronary bifurcation intervention. The rate of SB occlusion was significantly higher in high-risk group (V-RESOLVE score ≥12, rate of SB occlusion: 16.7%) than that in non-high-risk group (V-RESOLVE score ≤11, rate of SB occlusion: 4.3%) (p<0.01). V-RESOLVE score makes precision medicine possible in the daily practice of coronary bifurcation intervention for its easy use. The development, validation and calculation methods are detailed in our previous study.14 The V-RESOLVE score is calculated by using a dedicate app, which is available in both the iTunes store and Google Play Store. Only patients with V-RESOLVE score ≥12 would be enrolled.
Study population
A total of 566 patients with coronary bifurcation lesions (at high risk of SB occlusion), requiring PCI with stent implantation, are studied. V-RESOLVE score ≥12 points are defined as lesions with high risk of SB occlusion. This implies the application of only few angiographic exclusion criteria (table 1). All patients provide written informed consent.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
Clinical inclusion criteria:
|
Clinical exclusion criteria:
|
Angiographic inclusion criteria:
|
Angiographic exclusion criteria:
|
PCI, percutaneous coronary intervention.
Hospitals selection
A total of 21 hospitals from 10 provinces (Beijing, Shanghai, Guangdong, Shanxi, Heilongjiang, Jilin, Liaoning, Guangxi, Hunan and Hebei, detailed in online supplementary file) are chosen. The annual PCI volume of each of these hospitals is ≥800. Only operators with a minimum annual volume of 200 cases are allowed to participate in the PCI procedure. All these interventionalists are skilled in coronary bifurcation PCI and qualified to perform both conventional and intentional strategies.
bmjopen-2017-016044supp001.pdf (457.3KB, pdf)
Investigator training
All investigators received comprehensive training on the standard definition of elements, protocol, app using, calculation of V-RESOLVE score, randomisation, standard procedure of PCI and data management.
Although there are only six variables in the V-RESOLVE score, intraobserver and interobserver variability for visual estimation is always a question for every visual score system and is also a major concern of us. To minimise the intraobserver and interobserver variability in the calculation of V-RESOLVE score, all investigators have undergone an extensive training session by a group of experienced technicians from the angiographic core laboratory in Fuwai Hospital on 13 August 2016. The training session included: (1) calculate the V-RESOLVE score of low-risk and high-risk bifurcation lesions and (2) a comprehensive review of bias, discrepancies and pitfalls related to these cases. The investigator interobserver agreement was found to be substantial or greater (Fleiss Kappa >0.60) after training. Once the investigators are not sure that the V-RESOLVE score is ≥12 points or not, we recommend them to send the cineangiograms by internet to the angiographic core laboratory in Fuwai Hospital, where cineangiograms would be assessed by two experienced technicians together, and the V-RESOLVE score was generated by consensus.
Patient enrolment and randomisation
Subjects must be ≥18 years and ≤75 years of age at the time of enrolment in the study. Coronary angiography would be performed to confirm that angiographic inclusion criteria are met. Then, wiring and predilation would be performed at the discretion of the interventional cardiologists in the conventional manner. A mobile app specialised for V-RESOLVE calculation will be used to calculate the V-RESOLVE score after predilation. Bifurcation lesions with V-RESOLVE score of ≥12 points will be enrolled. Patients that meet all the inclusion criteria and have no exclusion criteria would be included in this study. Patient enrolment has been started on 1 December 2016 and anticipated to be completed before December 2017.
Patient randomisation will be performed centrally by internet after signing an informed consent form. The randomisation will be stratified by the diameter of SB (diameter of SB <2.5 mm and ≥2.0 mm vs diameter of SB ≥2.5 mm), with a randomisation ratio of 1:1 to either conventional strategy group or intentional strategy group.
Intervention and procedure
PCI is undertaken via the access site of operators’ choice. Coronary angioplasty is performed in the conventional manner and coronary stents or other procedures/devices are used only when required. The administration of periprocedural antiplatelet and antithrombotic medications is based on the operator’s discretion and current guidelines. Intravenous unfractionated heparin is used to maintain an activated clotting time between 250 s and 300 s through the whole procedure. Cardiac enzymes (creatine kinase–myocardial band (CK–MB) and troponin) are dynamically measured until 48 hours postprocedure. Lifelong aspirin (100 mg/d) is prescribed to all patients. At least 12 months of clopidogrel (75 mg/d) would be recommended to all patients.
Conventional strategy group
Patients randomised to the conventional strategy group would undergo either jailed wire technique (diameter of SB <2.5 mm and ≥2.0 mm) or provisional two-stent strategy (diameter of SB ≥2.5 mm).
Jailed wire technique
Both MV and SB are wired, with lesion preparation at the operator’s discretion. The MV is stented with wire protection in SB. The SB is not further treated unless there is threatened SB closure, severe ostial pinching of SB (>90%), TIMI flow grade decrease in SB, or SB dissection greater than type A. If one of these criteria exists, the SB would be rewired and a kissing balloon inflation is undertaken with anatomically appropriate sizing for each vessel.
Provisional two-stent strategy
Both vessels are wired, with lesion preparation and MV stenting the same as the jailed wire technique. Provisional T stenting of the SB could be undertaken if one of the following criteria exists after SB rewiring and a kissing balloon inflation is undertaken: threatened SB closure, severe ostial pinching of SB (>90%), TIMI flow grade decrease in SB, or SB dissection greater than type A.
Intentional strategy group
In the present trial, we would enrol high-risk SB with diameter ≥2.0 mm, which would critically impact the prognosis. However, elective two-stent strategy is not appropriate for all SB with diameter ≥2.0 mm. Thus, we use two aggressive strategies in intentional strategy group: jailed balloon technique (for SB with diameter <2.5 mm and ≥2.0 mm) or elective two-stent strategy (for SB with diameter ≥2.5 mm).
Jailed balloon technique
The technique has been detailed in previous studies.4 5 To be brief, vessel wiring and lesion preparation are the same as the jailed wire technique. A balloon that is appropriately sized to approximate the reference vessel diameter (RVD) of SB is advanced into the SB. A stent is then advanced into correct position over the target lesion in the MV. To prevent entrapment of the SB balloon, the proximal marker of the balloon is positioned approximately 2 mm proximal to the MV stent. Adequate length of balloon is advanced into SB to project the ostium. Then, the stent in MV is deployed to nominal pressures, jailing the SB balloon and wire. If the SB is not compromised, then the jailed SB balloon is inflated to low pressure (<3 atmospheres), deflated, and the SB wire and balloon removed. Then the SB is rewired, followed by mandatory proximal optimisation technique.
However, if there is TIMI flow grade decrease in SB, the balloon is inflated to try to reopen the SB. After the SB is rewired, the SB balloon is removed. Ballooning or T stenting of the SB could be undertaken. POT is mandated to achieve good apposition of the proximal MV stent after the SB is reopened. The wire in SB will not be removed until the POT is completed.
No matter there is SB compromise or not, final kissing balloon technique could be performed at the discretion of the interventional cardiologists.
Elective two-stent strategy
Patients in this subgroup would undergo crush procedure (eg, Crush, Balloon Crush or DK-Crush)16–18 or any other elective two-stent strategy like Culotte and T stent,19 20 which stenting SB before MV stenting. These techniques were detailed in previous studies.16–20
For both the conventional and intentional strategy groups, proximal or distal dissections could be treated with further stenting at any stage. Post-dilations could be performed to optimise stent expansion. In all cases, an additional vessel with other lesions could be treated if required.
Primary and secondary endpoint(s)
The primary endpoint is SB occlusion, which is defined as any decrease in TIMI flow grade or absence of flow in SB after MV stent well opposed. For lesions underwent conventional strategy, TIMI flow grade is assessed immediately after the MV stent is deployed and post-dilation (if post-dilation is performed), then, the SB could be further treated if required. For lesions underwent jailed balloon technique, TIMI flow grade is assessed after POT is performed. For lesions underwent elective two-stent strategy, TIMI flow grade is assessed immediately after the MV stent is deployed and post-dilation (if post-dilation is performed), then rewiring the SB or final kissing balloon is performed if required.
The secondary endpoints are: (1) the elevation of biomarkers of periprocedural myocardial injury (CK–MB and troponin); periprocedural MI is defined as biomarkers elevation ≥10 × upper reference limit (URL) for CK–MB and/or ≥70 × URL for troponin21 and (2) 12-month major adverse cardiac events (MACE, including all-cause death, all MIs and target vessel revascularisation).
Follow-up
Subjects had either a telephone call or clinic visit at 30 days (±7 days), 3 months (±14 days), 6 months (±14 days) and 12 months (±30 days) by the enrolling site for outcome evaluation. For all patients, MACE at 12 months will be reported. MACE will be defined as a composite of all-cause death, all MIs (defined by the Third Universal Definition22) and target vessel revascularisation (defined by the Academic Research Consortium23).
Data collection
Profession trained staff who are independent of patient treatment will be responsible for data collection and entering. The data collected for each new CIT-RESOLVE patient include baseline information, sociodemographic characteristics, symptoms and signs of the presenting coronary disease, medical history, biomarker findings (CK–MB and troponin activity will be determined by using an immunoinhibition assay and confirmed by mass spectrometry), electrocardiography, and treatments administered prior to admission during hospitalisation. Final diagnosis, major in-hospital clinical events (death, periprocedural MI, major bleeding and stroke) and discharge status will also be recorded.
Baseline and procedural coronary angiography will be reviewed and analysed by physicians and interventionalists to calculate the V-RESOLVE score. Coronary angiography findings, including bifurcation location, baseline and post-MV stenting TIMI flow grade in MV and SB, will be recorded. Procedural characteristics including interventional strategy, the presence of jailed wire/balloon and successful final kissing or not will be collected. All investigators are required to collect, recheck and input all these data and submit the completed electronic case report form on the patient’s discharge or death. The investigation scheduling is detailed in table 2.
Table 2.
Investigation scheduling
| Schedule of investigations | Baseline (pre-PCI) |
Procedure | Postprocedure/ discharge | 30 days (±7 days) |
3 months (±14 days) |
6 months (±14 days) |
12 months (±30 days) |
| Visit or phone contact | Visit or phone contact | Visit or phone contact | Visit or phone contact |
||||
| Inclusion/exclusion criteria | • | ||||||
| Informed consent | • | ||||||
| History and risk factors | • | ||||||
| Physical examination | • | ||||||
| Anginal status | • | • | • | • | • | • | |
| Recording of medications | • | • | • | • | • | • | |
| 12-lead electrocardiography | •† | •‡ | |||||
| Cardiac enzymes (CK–MB and troponin) | •§ | •¶ | |||||
| Serious adverse events** | • | • | • | • | • | • | |
| V-RESOLVE score calculation | • |
• Procedure need to be performed or data need to be collected.
†Electrocardiography (ECG) at time of screening should be performed within 72 hours prior to PCI procedure.
‡ECG within 24 hours postprocedure or at discharge, whichever comes first.
§Cardiac biomarkers per standard of care and local practice is drawn prior to the index PCI procedure (within 24 hours prior to PCI).
¶CK–MB and troponin in the postprocedure hospitalisation period should be taken approximately 3–6 hours postprocedure). If cardiac enzymes are elevated (according to local upper limit of normal), serial measurements of cardiac enzymes must be taken until a decline is noted.
**For all revascularisations (including stent thrombosis and so on), the angiogram must be sent to the monitor organisation.
Note: In the event of undercurrents illnesses, interventions, adverse events or treatment failure, effort should be made to complete the required observations as much as possible.
CK–MB, creatine kinase–myocardial band; PCI, percutaneous coronary interventions.
One follow-up survey (by outpatient clinic visit or telephone) will be conducted at 12 months after discharge, to collect information on medications, MACE and any rehospitalisations after discharge.
Statistical considerations
Sample size calculations
Sample size parameters for the primary endpoint:
A 1:1 treatment allocation ratio of intentional strategy group and the conventional strategy group.
A two-side significance level (alpha) of 0.05.
Eighty per cent power to show differences in the rate of SB occlusion between intentional strategy group and conventional strategy group.
The rate of SB occlusion in intentional strategy group: 4.0%.
The rate of SB occlusion in conventional strategy group: 10.0%.
The primary endpoint would be reached immediately after the MV stenting; therefore, the attrition rate is 0%.
Sample size formula:
The 10% rate of SB occlusion in conventional strategy group is based on the V-RESOLVE study.15 It is reasonable to assume that, with an intentional strategy for bifurcation lesions with V-RESOLVE score ≥12 points, the rate of SB occlusion would decrease to 4% in intentional strategy group. Thus, the present study requires 283 subjects in intentional strategy group and 283 in conventional strategy group and the total number will be 566.
Analysis plan
The statistical analyses of the full analysis set will follow the intention-to-treat (ITT) principle. The ITT set will consist of all subjects who signed the written informed consent and are randomised, regardless which strategy was selected. The primary analysis is a superiority ITT analysis of the primary clinical endpoint. Normal approximation test for the difference between two proportions (pooled proportion) or Fisher’s exact test (if applicable) will be used to test the two-sided hypothesis of superiority in proportions. If the p value from the two-sided test is <0.05, the intentional strategy (test) will be concluded to be superior to conventional strategy. If required, an additional analysis of the per-protocol population will be conducted of the primary and secondary endpoints.
The conventional χ2 test or Fisher exact test will be used for the analysis of categorical variables. The treatment group differences will be evaluated with Student’s t-test or Wilcoxon rank sum scores for continuous variables. The two strategies will be compared by Kaplan-Meier estimates for survival analysis. Statistical significance will be declared if the two-sided p value is <0.05. All analyses will be performed with the use of the statistical programme SAS V.9.4.
Discussion
During coronary bifurcation intervention, one of the most serious complications is SB occlusion. Keeping the SB open is the major principle during PCI. However, no previous randomised trials tried to address the problem of decreasing SB occlusion rate in patients with high risk of SB occlusion. The intentional strategy, which is more aggressive in SB protection, is thought to have lower SB occlusion rate. However, there is no concrete evidence confirming that intentional strategy is associated with significant reduction of SB occlusion rate compared with conventional strategy in patients with high risk of SB occlusion. CIT-RESOLVE is the leading randomised trial that attempts to clarify this issue. To the best of our knowledge, CIT-RESOLVE will be the first trial that (1) enrols high-risk patients by using an inclusion criteria of SB occlusion risk (V-RESOLVE score ≥12 points) and (2) compares the rate of SB occlusion between intentional strategy and conventional strategy in patients with high risk of SB occlusion.
Series randomised clinical trials have attempted to address the problem of whether bifurcation lesions require stenting both the MV and SB or not.2 6 19 24–33 However, the results of previous studies remain controversial: the BBC ONE study showed significant lower incidence of MACE in simple strategy group,29 while the Double Kissing Crush (DKCRUSH) -II study showed a significant reduction of target lesion revascularisation and target vessel revascularisation in DK crush group.6 Most of the randomised clinical trials performed randomisation of all bifurcation lesions by using computer-generated random sequence, totally ignored the individual lesion anatomical characteristics and risk factors of SB occlusion. Thus, a substantial part of bifurcation lesions may not undergo proper intervention strategy though some patients have crossed over to another group. This may be the major reason why the results of previous studies remain controversial.
Previous studies enrolled patients by using the inclusion criteria of either unselected bifurcation lesions, specific Medina classifications or true bifurcation lesions. However, neither ‘Medina classification’ nor ‘true bifurcation lesion’ could predict the risk of SB occlusion accurately.34 35 The SB occlusion risk is not considered as an important criterion when enrolling patients. CIT-RESOLVE is the first trial that only enrols high-risk patients by using a risk prediction tool (V-RESOLVE score ≥12 points).
Numerous classifications and definitions of coronary bifurcation lesions have been proposed to simplify the hard topic of bifurcation lesion in interventional cardiology.36–45 Among them, ‘Medina classification’ as well as ‘true bifurcation lesion’ are straightforward and widely used. However, none of these classifications or definitions could accurately predict the risk of SB occlusion.35 One of our previous researches has shown that ‘true bifurcation lesion’ could not be regarded as an independent predictor of SB occlusion.34 RESOLVE score and V-RESOLVE score is the first attempt to stratify the risk of SB occlusion during coronary bifurcation intervention. V-RESOLVE score, which contains six independent predictors of SB occlusion, is a validated score system to evaluate the risk of SB occlusion14 and a useful tool for risk prediction of SB occlusion in the present study. V-RESOLVE score ≥12 points is considered as high risk in SB occlusion, which may trigger interventional SB protection, and set as one of the inclusion criteria of CIT-RESOLVE trial.
The intentional strategy is more aggressive in SB protection: jailed wire may help SB reopen; stenting the SB before MV stenting may avoid SB occlusion. Thus, the intentional strategy is thought as a more suitable strategy for high-risk bifurcation lesion. CIT-RESOLVE is the leading trial that intends to investigate if intentional strategy could decrease the rate of SB occlusion in patients with high risk of SB occlusion. Comparing the rate of SB occlusion between intentional and conventional strategy would provide evidence for interventionalists in strategy selection when treating bifurcation with high risk of SB occlusion. Twelve-month follow-up would investigate if SB occlusion could impact the clinical outcome directly.
One limitation of the trial design is that not all high-risk bifurcation lesions are included in the present study. When treating left main diseases, left anterior descending artery or left circumflex artery occlusion may lead to serious outcome, thus, left main diseases are excluded in the consideration of ethic. Also, in case of acute MI of which the culprit vessel located at the LAD, the bifurcation lesion (LAD/diagonal branch (RVD >2.5 mm)), which is proximal to occluded LAD segment, is excluded. Another limitation is that jailed balloon technique, which has not been proven by randomised clinical trials and widely used in clinical practice, is used in the interventional group. Although jailed balloon technique has been reported to be associated with very low rate of SB occlusion,4 its effect in SB protection warrant further studies. In future studies, we would compare the rate of SB occlusion between provisional two-stent strategy and elective two-stent strategy in patients at high risk of SB occlusion.
Conclusion
The CIT-RESOLVE study is the first large randomised trial that enrols only high-risk patients by using an inclusion criteria of SB occlusion risk (V-RESOLVE score ≥12 points), and it has sufficient power to assess the effect of intentional strategy in decreasing the SB occlusion rate in patients at high risk of SB occlusion.
CIT-RESOLVE study group
Principal investigator: KD (Fuwai Hospital and National Center for Cardiovascular Diseases). Co-principal investigator: BX (Fuwai Hospital and National Center for Cardiovascular Diseases). Coordinating center: Fuwai Hospital and National Center for Cardiovascular Diseases, Beijing, China. Advisory Chairmen: Yuejin Yang (Fuwai Hospital and National Center for Cardiovascular Diseases), Shaoliang Chen (Nanjing First Hospital and Nanjing Medical University) and AJK (Columbia University Medical Center and New York Presbyterian Hospital).
Supplementary Material
Footnotes
Contributors: All authors have contributed to the conception, critical review and revision process and have offered final approval of this manuscript.
Funding: The CITCRESOLVE trial is physician initiated and supported by Investigator Sponsored Studies (Abbott Cardiovascular, Study No.: CORC10498) and Peking Union Medical College Youth Fund and Fundamental Research Funds for the Central Universities (grant number: 3332016130). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript and its final contents.
Competing interests: None declared.
Patient consent: The present manuscript is protocol, no patient information is included.
Ethics approval: The Ethics Committee of the Cardiovascular Institute and Fuwai Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The present manuscript is protocol, there is no additional unpublished data.
References
- 1. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126–30. 10.1001/jama.293.17.2126 [DOI] [PubMed] [Google Scholar]
- 2. Steigen TK, Maeng M, Wiseth R, et al. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation 2006;114:1955–61. 10.1161/CIRCULATIONAHA.106.664920 [DOI] [PubMed] [Google Scholar]
- 3. Latib A, Colombo A. Bifurcation disease: what do we know, what should we do? JACC Cardiovasc Interv 2008;1:218–26. 10.1016/j.jcin.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 4. Singh J, Patel Y, Depta JP, et al. A modified provisional stenting approach to coronary bifurcation lesions: clinical application of the "jailed-balloon technique". J Interv Cardiol 2012;25:289–96. 10.1111/j.1540-8183.2011.00716.x [DOI] [PubMed] [Google Scholar]
- 5. Burzotta F, Trani C, Sianos G. Jailed balloon protection: a new technique to avoid acute side-branch occlusion during provisional stenting of bifurcated lesions. Bench test report and first clinical experience. EuroIntervention 2010;5:809–13. 10.4244/EIJV5I7A135 [DOI] [PubMed] [Google Scholar]
- 6. Chen SL, Santoso T, Zhang JJ, et al. A randomized clinical study comparing double kissing crush with provisional stenting for treatment of coronary bifurcation lesions: results from the DKCRUSH-II (Double Kissing Crush versus Provisional Stenting Technique for treatment of coronary bifurcation lesions) trial. J Am Coll Cardiol 2011;57:914–20. 10.1016/j.jacc.2010.10.023 [DOI] [PubMed] [Google Scholar]
- 7. Généreux P, Kumsars I, Lesiak M, et al. A randomized trial of a dedicated bifurcation stent versus provisional stenting in the treatment of coronary bifurcation lesions. J Am Coll Cardiol 2015;65:533–43. 10.1016/j.jacc.2014.11.031 [DOI] [PubMed] [Google Scholar]
- 8. Abdel-Latif A, Moliterno DJ. Bifurcation stenting techniques and outcomes in patients with stable coronary artery disease: more evidence suggesting simpler is safer. JACC Cardiovasc Interv 2015;8:561–3. 10.1016/j.jcin.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 9. Dou K, Zhang D, Xu B, et al. An angiographic tool for risk prediction of side branch occlusion in coronary bifurcation intervention: the RESOLVE score system (Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion). JACC Cardiovasc Interv 2015;8:39–46. 10.1016/j.jcin.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 10. Kralev S, Poerner TC, Basorth D, et al. Side branch occlusion after coronary stent implantation in patients presenting with ST-elevation myocardial infarction: clinical impact and angiographic predictors. Am Heart J 2006;151:153–7. 10.1016/j.ahj.2005.01.034 [DOI] [PubMed] [Google Scholar]
- 11. Aliabadi D, Tilli FV, Bowers TR, et al. Incidence and angiographic predictors of side branch occlusion following high-pressure intracoronary stenting. Am J Cardiol 1997;80:994–7. 10.1016/S0002-9149(97)00591-2 [DOI] [PubMed] [Google Scholar]
- 12. Hahn JY, Chun WJ, Kim JH, et al. Predictors and outcomes of side branch occlusion after main vessel stenting in coronary bifurcation lesions: results from the COBIS II Registry (COronary BIfurcation stenting). J Am Coll Cardiol 2013;62:1654–9. 10.1016/j.jacc.2013.07.041 [DOI] [PubMed] [Google Scholar]
- 13. Muramatsu T, Onuma Y, García-García HM, et al. Incidence and short-term clinical outcomes of small side branch occlusion after implantation of an everolimus-eluting bioresorbable vascular scaffold: an interim report of 435 patients in the ABSORB-EXTEND single-arm trial in comparison with an everolimus-eluting metallic stent in the SPIRIT first and II trials. JACC Cardiovasc Interv 2013;6:247–57. 10.1016/j.jcin.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 14. Dou K, Zhang D, Xu B, et al. An angiographic tool based on visual estimation for risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion: the V-RESOLVE score system. EuroIntervention 2016;11:e1604–e1611. 10.4244/EIJV11I14A311 [DOI] [PubMed] [Google Scholar]
- 15. Colombo A, Ruparelia N. When you ask yourself the question "should I protect the side branch?": the answer is "yes". JACC Cardiovasc Interv 2015;8:47–8. 10.1016/j.jcin.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 16. Chen SL, Ye F, Zhang JJ, et al. [DK crush technique: modified treatment of bifurcation lesions in coronary artery]. Chinese Med J 2005;118:1746–50. [PubMed] [Google Scholar]
- 17. Lim PO, Dzavík V. Balloon crush: treatment of bifurcation lesions using the crush stenting technique as adapted for transradial approach of percutaneous coronary intervention. Catheter Cardiovasc Interv 2004;63:412–6. 10.1002/ccd.20179 [DOI] [PubMed] [Google Scholar]
- 18. Colombo A, Moses JW, Morice MC, et al. Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation 2004;109:1244–9. 10.1161/01.CIR.0000118474.71662.E3 [DOI] [PubMed] [Google Scholar]
- 19. Adriaenssens T, Byrne RA, Dibra A, et al. Culotte stenting technique in coronary bifurcation disease: angiographic follow-up using dedicated quantitative coronary angiographic analysis and 12-month clinical outcomes. Eur Heart J 2008;29:2868–76. 10.1093/eurheartj/ehn512 [DOI] [PubMed] [Google Scholar]
- 20. Sheiban I, Albiero R, Marsico F, et al. Immediate and long-term results of "T" stenting for bifurcation coronary lesions. Am J Cardiol 2000;85:1141–4. 10.1016/S0002-9149(00)00712-8 [DOI] [PubMed] [Google Scholar]
- 21. Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563–70. 10.1016/j.jacc.2013.08.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98. 10.1016/j.jacc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 23. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 24. Maeng M, Holm NR, Erglis A, et al. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions: nordic Bifurcation Study 5-year follow-up results. J Am Coll Cardiol 2013;62:30–4. 10.1016/j.jacc.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 25. Sirker A, Sohal M, Oldroyd K, et al. The impact of coronary bifurcation stenting strategy on health-related functional status: a quality-of-life analysis from the BBC one (British Bifurcation Coronary; Old, New, and Evolving strategies) study. JACC Cardiovasc Interv 2013;6:139–45. 10.1016/j.jcin.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 26. Song YB, Hahn JY, Song PS, et al. Randomized comparison of conservative versus aggressive strategy for provisional side branch intervention in coronary bifurcation lesions: results from the SMART-STRATEGY (Smart Angioplasty Research Team-Optimal Strategy for Side Branch intervention in coronary bifurcation lesions) randomized trial. JACC Cardiovasc Interv 2012;5:1133–40. 10.1016/j.jcin.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 27. Behan MW, Holm NR, Curzen NP, et al. Simple or complex stenting for bifurcation coronary lesions: a patient-level pooled-analysis of the nordic bifurcation study and the British bifurcation coronary study. Circ Cardiovasc Interv 2011;4:57–64. 10.1161/CIRCINTERVENTIONS.110.958512 [DOI] [PubMed] [Google Scholar]
- 28. Lin QF, Luo YK, Lin CG, et al. Choice of stenting strategy in true coronary artery bifurcation lesions. Coron Artery Dis 2010;21:345–51. 10.1097/MCA.0b013e32833ce04c [DOI] [PubMed] [Google Scholar]
- 29. Hildick-Smith D, de Belder AJ, Cooter N, et al. Randomized trial of simple versus complex drug-eluting stenting for bifurcation lesions: the british bifurcation coronary study: old, new, and evolving strategies. Circulation 2010;121:1235–43. 10.1161/CIRCULATIONAHA.109.888297 [DOI] [PubMed] [Google Scholar]
- 30. Jensen JS, Galløe A, Lassen JF, et al. Safety in simple versus complex stenting of coronary artery bifurcation lesions. the nordic bifurcation study 14-month follow-up results. EuroIntervention 2008;4:229–33. 10.4244/EIJV4I2A41 [DOI] [PubMed] [Google Scholar]
- 31. Colombo A, Bramucci E, Saccà S, et al. Randomized study of the crush technique versus provisional side-branch stenting in true coronary bifurcations: the CACTUS (Coronary bifurcations: application of the Crushing Technique using Sirolimus-Eluting stents) Study. Circulation 2009;119:71–8. 10.1161/CIRCULATIONAHA.108.808402 [DOI] [PubMed] [Google Scholar]
- 32. Ferenc M, Gick M, Kienzle RP, et al. Randomized trial on routine vs. provisional T-stenting in the treatment of de novo coronary bifurcation lesions. Eur Heart J 2008;29:2859–67. 10.1093/eurheartj/ehn455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan M, de Lezo JS, Medina A, et al. Rapamycin-eluting stents for the treatment of bifurcated coronary lesions: a randomized comparison of a simple versus complex strategy. Am Heart J 2004;148:857–64. 10.1016/j.ahj.2004.05.029 [DOI] [PubMed] [Google Scholar]
- 34. Park TK, Park YH, Song YB, et al. Long-Term clinical outcomes of true and Non-True bifurcation lesions according to Medina classification- Results from the COBIS (COronary BIfurcation stent) II registry. Circ J 2015;79:1954–62. 10.1253/circj.CJ-15-0264 [DOI] [PubMed] [Google Scholar]
- 35. Chen X, Zhang D, Yin D, et al. Can "true bifurcation lesion" actually be regarded as an independent risk factor of acute side branch occlusion after main vessel stenting?: A retrospective analysis of 1,200 consecutive bifurcation lesions in a single center. Catheter Cardiovasc Interv 2016;87(Suppl 1):554–63. 10.1002/ccd.26403 [DOI] [PubMed] [Google Scholar]
- 36. Medina A, Suárez de Lezo J, Pan M. [A new classification of coronary bifurcation lesions]. Rev Esp Cardiol 2006;59:183. [PubMed] [Google Scholar]
- 37. Y-Hassan S, Lindroos MC, Sylvén C. A novel descriptive, intelligible and ordered (DINO) classification of coronary bifurcation lesions. review of current classifications. Circ J 2011;75:299–305. [DOI] [PubMed] [Google Scholar]
- 38. Movahed MR, Stinis CT. A new proposed simplified classification of coronary artery bifurcation lesions and bifurcation interventional techniques. J Invasive Cardiol 2006;18:99–204. 10.1016/j.carrev.2006.03.040 [DOI] [PubMed] [Google Scholar]
- 39. Lefèvre T, Louvard Y, Morice MC, et al. Stenting of bifurcation lesions: classification, treatments, and results. Catheter Cardiovasc Interv 2000;49:274–83. [DOI] [PubMed] [Google Scholar]
- 40. Popma J, Bashore T. Qualitative and quantitative angiography—Bifurcation lesions. Textbook of interventional cardiology. Philadelphia: WB Saunders, 1994:1055–8. [Google Scholar]
- 41. George BS, Myler RK, Stertzer SH, et al. Balloon angioplasty of coronary bifurcation lesions: the kissing balloon technique. Cathet Cardiovasc Diagn 1986;12:124–38. 10.1002/ccd.1810120212 [DOI] [PubMed] [Google Scholar]
- 42. Tsuchida K, Colombo A, Lefèvre T, et al. The clinical outcome of percutaneous treatment of bifurcation lesions in multivessel coronary artery disease with the sirolimus-eluting stent: insights from the arterial revascularization therapies Study part II (ARTS II). Eur Heart J 2007;28:433–42. 10.1093/eurheartj/ehl539 [DOI] [PubMed] [Google Scholar]
- 43. RDS. Bifurcation lesions. the manual of interventional cardiology. 2001:233–43.
- 44. Dauerman HL, Higgins PJ, Sparano AM, et al. Mechanical debulking versus balloon angioplasty for the treatment of true bifurcation lesions. J Am Coll Cardiol 1998;32:1845–52. 10.1016/S0735-1097(98)00488-4 [DOI] [PubMed] [Google Scholar]
- 45. Al Suwaidi J, Berger PB, Rihal CS, et al. Immediate and long-term outcome of intracoronary stent implantation for true bifurcation lesions. J Am Coll Cardiol 2000;35:929–36. 10.1016/S0735-1097(99)00648-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-016044supp001.pdf (457.3KB, pdf)