Abstract
Objectives
The aim of the present study was to estimate the incidence of acute mountain sickness (AMS) and address the changes in arterial oxygen saturation (SaO2) and heart rate (HR) in native Tibetans who reascend to the high-altitude city of Lhasa (3658 m) after a 7-year stay at low altitude.
Methods
We followed two cohorts of students aged 17–21 years (859 Native Tibetan and 801 Han Chinese), travelling from lowland China until 3 days after their arrival in highland city of Lhasa. Questionnaire information of the symptoms of AMS using the Lake Louise Scoring System, resting SaO2 and HR were assessed both before leaving the lowland and after arriving in Lhasa. Linear regression was performed to compare changes in SaO2 and HR levels from low to high altitude in Tibetan and Han Chinese.
Results
New cases of AMS occurred in only 1.2% (95% CI 0.4% to 2.0%) of the Tibetan students who came to Lhasa by train compared with 32.7% (95% CI 28.0% to 37.3%) and 42.9% (95% CI 38.0% to 47.7%) of the Han Chinese students who came to Lhasa by train and by air, respectively. Tibetan students had less changes in SaO2 (−2.95 percentage points, 95% CI −3.24% to −2.65%) and HR (10.89 beats per minute (bpm), 95% CI 9.62 to 12.16 bpm) from low to high altitude compared with Han Chinese students, although measurements did not differ between the two groups when measured at low altitude.
Conclusions
Healthy Tibetans are mostly protected against AMS and primarily maintain their good adaptation to high altitude, even after a long period of stay at low altitude.
Keywords: acute mountain sickness, oxygen saturation, heart rate, tibetan, re-ascend
Strengths and limitations of this study.
This is the first population-based study addressing the occurrence of acute mountain sickness and acute changes in arterial oxygen saturation and heart rate levels in a large cohort of Tibetans who after living at low altitude for 7 years returned to high altitude in Tibet at the same point in time.
The study was able to compare results among Native Tibetan students with similar results from a cohort of Han Chinese students well matched for characteristics like age, height and weight travelling from the same lowland area to the same high altitude.
Only a few simple physiological parameters suitable for field studies were measured affecting the possibility to address the mechanism behind physiological adaptation in depth.
The data collection for the two cohorts took place in different years, but both studies were conducted in the warm season, and the same date collection procedure was used for both groups reducing the chance of bias caused by differences in environmental conditions and data collection methods.
There were some losses to follow-up among the Tibetan students, but we did not find any differences in baseline characteristics between Tibetans lost or not lost to follow-up reducing the chance of selection bias.
Introduction
Exposure to high altitude may lead to acute mountain sickness (AMS), which usually manifests itself with non-specific symptoms such as headache, dizziness, loss of appetite, nausea, vomiting, insomnia and fatigue.1–5 Although AMS is generally benign and self-limiting, in some cases it may progress to more serious conditions like high-altitude cerebral oedema (HACE).1
Studies of AMS among lowlanders have shown signs of some persistency of acclimatisation which lead to reduction in the incidence and severity of AMS from prior high-altitude exposure,6–9 and this residual acclimatisation from prior exposure will be lost over a few days to weeks after a return to low altitude.8 10–12 Since most high-altitude natives live in high altitude, and usually only descend to low altitude for short periods, little information is available as to what extent and for how long a residual adaptation will persist. Findings from some previous studies among high-altitude natives of Bolivia13 and Peru5 indicate a reduction of adaptation when returning to high altitude after a short stay at low altitude. Among Tibetan highlanders, there are occasional reports of an AMS incidence of zero, usually upon ascending from a high to higher altitude or after only a short time stay at a low altitude.9 14 15 However, the effect of exposure to low altitudes on AMS among Tibetans reascending to high altitudes is still unclear, especially after long stay at low altitudes.
The exact mechanism of AMS is unknown, but hypoxaemia which refers to a lowered oxygen level in the blood is proposed as a principal cause of AMS,16–20 making arterial oxygen saturation (SaO2) an interesting measure for addressing acute acclimatisation/adaptation to hypoxic exposure. Furthermore, a marked increase in peripheral sympathetic activity which can be reflected by heart rate (HR) has also been indicated as a consequence of high-altitude exposure.21 22 Previous studies have mainly paid attention to comparisons of SaO2 and HR between indigenous populations at specific altitudes.4 9 14 23 24 As far as we know, no study has addressed acute changes of SaO2 and HR after ascent from low to high altitudes among native highlanders.
Tibetan students who attended middle school in lowland China provided a unique opportunity to study AMS and changes in SaO2 and HR among Tibetans reascending to high altitudes after long stay at low altitudes. A comparison of these parameters between native Tibetans and Han Chinese with matched characteristics may contribute to the understanding of high-altitude adaptation among Tibetans. Therefore, in the present study we have collected questionnaire information about AMS symptoms and measures of SaO2 and HR in 17–21-year-old Tibetan and Han Chinese students both at low and high altitudes. The Tibetan students had been living at low altitude for 7 years, while the Han Chinese students were born and raised at low altitude China and had never been to high altitude before. Data were collected shortly before both groups left lowland China, and when the same students arrived in Lhasa at 3658 m. The aim of the present study was to investigate the incidence of AMS and assess changes in SaO2 and HR in a population of native Tibetan students returning to Lhasa after a 7-year stay at low altitude. We hypothesised that native Tibetans would have a lower risk of developing AMS and less changes in SaO2 and HR after a long-term stay at low-altitude areas compared with Han Chinese lowland residents.
Materials and methods
Setting
The data collections were carried out in seven different cities in lowland China 7 days prior to departure and on the third day after the ascent to Lhasa. The seven lowland cities were Beijing (32 metres above sea level (MASL)), Tianjin (5 MASL), Chengdu (506 MASL), Nantong (5 MASL), Wuhan (23 MASL), Yueyang (57 MASL) and Shijiazhuang (81 MASL). The cities were chosen because they all had educational institutions with Tibetan students planning to go home after ending their education, and with Han Chinese students planning to go to Lhasa to continue their education. Lhasa, the capital city of the Tibet Autonomous Region, People’s Republic of China, is located at an elevation of 3658 MASL, which makes it one of the highest situated cities in the world.
Study samples
We included two populations: Native Tibetan students who stayed in the lowland area for 7 years and Han Chinese students who were born and raised in the lowlands without any former exposure to high altitude.
Tibetan students
In total, 7 out of 12 middle schools for Tibetans in lowland China were randomly selected. All senior students from the selected schools who were about to graduate in June 2012 were invited to participate, with the data collection taking place from May to June 2012. Of the 859 eligible students, all students had their SaO2 and HR measured, and returned completed self-assessment questionnaires (lowland questionnaire) to field workers at their schools before leaving for high-altitude areas. The same students received a new questionnaire (highland questionnaire) at the Lhasa railway station since all of them travelled to Tibet by train, which was organised by their school administrations. All students were asked to deliver the highland questionnaire to field workers and measured the SaO2 and HR on the third day after their arrival in Lhasa. For reasons unknown, 120 students did not return the highland questionnaire and 8 questionnaires were incompletely filled out, leaving 731 (85.1%) highland questionnaires for the analysis. Moreover, 158 students did not attend the SaO2 and HR measurements on the third day (response rate 81.6%).
Han Chinese students
The students came from the same lowland area where the Tibetan students had studied and were going to study at Tibet University. Data collection took place from August to September 2014. Of the 810 eligible students, all students received the lowland questionnaires via post before they ascended to Lhasa and were asked to return it to field workers when they registered at Tibet University. They were also asked to visit field workers for measuring their SaO2 and HR at the same place in the seven lowland cities as where the Tibetan students had their measurements taken before they ascended to Lhasa. For unknown reasons, 227 students did not attend the SaO2 and HR measurements (response rate 72.0%). Moreover, 11 students did not return the lowland questionnaires because of misplacement (response rate 98.6%). Since all students live in campus during their study at Tibet University, we had the chance to follow the students and measure their SaO2 and HR, and ask them to fill in the highland questionnaire on the third day after their arrival in Lhasa. One student refused, and eight were excluded because they had already been in Lhasa more than 3 days before the data collection took place. Thus, information from the highland questionnaire and measurements of SaO2 and HR from 801 students was included in the analysis of high-altitude information (response rate of 98.9%).
It takes about 2 hours with a cabin pressure equivalent to 2400 m by air from lowland China to Lhasa. Trains that go to Lhasa have only one route which is from Xining to Glomud (10 hours, average altitude 2906 m, range 2261–3698 m) and from Glomud to Lhasa (14 hours, average altitude 4251 m, range 2808–5072 m).
A written consent form about the study was given on the first page of the questionnaire, and the participants were informed that they could withdraw from the study for any reason at any time without any negative consequences.
Questionnaire
Two questionnaires based on the Lake Louise-AMS scoring system were developed and used in the data collection: one lowland and one highland questionnaire. The lowland questionnaires included questions on AMS-related symptoms, and the aim was to identify the basic health conditions and symptoms before the students ascended to high altitude.
In addition to including the same questions as in the lowland questionnaire, the highland questionnaire was designed to obtain data concerning gender, age, ethnicity, height, weight, type of transport to Lhasa, altitude of permanent residence, previous exposure to high altitude, self-reported health condition, history of high-altitude illness, use of prophylactic medicine, awareness of altitude sickness and smoking habits. Body mass index (BMI) was calculated as body weight (kg) divided by height (m) squared.
The AMS-related questions are based on a Chinese version of the original Lake Louise Score System (LLSS) questionnaire.25 The Chinese version has been used in several earlier studies on AMS.9 15 24 26 27 Before the questionnaire was used for the current study, we translated back the questions into English and it did not reveal any discrepancy with the original English version. The LLSS consists of a self-reported assessment of five AMS symptoms: headache, lassitude or fatigue, gastrointestinal distress (loss of appetite, nausea or vomiting), dizziness and insomnia.25 All symptoms were graded from 0 to 3, which was indicative of no, mild, moderate or severe symptoms, respectively. A diagnosis of AMS was defined as the presence of headache, at least one of the other symptoms and a total LLS ≥4.28 The participants were also asked ‘when did the symptoms first begin?’. The response options included ‘<12 hours’, ‘12–24 hours’, ‘25–48 hours’ and ‘>48 hours after arrival’.
Measurements of SaO2 and HR
The SaO2 and HR were measured at low altitude and then again on the third day after arrival in Lhasa. SaO2 and HR were measured by finger pulse oximetry (Nellcor, NPB-40, California, USA) with the probe placed on the index finger of the left hand in a sitting position after a 15 min rest. Values were observed three times at 30 s intervals, and the mean of three readings was recorded for data analysis. Furthermore, all measurements were conducted by the same field workers using the same equipment. No smoking was permitted within 2 hours before the measurements.
Statistical analysis
The descriptive statistics are presented as the frequencies and mean with a SD for each variable. The χ2 tests were performed for comparing categorical variables and a Student’s t-test were used for comparing continuous variables. One-way analysis of variance (ANOVA) was performed for comparing the differences of SaO2 and HR between Tibetan and Han Chinese students by different transportations. We used a linear regression model to check the changes from low altitude to high altitude of SaO2 and HR for Tibetan and Han Chinese. We put SaO2 and HR as dependent variables and altitude and ethnicity as explanatory variables, and added an interaction term between altitude and ethnicity. Associations were considered statistically significant at p<0.05, and analyses were carried out using SPSS V.24 for Windows.
Results
Population characterisation
Table 1 shows that the population characteristics of native Tibetans and Han Chinese did not differ regarding sex, age, height, weight and BMI. However, Tibetans were more frequent smokers. Of the Tibetan students, 152 (17.7%) had visited Tibet once, 326 (38.0%) twice, 192 (22.4%) three times, 93 (10.8%) four times and 96 (11.2%) five times or more during their 7 years of study in lowland China. Each visit lasted 30 days or less, except for one visit of 2 months after their graduation from junior middle school. None of the Tibetan students had been exposed to high altitude in the preceding 3 months before the baseline data collection. All Han Chinese students had permanent residence below 2000 m and had never been at high altitude before. Approximately half of them (49.3%) came to Lhasa by train and the rest by air.
Table 1.
Population characteristics of 17–21-year-old native Tibetan and Han Chinese students in Lhasa
| Native Tibetan | Han Chinese | ||
| By train (n=859) | By train (n=395) | By air (n=406) | |
| Male (%) | 425 (49.5) | 198 (50.1) | 215 (53.0) |
| Age, years (SD) | 18.89 (0.88) | 18.94 (0.98) | 18.96 (0.94) |
| Height, cm (SD) | 167.20 (7.08) | 166.99 (7.85) | 167.62 (7.72) |
| Weight, kg (SD) | 59.88 (7.75) | 60.15 (10.07) | 60.34 (10.33) |
| Body mass index, kg/m2 (SD) | 21.37 (1.98) | 21.51 (2.82) | 21.39 (2.76) |
| Smoking, yes (%) | 140 (19.3) | 28 (7.1)* | 26 (6.4)† |
Data are presented as frequencies (%) and means (SD). Data were analysed using χ2 test for comparison of categorical variables, and one-way analysis of variance for comparison of continuous variables.
*p<0.05 native Tibetan versus Han Chinese by train.
†p<0.05 native Tibetan versus Han Chinese by air.
Incidence of AMS
A total of nine Tibetan students (1.0%) had symptoms qualifying them as having AMS-like symptoms at baseline, while the corresponding number for the Han Chinese students was 12 (1.5%). Excluding students with AMS-like symptoms at baseline, a total of 9 (1.2%) Tibetan and 303 (37.8%) Han Chinese students developed AMS (headaches and an LLS ≥4) within the third day after arrival in Lhasa. Among the nine Tibetans free from AMS-like symptoms at baseline, and who developed AMS after arriving in Lhasa, eight (88.9%) reported having influenza and one sinusitis before they left for Lhasa. For the Han Chinese students who developed AMS, 7% reported influenza, 5% tonsillitis and 3% pollen allergy before their ascent to high altitude, whereas the rest did not report any diseases. For the nine Tibetan students that were categorised as having AMS, eight reported symptom start within 24 hours after arrival. For most of the Han Chinese students (81.7%) who were categorised as having AMS, the onset of symptoms occurred within the first 24 hours after arriving in Lhasa. Table 2 compares the incidence of AMS, average SaO2 and HR between Native Tibetan and Han Chinese students. Han Chinese students were stratified into groups according to whether they travelled to Lhasa by plane or train. Before their ascent, there were no statistically significant differences between Tibetan and Han Chinese students regarding prevalence of AMS-like symptoms and means of HR. Both Tibetan students and Han Chinese students who came to Lhasa by train had higher SaO2 than Han Chinese students by plane. However, there was no statistically significant differences between Tibetan and Han Chinese students regarding overall means of SaO2 (Tibetan vs Han Chinese: 99.2% vs 99.1%, p>0.05). On the third day after arrival in Lhasa, Han Chinese students who flew to Lhasa had a higher incidence of AMS compared with those who came to Lhasa by train (p<0.05). Both groups had a higher incidence of AMS, a lower SaO2 and a higher HR compared with native Tibetans (p<0.05). The incidence of AMS and the means of SaO2 and HR were not statistically significantly related to the number of visits to Tibet during the 7 years of study in lowland China among Tibetan students (see online supplementary table 1).
Table 2.
Incidence of acute mountain sickness (AMS) and means of arterial oxygen saturation (SaO2) and heart rate (HR) with 95% CI in 17–21-year-old native Tibetan and Han Chinese students
| Native Tibetan | Han Chinese | |||||
| By train (n=859) | By train (n=395) | By air (n=406) | ||||
| Lowland | ||||||
| AMS-like symptoms | 0.011 | (0.004–0.017) | 0.015 | (0.003–0.027) | 0.015 | (0.003–0.027) |
| SaO2 (%) | 99.2 | (99.1–99.3) | 99.2‡ | (99.0–99.4) | 98.9§ | (98.7–99.1) |
| HR (bpm*) | 72.1 | (71.6–72.5) | 71.6 | (70.5–72.7) | 71.1 | (70.1–72.1) |
| Highland | ||||||
| AMS | 0.012† | (0.004–0.020) | 0.327‡ | (0.280–0.373) | 0.429§ | (0.380–0.477) |
| SaO2 (%) | 91.1† | (90.8–91.3) | 88.1 | (87.9–88.3) | 87.9§ | (87.6–88.1) |
| HR (bpm*) | 72.7† | (72.1–73.2) | 82.2 | (81.2–83.2) | 83.5§ | (82.4–84.5) |
*For Han Chinese students, the results are stratified according to the type of transportation from lowland China to Lhasa.
†p<0.05 native Tibetan versus Han Chinese by train.
‡p<0.05 Han Chinese by train versus Han Chinese by air.
§p<0.05 native Tibetan versus Han Chinese by air.
bpm, beats per minute.
Data were analysed using one way analysis of variance for comparison of the differences of SaO2 and HR between native
Tibetan and Han Chinese by different transpotation. X2 test was performed for comparing incidence of AMS between
native Tibetan and Han Chinese by different transportation.
bmjopen-2017-016460supp001.pdf (158.5KB, pdf)
Some Tibetans were lost in the follow-up, and some Han Chinese students did not participate in parts of the data collection at baseline. Therefore, we also compared the AMS incidence, SaO2 and HR levels between Tibetan students and Han Chinese students with information from both the lowland and highland data collection. The results show similar contrasts between the population groups, as shown in table 2 (results not presented). No HACE and high altitude pulmonary edema (HAPE) were reported during the data collection time in both groups.
Symptoms of AMS
AMS-like symptoms were rare and of similar frequency in Tibetan and Han Chinese at low altitude, except that Han Chinese who came to Lhasa by air had more sleeping difficulties. After the ascent to high altitude, both the Han Chinese students arriving by train and by plane had statistically significant higher incidence of all the five AMS-related symptoms compared with the Tibetan students (p<0.05) (table 3). A total of 452 Tibetan students (61.8%) and 682 Han Chinese students (85.1%) reported at least one AMS-related symptom. Headache was the most frequently reported symptom, followed by fatigue, dizziness, insomnia and gastrointestinal symptoms, both in Tibetans and Han Chinese (table 3). The mean overall LLSS scores differed significantly with 1.31±1.46 among Tibetans compared with 3.15±2.56 and 3.96±2.76 among Han Chinese arriving by train or plane (p<0.05 respectively).
Table 3.
Incidence of acute mountain sickness-related symptoms in 17–21-year-old native Tibetan and Han Chinese students after arrival in Lhasa
| Symptoms | Headache n (%) |
Gastrointestinal symptoms n (%) |
Fatigue n (%) |
Dizziness n (%) |
Difficulty sleeping n (%) |
|
| Tibetan by train | None | 449 (61.0) | 683 (93.1) | 523 (71.2) | 599 (81.4) | 670 (91.2) |
| Mild | 241 (32.7) | 42 (5.7) | 148 (20.1) | 119 (16.2) | 30 (4.1) | |
| Moderate | 44 (6.0) | 6 (0.8) | 45 (6.1) | 13 (1.8) | 23 (3.1) | |
| Severe | 2 (0.3) | 3 (0.4) | 19 (2.6) | 5 (0.7) | 12 (1.6) | |
| Han Chinese by train | None | 178 (45.1) | 268 (67.8) | 183 (46.3) | 204 (51.6) | 239 (60.5) |
| Mild | 166 (42.0) | 97 (24.6) | 137 (34.7) | 164 (41.5) | 85 (21.5) | |
| Moderate | 32 (8.1) | 19 (4.8) | 51 (12.9) | 24 (6.1) | 40 (10.1) | |
| Severe | 19 (4.8) | 11 (2.8) | 24 (6.1) | 3 (0.8) | 31 (7.8) | |
| Han Chinese by air | None | 118 (29.1) | 251 (61.8) | 132 (32.5) | 148 (36.5) | 222 (54.7) |
| Mild | 202 (49.8) | 114 (28.1) | 166 (40.9) | 205 (50.5) | 106 (26.1) | |
| Moderate | 69 (17.0) | 37 (9.1) | 78 (19.2) | 49 (12.1) | 50 (12.3) | |
| Severe | 17 (4.2) | 4 (1.0) | 30 (7.4) | 4 (1.0) | 28 (6.9) |
Data are presented as number with frequencies (%).
Resting SaO2 and HR
There was no difference in SaO2 and HR levels between Tibetan and Han Chinese students at low altitude (table 2). After arrival at high altitude, Tibetan students had a significantly higher SaO2 and lower HR compared with Han Chinese students. This was also the case when comparing Tibetan and Han Chinese students with and without AMS (figures 1 and 2). The SaO2 was decreased in both Tibetan and Han Chinese students after arrival at high altitude. On average, the decrease was 8.1% in Tibetans (99.2% to 91.1%)%) and 11.1% in Han Chinese (99.1% to 88.0%)%). The HR among Tibetans on the third day after arrival at high altitude was similar to the values observed at low altitude (72.1 beats per minute (bpm) vs 72.7 bpm), while on average the HR in Han Chinese increased by 11.5 bpm after arrival in Lhasa (71.3−82.8 bpm). The changes in SaO2 (−11.2by train vs −11.1by air, p=0.82) and HR (10.3by train vs 12.5by air, p=0.07) were not statistically significantly different when comparing Han Chinese students arriving in Lhasa by train or plane. The linear regression model revealed that Tibetan students had less changes in SaO2 (−2.95 per cent points, 95% CI −3.24% to −2.65%) and HR (10.89 bpm, 95% CI 9.62 to 12.16 bpm) from low to high altitude compared with Han Chinese. Participants with AMS also had a significantly lower average SaO2 than those without AMS after arrival in Lhasa in both groups (figure 1). The HR was significantly higher in AMS subjects than non-AMS subjects in Han Chinese, while for Tibetans, there was a similar but not statistically significant trend (figure 2).
Figure 1.
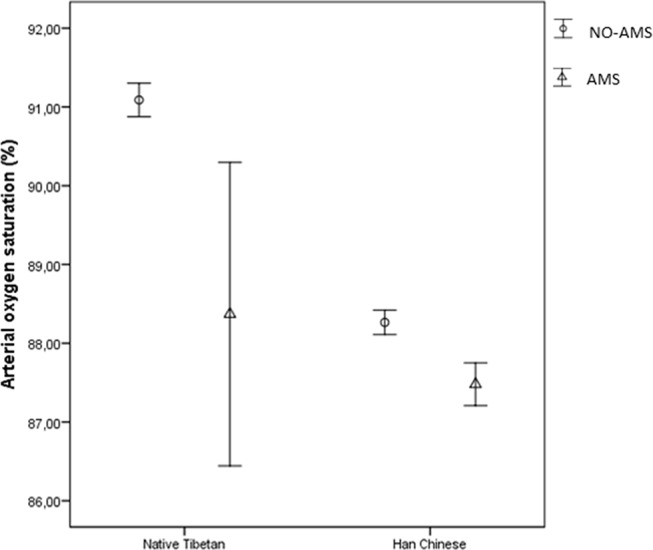
Mean of oxygen saturation (%) with 95% CI in acute mountain sickness (AMS) and non-AMS subjects on the third day after arrival in Lhasa by different ethnicity.
Figure 2.
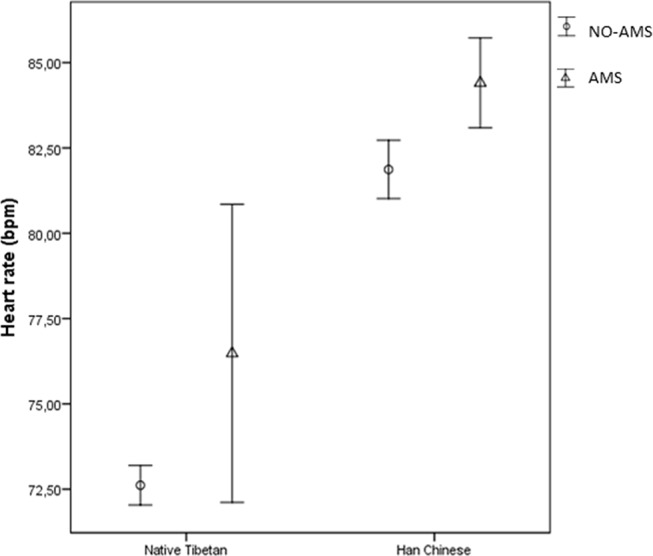
Mean of heart rate (beats per minute (bpm)) with 95% CI in acute mountain sickness (AMS) and non-AMS subjects on the third day after arrival in Lhasa by different ethnicity.
Discussion
The Tibetan students clearly had a lower incidence of AMS and less AMS-related symptoms compared with the Han Chinese students. Furthermore, the Tibetans had significantly less changes in SaO2 and HR levels than the Han Chinese students after a 3-day stay at 3658 m in Lhasa, even if they had similar levels before leaving lowland China.
The significantly lower incidence of AMS among Tibetan compared with Han Chinese students found in this study is not surprising as it is well-known that native Tibetans have a better adaptation to high altitude than any other population.1 24 29 Nonetheless, there are few population-based studies that have actually confirmed that Tibetans remain adapted to high altitude, and are protected against the development of AMS if high-altitude exposure is discontinued by stays at low altitude, especially after long-term stays. Some studies among train passengers,15 construction workers9 and mountaineers30 have reported that Tibetans are completely free from AMS. However, these studies were conducted in selected populations moving from a high to higher altitude, or reascending to high altitude after a short stay at low altitude. To the best of our knowledge, this is the first population-based study addressing the occurrence of AMS, and the changes in SaO2 and HR levels in Tibetans who lived at a low altitude for 7 years and then returned to a high altitude.
We also had the possibility to compare our results to findings from a population of Han Chinese students with similar characteristics who had never been at high altitude before arriving in Lhasa. It appears that after a long-term stay at low altitude, Tibetans were clearly less susceptible to AMS than lowland Han Chinese, also when compared with subgroups of Han Chinese students who travelled by air or used the same mode of transportation as the Tibetans did, namely by train. Short visits to high altitude during a long-term stay at low altitude did not seem to influence the Tibetan’s degree of adaptation. We could not claim that Tibetans were entirely immune against developing AMS since AMS-related symptoms were reported by a substantial proportion of Tibetans. However, the incidence and severity of the symptoms were clearly lower in Tibetan than in Han Chinese students. Additionally, examining the data of the nine Tibetan students who developed AMS, we found that eight of them reported having influenza shortly before leaving low altitude. It has been suggested that normal acclimatisation could be disturbed by respiratory infections.31–33 In addition, such infections may also lead to AMS-like symptoms, and through that increase the potential for a misdiagnosis with AMS.31 Overall, our findings support the idea that healthy Tibetans after a long-term stay at low altitude are primarily protected against the development of AMS when reascending to high altitude.
Tibetans also showed a different reaction to high altitude than Han Chinese students when it came to levels of SaO2 and HR. Before their ascent, there were no signs of difference in these measurements between the two populations. After their arrival at high altitude, Tibetans had less change in SaO2 and HR levels than Han Chinese, which may indicate a more favourable reaction to acute altitude exposure. Since hypoxia plays a key role in the development of AMS, measuring SaO2 and HR has been suggested as simple indicators of adaptation/acclimatisation to high altitudes and impending AMS.33 34 This is motivated by the fact that lowered oxygen levels in the blood are clearly related to AMS at high altitude.16 18 Likewise, individuals with a higher HR at rest have been reported to be at risk of developing AMS at high altitude.9 It therefore seems reasonable to suggest that genetic factors involved in long-term adaptation to high altitude in Tibetans have contributed to our findings, even if we did not have the possibility to explore this. As opposed to acclimatisation among Han Chinese, which occurs because of an immediate physiological response to a changing environment, the ‘high-altitude adaptation’ among Tibetans has developed in long-term physiological responses to a high-altitude hypobaric hypoxic environment with heritable behavioural and genetic changes.24 A study from second-generation Tibetan lowlanders35 provides evidence of adaptation with regard to acute reaction to high-altitude exposure. The study did not measure AMS, but Tibetans born and living in Kathmandu (1300 m) exhibited a greater aerobic working capacity than Caucasian lowlanders when exposed to high altitude. This indicates that the adaptation to acute exposure to high altitude among Tibetans has not changed over one generation and could be linked to unique adaptation genes.35 Several recent genomic studies14 36–38 in native Tibetan highlanders have revealed that some genes are associated with high-altitude adaptation. However, these genes have been related to chronic mountain sickness, and as far as we know, no direct evidence between these genes and AMS has been proposed.38 39 Although it seems likely that genetics plays a role in the development of AMS, we cannot fully decide whether the observed differences between Tibetan and Han Chinese students can be explained by genetic background. It has been reported that lowlanders with a pre-exposure to high altitude may establish some degree of physiological adaptation, thus resulting in a reduction in the incidence and severity of AMS when they reascend to high altitude.7–9 Hence, the previous length of stay in high altitude may also have contributed to the Tibetan students’ adaptation to high altitude. Taken together, both genetic and physiological factors may have contributed to a better adaptation to high altitude in the Tibetan than Han Chinese, also after a long-term stay at low altitude.
In the present study, some Tibetan students were lost to follow-up, and some Han Chinese students did not participate in parts of the data collection at baseline. If the subjects who did not complete the study had different characteristics compared with the total sample, the incidence of AMS could then be overestimated or underestimated. However, we did not find any low-altitude differences in characteristics between the Tibetans lost or not lost to follow-up at altitude which we believe is an argument against expecting large difference in AMS among those lost and not lost to follow-up. For the Han Chinese, the collected data were quite complete except for some missing information about baseline SaO2 and HR which we do not believe have affected the main finding as these individuals were quite comparable to those with complete information. Therefore, we believe that our findings reflect AMS conditions and relations between Tibetan and Han Chinese students in general as the samples were randomly selected. Some participants may forget about their health problems. However, we believe that most people will remember which health problems they have experienced during the last three days. Furthermore, in the present study, the participants had been informed about the purpose of the study and knew that they were going to answer to questions about AMS symptoms after arrival which is a further argument against substantial recall bias. The different ascent profiles may also have influenced AMS incidence in our study. Consequently, we also compared Tibetan with Han Chinese students who had arrived in Lhasa by train. The results still showed that Tibetans clearly had a lower incidence of AMS, a less changes in SaO2 and HR levels than Han Chinese students. The two cohort studies took place in different years, but both studies were conducted in the warm season, and thus the effect of temperature on AMS, SaO2 and HR was almost the same. Both the baseline measurements in Tibetan and Han Chinese were conducted 1 week before leaving low altitude. As a result, the influence of influenza on reported AMS symptoms at high altitude may have been different if baseline measures were registered just before the participants left. Practical reasons made this difficult, but the same data collection procedure for both groups should reduce the chance of biased comparisons between the groups. To obtain representative measurements, SaO2 and HR were measured three times at 30 s intervals, and the same pulse oximeter was used throughout the data collection period by the same field workers.
Conclusion
The significantly lower incidence of AMS, less change in SaO2 and HR levels in Tibetan than Han Chinese students favours the view that healthy Tibetans are mostly protected against AMS and maintain their good adaptation to high altitude, even after a long-term stay at low altitude. It is likely that both genetic and physiological factors contributed to this, but based on the available data this study could not address this issue further.
Supplementary Material
Acknowledgments
The authors thank all the students who participated in this survey and colleagues at the Tibet University Medical College who gave great support in the data collection process.
Footnotes
Contributors: PN, EB and GGLZ designed the study. GGLZ, LBSZ and colleagues collected the data. GGLZ analysed and drafted the manuscript. HS provided expert statistical advice. PN, EB and WTY provided professional advice and technical support. All authors read and approved the final version of the manuscript.
Funding: This study was supported by the National 973 Program of China grants 2012CB518202 to TW, Zhufeng Scholar Program of Tibet University (to Gonggalanzi) and the Network for University Cooperation Tibet-Norway.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study was approved by the Medical Ethics Committee of Fu-kang Obstetrics & Gynecology and Children’s Hospital in Lhasa, Tibet, China. Norwegian Regional Committees for Medical and Health Research Ethics also approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Basnyat B, Murdoch DR. High-altitude illness. Lancet 2003;361:1967–74. [DOI] [PubMed] [Google Scholar]
- 2. Rodway GW, Hoffman LA, Sanders MH. High-altitude-related disorders--Part I: pathophysiology, differential diagnosis, and treatment. Heart Lung 2003;32:353–9. 10.1016/j.hrtlng.2003.08.002 [DOI] [PubMed] [Google Scholar]
- 3. Hackett PH, Roach RC. High-altitude illness. N Engl J Med 2001;345:107–14. 10.1056/NEJM200107123450206 [DOI] [PubMed] [Google Scholar]
- 4. Gallagher SA, Hackett PH. High-altitude illness. Emerg Med Clin North Am 2004;22:329–55. viii. doi 10.1016/j.emc.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 5. Hultgren HN, Spickard WB, Hellriegel K, et al. . High altitude pulmonary edema. Medicine 1961;40:289–313. 10.1097/00005792-196109000-00002 [DOI] [PubMed] [Google Scholar]
- 6. Lyons TP, Muza SR, Rock PB, et al. . The effect of altitude pre-acclimatization on acute mountain sickness during reexposure. Aviat Space Environ Med 1995;66:957–62. [PubMed] [Google Scholar]
- 7. Farias JG, Osorio J, Soto G, et al. . Sustained acclimatization in Chilean mine workers subjected to chronic intermittent hypoxia. High Alt Med Biol 2006;7:302–6. 10.1089/ham.2006.7.302 [DOI] [PubMed] [Google Scholar]
- 8. West RS JB, Milledge JS. commuting to high altitude for commercial and other activities High Altitude Medicine and Physiology. 2007. published Online First: 370. [Google Scholar]
- 9. Wu TY, Ding SQ, Liu JL, et al. . Reduced incidence and severity of acute mountain sickness in Qinghai-Tibet railroad construction workers after repeated 7-month exposures despite 5-month low altitude periods. High Alt Med Biol 2009;10:221–32. 10.1089/ham.2009.1012 [DOI] [PubMed] [Google Scholar]
- 10. Muza SR, Beidleman BA, Fulco CS. Altitude preexposure recommendations for inducing acclimatization. High Alt Med Biol 2010;11:87–92. 10.1089/ham.2010.1006 [DOI] [PubMed] [Google Scholar]
- 11. Chapman RF, Laymon Stickford AS, Lundby C, et al. . Timing of return from altitude training for optimal sea level performance. J Appl Physiol 2014;116:837–43. 10.1152/japplphysiol.00663.2013 [DOI] [PubMed] [Google Scholar]
- 12. Meyer LB, Pace N, Vaughan BE. Erythrolysis on return of altitude acclimatized individuals to sea level. J Appl Physiol 1956;9:141–4. [DOI] [PubMed] [Google Scholar]
- 13. Antezana G, Leguia G, Guzman M, et al. . Hemodynamic study of high altitude pulmonary edema (12. 200feet) 1982:232–41. [Google Scholar]
- 14. Weitz CA, Liu JC, He X, et al. . Responses of Han migrants compared to Tibetans at high altitude. Am J Hum Biol 2013;25:169–78. 10.1002/ajhb.22368 [DOI] [PubMed] [Google Scholar]
- 15. Wu TY, Ding SQ, Zhang SL, et al. . Altitude illness in Qinghai–Tibet railroad passengers. High Alt Med Biol 2010;11:189–98. 10.1089/ham.2009.1047 [DOI] [PubMed] [Google Scholar]
- 16. Loeppky JA, Icenogle MV, Charlton GA, et al. . Hypoxemia and acute mountain sickness: which comes first? High Alt Med Biol 2008;9:271–9. 10.1089/ham.2008.1035 [DOI] [PubMed] [Google Scholar]
- 17. Rupp T, Jubeau M, Millet GY, et al. . The effect of hypoxemia and exercise on acute mountain sickness symptoms. J Appl Physiol 2013;114:180–5. 10.1152/japplphysiol.00769.2012 [DOI] [PubMed] [Google Scholar]
- 18. Karinen HM, Peltonen JE, Kähönen M, et al. . Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent. High Alt Med Biol 2010;11:325–32. 10.1089/ham.2009.1060 [DOI] [PubMed] [Google Scholar]
- 19. Windsor JS, Rodway GW. Con: pulse oximetry is useful in predicting acute mountain sickness. High Alt Med Biol 2014;15:442–3. 10.1089/ham.2013.1140 [DOI] [PubMed] [Google Scholar]
- 20. Burtscher M, Flatz M, Faulhaber M. Prediction of susceptibility to acute mountain sickness by SaO2 values during short-term exposure to hypoxia. High Alt Med Biol 2004;5:335–40. 10.1089/ham.2004.5.335 [DOI] [PubMed] [Google Scholar]
- 21. Karinen HM, Uusitalo A, Vähä-Ypyä H, et al. . Heart rate variability changes at 2400 m altitude predicts acute mountain sickness on further ascent at 3000-4300 m altitudes. Front Physiol 2012;3:336 10.3389/fphys.2012.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hainsworth R, Drinkhill MJ, Rivera-Chira M. The autonomic nervous system at high altitude. Clin Auton Res 2007;17:13–19. 10.1007/s10286-006-0395-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naeije R. High-altitude adaptation: where Tibetans and Han Chinese agree. Exp Physiol 2014;99:1593–4. 10.1113/expphysiol.2014.082677 [DOI] [PubMed] [Google Scholar]
- 24. Wu T, Li S, Ward MP. Tibetans at extreme altitude. Wilderness Environ Med 2005;16:47–54. 10.1580/PR04-04.1 [DOI] [PubMed] [Google Scholar]
- 25. Bp RRC, Hackett PH, Oelz O. The Lake Louise acute mountain sickness scoring system In: Sutton JR, Houston CS, Coates G, eds Hypoxia and Mountain Medicine: proceeding of the International hypoxia Symposium. 1993:272–4. [Google Scholar]
- 26. Wu T. The Qinghai-Tibetan plateau: how high do Tibetans live? High Alt Med Biol 2001;2:489–99. 10.1089/152702901753397054 [DOI] [PubMed] [Google Scholar]
- 27. Wu TY, Ding SQ, Liu JL, et al. . Who are more at risk for acute mountain sickness: a prospective study in Qinghai-Tibet railroad construction workers on Mt. Tanggula. Chin Med J 2012;125:1393–400. [PubMed] [Google Scholar]
- 28. Maggiorini M, Müller A, Hofstetter D, et al. . Assessment of acute mountain sickness by different score protocols in the swiss Alps. Aviat Space Environ Med 1998;69:1186–92. [PubMed] [Google Scholar]
- 29. Hackett PH RD. Acute mountain sickness. 5, 1983. [Google Scholar]
- 30. Sampson JB, Cymerman A, Burse RL, et al. . Procedures for the measurement of acute mountain sickness. Aviat Space Environ Med 1983;54(12 Pt 1):1063–73. [PubMed] [Google Scholar]
- 31. Bailey DM, Davies B, Castell LM, et al. . Symptoms of infection and acute mountain sickness; associated metabolic sequelae and problems in differential diagnosis. High Alt Med Biol 2003;4:319–31. 10.1089/152702903769192278 [DOI] [PubMed] [Google Scholar]
- 32. Schneider M, Bernasch D, Weymann J, et al. . Acute mountain sickness: influence of susceptibility, preexposure, and ascent rate. Med Sci Sports Exerc 2002;34:1886–91. 10.1097/00005768-200212000-00005 [DOI] [PubMed] [Google Scholar]
- 33. Roach RC, Greene ER, Schoene RB, et al. . Arterial oxygen saturation for prediction of acute mountain sickness. Aviat Space Environ Med 1998;69:1182–5. [PubMed] [Google Scholar]
- 34. Burtscher M, Szubski C, Faulhaber M. Prediction of the susceptibility to AMS in simulated altitude. Sleep Breath 2008;12:103–8. 10.1007/s11325-007-0131-0 [DOI] [PubMed] [Google Scholar]
- 35. Marconi C, Marzorati M, Grassi B, et al. . Second generation Tibetan lowlanders acclimatize to high altitude more quickly than Caucasians. J Physiol 2004;556(Pt 2):661–71. 10.1113/jphysiol.2003.059188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beall CM. Detecting natural selection in high-altitude human populations. Respir Physiol Neurobiol 2007;158(2-3):161–71. 10.1016/j.resp.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 37. Guo LI, Zhang J, Jin J, et al. . Genetic variants of endothelial PAS domain protein 1 are associated with susceptibility to acute mountain sickness in individuals unaccustomed to high altitude: A Nested Case-Control Study. Exp Ther Med 2015;10:907–14. 10.3892/etm.2015.2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MacInnis MJ, Koehle MS. Evidence for and against genetic predispositions to acute and chronic altitude illnesses. High Alt Med Biol 2016;17:281–93. 10.1089/ham.2016.0024 [DOI] [PubMed] [Google Scholar]
- 39. Simonson TS, McClain DA, Jorde LB, et al. . Genetic determinants of Tibetan high-altitude adaptation. Hum Genet 2012;131:527–33. 10.1007/s00439-011-1109-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-016460supp001.pdf (158.5KB, pdf)


