Abstract
Background:
Oxidative stress plays an important role in the pathogenesis of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. Flavonoids exert their antioxidant effects by neutralizing all types of oxidizing radicals including the superoxide and hydroxyl radicals. Passiflora incarnata Linn. (Passifloraceae) is an important plant used in Ayurveda for the treatment of various disorders of the CNS and is a rich source of flavonoids.
Aim:
In the present study, we investigated the antioxidant, antiparkinsonian, and memory enhancing activity of flavonoid rich n-butanol extract of P. incarnata flowers (BEPIF).
Materials and Methods:
Antioxidant activity was assessed using DPPH and hydrogen peroxide scavenging assay. The antiparkinsonian activity was evaluated using haloperidol induced catalepsy and tacrine induced vacuous chewing movement and memory enhancing activity was assessed using elevated plus maze and object recognition test.
Statistical Analysis:
The results were analyzed by Analysis of Variance test followed by Dunnett’s test.
Results:
Administration of BEPIF decreased transfer latency on day 2 and 9 significantly in elevated plus maze test and showed a significant increase in discrimination index in the object recognition test which is suggestive of its cognitive improvement action. Pretreatment with BEPIF showed a significant reduction in the haloperidol induced catalepsy and the tacrine induced jaw movements which are suggestive of its antiparkinsonian activity. In DPPH and H2O2 scavenging assay, BEPIF exhibited significant free radical scavenging activity.
Conclusions:
It can be concluded that the butanolic extract of P. incarnata flowers has significant antiparkinsonian and cognition enhancing activity which may be associated with its antioxidant potential. Thus, P. incarnata flowers may be employed in treatment of dementia and parkinsonism.
Keywords: Antioxidant, haloperidol, Passifloraincarnata, tacrine
Introduction
Neurodegenerative disorders are a diverse group of diseases of the nervous system. Disorders such as Alzheimer’s and Parkinson’s disease account for a significant and increasing amount of morbidity and mortality in the developed world. Neurodegenerative dementias and movement disorders are becoming more common mainly as a result of increased life expectancy and changing population demographics.[1] Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by neuronal degeneration and cognitive deterioration, especially in the elderly.[2,3] Parkinson’s disease (PD) is the most common neurodegenerative movement disorder with classical clinical manifestations of tremors, akinesia, muscle rigidity, and postural instability. There is a growing body of evidence that nigral neurons may be damaged by free radicals in this disorder. Free radicals are thought to be produced locally within the basal ganglia and lead to progressive damage and death of substantia nigra neurons in susceptible individuals.[4,5] Oxidative stress has been implicated in the pathogenesis of AD and PD by the finding of several characteristics, such as enhanced lipid peroxidation in specific areas of the brain in postmortem studies.[2,3]
Plant extracts and their constituents act as a natural source of antioxidants. The antioxidant activity of several plant extracts is due to several secondary metabolites especially phenolic compounds such as flavonoids, alkaloids and tannins. The potent antioxidant activity of flavonoids may be the most important function of flavonoids responsible for their actions in the body.[6] There are several studies suggesting neuroprotective effect of flavonoids.[7,8,9]
Passiflora incarnata Linn. (Passifloraceae) commonly known as passion flower has been used for the treatment of anxiety, insomnia, epilepsy, muscular spasms, and many other allied diseases.[10] The Passion flowers contain flavonoids (0.25%) such as quercetin, vitexin, isovitexin and phenolic compounds, Indole alkaloids (0.1%) such as harman, harmin, harmalin, harmol, harmalol and also cyanogenic glycosides.[11] Harmine and harmaline alkaloids are reported to be effective anti-parkinsonism compounds.[12] Hence the present study was designed to investigate antioxidant, antiparkinsonian and memory enhancing activity of flavonoid rich n-butanol extract of P. incarnata flowers. Antioxidant activity was assessed using DPPH and hydrogen peroxide scavenging assay. The antiparkinsonian activity was evaluated using haloperidol induced catalepsy and tacrine induced vacuous chewing movement and memory enhancing activity was assessed using elevated plus maze and object recognition test.
Materials and Methods
Plant material
The fresh flowers of P. incarnata were collected in the months of June, July, and August from a nursery in Pune, Maharashtra, India. The plant was identified and authenticated by Dr. Dinesh Shirodkar, Botanical Survey of India (BSI), Pune. A voucher specimen was deposited in BSI (Voucher Specimen No: PASSIN 3).
Preparation of extracts
Shade dried flowers (1000 g) were powdered and macerated with ethanol for 48 hours. The extract was concentrated under vacuum and evaporated to dryness. The extract was suspended in water and extracted successively with hexane, chloroform, ethyl acetate and n-butanol. n-butanol extract of P. incarnata flower (BEPIF) was used for further study.
Drugs and chemicals
Piracetam was obtained from Alkums Drugs and Pharmaceutical Ltd, Haridwar. Chrysin was obtained from Sigma-Aldrich (St. Louis, MO, USA). Levodopa was obtained from Alembic Ltd, Vadodara. Ethanol, hexane, chloroform, ethyl acetate and n-butanol used in this study were procured from S.D. Fine Chem. Limited, Mumbai, Maharashtra, India.
Animals
Swiss albino mice (25 – 30 g) and Sprague Dawley rats (120-150 g) of either sex were used. Animals were housed under standard conditions of temperature (24 ± 2°C) and relative humidity (30-70%) with a 12:12 hr light: dark cycle. The animals were fed with standard pellet diet and water ad libitum. The Institutional Animal Ethical Committee approved the protocols.
Qualitative phytochemical analysis
The screening for presence of phytoconstituents was carried out as per standard procedure described.[13]
Quantitative phytochemical estimation
Total phenolic content determination
The total phenolic content of BEPIF was determined by using the Folin–Ciocalteu assay.[14] Total phenolic content of BEPIF was expressed as mg Gallic Acid Equivalents. (GAE)/100 g dry weight. All samples were analyzed in triplicate.
Total flavonoid assay
Total flavonoid content was measured by the aluminum chloride colorimetric assay.[14] The total flavonoid content of BEPIF was expressed as mg Chrysin Equivalents (CE)/100 g dry weight. Samples were analyzed in triplicate.
Total alkaloid determination
Total alkaloid content was measured by Bromocresol Green colorimetric assay.[15] The total alkaloid content was expressed as mg Atropine Equivalent/100 gm of dry weight. All samples were analyzed in triplicate.
Assessment of nootropic activity
Elevated plus maze test
An elevated plus maze consisting of two open arms (36 × 6 cm) and two enclosed arms (35 × 6 × 15 cm) was used. The maze was elevated at a height of 45 cm. Mice were placed individually at the end of the open arm and the time taken by the animal to enter into either of the enclosed arms (transfer latency, TL) was recorded. On the first day, the mice were allowed to explore the plus maze for 5 min and sent back to home cage after the first trial. After 24 hours, mice were placed again in the elevated plus maze individually as before and the TL was noted again. TL measured on first day served as a parameter for acquisition. TL was expressed as retention scores after 24 hours or one week for each mouse by calculating the ‘inflexion ratio’ (IR).
Inflexion ratio = (L1 – L0)/L0
Where L0 = transfer latency after 24 hours on day 2/after a week on day 9 in seconds.
L1 = transfer latency on day 1 in seconds.
The mice were treated with vehicle, BEPIF (150 and 300 mg/kg, i.p.) and Piracetam (100 mg/kg, i.p.) 30 minutes before first trial. Each group consisted of 6 animals.[16]
Object recognition test
The apparatus consisted of plywood (70 × 60 × 30 cm) with a grid floor that could be easily cleaned with hydrogen peroxide after each trial. The apparatus was illuminated by a 40 W lamp suspended 50 cm above the box. The objects to be discriminated were also made of plywood in two different shapes of 8 cm height colored black.
The day before test, mice were allowed to explore the box (without any object) for two min. On the day of the test in the first trial (T1) two identical objects were placed in two opposite corners of the box and the amount of time taken by each mouse to complete 20 sec of object exploration was recorded. Exploration was considered directing the nose at a distance <2 cm to the object and/or touching it with the nose. During the second trial (T2, 90 min after T1) one of the objects presented in trial T1 was replaced by new object and the mice were left in the box for 5 min. The time spent in exploration of familiar (F) and the new object (N) were recorded separately and discrimination index (D) was calculated (N-F/N+F). Care was taken to avoid place preferences and olfactory stimuli by randomly changing the role (familiar or new object) and the position of the two objects during T2 and cleaning the apparatus with hydrogen peroxide.
The mice were treated with vehicle, BEPIF (150 and 300 mg/kg, i.p.) and Piracetam (100 mg/kg, i.p.) 30 minutes before the first trial. The second trial was performed 90 min after the first trial. Each group consisted of 6 animals.[17]
Assessment of anti-parkinsonian activity
Haloperidol induced catalepsy
Adult Swiss albino mice (25-30 g) were divided into four groups of six each. Mice were pretreated with vehicle, BEPIF (150 and 300 mg/kg, i.p.), and L-DOPA (30 mg/kg, i.p.) 30 min before haloperidol (1 mg/kg, i.p.). The duration of catalepsy was measured at 0, 30, 60, 90, 120, and 150 min after haloperidol administration using bar test. Both the forepaws of the animals were placed on a wooden bar elevated 3 cm above the ground. The cutoff time (time for which animal was placed on elevated bar) was 300 seconds.[18,19]
Tacrine induced jaw movements
Rats were divided into four groups and treated with vehicle, BEPIF (150 and 300 mg/kg, i.p.) and L-DOPA. After 20 min, tacrine (2.5 mg/kg i.p.) was administered and the number of tremulous jaw movements and orofacial bursts were measured for 60 min as described by Kasture et al.[20]
Antioxidant activity
1, 1-diphenyl-2-picrylhydrazyl assay
The free radical scavenging activity of the extract was measured in terms of hydrogen donating or radical scavenging ability using the stable free radical DPPH. 0.1 mM solution of DPPH in methanol was prepared and 1 ml of this solution was added to 3 ml of extract solution in water at various concentrations (2 – 1000 μg/ml). The mixture was incubated for 45 min at room temperature and the absorbance was measured at 517 nm against the corresponding blank solution. Ascorbic acid was used as reference standard. Percentage inhibition of DPPH free radical was calculated using the following equation:
DPPH Scavenged (%) = [(Ac - At)/Ac] ×100
Where Ac is the absorbance of the control, and At is the absorbance of the extract or reference standard. The antioxidant activity was expressed as IC50. The IC50 value was defined as the concentration in μg/ml of the extract that inhibits the formation of DPPH radicals by 50%.[21]
H2O2 scavenging activity
A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). 3.4 ml (16 – 1000 μg/ml) extract in phosphate buffer were added to H2O2 (0.6 ml, 40 mM). Absorbance was determined at 230 nm after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide. The percentage of H2O2 scavenging of extract and ascorbic acid (standard compound) was calculated as:
% H2O2 Scavenged = [(Ac-At)/Ac] ×100
Where Ac is the absorbance of the control, and At is the absorbance of the extract or standard. The antioxidant activity was expressed as IC50.[22]
Statistical analysis
Results were expressed as mean ± SEM. Significant differences between groups were determined by analysis of variance test followed by Dunnett’s test.
Results
Qualitative phytochemical screening
The preliminary phytochemical screening of BEPIF showed the presence of tannins, glycosides, alkaloids, flavonoids and phenolic compounds.
Quantitative phytochemical estimation
Total phenolic content of BEPIF was found to be 26.85 mg Gallic acid equivalent/100 g of dry weight. The total flavonoid content of BEPIF was found to be 83.78 mg Chrysin equivalent/100 g of dry weight. The total alkaloid content of BEPIF was found to be 45 mg atropine equivalent/100 g of dry weight [Table 1].
Table 1.
Quantitative phytochemical estimation

Assessment of nootropic activity
In the EPM test, BEPIF (150 mg/kg, i.p.) showed significant decrease in TL on day 2 (P < 0.01) and day 9 (P < 0.001) compared to day 1. BEPIF (300 mg/kg, i.p.) showed significant decrease in TL on day 2 and 9 (P < 0.001) with significant increase in IR on day 2 (P < 0.001) and day 9 (P < 0.05) compared to vehicle treated group. Thus, BEPIF (150 and 300 mg/kg) showed significant increase in inflexion ratio indicating facilitatory action on learning and memory [Figures 1 and 2].
Figure 1.
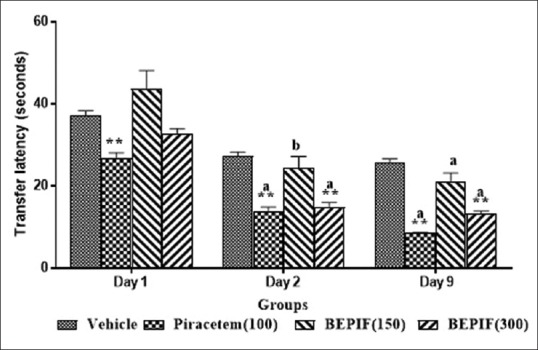
Effect of n-butanol extract of Passiflora incarnata flowers on inflexion ratio in elevated plus maze test; n = 6, values are mean ± SEM, **P < 0.001 (One way ANOVA followed by Dunnett’s test) compared to vehicle treated group; a P < 0.001 (Student t test) compared to day 1; b P < 0.01 (Student t test) compared to day 1
Figure 2.
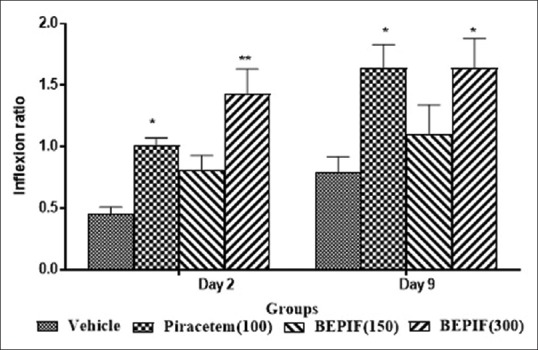
Effect of n-butanol extract of Passiflora incarnata flowers on inflexion ratio in elevated plus maze test; n = 6, *P < 0.05 compared to vehicle treated group (One way ANOVA followed by Dunnett’s test). **P < 0.001 compared to vehicle treated group (One way ANOVA followed by Dunnett’s test)
In the object recognition test, the mice spent more time to explore the objects in the first trial (T1 session). In the second trial (T2 session), when a new object replaced a familiar object, BEPIF at a dose of 150 and 300 mg/kg and piracetam at a dose of 100 mg/kg significantly (P < 0.001) reduced the time to explore the familiar object as compared with the time to explore the new object [Figure 3]. Moreover, BEPIF (150 and 300 mg/kg) also showed significant increase in discrimination index (P < 0.001) [Figure 4]. Thus, the results of elevated plus maze and object recognition test confirmed the nootropic potential of BEPIF.
Figure 3.
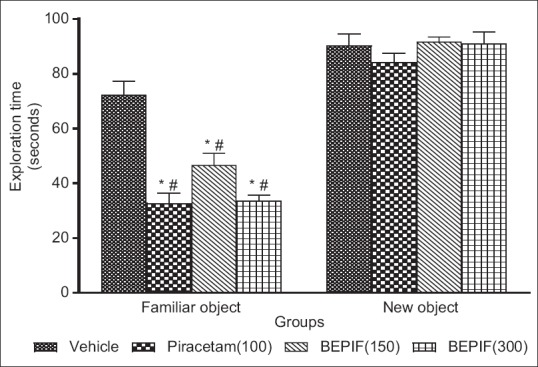
Effect of BEPIF on exploration time in ORT test; n=6, *P<0.001 compared to vehicle treated group (One way ANOVA followed by Dunnett’s test), #P<0.001 compared to vehicle treated group (Student t test)
Figure 4.
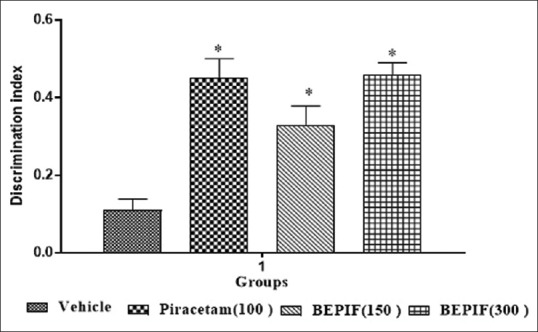
Effect of n-butanol extract of Passiflora incarnata flowers on discrimination index in object recognition task test; n = 6, *P < 0.001 compared to vehicle treated group (One way ANOVA followed by Dunnett’s test)
Antiparkinsonian activity
Antiparkinsonian activity was studied using haloperidol induced catalepsy and tacrine induced jaw movements. In the present study, haloperidol produced maximum catalepsy after 120 minutes in vehicle treated animals. Pretreatment with BEPIF (150 mg/kg and 300 mg/kg, i.p.) showed a significant (P < 0.001) reduction in the duration of haloperidol induced catalepsy [Figure 5].
Figure 5.
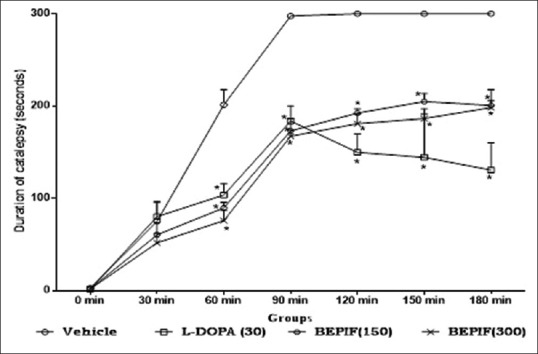
Effect of n-butanol extract of Passiflora incarnata flowers on haloperidol induced catalepsy; n = 6, *P < 0.001 compared to vehicle treated group (One way ANOVA followed by Dunnett’s test)
In tacrine induced jaw movement model, maximum number of tremulous jaw movements were observed during 30-40 min interval. Pretreatment with BEPIF (150 and 300 mg/kg) significantly reduced the tacrine induced jaw movements. BEPIF pretreatment also reduced the number of bursts induced by tacrine [Figures 6 and 7]. Thus the results of haloperidol induced catalepsy and tacrine induced jaw movements models confirmed the antiparkinsonian activity of BEPIF.
Figure 6.
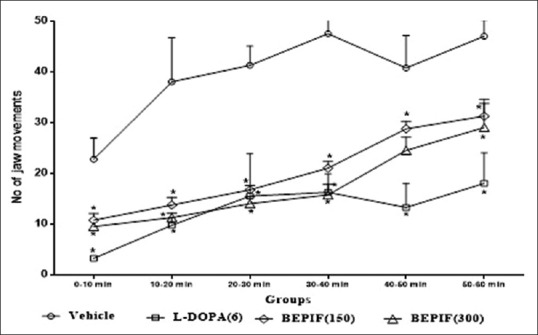
Effect of n-butanol extract of Passiflora incarnata flowers on number of jaw movements; n = 6, *P < 0.001 compared to vehicle treated group (One way ANOVA followed by Dunnett’s test)
Figure 7.
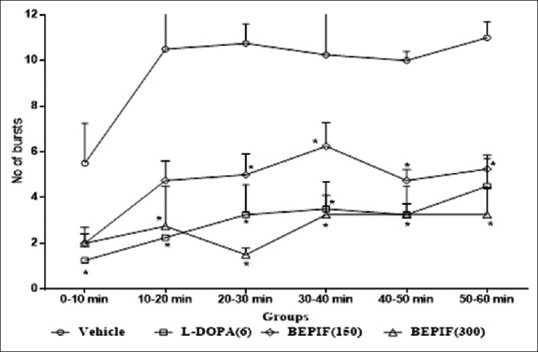
Effect of n-butanol extract of Passiflora incarnata flowers on number of bursts; n = 6, *P < 0.001 compared to vehicle treated group (One way ANOVA followed by Dunnett’s test)
Antioxidant activity
The IC50 value of BEPIF by DPPH free radical scavenging and the H2O2 scavenging method was found to be 35.37 and 29.51 μg/ml respectively [Figures 8 and 9]. The free radical scavenging activity of BEPIF was found to be comparable to ascorbic acid.
Figure 8.
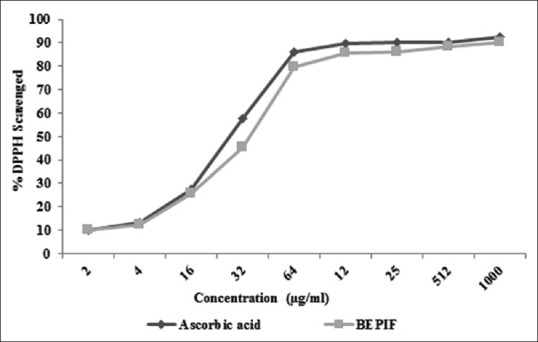
DPPH scavenging activity of n-butanol extract of Passiflora incarnata flowers
Figure 9.
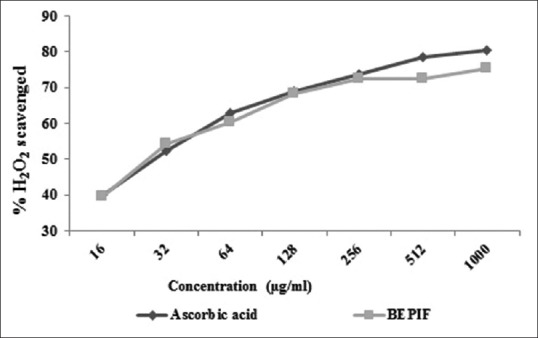
H2O2 scavenging activity of n-butanol extract of Passiflora incarnata flowers
Discussion
The high lipid content of nervous tissue, coupled with its high metabolic activity, makes it particularly susceptible to oxidative damage. Greater concentration of iron may be essential, particularly during brain development, but its presence also leads to oxidative stress via the iron-catalyzed formation of reactive oxygen species. In addition, the brain regions that are rich in catecholamines are very much vulnerable to free radical generation. The catecholamines such as adrenaline, noradrenaline and dopamine can spontaneously break down (auto-oxidize) to free radicals, or can be metabolized to radicals by the endogenous enzymes such as monoamine oxidase.[23] One such region of the brain is the substantia nigra.[24] Various studies have shown that antioxidants, both endogenous and dietary, can protect nervous tissue from damage by oxidative stress.[5,25] The facts till date for oxidative stress in PD, AD and other neurodegenerative diseases is strongly persuasive. Clinical studies show that a number of events associated with Alzheimer’s are capable of stimulating production of free radicals and depletion of antioxidant levels. Patients with Parkinson’s also have reduced glutathione levels and free radical damage is found in the form of increased lipid peroxidation and oxidation of DNA bases.[3]
Plant extracts and their constituents as a natural source of antioxidants have been extensively used in phytotherapy since ancient times.[6] Flavonoids belong to a group of natural substances with variable phenolic structures and are found in fruits, vegetables, grains, flowers, tea, and wine. Flavonoids can prevent injury caused by ROS in various ways. One way is the direct scavenging of free radicals. Flavonoids are oxidized by radicals, resulting in a more stable, less-reactive radical. Selected flavonoids can directly scavenge superoxides, whereas other flavonoids can scavenge the highly reactive oxygen-derived radical peroxy nitrite.[26]
It is apparent from the present study that the BEPIF possesses antioxidant activity as shown by significant DPPH scavenging and H2O2 scavenging ability. The naturally occurring phenolic compounds have free radical scavenging properties due to their hydroxyl groups. In the present study, quantitative phytochemical estimation of BEPIF showed presence of high flavonoid content. Thus, the antioxidant potential of BEPIF may be attributed to the presence of phenolic compounds, mainly flavonoids.
Neuroleptic-induced catalepsy is a robust behavioral method for studying antiparkinsonian activity and its modulation by various neurotransmitters. Drugs useful in the treatment of parkinsonism inhibit haloperidol induced catalepsy.[19] The use of haloperidol has been associated with an increased level of oxidative stress in the brain. This evidence suggests a possible role for antioxidants in the treatment of haloperidol-induced catalepsy.[27] In this study, BEPIF significantly reduced the duration of haloperidol induced catalepsy. In addition, BEPIF also significantly reduced the number of jaw movements induced by tacrine, a widely used animal model of parkinsonian tremors. Accordingly, it can be concluded that BEPIF has protective effect in Parkinson’s disease.
Oxidative stress has long been thought to play a major role in the pathogenesis of AD. Certain plant products and diet rich in antioxidants are said to be neuroprotective and hence may have a role in improving cognition in aging and neurodegenerative diseases.[28] In the present study, BEPIF showed significant cognitive improvement as shown by the decrease in transfer latency in EPM test and increase in discrimination index in ORT test. Thus, BEPIF has a neuroprotective effect and hence may have a role in improving cognition.
Conclusion
The above results conclude that BEPIF possesses neuroprotective effect as evident from its antiparkinsonian and memory enhancing activity. Further, it has been observed that BEPIF possesses significant antioxidant activity which may be due to its high phenolic and flavonoid content. Thus, it may be concluded that butanolic extract of Passiflora incarnata flowers has neuroprotective effect in neurodegerarive diseases such as Alzheimer’s and Parkinson’s disease which is mediated through its antioxidant potential.
Financial support and sponsorship
Authors would like to thank Savitribai Phule Pune University for financial support and the management of SCES’s Indira College of Pharmacy, Pune – 411 033, Maharashtra, India for providing laboratory facilities.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors would like to thank Savitribai Phule Pune University for financial support and the management of SCES’s Indira College of Pharmacy, Pune-411033, Maharashtra, India for providing laboratory facilities.
References
- 1.Skovronsky DM, Lee VM, Trojanowski JQ. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol. 2006;1:151–70. doi: 10.1146/annurev.pathol.1.110304.100113. [DOI] [PubMed] [Google Scholar]
- 2.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–75. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, Sharad S, Kapur S. Free radicals and oxidative stress in neurodegenerative diseases: Relevance of dietary antioxidants. J Indian Acad Clinl Med. 2004;5:218–25. [Google Scholar]
- 4.Mario C, Neville V. The potential role of dietary polyphenols in Parkinson's disease. Malta Med J. 2011;23:52–5. [Google Scholar]
- 5.Ciccone CD. Free-radical toxicity and antioxidant medications in Parkinson's disease. Phys Ther. 1998;78:313–9. doi: 10.1093/ptj/78.3.313. [DOI] [PubMed] [Google Scholar]
- 6.Awaad AS, AL-Jaber NA. Antioxidant Natural Plant. RPMP Ethnomed Source Mech. 2010;27:1–35. [Google Scholar]
- 7.Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, et al. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. Saboten. Brain Res. 2003;965:130–6. doi: 10.1016/s0006-8993(02)04150-1. [DOI] [PubMed] [Google Scholar]
- 8.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3:115–26. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbasi E, Nassiri-Asl M, Shafeei M, Sheikhi M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem Biol Drug Des. 2012;80:274–8. doi: 10.1111/j.1747-0285.2012.01400.x. [DOI] [PubMed] [Google Scholar]
- 10.The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products. New Delhi: CSIR; 2001. [Google Scholar]
- 11.Dhawan K, Kumar S, Sharma A. Passiflora: A review update. J Ethnopharmacol. 2004;94:1–23. doi: 10.1016/j.jep.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Jean PH, Richard CB. Parkinson's Disease: Medications. Florida, USA: The National Parkinson Foundation, Inc; 1999. [Google Scholar]
- 13.Trease GE, Evans MC. Textbook of Pharmacognosy. London: Balliere Tindall; 2002. p. 220. [Google Scholar]
- 14.Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall. 2005;40:255–60. [Google Scholar]
- 15.Shamsa F, Monsef HR, Ghamooshi R. Spectrophotometric determination of total alkaloids in Peganum harmala L. using bromocresol green. Res J Phytochem. 2007;1:79–82. [Google Scholar]
- 16.Chintawar SD, Somani RS, Kasture VS, Kasture SB. Nootropic activity of Albizzia lebbeck in mice. J Ethnopharmacol. 2002;81:299–305. doi: 10.1016/s0378-8741(02)00140-x. [DOI] [PubMed] [Google Scholar]
- 17.Bartolini L, Casamenti F, Pepeu G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol Biochem Behav. 1996;53:277–83. doi: 10.1016/0091-3057(95)02021-7. [DOI] [PubMed] [Google Scholar]
- 18.Ferré S, Guix T, Prat G, Jane F, Casas M. Is experimental catalepsy properly measured? Pharmacol Biochem Behav. 1990;35:753–7. doi: 10.1016/0091-3057(90)90354-k. [DOI] [PubMed] [Google Scholar]
- 19.Nair V, Arjuman A, Dorababu P, Gopalakrishna HN, Chakradhar Rao U, Mohan L, et al. Effect of NR-ANX-C (a polyherbal formulation) on haloperidol induced catalepsy in albino mice. Indian J Med Res. 2007;126:480–4. [PubMed] [Google Scholar]
- 20.Kasture S, Pontis S, Pinna A, Schintu N, Spina L, Longoni R, et al. Assessment of symptomatic and neuroprotective efficacy of Mucuna pruriens seed extract in rodent model of Parkinson's disease. Neurotox Res. 2009;15:111–22. doi: 10.1007/s12640-009-9011-7. [DOI] [PubMed] [Google Scholar]
- 21.Jiin-Tzong G, Hui-Lien L, Shu-Hsiu C. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J Food Drug Anal. 2001;9:96–101. [Google Scholar]
- 22.Ranju P, Kundlik G, Nidhi S, Hussain MM, Thirumoorthy N. Antioxidant and free radical scavenging activity of ethanolic extract of Morinda citrifolia . Ann Biol Res. 2011;2:127–31. [Google Scholar]
- 23.Perry G, Sayre LM, Atwood CS, Castellani RJ, Cash AD, Rottkamp CA, et al. The role of iron and copper in the aetiology of neurodegenerative disorders: Therapeutic implications. CNS Drugs. 2002;16:339–52. doi: 10.2165/00023210-200216050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Contestabile A. Oxidative stress in neurodegeneration: Mechanisms and therapeutic perspectives. Curr Top Med Chem. 2001;1:553–68. doi: 10.2174/1568026013394723. [DOI] [PubMed] [Google Scholar]
- 25.Bizimenyera ES, Aderogba MA, Eloff JN, Swan GE. Potential of neuroprotective antioxidant-based therapeutics from Peltophorum africanum sond. (Fabaceae) Afr J Tradit Complement Altern Med. 2006;4:99–106. doi: 10.4314/ajtcam.v4i1.31199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S, et al. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–35. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 27.Rasheed AS, Venkataraman S, Jayaveera KN, Fazil AM, Yasodha KJ, Aleem MA, et al. Evaluation of toxicological and antioxidant potential of Nardostachys jatamansi in reversing haloperidol-induced catalepsy in rats. Int J Gen Med. 2010;3:127–36. doi: 10.2147/ijgm.s9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijayalakshmi Adiga S, Bhat P, Chaturvedi A, Bairy KL, Kamath S, et al. Evaluation of the effect of Ferula asafoetida linn. Gum extract on learning and memory in Wistar rats. Indian J Pharmacol. 2012;44:82–7. doi: 10.4103/0253-7613.91873. [DOI] [PMC free article] [PubMed] [Google Scholar]


