Summary
Multiple endocrine neoplasia type 1 (MEN1) is a rare hereditary tumoral syndrome, featured by a combination of neoplasms of various endocrine and nonendocrine tissues. Approximately 33% of MEN1-related deaths are due to the malignant behaviour of well-differentiated neuroendocrine tumors (NETs), for which a preventive surgical treatment is not feasible. Somatostatin analogues (SSA) have been employed in the treatment of NETs in the stage of advanced or metastatic disease, in order to control the growth and secretion of tumor lesions. A longitudinal, open label study named “LARO-MEN1” was undertaken in order to assess whether early medical treatment with long-acting SSA could act as a preventive approach in small MEN1-related gastroenteropancreatic (GEP) NETs.
Thirty consecutive patients affected by MEN1 were screened and 8 patients with small (<2 cm) NETs and abnormal laboratory values of at least one of the GEP hormones were administered octreotide acetate slow-release formulation (LAR) (10 mg i.m. every 28 days).
Octreotide LAR was effective in decreasing GEP hormones and overall safe in the majority of patients up to six years of treatment, maintaining the disease stable also in terms of tumor size.
The positive outcomes of this study in MEN1 patients reinforce the results obtained in advanced NETs on the use of SSA, opening to the opportunity for preventive use of octreotide LAR, aimed to delay or even avoid surgery in these patients.
Keywords: MEN1, somatostatin analogs, neuroendocrine tumors, somatostatin receptors
Introduction
Multiple endocrine neoplasia type 1 (MEN1; MIM #131100) is a rare hereditary cancer syndrome transmitted to offsprfing in an autosomal dominant manner, featured by a combination of more than 20 different types of endocrine and non-endocrine tumors (Table 1). A practical definition of MEN1 is the occurrence of at least two of the three main “classical” MEN1-related endocrine neoplasias: multiglandular parathyroid tumors, anterior pituitary tumors and neuroendocrine tumors (NETs) of the gastro-entero-pancreatic (GEP) tract (1, 2).
Table 1.
Endocrine and non-endocrine tumor types and their prevalence in MEN1 syndrome.
| Tumor Type | Tumor Subtype | Hormone | Prevalence in MEN1 Syndrome | |
|---|---|---|---|---|
|
| ||||
| Parathyroid | NA1 | PTH | 100% have primary hyperparathyroidism by age 50 yrs 2 | |
|
| ||||
| Anterior pituitary | Functioning | Prolactinoma (PRLoma) | ~10–60%3 have anterior pituitary tumors | Most commonly seen anterior pituitary tumor subtype |
|
|
|
|||
| Growth hormone (GH)-secreting | 5% of anterior pituitary tumors 4 | |||
|
|
|
|||
| GH/PRL-secreting | 5% of anterior pituitary tumors 4 | |||
|
|
|
|||
| ACTH-secreting | 2% 4 | |||
| TSH-secreting | Rare 5 | |||
|
| ||||
| Nonfunctioning | - | |||
|
| ||||
| Well-differentiated endocrine | Functioning | Gastrinoma | Accounts for 40% of well-differentiated endocrine tumors 6 | |
|
| ||||
| Insulinoma | 10% 4 | |||
|
| ||||
| Glucagonoma | 2% 4 | |||
|
| ||||
| VIPoma | 2% 4 | |||
|
| ||||
| Nonfunctioning | - | 55% | ||
|
| ||||
| Carcinoid | Bronchial | No | 10% | |
|
| ||||
| Thymic | No | |||
|
| ||||
| Adrenocortical | Functioning | Cortisol-secreting | ~20–40% have adrenocortical tumors | Rare |
|
|
|
|||
| Aldosterone-secreting | Rare | |||
|
|
|
|||
| Nonfunctioning | - | |||
|
| ||||
| Skin | Lipomas | - | (30%) | |
| Angiofibromas | - | (85%) | ||
| Collagenomas | - | (70%) | ||
|
| ||||
| Nervous Central System | Meningiomas | - | (~8%) | |
| Ependymomas | - | (1%) | ||
|
| ||||
| Others | Leiomyoma | - | 1% | Rare |
| Pheochromocytoma | Epinephrine, Norepinephrine | <1% | Rare | |
The age-dependent penetrance for the clinical features rises above 50% by age 20 years and more than 95% by age 40 years (3, 4). In a recent study, a high penetrance of nonfunctioning pancreatic neuroendocrine tumors in 15- to 20-year-old MEN1 patients has been demonstrated, suggesting that a periodic surveillance is advised in this group of subjects (5).
Approximately one third of deaths in MEN1 patients are caused by the malignant behavior of NETs for which a preventive surgical treatment is not advisable (except for prophylactic thymectomy for thymic carcinoid during parathyroid surgery) (6). This is mainly due to two reasons: 1) the sites of involvement by tumors (pancreas, duodenum and lungs) are difficult to evaluate in terms of early detection of the initial lesions; and 2) they cannot be subjected to prophylactic ablative surgery (2).
Well-differentiated neuroendocrine tumors occur in 30–80% of MEN1 patients, ranging from micro-to macro-adenomas, and invasive metastatic carcinomas. They are generally multicentric neoplasms, either functioning [gastrinomas (40%), insulinomas (10%), VIPomas, Glucagonomas (<2%)] or nonfunctioning (1, 2). The latter are more frequent (55%) than previously believed and are usually clinically silent or possibly exert a mass effect (7).
Gastrinoma(s) (40% of MEN1 cases) can give rise to Zollinger Ellison syndrome (ZES) due to increased gastrin secretion from multiple, small (<1 cm diameter) NETs, located mainly in the duodenal submucosal (8–10). The average age of ZES onset is 41 yr, one decade earlier than the non-syndromic counterpart, with an average delay in its diagnosis of 3–5 yr or 5–9 yr, according to the considered clinical series, from its onset (11).
Recent studies have shown that almost two thirds of patients with MEN1 die for MEN-related causes and in 40–45% the main cause of death is represented by pancreatic NETs (pNETs) (12, 13). It has been reported that approximately 50% of MEN1 gastrinomas have metastasized prior to diagnosis, mainly to regional lymph nodes and less frequently to the liver. The occurrence of liver metastases has a poor prognosis and reduced survival rate (1, 14). Twenty-five percent of people with MEN1/ZES syndrome do not have a family history of MEN1 syndrome (10). Pancreatic gastrinomas, which are rare in MEN1, are more aggressive than duodenal gastrinomas and frequently metastasize to the liver (15).
The diagnosis of gastro-intestinal NETs is multimodal, based on clinical symptoms, hormone levels, radiological and nuclear imaging (16). Imaging techniques, such as CT, MRI and endoscopic ultrasound (EUS) are currently used to determine the location of the primary tumor and for staging of the disease. Scintigraphy with 111In-labeled octreotide (octreoscan) displays a high sensitivity in detecting NETs and estimating their size, since NETs often express somatostatin 2 (sst2) and sst5 receptors (17, 18). Nonetheless, recently introduced techniques of functional imaging such as positron emission tomography with gallium 68 DOTANOC show greater sensitivity and specificity for NETs, although their use in MEN1 is not yet defined as screening method in asymptomatic patients (13, 19).
The management of MEN1-related small and asymptomatic NETs is still debated (20). MEN1-related NETs present peculiar characteristics if compared to the sporadic counterpart, such as a younger age of onset, multifocality and simultaneous presence of other tumors. Therefore, applying in MEN1-related NETs similar therapeutic approaches requires caution (20). There is no universal consensus on the indications for entero-pancreatic surgery for patients with MEN1-related NETs. The size cut-off for surgery has been differently set at 1, 2 or 3 cm as main diameter, as indicated by different studies (21–26). According to recently published consensus guidelines by the European Neuroendocrine Tumor Society (ENETS), surgery is not recommended in gastrinomas less than 2 cm and non-functioning pNETs detected in functional studies, while surgical treatment is reserved for lesions greater than 2 cm (13). A surgical approach should be advised for patients with either sporadic or MEN1-related insulinomas in the absence of non-resectable metastatic lesions (13).
While some studies have shown that well differentiated NETs in general are very slowly growing tumors and remain quite stable over time (27, 28), the prognosis of MEN1-related NETs remain uncertain (13). Thus, the management of smaller neuroendocrine lesions in MEN1 is still a matter of controversy (20).
If surgery is not chosen or not possible, the medical treatment of MEN1 GEP-NETs may include: proton pump inhibitors (for the control of ZES-related symptoms) and somatostatin analogues (SSAs). Chemotherapy is usually limited to cases of metastatic disease, and yields poor results and significant side effects (29). Interferon-alpha produces a symptomatic response in 40–60% of patients, a biochemical response in 30–60%, and reduced tumor size in 10–15% (30). SSAs have been acknowledged as the treatment of choice in functioning NETs for symptoms control (31, 32). Two recently published randomized trials have also assessed the antiproliferative effects of long-acting SSAs in NETs in advanced stage of disease, having as primary endpoint the progression-free survival. The Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PRO-MID study) and the Controlled Study of Lanreotide Antiproliferative Response in Neuroendocrine Tumors (CLARINET study) have demonstrated the efficacy and long-term safety of long-acting octreotide or a gel formulation of lanreotide, respectively, in terms of prolonged progression-free survival in patients with non-functioning, somatostatin receptor-positive, metastatic, well- or moderately differentiated sporadic NETs (33–36).
Despite of the availability of different non-surgical approaches for NETs, including SSAs, no similar randomized trials have been pursued focusing on patients with MEN1-related NETs, aiming to demonstrate the efficacy of medical treatments such as SSAs in terms of symptoms controls and tumor progression (20). Similarly, no study on dose optimization of SSAs has been carried out so far in patients with MEN1.
In patients with MEN1, an initial experience in a small group of patients with advanced gastrinomas showed the efficacy of octreotide in terms of reduction of symptoms and hypersecretion (37). Recently, a retrospective experience on the use of SSAa in early, MEN1-related duodeno-pancreatic NETs has shown that treatment with SSAs (treatment duration 12–75 months) is effective in maintaining a stable disease in 80% of patients, with even an effect on tumor growth in 10% of patients (38).
Since no longitudinal, intervention studies assessing the usefulness of medical treatment in early neuroendocrine lesions are available thus far and in order to contribute to the research in this interesting area, a preventive treatment study was undertaken in patients with MEN1-related early, non metastatic NETs, that would allow the definition of a common clinical protocol for selected patients with a diagnosis of MEN1 and small asymptomatic NETs.
Materials and methods
Study Design
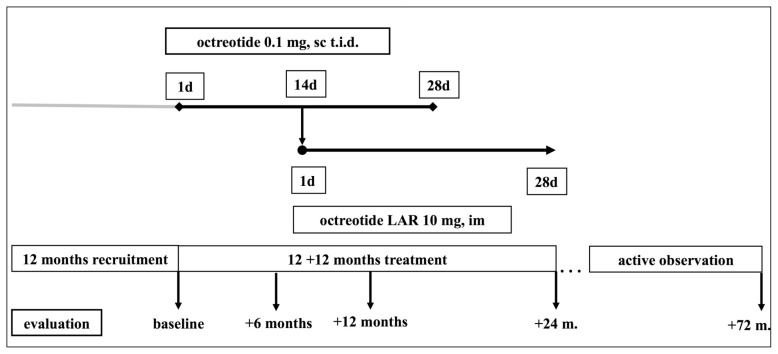
The study, also referred to as the Long Acting Release octreotide-MEN1 (LARO-MEN1) study, consists of a clinical prospective, not comparative, open label trial with an open label design to evaluate the activity and safety of octreotide acetate slow-release formulation (LAR) in patients with MEN1-NETs with abnormal laboratory values and no indication for surgery. The study was designed to include: 12 months recruitment, 12 months active treatment, and 12 months of observation in a prospective, not comparative, open label design. The possibility to expand the study to 24 months of treatment was included in the application to the Internal Review Board (IRB). The study was approved by the IRB of the University Hospital of Florence and an informed consent was obtained from all participants.
Study Population
Eight (out of 30 screened) consecutive patients of both sexes, aged >18 years (age range 30–62 years), evaluated in the period 2005–2006 in the Unit of Bone and Mineral Metabolism of the University Hospital of Florence (Italy), were selected on the basis of all of the following criteria: 1) a diagnosis of MEN1, confirmed by history and genetic testing; 2) a duodenal or pancreatic neuroendocrine tumor less than 2 cm in diameter, as assessed by ultrasound and/or CT scan, not indicated for surgery; 3) abnormal levels of PP (>85.8 pg/ml); 4) abnormal values of at least one of the following biochemical parameters: gastrin, glucagon, insulin, VIP, somatostatin, histamine and chromogranin A; 5) positive Octreoscan lesions (as expressing ss2 and ss5 receptors). In this regard, octreoscan was considered as the gold standard for functional imaging of NETs at the time of the development of the study protocol. Exclusion criteria were: abnormal hematocrit, liver function tests (ALT, AST, alkaline phosphatase and total bilirubin above the standard range), and renal function (blood urea nitrogen and creatinine levels above the standard range); 6) performance ECOG status <2.
Intervention
Octreotide acetate, a synthetic octapeptide, is a long-acting, somatostatin analogue. In fact, the short half-life of natural somatostatin would require continuous infusion to maintain active plasma concentrations, while octreotide, having a half-life of 80–100 minutes (about 30 times higher than that of natural somatostatin) can be administered intermittently (39). After baseline assessment, treatment with subcutaneous (sc) octreotide administered every 8 hours was begun, with the aim of improving patient adherence to therapy. After a run-in period of 14 days with sc octreotide, which was withdrawn 14 days afterwards, a slow-release formulation of octreotide LAR 10 mg was administered intramuscularly (im) every 28 days. This dosage regimen was chosen as appropriate since no evidence of the superior effectiveness of greater dosages of somatostatin analogs in NETs in general and MEN1-related NET, in particular, was available at the time of the study design. Octreotide LAR treatment, which was initially planned to last for 1 year, was extended for another 12 months and then maintained afterwards (up to 72 months) (Table 2). A schematic representation of the intervention is reported in Figure 1.
Table 2.
Baseline characteristics of the patients enrolled in the LARO-MEN1 study and biochemical and morphological follow-up.
| Patient # | Sex | Age (years) | NET foci at baseline | PP (pg/ml, <85.8) | Glucagon (pg/ml, 60–209) | NET max diameter (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline | +12 m. | +24 m. | + 48 m. | + 72 m. | baseline | +12 m. | +24 m. | + 48 m. | + 72 m. | baseline | +12 m. | +24 m. | + 48 m. | +72 m. | ||||
| 1 | F | 33 | Multiple | 102 | 63.8 | 33.3 | 35.6 | 30.8 | 252 | 138 | 82.8 | 108 | 156 | <10 | <10 | <10 | <10 | <10 |
| 2 | F | 30 | Single | 197 | 33.3 | 16.0 | 28 | 68 | 235 | 127 | 98 | 100 | 110 | 10 | 10 | 10 | 10 | 10 |
| 3 | F | 34 | Single | 116 | 8.96 | 5.11 | 6 | 4.5 | 304,2 | 209 | 174 | 135 | 155 | 10 | 10 | 10 | 10 | 10 |
| 4 | M | 34 | Multiple | 122 | 72 | 66.1 | 70 | 62.4 | 41, 5§ | 18.2§ | 9.9§ | 9.5 | 8.7 | 12 | 12 | 12 | 12 | 12 |
| 5* | M | 62 | Single | 562 | NA | NA | NA | NA | 362 | NA | NA | NA | NA | 18 | NA | NA | NA | NA |
| 6 | M | 31 | Multiple | 451 | 67.7 | 108 | 99 | 81 | 352 | 145 | 168 | 152 | 146 | <10 | <10 | <10 | <10 | <10 |
| 7** | F | 35 | Multiple | 98 | NA | NA | NA | NA | 257 | NA | NA | NA | NA | 15 | NA | NA | NA | NA |
| 8 | F | 36 | Single | 202 | 75.7 | 37.6 | 41 | 33 | 259 | 199 | 166 | 131 | 103 | 17 | 17 | 17 | 17 | 17 |
treatment with octreotide discontinued at +14 days because of adverse event (diffuse abdominal pain)
treatment with octreotide LAR discontinued at 3 months because of the occurrence of insulinoma requiring surgery
somatostatin (pg/ml, <37)
PP=pancreatic polipeptide
NA=not assessed
Figure 1.
Schematic representation of the study design. After a recruitment period of 12 months, eligible subjects were evaluated and initially treated with octreotide 0.1 mg administered subcutaneously every 8 hours (i.e. three times daily, t.i.d.). At +14 days octreotide LAR was begun, with an overlap of 14 days with octreotide LAR, which was discontinued at +28 days. Subjects had been followed up for 12 months under octreotide LAR treatment. The original study, as registered by the local IRB, was extended to 24 months, as detailed in the text. Then, an active observation period up to 72 months ensued.
Evaluation
Baseline assessment included: clinical examination, abdomen ultrasound and/or contrast-enhanced CT scan, and determination of PP and other neuroendocrine tumors, as listed above. Clinical and biochemical follow-up was initially planned every 6 months, up to 12 months, with yearly re-assessment of tumor lesion by CT scan in the extension of the study. Radiological antitumoral response was evaluated taking advantage of the Response Evaluation Criteria in Solid Tumors (R.E.C.I.S.T.), as previously described (40). Biochemical, hormonal and instrumental evaluation included assessment of parathyroid and pituitary function, the results of which are not shown in this paper.
Baseline characteristics of the patients
All patients had pNETs. Four out of eight patients (50%) had multifocal pancreatic lesions. Maximum diameter of the lesions ranged from less than 10 mm to 18 mm. Regarding additional GEP markers, glucagon was increased in 7/8 subjects, while somatostatin was high in one patient (Table 2). No patient showed NET-related symptoms at baseline. Seven out of eight patients included in the study (87.5%) had primary hyperparathyroidism, which had been successfully treated with subtotal parathyroidectomy before enrollment; 4 patients (50%) suffered from prolactin-secreting pituitary microadenoma under cabergoline treatment; 1 patient was surgically treated for prolactin/ACTH secreting pituitary microadenoma. Patient #3 displayed cutaneous fibromatosis of the back, and recurrent acute pancreatitis developed after duodenoce-falopancreasectomy for a gastrinoma, as additional manifestations.
Objectives
The primary objective of this study was to assess the activity of octreotide LAR in patients with MEN1-related NETs in terms of a reduction or normalization of PP and/or the reduction or disappearance of tumor lesions. Secondary objectives were to evaluate the changes in other gastrointestinal hormones related to MEN1 syndrome and assess the safety and tolerability of the drug.
Results
The main results of the study are reported in Table 2. All MEN1 patients selected agreed to participate in the LARO-MEN1 study.
Octreotide LAR treatment was well tolerated overall, with the exception of one patient (#5), for whom it was discontinued after a few days due to a side effect consisting of diffuse abdominal pain under sc octreotide. The pain resolved after the withdrawal of the drug, but clearly prevented further administration of octreotide LAR. Treatment with octreotide LAR was discontinued after 3 months in patient #7, in whom the occurrence of a hypoglycemic crisis led to the diagnosis of insulinoma which required elective surgery (duodenoce-falopancreasectomy).
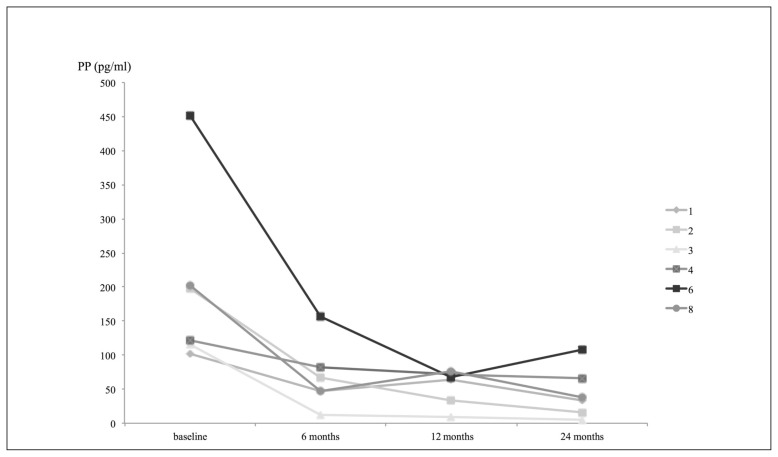
A complete response in terms of activity by secreting tumor tissue was obtained in all patients, with normalization of the PP values and other GEP hormones (glucagon and somatostatin) altered at baseline, both in basal conditions (Table 2) and during stimulation with secretin test (data not shown). The normalization of PP levels occurred as early as 6 months after the beginning of therapy with octreotide LAR for the majority of patients (5/6, 83.3%), and was maintained for up to 12 months (Figure 2). Regarding the efficacy of the drug in terms of anti-tumoral response, the maximum diameter of the pancreatic lesions remained unchanged, as assessed by CT scan at one year. Patients remained asymptomatic for the whole study period. In patient #3, the complete disappearance of nodular skin lesions and resolution of recurrent pancreatitis were documented after just 2 months of octreotide LAR.
Figure 2.
Trend of pancreatic polypeptide (PP) in patients under monthly treatment with octreotide LAR.
Based on the extremely positive results obtained after the first year of treatment, both in terms of efficacy and tolerability of the drug, an extension of the study for an additional 12 months was obtained. In the second year of treatment with octreotide LAR, pancreatic lesions and hormonal markers (both at baseline and after stimulation test) remained stable, and no side effects were observed (Table 2).
All the subjects who completed the two-year follow-up were maintained under octreotide LAR treatment (median followup 6.5 years), with the exception of one (patient #2), for whom the drug was discontinued because of the occurrence of gallbladder stones after 2 years of treatment, a known side effect of octreotide analog treatment (Table 2). All the other patients had a stable disease in terms of antitumoral response and biomarkers. In particular, the diameter of the main pancreatic lesion remained unchanged, without occurrence of new lesions other than the ones observed at baseline. So far, in patient #1, who displayed a multifocal NET at baseline, only the tumor with the maximum diameter was detectable in the CT-scan performed after 7 years of treatment with Otreotide LAR. No additional long-term side effects have been observed over the entire period of intervention.
Discussion
Neuroendocrine tumors of the gastroenteropancreatic system comprise a rare group of malignant neoplasm. Nonetheless, they are a common finding in subjects with MEN1 and represent the major MEN-related cause of death in this group of subjects. Somatostatin analogues have been shown to be very useful for symptomatic and biochemical improvement in patients with GEP-NETs. The mechanisms of these effects are not completely understood, but recent evidences indicate that they rely, at least in part, on the inhibition of proliferative signaling pathways, activation of apoptosis, and modulation of angiogenesis (16, 41, 42). Data from international clinical trials have demonstrated the efficacy of octreotide LAR in controlling symptoms related to hormonal hypersecretion by functioning NETs and carcinoid syndrome, but also to control and slow down tumor growth even in functioning tumors in progression (PROMID and CLARINET studies) (33–36), with an extension of progression-free survival, while preclinical and clinical studies provide conflicting results on their antitumor effect in asymptomatic lesions discovered during screening procedures in inherited syndromes (20).
Only a few small, inhomogeneous, mainly retrospective studies have evaluated the effect of treatment with SSAs in MEN1 subjects with GEP-NETs (37, 38), so that no definitive conclusion nor recommendation can be drawn on the use/effect of these drugs on these patients, with the exception of the control of the symptoms and the consequential improvement of the physical conditions of patients.
The pilot, open-label study hereby presented is the first longitudinal, intervention study, which has assessed the efficacy of octreotide LAR, a well-tolerated drug of proven safety, in the early treatment of GEP-NETs in a small group of patients with MEN1, with the aim to delay or avoid surgery. These subjects have been strictly followed for up to a pre-planned period of 12–24 months. They were subsequently maintained under the same treatment and observed up to 72 months afterwards by means of biochemical and instrumental assessment. The outcomes of this study are in agreement with the results of several large clinical trials, which have shown the efficacy of somatostatin analogues in the treatment of sporadic and advanced NETs (33–36) and reinforce data of the retrospective study by Ramundo et al. on a larger group of patients with early MEN1-related NETs (20 subjects) treated with octreotide LAR 30 mg administered i.m. every 28 days as first-line therapy for a mean follow-up period of about 40 months (38). The fact that in the LARO-MEN1 study no disease progression was observed administering the minimal dose of 10 mg i.m. every 28 days might challenge the concept that in MEN1 syndrome the more is the better. Nonetheless, further trials are needed regarding this subject.
Genetic testing has decreased the morbidity and mortality associated with MEN1. Indeed, a differential detection of endocrine tumors leads to an early diagnosis as demonstrated in a multicenter study comparing MEN1 carriers born in the second half of the twentieth century versus the ones of the same age born in the first half (43). Moreover, a prospective clinical study in carriers of a MEN1 mutation revealed that it is possible to find biochemical evidence of tumor, on average, 10 years before the clinical symptoms occur, allowing early pharmacological and/or surgical intervention. Thus, genetically positive individuals should be subject to targeted surveillance for early detection of potentially malignant neuroendocrine tumors, the presence of which increases morbidity and mortality related to the syndrome (44).
Octreotide LAR can be proposed as a first-line, early medical treatment for both sporadic and familial NETs, in a prophylactic way.
In our series of MEN1 patients selected for having an asymptomatic NET, still with positive biomarkers of the disease, treatment with octreotide LAR has been demonstrated to be safe, well tolerated and overall effective in preventing the progression of the lesions and keeping patients asymptomatic. Indeed, in the pre-planned one year of treatment, the drug was promptly interrupted in only one patient for the occurrence of mild side effects and in another patient for the occurrence of symptoms related to insulinoma. In the latter subject, a more severe disease could be hypothesized from the beginning, because of high baseline serum levels or neuroendocrine biomarkers. Further studies are needed in order to establish baseline cut-off values above which a careful follow-up for detection of symptomatic disease should be advised, even under treatment with SSAs. In the second year extension trial, NETs remained stable in all patients under octreotide LAR administered at a standard dose from a clinical, biochemical and morphological point of view. In the long-term, a patient developed a known side effect of SSAs (gallbladder stones), which was easily detected during proper imaging follow-up and appropriately treated. In patient #5, who discontinued treatment early because of diffuse abdominal pain, signs of disease progression were observed in the long-term, because of the appearance of other pancreatic tumor foci and an increase of serum levels of neuroendocrine markers.
After the two-year completion of this longitudinal study, other MEN1 patients with similar characteristics have been placed under long-term, monthly, anti-proliferative octreotide LAR treatment (data not shown), which has been confirmed as a safe and effective treatment for NETs not meeting criteria for surgery. Since octreotide LAR is widely used in malignant carcinoid syndrome, this medical management could also be extended to patients with pulmonary carcinoids within the MEN1 syndrome. Indeed, a complete regression of other MEN1-associated benign lesions (skin neurofibromas) was observed in one patient under octreotide LAR treatment.
The relatively small number of enrolled subjects, although recruited in a single referral center in a relatively short period of time and according to stringent criteria, the initial limited planned length of the intervention, and the absence of information on the baseline histological grading of the pNETs confirming the supposed low replicative potential in our series, constitute obvious limits to the LARO-MEN1 study. In this respect, more extensive multicentric, placebo-controlled, intervention trials with progression-free survival as primary objective, as well as studies on dose optimization of SSAs are needed in order to establish the efficacy of SSAs for clinical use in individuals with early MEN1-related GEP-NETs. Only through multicentric, well characterized and homogenous clinical series will it be possible to give a more clear answer regarding the potential beneficial effects, both clinical and quality of life, that such molecules may offer to patients with MEN1-related NETs, in particular for those who do not have the criteria for a surgical solution of the disease, which is now reserved for bigger lesions and more aggressive forms.
In the future, the use of drugs directly modulating key pathways important in MEN1-related tumorigenesis (AKT mTOR pathways, microRNA overexpression), alone or in combination, could offer a new therapeutic opportunity in MEN1-related tumors.
In conclusion, this study is the first longitudinal, open-label, intervention trial, which has demonstrated that long-acting SSAs are a safe and effective treatment for subjects positive to EN1 screening with asymptomatic, small sized GEP-NETs not requiring surgery.
Acknowledgments
This work was supported by an unrestricted grant from Fondazione Italiana Ricerca Malattie Ossee (F.I.R.M.O.) Fondazione Raffaella Becagli.
Footnotes
Conflict of interest
C.F., C.L., M.L., and G.F. declare that have no conflict of interest to disclose in general and regarding this paper.
B.M.L. declares that has received grants and consulting fees from Alexion, Abiogen Pharma, Amgen, Bruno Farmaceutici, Eli Lilly, MSD, NPS, Shire, SPA, Servier.
References
- 1.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA, Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocr Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. (2001) [DOI] [PubMed] [Google Scholar]
- 2.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. Endocrine Society Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrin Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 3.Burgess JR, Greenaway TM, Shepherd JJ. Expression of the MEN1 gene in a large kindred with multiple endocrine neoplasia type I. J Intern Med. 1998;243:465–470. doi: 10.1046/j.1365-2796.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- 4.Marx S, Spiegel AM, Skarulis MC, Doppman JL, Collins FS, Liotta LA. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med. 1998;129:484–494. doi: 10.7326/0003-4819-129-6-199809150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves TD, Toledo RA, Sekiya T, Matuguma SE, Maluf Filho F, Roch MS, Siqueira SA, Glezer A, Bronstein MD, Pereira MA, Jureidini R, Bacchella T, Machado MC, Toledo SP, Lourenco DM., Jr Penetrance of functioning and nonfunctioning pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 in the second decade of life. J Clin Endocrin Metab. 2014;99:E89–96. doi: 10.1210/jc.2013-1768. [DOI] [PubMed] [Google Scholar]
- 6.Doherty GM, Olson JA, Frisella MM, Lairmore TC, Wells SA, Jr, Norton JA. Lethality of multiple endocrine neoplasia type 1. World J Surg. 1998;22:581–585. doi: 10.1007/s002689900438. [DOI] [PubMed] [Google Scholar]
- 7.Thomas-Marques L, Murat A, Delemer B, Penfornis A, Cardot-Bauters C, Baudin E, Niccoli-Sire P, Levoir D, Choplin Hdu B, Chabre O, Jovenin N, Cadiot G Groupe des Tumeurs Endocrines (GTE) Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am J Gastroenterol. 2006;101:266–273. doi: 10.1111/j.1572-0241.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 8.Pipeleers-Marichal M, Somers G, Willems G, Foulis A, Imrie C, Bishop AE, Polak JM, Hacki WH, Stamm B, Heitz PU, Path FRC, Kloppel G. Gastrinomas in the duodenum of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. New Engl J Med. 1990;322:723–727. doi: 10.1056/NEJM199003153221103. [DOI] [PubMed] [Google Scholar]
- 9.Townsend CM, Jr, Thompson JC. Gastrinoma. Semin Surg Oncol. 1990;6:91–97. doi: 10.1002/ssu.2980060207. [DOI] [PubMed] [Google Scholar]
- 10.Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. New Engl J Med. 1999;341:653–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 11.Norton JA, Melcher ML, Gibril F, Jensen RT. Gastric carcinoid tumors in multiple endocrine neoplasia-1 patients with Zollinger-Ellison syndrome can be symptomatic, demonstrate aggressive growth, and require surgical treatment. Surgery. 2004;136:1267–1274. doi: 10.1016/j.surg.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1patients with or without pancreatic endocrine tumors. Medicine (Baltimore) 2013;92:135–181. doi: 10.1097/MD.0b013e3182954af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Kloppel G, Reed N, Kianmanesh R, Jensen RT all other Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fendrich V, Langer P, Waldmann J, Bartsch DK, Rothmund M. Management of sporadic and multiple endocrine neoplasia type 1 gastrinomas. Brit J Surg. 2007;94:1331–1341. doi: 10.1002/bjs.5987. [DOI] [PubMed] [Google Scholar]
- 15.Anlauf M, Garbrecht N, Henopp T, Schmitt A, Schlenger R, Raffel A, Krausch M, Gimm O, Eisenberger CF, Knoefel WT, Dralle H, Komminoth P, Heitz PU, Perren A, Kloppel G. Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological features. World J Gastroentero. 2006;12:5440–5446. doi: 10.3748/wjg.v12.i34.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberg KE. Gastrointestinal neuroendocrine tumors. Ann Oncol. 2010;21:72–80. doi: 10.1093/annonc/mdq290. [DOI] [PubMed] [Google Scholar]
- 17.Falconi M, Plockinger U, Kwekkeboom DJ, Manfredi R, Korner M, Kvols L, Pape UF, Ricke J, Goretzki PE, Wildi S, Steinmuller T, Oberg K, Scoazec JY Frascati Consensus Conference, European Neuroendocrine Tumor Society. Well-diffentiated pancreatic non functioning tumors/carcinoma. Neuroendocrinology. 2006;84:196–211. doi: 10.1159/000098012. [DOI] [PubMed] [Google Scholar]
- 18.Shi W, Johnston CF, Buchanan KD, Ferguson WR, Laird JD, Crothers JG, McIlrath EM. Localization of neuroendocrine tumours with [In-111] DTPA-octreotide scintigraphy (Octreoscan): a comparative study with CT and MR imaging. QJM-Int J Med. 1998;91:295–301. doi: 10.1093/qjmed/91.4.295. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosini V, Campana D, Tomassetti P, Fanti S. S 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur J Nucl Med Mol Imaging. 2012;39:S52–60. doi: 10.1007/s00259-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 20.Yates CJ, Newey PJ, Thakker RV. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 2015;3:895–905. doi: 10.1016/S2213-8587(15)00043-1. [DOI] [PubMed] [Google Scholar]
- 21.Cadiot G, Vuagnat A, Doukhan I, Murat A, Bonnaud G, Delemer B, Thiefin G, Beckers A, Veyrac M, Proye C, Ruszniewski P, Mignon M. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Gastroenterology. 1999;116:286–293. doi: 10.1016/s0016-5085(99)70124-1. [DOI] [PubMed] [Google Scholar]
- 22.Wiedenmann B, Jensen RT, Mignon M, Modlin CI, Skogseid B, Doherty G, Oberg K. Preoperative diagnosis and surgical management of neuroendocrine gastroenteropancreatic tumors: general recommendations by a consensus workshop. World J Surg. 1998;22:309–318. doi: 10.1007/s002689900387. [DOI] [PubMed] [Google Scholar]
- 23.Lowney JK, Frisella MM, Lairmore TC, Doherty GM. Pancreatic islet cell tumor metastasis in multiple endocrine neoplasia type 1: correlation with primary tumor size. Surgery. 1998;124:1043–1048. doi: 10.1067/msy.1998.92561. [DOI] [PubMed] [Google Scholar]
- 24.Marx SJ. Multiple endocrine neoplasia type 1. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. edn 8. McGraw-Hill; New York: 2001. pp. 943–966. (2001) [Google Scholar]
- 25.Skogseid B, Oberg K, Eriksson B, Juhlin C, Granberg D, Akerstrom G, Rastad J. Surgery for asymptomatic pancreatic lesion in multiple endocrine neoplasia type I. World J Surg. 1996;20:872–877. doi: 10.1007/s002689900133. [DOI] [PubMed] [Google Scholar]
- 26.Tonelli F, Fratini G, Nesi G, Tommasi MS, Batignani G, Falchetti A, Brandi ML. Pancreatectomy in multiple endocrine neoplasia type 1-related gastrinomas and pancreatic endocrine neoplasias. Ann Surg. 2006;244:61–70. doi: 10.1097/01.sla.0000218073.77254.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DBJ. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 28.Partelli S, Tamburrino D, Lopez C, Albers M, Milanetto AC, Pasquali C, Manzoni M, Toumpanakis C, Fusai G, Bartsch D, Falconi M. Active Surveillance versus Surgery of Nonfunctioning Pancreatic Neuroendocrine Neoplasms ≤2 cm in MEN1 Patients. Neuroendocrinology. 2016;103:779–786. doi: 10.1159/000443613. [DOI] [PubMed] [Google Scholar]
- 29.Kulke MH. Neuroendocrine tumors: is there a standard treatment? Gastrointest Cancer Res. 2008;2:152–153. [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson B, Kloppel G, Krenning E, Ahlman H, Plockinger U, Wiedenmann B, Arnold R, Auernhammer C, Korner M, Rindi G, Wildi S Frascati Consensus Conference participants. Consensus guidelines for the management of patients with digestive neuroendocrine tumors-well-differentiated jejuna-ileal tumor/carcinoma. Neuroendocrinology. 2008;87:8–19. doi: 10.1159/000111034. [DOI] [PubMed] [Google Scholar]
- 31.Di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L, Zilembo N, Di Leo A. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;72:402–408. doi: 10.1002/(SICI)1097-0142(19960115)77:2<402::AID-CNCR25>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 33.Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Blaker M, Harder J, Arnold C, Gress T, Arnold R PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study. Group J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 34.Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Muller HH, Arnold R PROMID Study Group. Placebo Controlled, Double Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results on Long Term Survival. Neuroendocrinology. 2016 doi: 10.1159/000443612. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedlačkova E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P. CLARINET Investigators: Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 36.Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedlačkova E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Gomez-Panzani E, Ruszniewski P. CLARINET Investigators: Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23:191–199. doi: 10.1530/ERC-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess JR, Greenaway TM, Parameswaran V, Shepherd JJ. Octreotide improves biochemical, radiologic, and symptomatic indices of gastroenteropancreatic neoplasia in patients with multiple endocrine neoplasia type 1 (MEN-1) Implications for an integrated model of MEN-1 tumorigenesis. Cancer. 1999;86:2154–2159. [PubMed] [Google Scholar]
- 38.Ramundo V, Del Prete M, Marotta V, Marciello F, Camera L, Napolitano V, De Luca L, Circelli L, Colantuoni V, Di Sarno A, Carratu AC, de Luca di Roseto C, Colao A, Faggiano A Multidisciplinary Group for Neuroendocrine Tumors of Naples. Impact of long-acting octreotide in patients with early-stage MEN1-related duodeno-pancreatic neuroendocrine tumours. Clin Endocrinol. 2014;80:850–855. doi: 10.1111/cen.12411. [DOI] [PubMed] [Google Scholar]
- 39.Lamberts SWJ, Vanderlely AJ, de Herder WW, Hofland LJ. Octreotide New Engl J Med. 1996:334246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 40.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline. Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Florio T. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front Biosci. 2008;13:822–840. doi: 10.2741/2722. [DOI] [PubMed] [Google Scholar]
- 42.Reubi JC, Schonbrunn A. Illuminating somatostatin analog action at neuroendocrine tumor receptors. Trends Pharmacol Sci. 2013;34:676–688. doi: 10.1016/j.tips.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machens A, Schaaf L, Karges W, Frank-Raue K, Bartsch DK, Rothmund M, Schneyer U, Goretzki P, Raue F, Dralle H. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol. 2007;67:613–622. doi: 10.1111/j.1365-2265.2007.02934.x. [DOI] [PubMed] [Google Scholar]
- 44.Lairmore TC, Piersall LD, DeBenedetti MK, Dilley WG, Mutch MG, Whelan AJ, Zehnbauer B. Clinical genetic testing and early surgical intervention in patients with multiple endocrine neoplasia type 1 (MEN 1) Ann Surg. 2004;239:637–645. doi: 10.1097/01.sla.0000124383.98416.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]