Summary
ROS are highly reactive molecules which consist of a number of diverse chemical species, including radical and non-radical oxygen species. Oxidative stress occurs as a result of an overproduction of ROS not balanced by an adequate level of antioxidants. The natural antioxidants are: thiol compounds among which GSH is the most representative, and non-thiol compounds such as polyphenols, vitamins and also various enzymes.
Many diseases have been linked to oxidative stress including bone diseases among which one of the most important is the osteoporosis. The redox state changes are also related to the bone remodeling process which allows the continuous bone regeneration through the coordinated action of bone cells: osteoclasts, osteoblasts and osteocytes. Changes in ROS and/or antioxidant systems seem to be involved in the pathogenesis of bone loss. ROS induce the apoptosis of osteoblasts and osteocytes, and this favours osteoclastogenesis and inhibits the mineralization and osteogenesis. Excessive osteocyte apoptosis correlates with oxidative stress causing an imbalance in favor of osteoclastogenesis which leads to increased turnover of bone remodeling and bone loss. Antioxidants either directly or by counteracting the action of oxidants contribute to activate the differentiation of osteoblasts, mineralization process and the reduction of osteoclast activity. In fact, a marked decrease in plasma antioxidants was found in aged or osteoporotic women. Some evidence shows a link among nutrients, antioxidant intake and bone health. Recent data demonstrate the antioxidant properties of various nutrients and their influence on bone metabolism. Polyphenols and anthocyanins are the most abundant antioxidants in the diet, and nutritional approaches to antioxidant strategies, in animals or selected groups of patients with osteoporosis or inflammatory bone diseases, suggest the antioxidant use in anti-resorptive therapies for the treatment and prevention of bone loss.
Keywords: oxidative stress, antioxidants, bone remodeling, osteoporosis, antioxidant nutrient treatment
Introduction
The physiological intracellular redox state depends on the ratio between the levels of pro-oxidants, oxidizing agents (reactive oxygen species, ROS) and antioxidants (1, 2). ROS are highly reactive molecules, which consist of a number of diverse chemical species, including radical and non-radical oxygen species such as superoxide anion (O2−), hydroxyl radical (OH−) and hydrogen peroxide (H2O2). O2−, considered as the ‘primary’ ROS, can further interact with other molecules in order to generate ‘secondary’ ROS which are more aggressive, and they act either directly or prevalently through enzyme or metal-catalysed processes. ROS are produced during normal metabolism following the activation of various enzymes such as NADPH oxidase (membrane enzyme), superoxide dismutase (cytoplasmic enzyme) and various mitochondrial oxidases (3, 4). Indeed, a controlled increase of ROS level and in particular of H2O2 may have an important role in the transmission of intracellular signaling which regulate many fundamental cellular processes such as proliferation, differentiation, apoptosis, repair processes and inflammation (5, 6).
The natural antioxidants are: thiol compounds among which the most important and represented in animals is the glutathione (GSH, γ-glutamyl-cysteinyl-glycine), non-thiol compounds such as polyphenols, predominantly contained in various plants, vitamins such as ascorbic acid, alfatocopherol and vitamin A, as well as various enzymes capable to eliminate ROS such as catalase, and enzymes that use GSH as substrate (GSH-reductase, GSH-peroxidase etc.) (3). GSH, present in concentrations of 2–10 mM within cells, is the primary determinant of the cellular redox environment (7) and exists mainly as the biologically active reduced-thiol form. The oxidation of GSH to GSSG and subsequent decrease in the GSH/GSSG ratio is often associated with oxidative stress. Thus, the GSH/GSSG ratio is a simple and useful indicator of cellular redox state (8, 9). De novo GSH synthesis, GSSG reduction, and exogenous GSH uptake are crucial in the maintenance of cellular redox homeostasis, and GSH seems to be involved in signaling pathways being able to regulate the activity of transcription factors and protein through reactions of glutathionilation (2, 10, 11). Other thiol antioxidants are: Thioredoxin, Glutaredoxin, Cysteine (Cys) and the reduced form of Lipoic Acid (LA), dihydrolipoic acid (DHLA): the first two have catalitic-redox-active cysteines and catalize the reduction of protein mixed disulfides (3). Cys is the most abundant low-molecular thiols in extracellular fluids with concentrations ranging from 40 μM to 8–10 μM (12), and DHLA contains two thiol groups and is produced in almost every cell in small amounts. In vivo Cys and DHLA act directly on ROS and RNS (Reactive Nitrogen Species) as scavengers and by regeneration of other antioxidants such as vitamin C, E and GSH (13–15).
The physiological redox state is maintained in equilibrium by various factors and mechanisms that regulate the activity of ROS-producing enzymes and antioxidants. Oxidative stress occurs as a result of an overproduction of ROS not balanced by adequate levels of antioxidants (1, 2). This can be determined by both physiological events, such as aging and hormonal changes (decrease of estrogen) (16–19) and pathological events related to the production of inflammatory cytokines involved in many pathological processes, exogenous and endogenous toxins, radiation exposure and drug therapies (2, 6, 20).
Oxidative stress generates a cellular damage due to lipid oxidation, structural alteration of the membranes, oxidation of proteins and nucleic acids; the damage may extend to the organs and become systemic (21). Many diseases have been linked to oxidative stress including bone diseases among which one of the most important is the osteoporosis. Oxidative stress in postmenopausal osteoporosis, due to estrogen deficiency, has been related to the activation of NADPH oxidase and/or decreased synthesis of antioxidant enzymes and GSH levels (17, 18, 22, 23). Oxidative stress, in the elderly osteoporosis and in the secondary osteoporosis due to intestinal chronic diseases (IBD), is due to decrease of GSH levels and defensive antioxidant abilities (19, 20, 24) related also to reduced intestinal absorption of antioxidants contained in food. In osteoporosis secondary to bone inflammatory processes and prolonged therapy with steroidal anti-inflammatory drugs, oxidative stress is mainly due to the activation of enzymes which produce ROS (25, 26).
Oxidative stress in bone remodeling
The redox state changes are also related to the bone remodeling process which allows the continuous bone regeneration (25, 27). In fact, bone is a dynamic tissue that continuously renews itself throughout life by the coordinated action of three major types of bone cells: osteoclasts, osteoblasts and osteocytes (28, 29). The remodeling process is the result of interactions between these cells and multiple molecular agents, including hormones, growth factors and cytokines. It is a physiological process that follows a time sequence lasting approximately six months wherein osteoclasts eliminate old or damaged bone tissue which is subsequently replaced with new bone tissue formed by osteoblasts, while the osteocytes function in the transduction of signals necessary to sustain mechanical loads. Recently, new data support the central regulatory role of osteocytes in bone remodeling and thus in viability and functionality of bone, maintaining normal levels of mineralization and repairing microdamage and microfractures (30–32). Healthy bone is tightly regulated and maintained in order to prevent significant alterations in bone mass or mechanical strength after each remodeling cycle (29).
Indeed, oxidative stress alters bone remodeling process causing an unbalance between osteoclast and osteoblast activity, this can lead to metabolic bone diseases and contribute to the pathogenesis of skeletal system disorders including osteoporosis characterized by low bone mineral density and decrease in bone mass, which makes the bone weak and more prone to fracture (18, 33–36). Recent evidences in a limited number of clinical studies have shown that ROS and/or antioxidant systems can be involved in the pathogenesis of bone loss (36–39).
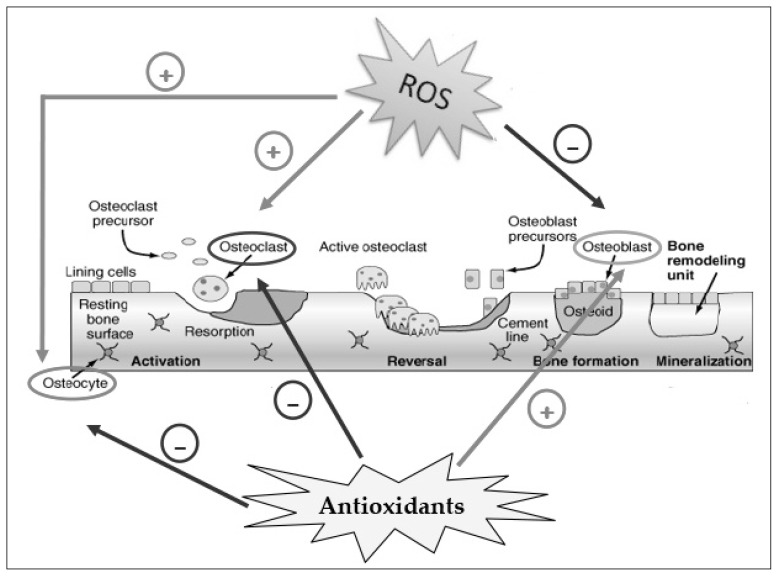
In fact, oxidative stress activates the differentiation of pre-osteoclasts in osteoclasts and strengthens the bone resorption (18, 40) (Figure 1). A significant increase in the number and activity of osteoclasts as well as in the tartrate-resistant acid phosphatase level was observed when H2O2 was added to cultures of human marrow mononuclear cells (36).
Figure 1.
Effect of ROS and antioxidants on the activity of osteoclasts, osteoblasts and osteocytes in bone remodeling. ROS activate osteoclast differentiation and osteocyte apoptosis (+), while inhibit osteoblast activity (−) inducing bone resorption; antioxidants activate osteoblast differentiation (+) and inhibit osteoclast activity and osteocyte apoptosis (−) inducing bone formation.
ROS induce the apoptosis of osteoblasts and osteocytes, cells localized in the bone matrix and derived from mature osteoblasts, thus favouring osteoclastogenesis (19, 30, 31, 41, 42) (Figure 1). In fact, ROS elicit a spectrum of responses ranging from proliferation, growth, differentiation arrest to cell death, by activating numerous signalling pathways. Indeed, mitogen-activated protein kinase (MAPKs) such as extracellular signal-regulated kinases (ERK1/2), c-Jun-N terminal kinase (JNK) and p38 MAPK are involved in osteoblast or osteocytes apoptosis (15, 43, 44). High levels of ROS block and reduce the osteoblast activity and differentiation, therefore the mineralization and osteogenesis (8, 45, 46) (Figure 1). These events increase bone remodeling turnover with consequent alteration and decrease in bone mass. Antioxidants have opposing effects, they contribute to the differentiation of osteoblasts and bone formation (8, 15, 34, 47), maintaining vital osteocytes which contribute to osteoblast activity and osteogenesis, while reduce the osteoclast differentiation and their activity (Figure 1).
There are several factors mainly produced by osteoblasts and osteocytes that regulate osteoclast and osteoblast activity and then bone remodeling, among these the most important are: the ligand of receptor activator of NF-kB (RANKL) and osteoprotegerin (OPG). Their expression is sensitive to increased oxidative status, that induces RANKL up-regulation and OPG down-regulation through the activation of protein kinases (ERK1/2, JNK etc.) and/or other factors which affect specific transcription factors (8, 15, 27). RANKL activates the differentiation and activity of osteoclasts by interacting with specific receptors in pre-ostoeclasts and mediates osteoclastogenesis and bone resorption; while OPG, produced by the activation of the signaling pathway Wnt/βcatenin, is a soluble receptor capable of binding and blocking RANKL, resulting in inhibition of osteoclast activity (30–32, 42, 48) The oxidative stress blocks the activation of osteoblasts and thus the production of OPG; under this condition, the action of RANKL prevails, and the differentiation and activity of osteoclasts are induced. Thereafter, the turnover of the bone remodeling process increases and this is indicated by an increase in RANKL/OPG ratio, that is, in fact, an index of the intensity of bone resorption (18, 49). The regulation of the RANKL/OPG ratio levels is responsible for the maintenance of balance between bone resorption and formation. Increased levels of this ratio are correlated to increased turnover of bone remodeling due to increased resorption rate without adequate and proper bone formation; this event has been related to the pathogenesis of various skeletal diseases, including various form of osteoporosis and bone diseases secondary to inflammation (31, 32, 48). The expression of RANKL and OPG is regulated by various hormones and cytokines, but a fundamental event in the initiation and regulation of the bone formation and remodeling is the apoptosis of osteoblasts and osteocytes (27, 32).
Experimental data show that excessive apoptosis of osteocytes is correlated to an increased oxidative status causing an imbalance in favor of osteoclastogenesis (15, 50–52). There are few data on the molecular mechanisms that regulate these processes, but many studies are currently focused on the regulatory activities of the osteocytes. These cells constitute 90% of the bone cell population and are embedded in the bone matrix. They have a morphology similar to neuronal cells with a central body and dendritic extensions thanks to which they communicate with each other, with other bone cells, with blood capillaries and nerve endings. They are mechanosensory cells (30, 41) and under physiological conditions, following a microdamage or other physical and hormonal signals, such as estrogen deficiency, mature osteocytes undergo apoptosis, and some data show that this is related to oxidative stress (42, 50, 51). Indeed, apoptotic osteocytes induce ligning cells to retract from the bone surface to form a suitable environment for the recruitment and activation of mature osteoclasts through formation and release of RANKL (32, 53). They also produce high levels of sclerostin and DKK1 which block OPG synthesis and release by Wnt/βcatenin pathway inhibition both in osteocytes and osteoblasts (30–32, 42). This event increases RANKL/OPG ratio promoting osteoclast activity, osteoblast apoptosis and bone degradation. Indeed, a microfracture can determine the breaking of the dendritic filaments and therefore the connections with other cells and blood vessels. This causes a deficient intake of O2, nutrients, hormones and factors essential for their viability inducing metabolic alterations, oxidative stress and osteocyte apoptosis which initiates the remodeling process and bone resorption (34, 41, 42, 52, 54).
Under physiological conditions, after the phase of bone resorption, in response to factors released from the bone matrix, the recruitment of osteoblast precursors and their differentiation into mature, bone-synthesizing cells occur. However, an excessive oxidative stress induces an abnormal apoptosis of osteocytes resulting in an imbalance of the remodeling process with consequent altered and deficient bone formation, as occurs in aging, glucocorticoid treatment, osteoporosis and other bone diseases related to oxidative stress (17, 26, 31, 55–57). Differently, vital osteocytes produce high levels of OPG and this contributes to the differentiation of osteoblasts and mineralization process (32, 50).
Antioxidants in bone remodeling and in bone loss
In vivo and in vitro data have shown that thiol and non-thiol antioxidants, directly and/or counteracting the effect of oxidants contribute to activation of osteoblast differentiation, mineralization process and reduction of osteoclast activity. All these antioxidants act as direct scavengers of ROS, but they also maintain high levels of GSH, in fact, together with GSH-reductase they contribute to the elimination of GSSG formed in the reduction reactions, maintaining normal levels of GSH/GSSG and the intracellular redox state (8, 14, 47). Some studies relate antioxidants to bone metabolism, in fact a marked decrease in plasma antioxidants was found in aged or osteoporotic rats and in aged or osteoporotic women (17, 19, 38). The loss of antioxidant leads to accelerated bone loss through the activation of a tumour necrosis factor alpha (TNFα)-dependent signalling pathway (18), and the administration of antioxidants such as vitamin C, E, N-acetyl-cysteine (NAC) and LA, has beneficial effects in individuals with osteoporosis (56–59). Administration of NAC or ascorbate in ovariectomized mice abolishes ovariectomy-induced bone loss, while l-buthionine-(S,R)-sulphoximine (BSO), a specific inhibitor of glutathione synthesis, causes substantial bone loss (49). LA has also beneficial effects in the maintenance of a healthy bone structure in rat ovariectomy and inflammation-mediated osteoporosis (60). Other data demonstrate that the administration of vitamin E is able to maintain bone mineral density in elderly men (39), and it promotes healing of osteoporotic fracture in ovariectomized rats inducing the bone regeneration (61).
However, few studies have been performed on the direct action of antioxidants on bone cell activity. As regards thiol antioxidants some data have been obtained by using NAC, GSH and LA. NAC, a cysteine analogue drug with many therapeutic applications, has a protective role in controlling oxidative stress against many cells including osteoblasts, and stimulates osteoblastic differentiation of mouse calvarial cells (8, 14, 47, 62). Other studies report that NAC inhibits oxidative stress induced apoptosis of osteoblastic cells, and this is mediated by GSH (63, 64), moreover, NAC prevents osteoclast formation, NF-κB activation and TNF-α expression involved in osteoclast activation (49). Indeed, GSH is involved in osteoblast and osteoclast differentiation as well as in osteoporosis and other bone diseases (14, 17, 65).
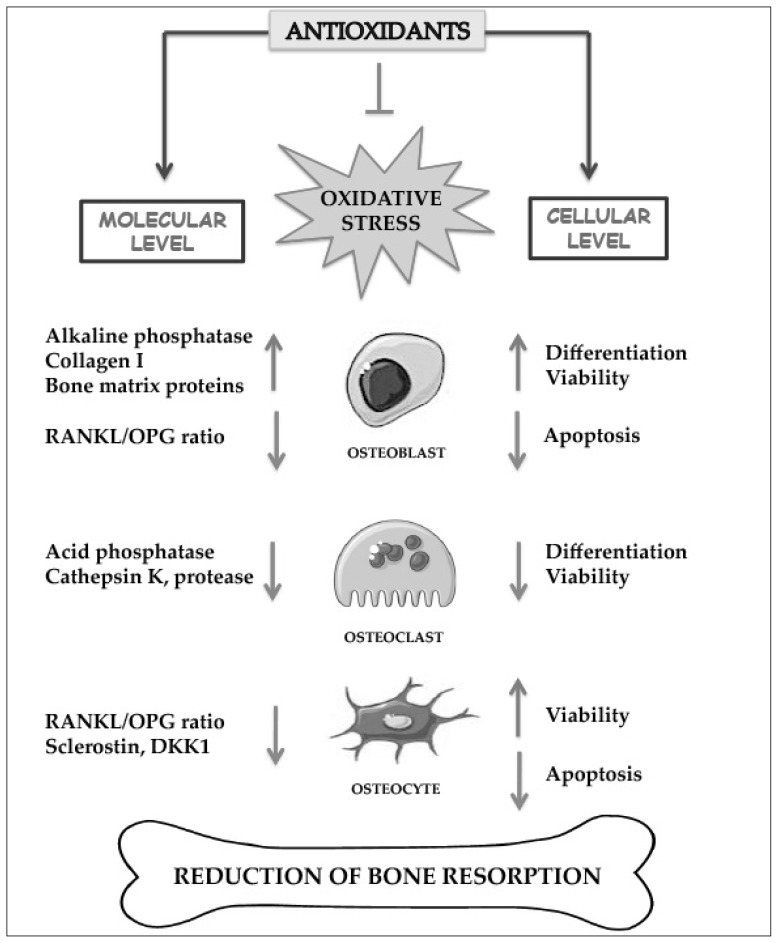
Data reported in human osteoblastic-like SaOS-2 cells, which represent a valuable model system for studying osteoblast functions and mineralization process, demonstrate that GSH and NAC increase alkaline phosphatase (ALP) activity and other osteogenic markers, such as RUNX-2 and osteocalcin, during the differentiation of these cells in mature osteoblasts (8). These antioxidants are able to restore osteoblastic mineralization due to oxidative stress induced both by GSH depletion, obtained by BSO (8) and by H2O2 treatment in bone marrow stromal cells isolated from rat femur (62). LA is also able to inhibit TNFα induced apoptosis of human bone marrow stromal cells in which JNK and NFkB are involved (66). It has also been demonstrated that high levels of GSH/GSSG ratio are important for osteoblast differentiation and mineralization process (8). Indeed, GSH redox state has been reported to play an important role in the differentiation and phenotype expression of some cell types including osteoblasts and osteoclasts (67). Moreover, GSH and NAC in SaOS-2 cells decrease RANKL/OPG levels, while, increase calcium levels and mineralization process (8). Therefore, these antioxidants promote bone formation and have an anti-osteoclastogenic action. LA also suppresses osteoclastogenesis in hBMSC by directly inhibiting RANKL-RANK mediated signals (13). Recent data have demonstrated the ability of GSH, NAC and LA to prevent osteocyte apoptosis and the increase of sclerostin and RANKL/OPG ratio levels induced by oxidative stress (15). This study is performed in a murine osteocyte-like cell line, MLO-Y4, which has similar phenotype and many characteristics of mature osteocytes and constitute a model to study osteocyte viability and apoptosis in response to microdamage and bone diseases (41, 52). This study was accomplished by inducing oxidative stress via starvation that, in part, mimics what happens following a microdamage at the level of osteocytes (34, 41, 42, 52, 54). Antioxidants and ROS mediate starvation-induced apoptosis and OPG expression by JNK signalling; whereas they regulate RANKL and sclerostin expression by both JNK and ERK1/2 activity (15). Moreover, it has been demonstrated that catalase, used as antioxidant, is able to inhibit the activation of TRAP-positive multinucleated giant cells induced by H2O2 treatment in cultures of primary human marrow cells (36). Other antioxidants inhibit TRAP, k-catepsin and protease activity in osteoclast cell lines (68, 69). In Figure 2 the effects of antioxidants against the action of oxidative stress at the molecular and cellular level are summarized. At the molecular level they prevent RANKL and sclerostin increase and OPG decrease, inhibiting the enhancement of the RANKL/OPG ratio in osteoblasts and osteocytes. Moreover, they inhibit the increase of bone acid phosphatase and protease activity which degrade the bone matrix in osteoclasts and induce alkaline phosphatase and matrix protein synthesis in osteoblasts. At the cellular level they counteract the excessive apoptosis of osteoblasts and osteocytes and reduce the differentiation and activity of osteoclasts.
Figure 2.
Effect of antioxidants on bone cells at molecular and cellular level in bone resorption.
Antioxidants inhibit and prevent oxidative stress and affect various enzymes, proteins and cytokines involved in bone remodeling.
Role of nutrients containing antioxidants in treatment and prevention of bone loss
Given the important role of ROS and oxidative stress in bone turnover, there is a considerable interest in the use of antioxidants in potential treatments for osteoporosis and bone inflammatory diseases. Different experimental protocols have been studied using either pharmacological or nutritional approaches. As regards nutritional approaches, epidemiological studies have provided evidence of a link between nutrient, antioxidant intake and bone health, and have led to investigations of the antioxidant properties of nutrients and their influence on bone metabolism.
Polyphenols and anthocyanins are the most abundant antioxidants in the diet and are widespread constituents of fruits, vegetables, cereals, dry legumes, chocolate, tea, coffee and wine. Experimental studies in animals or cultured cell lines have supported roles for polyphenols in the prevention of cardiovascular diseases, cancer, neurodegenerative diseases, diabetes or osteoporosis (70). Recent data demonstrate that nutritional approaches to antioxidant strategies in bone cells and/or in animals or selected groups of patients with osteoporosis or inflammatory bone diseases, could be useful for the treatment and prevention of bone loss. In particular, as regards resveratrol, this increases bone mineral density and bone ALP in osteoporotic obese man (71), and represents an effective therapeutic agent in eliminating oxidative stress and in preventing bone loss in ovariectomized and old rats (72). Similarly, dietary supplementation with green tea or Hypericum perforatum or blueberry, containing various types of polyphenols, attenuates trabecular bone loss, prevents loss of collagen in bone matrix, inhibits senescence pathways in osteoblastic cells and prevents osteoporosis in ovariectomized rats (73–76). Tea drinking is also associated with beneficial effects in maintaining bone density in old women and in menopausal women (77, 78). Other data show that green tea polyphenols mitigate bone loss of female rats with chronic inflammation (79), and blueberry extracts have protective effects in acute inflammation and collagen-induced arthritis in the rats (80). Interesting is the protective effect of dietary supplementation of Hypericum perforatum against the oxidative stress and the bone mass loss obtained in rats subjected to forced swimming, in this case it has been shown how antioxidants can induce beneficial effects when oxidative stress and active bone resorption is caused by excessive physical activity (81). Finally, it has been demonstrated in young rats that blueberry phenolic component promotes bone growth activating canonical Want signalling, and diets enriched with blueberries increase bone density mass through suppression of RANKL in stromal cells (82, 83). Indeed, it is note that accumulation of bone mineral during childhood and adolescence is a determining factor for the risk of osteoporosis in aging and in post-menopausal period (84, 85), and various data indicate that daily consumption of fruits or vegetables may be important in increasing the bone mass peak (86, 87).
Conclusions
In this review, it has been shown that changes in intracellular redox state occur during the physiological bone remodeling and that oxidative stress induces important alterations of the differentiation process and activity of bone cells including also the osteocytes. It is highlighted the regulatory role of osteocytes in bone remodeling process and that oxidative stress, related to many diseases including osteoporosis, promotes osteoclast resorption and bone loss through an excessive apoptosis of osteocytes. This is due to RANK signalling activation by increasing RANKL expression with the consequent increase of RANKL/OPG ratio and inhibition of osteoblastic activation and mineralization process. Many in vitro and in vivo experiments demonstrate that these events are regulated by redox-sensitive signalling pathways in which are involved MAPKs, β-catenin and NF-kB activity. On the contrary, antioxidants have an important role in maintaining a normal bone remodeling process and protecting bone health; in fact, they prevent and/or reduce inflammatory state and bone loss by inhibiting osteocyte apoptosis and mitigating osteoclast activity, consequently, they increase osteoblast activity and induce osteogenesis. Clinical studies show a positive correlation between low levels of antioxidants and bone loss, and this is also related to an increase of markers of bone resorption. Among the biological antioxidants the most important is GSH and different antioxidants act as direct ROS scavengers but also maintaining high levels of intracellular GSH. Moreover, various studies demonstrate also the important role of nutrient antioxidants in bone health, both in young people, in order to favour the formation of optimal peak bone mass, and in the elderly and in menopausal women in order to prevent bone loss, often associated to bone fracture, morbidity and mortality. Indeed, it has been proposed antioxidant use in anti-resorption therapies considering also that they are able to reduce the activity of osteoclasts without determining their destruction which can be important when it is need not only to reduce bone resorption but also to restore physiological bone remodeling. In fact, the anti-resorptive drugs, which are very powerful drugs, currently in use bisphosphonates and the antibody to RANKL, denosumab, block resorption factors, but at the same time reduce the vitality of osteoclasts and promote their apoptosis, breaking the two-way communication between these and osteoblasts, and enabling the restoration of a normal remodeling process (88). Thereafter, it could be interesting to design new therapeutic approaches which include antioxidant treatments for bone diseases related to oxidative stress and bone loss. Indeed, these could act on the redox balance in bone cells and on redox regulated factors and processes involved in bone turnover. However, further studies are needed to clarify the cellular and molecular mechanisms underlying the relationship among oxidative stress, antioxidants and bone metabolism.
Footnotes
Disclosure
All Authors declare that they have no conflicts of interest.
References
- 1.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Mochel NS, Seronello S, Wang SH, Ito C, Zheng JX, Liang TJ, Lambeth JD, Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catarzi S, Romagnoli C, Marcucci G, Favilli F, Iantomasi T, Vincenzini MT. Redox regulation of ERK1/2 activation induced by sphingosine 1-phosphate in fibroblasts: involvement of NADPH oxidase and platelet-derived growth factor receptor. Biochim Biophys Acta. 2011;1810:446–456. doi: 10.1016/j.bbagen.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romagnoli C, Marcucci G, Favilli F, Zonefrati R, Mavilia C, Galli G, Tanini A, Iantomasi T, Brandi ML, Vincenzini MT. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013;280:867–879. doi: 10.1111/febs.12075. [DOI] [PubMed] [Google Scholar]
- 9.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 10.Romagnoli C, Marcucci T, Picariello L, Tonelli F, Vincenzini MT, Iantomasi T. Role of N-acetylcysteine and GSH redox system on total and active MMP-2 in intestinal myofibroblasts of Crohn’s disease patients. Int J Colorectal Dis. 2013;28:915–924. doi: 10.1007/s00384-012-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iantomasi T, Favilli F, Catarzi S, Vincenzini MT. GSH role on platelet-derived growth factor receptor tyrosine phosphorylation induced by H2O2. Biochem Biophys Res Commun. 2001;280:1279–1285. doi: 10.1006/bbrc.2001.4274. [DOI] [PubMed] [Google Scholar]
- 12.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 13.Koh JM, Lee YS, Byun CH, Chang EJ, Kim H, Kim YH, Kim HH, Kim GS. Alpha-lipoic acid suppresses osteoclastogenesis despite increasing the receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio in human bone marrow stromal cells. J Endocrinol. 2005;185:401–413. doi: 10.1677/joe.1.05995. [DOI] [PubMed] [Google Scholar]
- 14.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 15.Fontani F, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: involvement of JNK and ERK1/2 signalling. Calcif Tissue Int. 2015;96:335–346. doi: 10.1007/s00223-015-9961-0. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 17.Sendur OF, Turan Y, Tastaban E, Serter M. Antioxidant status in patients with osteoporosis: a controlled study. Joint Bone Spine. 2009;76:514–8. doi: 10.1016/j.jbspin.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146:728–735. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 19.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR, Kaser A, Pines A, Dotan I. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684–94. doi: 10.1136/gut.2006.117382. [DOI] [PubMed] [Google Scholar]
- 21.Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 22.Bellanti F, Matteo M, Rollo T, De Rosario F, Greco P, Vendemiale G, Serviddio G. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol. 2013;1:340–346. doi: 10.1016/j.redox.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumi D, Hayashi T, Matsui-Hirai H, Jacobs AT, Ignarro LJ, Iguchi A. 17beta-estradiol inhibits NADPH oxidase activity through the regulation of p47phox mRNA and protein expression in THP-1 cells. Biochim Biophys Acta. 2003;1640:113–118. doi: 10.1016/s0167-4889(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 24.Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr Drug Metab. 2007;8:519–25. doi: 10.2174/138920007780866852. [DOI] [PubMed] [Google Scholar]
- 25.Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Bjelaković G, Beninati S, Pavlović D, Kocić G, Jevtović T, Kamenov B, Saranac LJ, Bjelaković B, Stojanović I, Basić J. Glucocorticoids and oxidative stress. J Basic Clin Physiol Pharmacol. 2007;18:115–127. doi: 10.1515/jbcpp.2007.18.2.115. [DOI] [PubMed] [Google Scholar]
- 27.Filaire E, Toumi H. Reactive oxygen species and exercise on bone metabolism: friend or enemy? Joint Bone Spine. 2012;79:341–346. doi: 10.1016/j.jbspin.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab. 2012;97:311–325. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–1033. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- 31.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94:25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46:1550–1555. doi: 10.1515/CCLM.2008.302. [DOI] [PubMed] [Google Scholar]
- 35.Mann V, Huber C, Kogianni G, Collins F, Noble B. The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone. 2007;40:674–684. doi: 10.1016/j.bone.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, Lee KW, Kang MI. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010;87:226–235. doi: 10.1007/s00223-010-9393-9. [DOI] [PubMed] [Google Scholar]
- 37.Yousefzadeh G, Larijani B, Mohammadirad A, Heshmat R, Dehghan G, Rahimi R, Abdollahi M. Determination of oxidative stress status and concentration of TGF-beta 1 in the blood and saliva of osteoporotic subjects. Ann N Y Acad Sci. 2006;1091:142–150. doi: 10.1196/annals.1378.062. [DOI] [PubMed] [Google Scholar]
- 38.Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 39.Ostman B, Michaelsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, Basu S. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radic Biol Med. 2009;47:668–673. doi: 10.1016/j.freeradbiomed.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Huh YJ, Kim JM, Kim H, Song H, So H, Lee SY, Kwon SB, Kim HJ, Kim HH, Lee SH, Choi Y, Chung SC, Jeong DW, Min BM. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2006;(7):1138–1146. doi: 10.1038/sj.cdd.4401793. [DOI] [PubMed] [Google Scholar]
- 41.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2013;54:264–271. doi: 10.1016/j.bone.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 44.Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB. Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem. 2012;287:978–988. doi: 10.1074/jbc.M111.294959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 46.Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39–46. doi: 10.1007/s10565-006-0018-z. [DOI] [PubMed] [Google Scholar]
- 47.Jun JH, Lee SH, Kwak HB, Lee ZH, Seo SB, Woo KM, Ryoo HM, Kim GS, Baek JH. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J Cell Biochem. 2008;103:1246–1255. doi: 10.1002/jcb.21508. [DOI] [PubMed] [Google Scholar]
- 48.Mulcahy LE, Taylor D, Lee TC, Duffy GP. RANKL and OPG activity is regulated by injury size in networks of osteocyte-like cells. Bone. 2011;48:182–188. doi: 10.1016/j.bone.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rochefort GY, Pallu S, Benhamou CL. Osteocyte: the unrecognized side of bone tissue. Osteoporos Int. 2010;21:1457–1469. doi: 10.1007/s00198-010-1194-5. [DOI] [PubMed] [Google Scholar]
- 51.Noble B. Microdamage and apoptosis. Eur J Morphol. 2005;42:91–8. doi: 10.1080/09243860500096248. [DOI] [PubMed] [Google Scholar]
- 52.Al-Dujaili SA, Lau E, Al-Dujaili H, Tsang K, Guenther A, You L. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J Cell Biochem. 2011;112:2412–2423. doi: 10.1002/jcb.23164. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014;64:132–137. doi: 10.1016/j.bone.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tami AE, Nasser P, Verborgt O, Schaffler MB, Knothe Tate ML. The role of interstitial fluid flow in the remodeling response to fatigue loading. J Bone Miner Res. 2002;17:2030–2037. doi: 10.1359/jbmr.2002.17.11.2030. [DOI] [PubMed] [Google Scholar]
- 55.Kikuyama A, Fukuda K, Mori S, Okada M, Yamaguchi H, Hamanishi C. Hydrogen peroxide induces apoptosis of osteocytes: involvement of calcium ion and caspase activity. Calcif Tissue Int. 2002;71:243–248. doi: 10.1007/s00223-001-1110-2. [DOI] [PubMed] [Google Scholar]
- 56.Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63:183–189. doi: 10.1007/s002239900512. [DOI] [PubMed] [Google Scholar]
- 57.Sanders KM, Kotowicz MA, Nicholson GC. Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early postmenopausal women: a pilot study. Transl Res. 2007;150:215. doi: 10.1016/j.trsl.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Morton DJ, Barrett-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. J Bone Miner Res. 2001;16:135–140. doi: 10.1359/jbmr.2001.16.1.135. [DOI] [PubMed] [Google Scholar]
- 59.Mainini G, Rotondi M, Di Nola K, Pezzella MT, Iervolino SA, Seguino E, D’Eufemia D, Iannicelli I, Torella M. Oral supplementation with antioxidant agents containing alpha lipoic acid: effects on postmenopausal bone mass. Clin Exp Obstet Gynecol. 2012;39:489–493. [PubMed] [Google Scholar]
- 60.Polat B, Halici Z, Cadirci E, Albayrak A, Karakus E, Bayir Y, Bilen H, Sahin A, Yuksel TN. The effect of alpha-lipoic acid in ovariectomy and inflammation-mediated osteoporosis on the skeletal status of rat bone. Eur J Pharmacol. 2013;718:469–474. doi: 10.1016/j.ejphar.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 61.Shuid AN, Mohamad S, Muhammad N, Fadzilah FM, Mokhtar SA, Mohamed N, Soelaiman IN. Effects of α-tocopherol on the early phase of osteoporotic fracture healing. J Orthop Res. 2011;29:1732–1738. doi: 10.1002/jor.21452. [DOI] [PubMed] [Google Scholar]
- 62.Ueno T, Yamada M, Igarashi Y, Ogawa T. N-acetyl cysteine protects osteoblastic function from oxidative stress. J Biomed Mater Res A. 2011;99:523–531. doi: 10.1002/jbm.a.33211. [DOI] [PubMed] [Google Scholar]
- 63.Tsukimura N, Yamada M, Aita H, Hori N, Yoshino F, Chang-Il Lee M, Kimoto K, Jewett A, Ogawa T. N-acetyl cysteine (NAC)-mediated detoxification and functionalization of poly(methyl methacrylate) bone cement. Biomaterials. 2009;30:3378–3389. doi: 10.1016/j.biomaterials.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 64.Yamada M, Ueno T, Minamikawa H, Sato N, Iwasa F, Hori N, Ogawa T. N-acetyl cysteine alleviates cytotoxicity of bone substitute. J Dent Res. 2010;89:411–416. doi: 10.1177/0022034510363243. [DOI] [PubMed] [Google Scholar]
- 65.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byun CH, Koh JM, Kim DK, Park SI, Lee KU, Kim GS. Alpha-lipoic acid inhibits TNF-alpha-induced apoptosis in human bone marrow stromal cells. J Bone Miner Res. 2005;20:1125–1135. doi: 10.1359/JBMR.050302. [DOI] [PubMed] [Google Scholar]
- 67.Kim JM, Kim H, Kwon SB, Lee SY, Chung SC, Jeong DW, Min BM. Intracellular glutathione status regulates mouse bone marrow monocyte-derived macrophage differentiation and phagocytic activity. Biochem Biophys Res Commun. 2004;325:101–108. doi: 10.1016/j.bbrc.2004.09.220. [DOI] [PubMed] [Google Scholar]
- 68.Lee SH, Kim JK, Jang HD. Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging. Int J Mol Sci. 2014;15:10605–10621. doi: 10.3390/ijms150610605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satue M, Arriero Mdel M, Monjo M, Ramis JM. Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem Pharmacol. 2013;86:1476–1486. doi: 10.1016/j.bcp.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 71.Ornstrup MJ, Harslof T, Kjar TN, Langdahl BL, Pedersen SB. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2014;99:4720–4729. doi: 10.1210/jc.2014-2799. [DOI] [PubMed] [Google Scholar]
- 72.Tou JC. Evaluating resveratrol as a therapeutic bone agent: preclinical evidence from rat models of osteoporosis. Ann N Y Acad Sci. 2015;1348:75–85. doi: 10.1111/nyas.12840. [DOI] [PubMed] [Google Scholar]
- 73.Shen CL, Yeh JK, Cao JJ, Chyu MC, Wang JS. Green tea and bone health: Evidence from laboratory studies. Pharmacol Res. 2011;64(2):155–161. doi: 10.1016/j.phrs.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.You MK, Kim DW, Jeong KS, Bang MA, Kim HS, Rhuy J, Kim HA. St. John’s Wort (Hypericum perforatum) stimulates human osteoblastic MG-63 cell proliferation and attenuates trabecular bone loss induced by ovariectomy. Nutr Res Pract. 2015;9:459–465. doi: 10.4162/nrp.2015.9.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devareddy L, Hooshmand S, Collins JK, Lucas EA, Chai SC, Arjmandi BH. Blueberry prevents bone loss in ovariectomized rat model of post-menopausal osteoporosis. J Nutr Biochem. 2008;19:694–699. doi: 10.1016/j.jnutbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Lazarenko OP, Blackburn ML, Badger TM, Ronis MJ, Chen JR. Blueberry consumption prevents loss of collagen in bone matrix and inhibits senescence pathways in osteoblastic cells. Age (Dordr) 2013;35:807–820. doi: 10.1007/s11357-012-9412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Devine A, Hodgson JM, Dick IM, Prince RL. Tea drinking is associated with benefits on bone density in older women. Am J Clin Nutr. 2007;86:1243–1247. doi: 10.1093/ajcn/86.4.1243. [DOI] [PubMed] [Google Scholar]
- 78.Shen CL, Chyu MC, Wang JS. Tea and bone health: steps forward in translational nutrition. Am J Clin Nutr. 2013;98:1694S–1699S. doi: 10.3945/ajcn.113.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen CL, Yeh JK, Cao JJ, Tatum OL, Dagda RY, Wang JS. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J Nutr Biochem. 2010;21:968–974. doi: 10.1016/j.jnutbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Figueira ME, Oliveira M, Direito R, Rocha J, Alves P, Serra AT, Duarte C, Bronze R, Fernandes A, Brites D, Freitas M, Fernandes E, Sepodes B. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed Pharmacother. 2016;83:1191–1202. doi: 10.1016/j.biopha.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 81.Seferos N, Petrokokkinos L, Kotsiou A, Rallis G, Tesseromatis C. Hypericum perforatum L. treatment restored bone mass changes in swimming stressed rats. Stomatologija. 2016;18:9–13. [PubMed] [Google Scholar]
- 82.Chen JR, Lazarenko OP, Wu X, Kang J, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J Bone Miner Res. 2010;25:2399–2411. doi: 10.1002/jbmr.137. [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Lazarenko OP, Kang J, Blackburn ML, Ronis MJ, Badger TM, Chen JR. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS One. 2013;8:e70438. doi: 10.1371/journal.pone.0070438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gilsanz V, Wren T. Assessment of bone acquisition in childhood and adolescence. Pediatrics. 2007;119:S145–149. doi: 10.1542/peds.2006-2023G. [DOI] [PubMed] [Google Scholar]
- 85.Lanham-New SA. Fruit and vegetables: the unexpected natural answer to the question of osteoporosis prevention? Am J Clin Nutr. 2006;83:1254–1255. doi: 10.1093/ajcn/83.6.1254. [DOI] [PubMed] [Google Scholar]
- 86.Novotny R, Daida YG, Grove JS, Acharya S, Vogt TM, Paperny D. Adolescent dairy consumption and physical activity associated with bone mass. Prev Med. 2004;39:355–360. doi: 10.1016/j.ypmed.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 87.Tylavsky FA, Holliday K, Danish R, Womack C, Norwood J, Carbone L. Fruit and vegetable intakes are an independent predictor of bone size in early pubertal children. Am J Clin Nutr. 2004;79:311–317. doi: 10.1093/ajcn/79.2.311. [DOI] [PubMed] [Google Scholar]
- 88.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]