Summary
Gorham-Stout disease – also called vanishing bone syndrome – is a rare bone disease characterized by a progressive intra-osseous proliferation of non-neoplastic vascular tissue resulting in massive osteolysis. Here, we report two clinical cases of Gorham-Stout disease. Case 1: a 56-year-old woman with 20 years of history of pain and swell in elbows, ankles and wrist. Then she was diagnosed as systemic lupus erythematosus (SLE) with glomerulonephritis type III. After other pathologies were ruled out Gorham-Stout disease was diagnosed. Intravenous zoledronic acid (5 mg) was indicated and after third infusion a progressive improvement of pain, mobility and daily activities were observed. Case 2: a 70-years-old man with a history of pain and limited motion in the left shoulder without X-ray abnormality. Six months later pathological fracture in the left humerus occurred and after ruled out other pathologies Gorham-Stout disease was diagnosed. Intravenous zoledronic acid (5 mg) was indicated and a good response was observed after the first infusion. Nowadays just over 200 cases were reported. Gorham-Stout disease was reported in different bones, at different age presentation and severe physical deformities, disabilities, and life-threatening complications can occur. Two cases of Gorham-Stout disease with good response to zoledronic acid was reported in this article.
Keywords: Gorham-Stout disease, massive osteolysis, zoledronic acid
Introduction
Gorham-Stout disease – also called vanishing bone syndrome – is a rare bone disease characterized by a progressive intra-osseous proliferation of non-neoplastic vascular tissue resulting in massive osteolysis of the adjacent bone. It has been described in the literature under different names. The first case was reported as a rare osteolisys by Jackson in 1838 (1). In 1954 Gorham described two cases of massive osteolysis (2). One year later Gorham and Stout described this entity as a massive osteolysis and its relation to hemangiomatosis. They described the radiological, clinical and histopatollogical characteristics of 24 cases (3). Actually there are just over 200 cases described in the literature. Gorham-Stout disease is progressive in most cases; however it can be self-limiting. It can affect hands, feet, humerus, clavicles, ribs, sternum, pelvis, femurs, shoulders and scapula, but can affect other sites as mandible, maxillofacial skeleton and spine (3, 4). Clinical manifestations depend on the affected site. Some patients show pain and swelling, limitation of motion and progressive weakness in the involved limb. The diagnostic involved clinical, radiologic and histologic features after ruled out inflammatory, infectious, neoplastic and endocrinological diseases.
Here, we report two cases of Gorham-Stout disease. Informed consent was obtained from both patients reported in this article.
Case 1
We report a 56-year-old woman with 20 years of history of pain and swell in elbows, ankles and wrist. These lesions were erroneously interpreted as vascular necrotic lesions and three elbow prosthesis were placed which were rejected. She also refers spontaneous fractures in humerus. In the last 6 years she had malar and photosensitivity rash, polyarthritis in metacarpophalangeals joints and wrists and oral ulcers. The immunological findings were anti-nuclear antibody (1/5180), anti-double-stranded DNA antibody and anti-Sm. Proteinuria was 1.5 g/24 h. Therefore, she was diagnosed as systemic lupus erythematosus (SLE) with glomerulonephritis type III and she was treated with prednisone 50 mg/day plus cyclophosphamide. After 3 months of cyclophosphamide treatment (cumulative dose 1.5 g) she was changed to mofetil mycofenolate (2 g/day). The remission of lupus nephritis was observed and the prednisone dose was tapered down to 5 mg/day for maintenance. Mofetil mycofenolate was maintenance for two years. However, she was greatly worsening her elbow pain and deformity was evident. Menopause at 51 years. At the age of 54 she was referred to our department because of severe pain in both elbows. The patient has marked impairment in her quality of life which requires care of others for activities of daily life. Plain radiographs of hers elbows and ankles showed intensive disorganization, deformity and osteolysis (Figure 1). The 99mTc bone scan showed an increase uptake in elbows and ankles. A biopsy was performed and no evidence of neoplasic cells was found. Infectious disease was ruled out. A thorax-abdomen and pelvis computed axial tomography showed no evidence of pathology. The bone mineral metabolism showed: calcemia 8.4 mg/dl, phosphatemia: 4.0 mg/dl, 25(OH) vitamin D: 19 ng/ml, PTH 78.2 pg/ml, urea: 31 mg/dl, creatinine: 0.73 mg/dl, urinary deoxypyridinoline: 7.53 nM/mMol and all of the others biochemical determinations were in normal range. For pain, chronically opioid analgesics and NSAIDs were necessary. Therefore, Gorham-Stout disease was diagnosed in a patient with SLE and glomerulonefritis. The patient also has osteoporosis with a decrease of 12.3% on bone mass in the last two years (bone mineral density measured by DXA in lumbar spine L2–L4: 0.823 g/cm2, T-score: −3.1; Z-score: −2.4). The patient was treated with hidroxicloroquina, prednisone, calcium and vitamin D. Then an intravenous zoledronic acid (5 mg) was indicated. Because pain persistence, two additional zoledronic acid infusions were administrated at 3 and 9 months after the first infusion. After the second infusion the patient showed partial pain improvement and after the third infusion a progressive reduction of pain (visual analog scale 3/10) was observed without pain treatment requirement. In serial radiographs of affected joints an improvement was observed. The bone mineral metabolism after 12 months after treatment showed: calcemia 9.5 mg/dl, phosphatemia: 3.5 mg/dl, 25(OH) vitamin D: 42 ng/ml, PTH 51 pg/ml, urea: 25 mg/dl, creatinine: 0.75 mg/dl, urinary deoxypyridinoline: 6 nM/mMol, calciuria: 160 mg/dl. The bone mineral density after 1 year measured by DXA in lumbar spine was: L2–L4: 0.892 g/cm2, T-score: −2.6; Z-score: −1.9, with a gain of 8% in BMD. Clinical follow-up: 48 months.
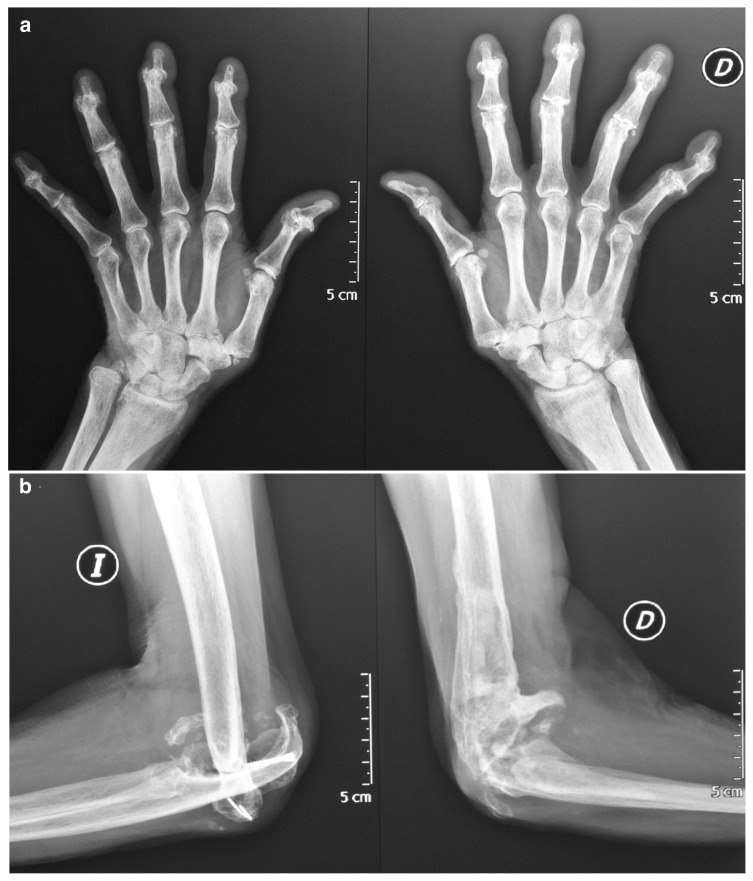
Figure 1.
a) Severe osteolysis and subluxation in bilateral distal phalanges. b) Severe osteolysis of the distal humerus, the radial head, and the olecranon in both elbows.
Case 2
We report a 70-year-old man with a history of pain (visual analog scale 10/10) and limited motion in the left shoulder without X-ray abnormality. Six months later pathological fracture in the left humerus occurs after slipping on the grass and fall. Plain radiographs showed diffuse osteolysis in the left scapula and acromion (Figure 2). A first biopsy did not found neoplastic cells and the diagnostic was uncertain. After 2 years of pain he was referred to our department. The patient has a history of prostatectomy because of prostate cancer at age 60. The bone mineral metabolism showed: calcemia 8.7 mg/dl, phosphatemia: 4.0 mg/dl, 25(OH) vitamin D: 20.7 ng/ml, PTH 41 pg/ml, urinary deoxypyridinoline: 5.3 nM/mMol. The 99mTc bone scan showed increased uptake in left scapula (Figure 2). Magnetic resonance image showed severe osteolysis and disorganization bone in left scapula and a second biopsy was performed. No evidence of neoplasic cells was found and bone tissue was replaced by vascularized dense connective tissue and focally hyaline. Infectious disease was ruled out. A thorax-abdomen and pelvis computed axial tomography showed no evidence of secondary pathology. Unilateral scapular Gorham-Stout disease was diagnosed. The BMD was in normal range. After vitamin D supplementation a treatment with intravenous zoledronic acid (5 mg) was indicated. Actually the patient has little pain (visual analog scale 2/10) and is under kinesiology treatment. The bone mineral metabolism showed calcemia 9.1 mg/dl, phosphatemia: 3.8 mg/dl, 25(OH) vitamin D: 41.5 ng/ml, PTH 36 pg/ml, urinary deoxypyridinoline: 5.6 nM/mMol. Clinical follow-up: 12 months.
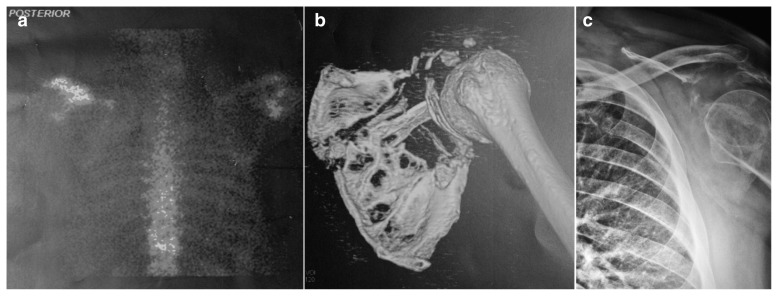
Figure 2.
Increased uptake in left scapula 99mTc bone scan (a, posterior view). Severe osteolysis in left scapula and acromion by computed tomography multislice with 3D reconstruction (b) and X-ray (c).
Discussion
Gorham-Stout disease is a rare bone disorder of uncertain etiology and unpredictable prognosis characterized by monostotics or polyostotics severe spontaneous bone resorption. It is characterized by local proliferation of small vascular or lymphatic channels which produce progressive bone destruction. Histopathologically, connective tissue and capillaries, fibrous tissue rich in blood vessels, marrow replaced by fibrous vascular tissue or abundant vascular channels can be observed. Also an hemangiomatosis association could be observed.
The disease affects frequently children or young men and women without family history association. In an extensive Gorham-Stout disease cases series it was found that 19.4% of the patients were greater than 40 years old (4). Progressive destruction of the carpal and tarsal bones usually occurs and other bones may also be involved. Chronic renal failure can be a component of this syndrome. The tubulointerstitial and glomerular changes are probably secondary to the severe vascular disease (5). Gorham reported different degree of bone resorption: complete (100%), moderate (over 50%), and slight (<50%). The milder cases show a partial single bone resorption while the severe cases progress until almost all of the osseous tissue has disappeared (3). In Gorham-Stout disease a severe osteolysis with an important bone disorganization and cortical alteration is observed. Regeneration of bone was reported only in one case (6). Diagnosis is often delayed because of laboratory parameters are usually within normal range. Inflammatory, infectious, neoplastic and endocrinological diseases must be ruled out.
Gorham-Stout disease has been classified into five groups. Type 1: hereditary multicentric osteolysis with dominant transmission. Type 2: hereditary multicentric osteolysis with recessive transmission. Type 3: non-hereditary multicentric osteolysis with nephropathy. Type 4: Gorham’s massive osteolysis associated with a vascular abnormality, angiomatosis or hemangiomatosis. Type 5: Winchester’s syndrome, autosomal recessive inheritance started in childhood with carpotarsal osteolysis without nephropathy (7).
Cystic angiomatosis is also a rare disease but more frequent than Gorham-Stout disease and may be a key differential diagnosis (8, 9). The histopathologically findings differ from Gorham-Stout disease and from others types of angiomatosis. Cystic angiomatosis is characterized by multifocal, well-defined, skeletal intramedullary cysts with a well-preserved cortical bone and without aggressive osteolysis. There is no periosteal reaction and the images reveal typical features. It varies in severity from mild forms with skeletal abnormalities to rare severe forms with visceral lymphangiomatosis. However, the pathogenesis between Gorham-Stout disease and cystic angiomatosis it would closely related.
Here, we report two cases of Gorham-Stout disease with atypical presentation. The first one associated with SLE and a second one in an older man. None of them had familial history of Gorham-Stout disease, osteolysis or angiomatosis. While renal involvement can be associated in patients with Gorham-Stout disease, in the first case a lupus nephritis was well documented. No radiographic disease progression was observed after three doses of zoledronic acid and the pain, mobility, daily activities were improved. Despite Gorham-Stout disease is associated with osteoporosis in some patients, in this case there are other causes as menopause, hyperparathyroidism secondary to vitamin D deficiency, glucocorticoid treatment and SLE.
The second case is Gorham-Stout disease in an older man which a severe scapular resorption. Although Gorham and Stout reported six cases in 1955, there are only 12 cases of scapular compromise reported (10–12). Although the previous X-ray did not reveal any abnormality, the patients have-severe pain and the osteolytic process increased rapidly before the fracture. Trauma has been mentioned as a triggering factor for osteolysis.
The treatment reported in the literature includes the use of medical treatment such as bisphosphonates, surgical intervention, radiotherapy and/or the combination of any them according the clinical case (13). However, there is no consensus about the most effective treatment approach. Bisphosphonates are choosing because its antiosteoclastic and antiangiogenic effect but there is no consensus about adequate doses or interval. Lehmann et al. reported a case treated with biphosphonates for 17 years with good response (14). Zoledronic acid treatment has been effective with no recurrence in young people (15, 16). Because of its antiangiogenic properties interferon alfa could be useful in Gorham-Stout disease with documented vessel proliferation with extended lymph-hemangiomatosis (13, 17). Radiotherapy acts accelerating sclerosis of the proliferating blood vessels and prevents re-growth of these vessels which have been found to be extremely radiosensitive. The different results obtained with radiotherapy are probably related to the different doses employed (18).
Nowadays just over 200 cases were reported. Gorham-Stout disease was reported in different bones, with different degree of resorption, at different age presentation and severe physical deformities, disabilities, and life-threatening complications. Unfortunately, the underlying cause remains unknown and, as a result, the therapeutic options are limited. Two cases of Gorham-Stout disease with good response to zoledronic acid were reported in this article.
Footnotes
Conflicts of interest
The Authors declare that they have no conflict of interest.
References
- 1.Jackson JBS. A boneless arm. Boston Med and Surg J. 1838:368–369. [Google Scholar]
- 2.Gorham LW, Wright AW, Shultz HH, Maxon FC., Jr Disappearing bones: a rare form of massive osteolysis; report of two cases, one with autopsy findings. Am J Med. 1954;17(5):674–682. doi: 10.1016/0002-9343(54)90027-3. [DOI] [PubMed] [Google Scholar]
- 3.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37-A(5):985–1004. [PubMed] [Google Scholar]
- 4.Hu P, Yuan XG, Hu XY, Shen FR, Wang JA. Gorham-Stout syndrome in mainland China: a case series of 67 patients and review of the literature. J Zhejiang Univ Sci B. 2013;14(8):729–735. doi: 10.1631/jzus.B1200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett WM, Houghton DC, Beals RC. Nephropathy of idiopathic multicentric osteolysis. Nephron. 1980;25(3):134–138. doi: 10.1159/000181769. [DOI] [PubMed] [Google Scholar]
- 6.Campbell J, Almond HG, Johnson R. Massive osteolysis of the humerus with spontaneous recovery. J Bone Joint Surg Br. 1975;57(2):238–240. [PubMed] [Google Scholar]
- 7.Hardegger F, Simpson LA, Segmueller G. The syndrome of idiopathic osteolysis. Classification, review, and case report. J Bone Joint Surg Br. 1985;67(1):88–93. doi: 10.1302/0301-620X.67B1.3968152. [DOI] [PubMed] [Google Scholar]
- 8.Najm A, Soltner-Neel E, Le Goff B, Guillot P, Maugars Y, Berthelot JM. Cystic angiomatosis, a heterogeneous condition: Four new cases and a literature review. Medicine (Baltimore) 2016;95(43):e5213. doi: 10.1097/MD.0000000000005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Knoch F, Grill F, Herneth AM, et al. Skeletal cystic angiomatosis with severe hip joint deformation resembling massive osteolysis. Arch Orthop Trauma Surg. 2001;121:48–58. doi: 10.1007/s004020100295. [DOI] [PubMed] [Google Scholar]
- 10.Sacristan HD, Portal LF, Castresana FG, Pena DR. Massive osteolysis of the scapula and ribs. A case report. J Bone Joint Surg Am. 1977;59(3):405–406. [PubMed] [Google Scholar]
- 11.Falcone G, Gusso MI, Pennisi M. A rare case of massive osteolysis (Gorham’s syndrome) Arch Putti Chir Organi Mov. 1989;37(2):447–54. [PubMed] [Google Scholar]
- 12.Glass-Royal M, Stull MA. Musculoskeletal case of the day. Gorham syndrome of the right clavicle and scapula. AJR Am J Roentgenol. 1990;154(6):1335–1336. doi: 10.2214/ajr.154.6.2110757. [DOI] [PubMed] [Google Scholar]
- 13.Ellati R, Attili A, Haddad H, Al-Hussaini M, Shehadeh A. Novel approach of treating Gorham-Stout disease in the humerus—Case report and review of literature. Eur Rev Med Pharmacol Sci. 2016;20(3):426–432. [PubMed] [Google Scholar]
- 14.Lehmann G, Pfeil A, Bottcher J, Kaiser WA, Fuller J, Hein G, Wolf G. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967–972. doi: 10.1007/s00402-008-0742-3. [DOI] [PubMed] [Google Scholar]
- 15.Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319–322. doi: 10.1016/j.ijporl.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Mignogna MD, Fedele S, Lo Russo L, Ciccarelli R. Treatment of Gorham’s disease with zoledronic acid. Oral Oncol. 2005;41(7):747–750. doi: 10.1016/j.oraloncology.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymphhemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47–50. doi: 10.1016/j.jpedsurg.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Handl-Zeller L, Hohenberg G. Radiotherapy of morbus Gorham-Stout:the biological value of low irradiation dose. Br J Radiol. 1990;63:206–208. doi: 10.1259/0007-1285-63-747-206. [DOI] [PubMed] [Google Scholar]