Summary
Background
Patients with squamous non-small-cell lung cancer that is refractory to multiple treatments have poor outcomes. We assessed the activity of nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor antibody, for patients with advanced, refractory, squamous non-small-cell lung cancer.
Methods
We did this phase 2, single-arm trial at 27 sites (academic, hospital, and private cancer centres) in France, Germany, Italy, and USA. Patients who had received two or more previous treatments received intravenous nivolumab (3 mg/kg) every 2 weeks until progression or unacceptable toxic effects. The primary endpoint was the proportion of patients with a confirmed objective response as assessed by an independent radiology review committee. We included all treated patients in the analyses. This study is registered with ClinicalTrials.gov, number NCT01721759.
Findings
Between Nov 16, 2012, and July 22, 2013, we enrolled and treated 117 patients. 17 (14·5%, 95% CI 8·7–22·2) of 117 patients had an objective response as assessed by an independent radiology review committee. Median time to response was 3·3 months (IQR 2·2–4·8), and median duration of response was not reached (95% CI 8·31–not applicable); 13 (77%) of 17 of responses were ongoing at the time of analysis. 30 (26%) of 117 patients had stable disease (median duration 6·0 months, 95% CI 4·7–10·9). 20 (17%) of 117 patients reported grade 3–4 treatment-related adverse events, including: fatigue (five [4%] of 117 patients), pneumonitis (four [3%]), and diarrhoea (three [3%]). There were two treatment-associated deaths caused by pneumonia and ischaemic stroke that occurred in patients with multiple comorbidities in the setting of progressive disease.
Interpretation
Nivolumab has clinically meaningful activity and a manageable safety profile in previously treated patients with advanced, refractory, squamous non-small cell lung cancer. These data support the assessment of nivolumab in randomised, controlled, phase 3 studies of first-line and second-line treatment.
Funding
Bristol-Myers Squibb.
Introduction
During immunosurveillance, the immune system is capable of recognising and destroying tumour cells; however, tumours can escape elimination by the immune system through activation of inhibitory feedback loops or (so-called immunological brakes) that are essential to avoid autoimmune events, and so can create barriers to T-cell activation and tumour rejection.1,2 The PD-1 pathway is one such inhibitory pathway, and its activation is exploited by several cancer types, including lung cancer. Inhibition of the PD-1 pathway is a novel therapeutic approach for treating cancer.3 Nivolumab, a fully human, IgG4 immune checkpoint inhibitor antibody, binds PD-1 on activated immune cells to disrupt PD-1 interaction with PD-L1 and PD-L2 ligands, thereby attenuating inhibitory signals and augmenting the host antitumour response.3 Nivolumab has anti-cancer activity against several tumour types, including non-small-cell lung cancer.4–7 In a phase 1 study5–7 of about 300 patients with advanced solid tumors, nivolumab treatment resulted in 22 (17%) of 129 patients with non-small-cell lung cancer achieving an objective response. Treatment with nivolumab also resulted in overall survival of 42% (95% CI 33–50) at 1 year, 24% (17–33) at 2 years, and 18% (11–25) at 3 years; similar results have been reported for non-squamous and squamous histological subtypes.5–7
Lung cancer is a major health burden, with more than 1·6 million new cases diagnosed per year and 1·3 million deaths per year.8 Most cases (85%) are non-small-cell lung cancer, consisting of non-squamous (70%) and squamous (30%) histological subtypes, and half of patients present with incurable metastatic disease.9–11 Prognosis for refractory squamous non-small-cell lung cancer is very poor, with median overall survival of between 4·0–6·5 months, 1-year survival of 6–18%, and 2-year survival of 3%.12–14
Recent advances in treatment for non-small-cell lung cancer have been restricted to patients with non-squamous disease, with little progress for the squamous subtype. This might be partly caused by the mutational complexity of squamous non-small-cell lung cancer, which limits the activity of the newly developed targeted therapies, and leaves systemic chemotherapy as the mainstay of treatment. Because of the absence of approved or efficacious treatments for patients with refractory squamous non-small-cell lung cancer, best supportive care or clinical trials remain the primary approaches for this population. Accordingly, we did CheckMate 063 to assess the therapeutic activity of nivolumab for patients with advanced, refractory squamous non-small-cell lung cancer.
Methods
Study design and participants
This study was an international, phase 2, single-arm trial at 27 sites (academic, hospital, and private cancer centres) in four countries: seven in France, three in Germany, three in Italy, and 14 in USA (one site in the USA enrolled patients but never treated them).
We included patients with histologically or cytologically documented stage IIIB or IV squamous non-small-cell lung cancer, and disease measurable by CT or MRI. Patients had to be aged 18 years or older, have an Eastern Cooperative Oncology Group performance status of 0 or 1, and have acceptable haematological, renal, and hepatic function (appendix). The following laboratory tests were done to assess patient eligibility: complete blood count with differential, serum chemistry tests, aspartate aminotransferase, alanine aminotransferase, total bilirubin, alkaline phosphatase, albumin, lactose dehydrogenase, thyroid stimulating hormone, free T3, free T4, hepatitis B surface antigen, and hepatitis C antibody or hepatitis C RNA.
Patients had to have had disease progression or recurrence after both a platinum doublet-based chemotherapy regimen and at least one additional systemic treatment (appendix). Patients with treated stable brain metastases who had neurologically returned to baseline for at least 2 weeks before enrolment were eligible. Exclusion criteria (appendix) included autoimmune disease, disorders requiring systemic immunosuppressive drugs, previous treatment with an antibody or drug specifically targeting T-cell co-stimulation or checkpoint pathways, positive test for HIV, hepatitis B, hepatitis C, or symptomatic interstitial lung disease (patients with a history of pneumonitis were not excluded). Previous cancer treatment, radiotherapy, or radiosurgery must have been completed at least 2 weeks before receiving the first dose of study drug. Patient selection was not based on estimated survival; typical median survival in this population is 4·0–6·5 months.12–14
The study protocol was approved by institutional review boards and independent ethics committees of each institute, and the study was done in accordance with international standards of good clinical practice. All patients or their legal representatives provided written informed consent before enrolment.
Procedures
Nivolumab (Bristol-Myers Squibb, Princeton, NJ, USA) was supplied as a solution for injection (100 mg; 10 mg/mL). Patients received nivolumab 3 mg/kg as an intravenous infusion every 2 weeks (1 cycle) until disease progression or unacceptable toxic effects. Treatment after progression was permitted if a patient had investigator-assessed clinical benefit (continuing disease or symptom control despite radiographic progression), stable performance status, and was tolerating nivolumab. This decision was taken based on evidence7,15 that some patients treated with immunotherapy might derive clinical benefit (response or prolonged disease stabilisation) despite initial evidence of progressive disease. The decision to continue treatment was at the discretion of the treating physician.
We based the dose and schedule of nivolumab on safety and activity data from a phase 1 study,6 which showed a similar proportion of objective responses in patients treated with 3 mg/kg and those treated with 10 mg/kg; both of these doses achieved better responses than with a 1 mg/kg dose. The safety profile was much the same for each dose and for different tumour types in the phase 1 trial.
Dose modifications of nivolumab were not permitted. Delays of nivolumab dose were allowed for protocol-defined reasons, including the occurrence of: grade 2 or worse non-skin treatment-related adverse events; grade 3 skin treatment-related adverse events; and grade 3 treatment-related laboratory abnormalities, with protocol-defined exceptions for lymphopenia, leucopenia, aspartate aminotransferase, alanine aminotransferase, or total bilirubin (appendix). Reasons for discontinuation from nivolumab treatment included the occurrence of grade 2 treatment-related uveitis or ocular toxic effects not responsive to treatment; grade 3 non-skin treatment-related adverse events or thrombocytopenia lasting more than 7 days, or grade 3 uveitis, pneumonitis, or hypersensitivity reaction of any duration; high-grade (eg, >5–10 times the upper limit of normal) liver function test abnormalities or concurrent increases of aspartate aminotransferase, alanine aminotransferase, or bilirubin; any grade 4 treatment-related adverse events or laboratory abnormalities, with exceptions for lymphopenia, leucopenia, or neutropenia for less than 7 days. Clinical and laboratory safety assessments were done for all treated patients. Adverse events were assessed at screening, every 2 weeks during treatment, and up to 100 days after receiving the last nivolumab dose. Severity of adverse events was graded on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).16 Immune-mediated events were defined as adverse events with potential immunological basis requiring frequent monitoring and potential intervention with immune suppression or endocrine treatment. Adverse events were managed with established safety guidelines.
Radiographic tumour assessments were done at screening, 8 weeks after the start of treatment, and every 6 weeks thereafter until disease progression or until discontinuation of nivolumab for patients treated beyond progression. Survival was assessed every 3 months after completion of treatment, until death or withdrawal of consent.
We measured PD-L1 protein expression in pre-treatment, formalin-fixed, paraffin-embedded tumour specimens with a validated, automated immunohistochemical assay (Dako, Carpinteria, CA, USA), with samples categorised as positive when tumour cell membranes were stained to any intensity in 1%, 5%, and 10% of cells in a section with a minimum of 100 assessable tumour cells. We used a cutoff of 5% to define PD-L1 positivity, on the basis of preliminary findings from a phase 1 study,6,7 and because the difference in responses between PD-L1 positive and PD-L1 negative patients was not greater when using a 1% cutoff than when using a 5% cutoff (appendix), prospectively defined in the statistical analysis plan. In cases for which the tissue sample was not optimally collected or prepared (ie, improperly fixed, contained sectioning artifacts such as folding, or were found not to contain tumour tissue) or for which PD-L1 expression could not be assessed, PD-L1 status was categorised as unevaluable.
Outcomes
The primary endpoint was the proportion of patients with a confirmed objective response (ie, the percentage of all treated patients with a best overall response of confirmed complete or partial response), as assessed by an independent radiology review committee (IRC) using Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1).17 Best overall response was defined as the best response designation recorded between the date of first dose and the date of initial objectively documented tumour progression per RECIST v1.1, or the date of subsequent therapy, whichever occurred first. Objective response was further characterised by the duration of response, defined as the time from first confirmed response to the date of initial radiographic progression for patients with complete response or partial response.
The secondary endpoint was the proportion of patients with investigator-assessed confirmed objective response using RECIST 1.1. Exploratory endpoints were to characterise pharmacokinetics of nivolumab and explore exposure-response (exposure safety and exposure efficacy) relationships with respect to selected safety and efficacy endpoints and to characterise immunogenicity of nivolumab, safety and tolerability of nivolumab, progression-free survival and overall survival of all treated patients, and association between the proportion of patients with an objective response rate and PD-L1 expression in all patients. Progression-free survival was defined as the time from the first dose of nivolumab to the date of first documented tumour progression or death from any cause. Overall survival was defined as the time from the date of first dose to the date of death from any cause or last known date alive for patients who were alive at the time of data analysis. Patients who died without a reported previous progression were considered to have progressed on the date of their death. Patients who neither progressed nor died were censored on the date of their last tumour assessment. Patients who received subsequent cancer treatment without a reported progression were censored at the date of the last tumour assessment before starting further treatment. Patients who did not have any on-study tumour assessments and did not die were censored on the date of the first dose of nivolumab. Patients who were not evaluable for best best overall response by the independent radiology review committee were recorded as having indeterminate best overall response. Patients without on-study scans were recorded as having best overall response not reported. These assessments were preplanned.
Statistical analysis
We designed the study to treat roughly 100 patients, such that the maximum width of the exact two-sided 95% CI was 20% when 10–50% of participants had an objective response. We did the safety and activity analyses in all treated patients, defined as those who received at least one dose of nivolumab. An initial data lock including investigator-assessed endpoints occurred on March 6, 2014. Data for endpoints assessed by the independent radiology review committee, overall survival, safety, and PD-L1 expression were updated on July 23, 2014.
We summarised objective response rates with binomial responses and their corresponding two-sided exact 95% CI by the Clopper-Pearson method.18 We assessed duration of response, progression-free survival, and overall survival by the Kaplan-Meier product-limit method. We also calculated median duration of response, progression-free survival, and overall survival with two-sided 95% CI by the per Brookmeyer and Crowley method.19 We used the Greenwood formula,20 to calculate overall survival and 95% CIs from the Kaplan-Meier estimates. The primary safety analysis was based on adverse events reported within 30 days of the last dose of nivolumab. We also assessed adverse events occurring within 100 days of the last nivolumab dose for consistency with the primary analysis. We did the statistical analyses with SAS (version 9.02).
This study is registered with ClinicalTrials.gov, number NCT01721759.
Role of the funding source
The funder provided the study drug and worked with investigators to design the study, collect and analyse data, and interpret the results. All authors made the decision to submit the report for publication, and all drafts of the report were prepared by the corresponding author with input from coauthors and editorial assistance from a professional medical writer, funded by the sponsor. Raw data were reviewed and made accessible to the authors and professional medical writers.
Results
From Nov 16, 2012, to July 22, 2013, we enrolled 140 patients, of whom 117 (84%) were treated with nivolumab (appendix). 23 enrolled patients were not treated for the following reasons: no longer met study eligibility criteria (n=20), died before treatment (n=2), or lost to follow-up (n=1). Table 1 shows the patients’ characteristics. The patient population was highly refractory, with almost two-thirds having previously received three or more systemic treatments. Progressive disease was the most common best response to the most recent previous treatment, with more than three-quarters of patients entering the study within 3 months of completing their most recent treatment (table 1). Half the patients had three or more baseline disease sites with at least one lesion (appendix). Four patients had brain metastasis at baseline. Median time from initial lung cancer diagnosis to nivolumab treatment was 1·7 years (IQR 1·1–2·6).
Table 1.
Baseline characteristics
| Patients (n=117) | |
|---|---|
| Age (years) | |
| Median (IQR) | 65 (57–71) |
| <75 | 101 (86%) |
| ≥75 | 16 (14%) |
|
| |
| Sex | |
| Male | 85 (73%) |
| Female | 32 (27%) |
|
| |
| Ethnic origin | |
| White | 99 (85%) |
| Black or African American | 11 (9%) |
| Asian | 2 (2%) |
| Other | 5 (4%) |
|
| |
| ECOG PS | |
| 0 | 26 (22%) |
| 1 | 91 (78%) |
|
| |
| Disease stage | |
| IIIB | 20 (17%) |
| IV | 97 (83%) |
|
| |
| History of smoking | 108 (92%) |
|
| |
| CNS metastasis | 2 (2%)* |
|
| |
| Previous systemic therapy | |
| Platinum-based therapy | 117 (100%) |
| Other | 117 (100%) |
| EGFR TKI | 39 (33%) |
| Experimental treatment | 13 (11%) |
|
| |
| Number of previous systemic treatments | |
| 2 | 41 (35%) |
| 3 | 52 (44%) |
| ≥4 | 24 (21%) |
|
| |
| Previous radiotherapy | 87 (74%) |
|
| |
| Best response to most recent previous treatment | |
| CR or PR | 5 (4%) |
| SD | 32 (27%) |
| Progressive disease | 71 (61%) |
| Unknown | 9 (8%) |
|
| |
| Months from completion of most recent previous regimen to treatment in this study | |
| <3 | 89 (76%) |
| 3–6 | 16 (14%) |
| >6 | 12 (10%) |
Data are n (%) unless otherwise stated. CR=complete response. ECOG PS=Eastern Cooperative Oncology Group performance status. TKI=tyrosine kinase inhibitor. CR=complete response. PR=partial response. SD=stable disease.
Two additional patients had CNS metastasis at baseline but were not included here because the characteristic was reported after the date of data analysis. Of the four patients with brain metastasis, two had received radiation treatment: one required on-study brain radiotherapy at day 35, because of increasing headache, and one had previous stereotactic radiosurgery.
A median of six doses (IQR 3·0–14·0) of nivolumab were administered, with a median treatment duration of 2·3 months (95% CI 1·4–2·8). The minimum follow-up for IRC-assessed response (calculated based on the date of last patient first visit to the date of last patient last visit) was 11·0 months, and the median follow-up for overall survival was 8·0 months (IQR 3·7–12·0). Disease progression was the most common reason for discontinuation (78 [67%] of 117 patients; appendix).
After discontinuing nivolumab, 28 (24%) of 117 patients received subsequent systemic cancer treatment, most commonly gemcitabine (12 [10%]), docetaxel (five [4%]), and vinorelbine (five [4%]). No patients received subsequent immunotherapy.
17 (14·5%, 95% CI 8·7–22·2) of 117 patients achieved a partial response according to the independent radiology review committee. 30 (26%, 18–35) of 117 patients had stable disease, including one unconfirmed responder (a patient who had at least 30% reduction in target lesion tumor burden, but without a subsequent scan confirming a response); 51 (44%, 34–53) had progressive disease, seven (6%, 2–12) had indeterminate best overall response, and 12 (10%, 5–17) were not reported. Patients who were not evaluable for imaging plus clinical best overall response were listed as having indeterminate best overall response. Patients without on-study scans were listed as having unreported best overall response (six patients died before disease assessment, three had investigator-assessed progressive disease, and one patient each was unreported because of early discontinuation because of toxic effects, clinical progression without CT scan, and no subsequent scan taken after cycle 1, day 1).
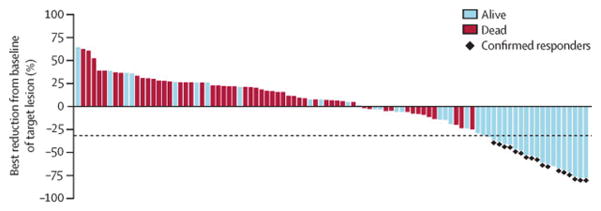
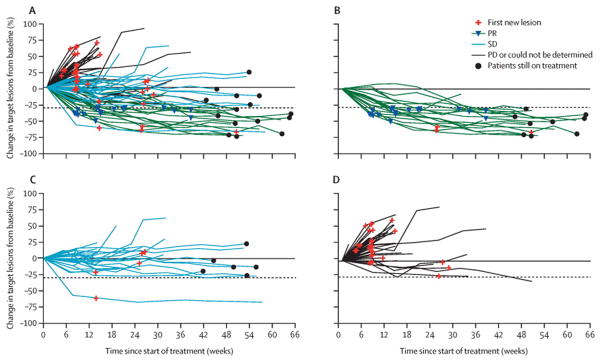
Eight (47%) of 17 confirmed responders had progressive disease as the best overall response to their previous treatment, and had started nivolumab within 3 months of completing their most recent treatment (appendix). Target tumour lesion burden was reduced by at least 50% for 11 (65%) of 17 responding patients (figure 1). 29 (69%) of 42 patients with any reduced tumour burden, including all patients with a confirmed partial response, were alive at the time of this analysis (figure 1). Some patients without evidence of tumour regression were also alive at the time of analysis. Two patients with non-target baseline CNS metastases had responses in their CNS lesions, one with a systemic best overall response of stable disease, and another with a partial response. Nivolumab treatment led to objective responses independent of age, sex, baseline performance status, region, ethnic origin, and number of previous treatments, with numerical differences noted in some subgroups probably attributable to small sample sizes (appendix). Figure 2 shows changes of target tumour burden over time as assessed per the IRC.
Figure 1. Best reduction of tumour size.
Data are based on the IRC assessment of survival status in July, 2014. Includes patients with complete target lesion data, a baseline assessment, and at least one on-treatment assessment before progression or start of subsequent treatment (n=95). The line at −30% indicates the threshold for objective response per RECIST v1.1.
Figure 2. Changes of target tumour burden over time as assessed per IRC.
Shows changes by RECIST response (A), in patients with an objective response (B), in patients with stable disease (C), and in patients with progressive disease or for whom response could not be determined (D). Includes patients with an evaluable response (n=100), who had a baseline assessment and at least one on-treatment tumour assessment. Tumour burden was measured as the sum of the longest diameters of target lesions. RECIST=Response Evaluation Criteria In Solid Tumors. SD=stable disease. PR=partial response. PD=progressive disease.
Median time to response was 3·3 months (IQR 2·2–4·8). 13 (77%) of 17 responding patients had ongoing responses at the time of analysis (eight on-treatment, five in follow-up), and median duration of response had not been reached (95% CI 8·3 months to not reached). 30 (26%) of 117 patients had stable disease as their best overall response, including one unconfirmed responder. Median duration of stable disease was 6·0 months (95% CI 4·7–10·9), with 20 patients progression-free at the time of analysis.
By investigator assessment, one (1%) patient had a confirmed complete response, and 14 (12%) had a confirmed partial response (12·8% [95% CI 7·4–20·3] achieved an objective response; appendix). The concordance rate between independent radiology review committee and investigator-assessed response was 92·2% based on the March 6, 2014, analysis. 22 (42%) of 52 patients continued nivolumab treatment after progression (appendix).
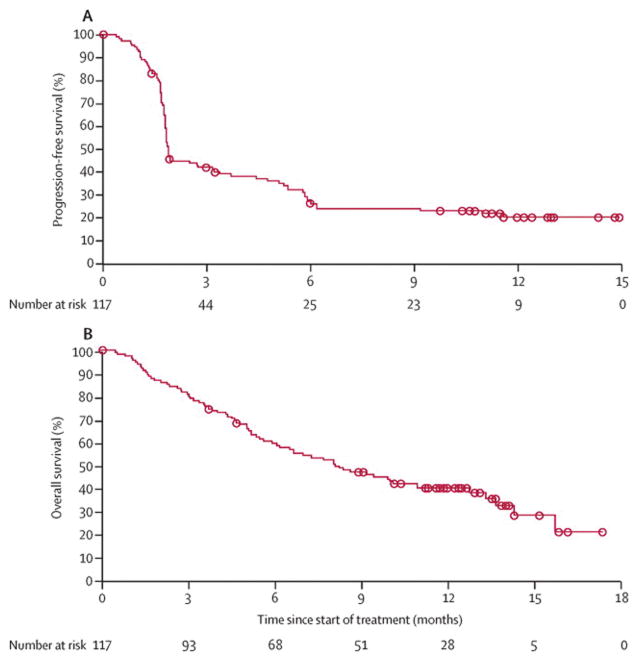
Median progression-free survival was 1·9 months (95% CI 1·8–3·2), with progression-free survival of 25·9% (18·0–34·6) at 6 months and 20·0% (12·7–28·5) at 1 year (figure 3A). Median overall survival was 8·2 months (95% CI 6·1–10·9) and overall survival at 1 year was 40·8% (31·6–49·7; figure 3B). At the time of analysis, 72 (62%) of 117 participants had died.
Figure 3. Kaplan-Meier analysis of progression-free survival (A) and overall survival (B).
Progression-free survival was IRC assessed.
We obtained pretreatment archival tumour samples for 86 (74%) of 117 participants, 76 (88%) of which could be assessed for PD-L1 expression. Median time from biopsy to start of nivolumab treatment for all collected tumour samples was 1·3 years (IQR 0·8–2·1). 25 (33%) of 76 patients had PD-L1-positive tumours (≥5% expression). Patients with PD-L1-positive tumours and those with PD-L1-negative tumours achieved an objective response, with more objective responses in patients with PD-L1-positive tumours (table 2). Almost a third of patients with unevaluable PD-L1 expression had an objective response (table 2). The appendix shows objective response by PD-L1 status using other cutoff s. Reductions in target tumour lesion burden were more common in patients with PD-L1-positive tumours (13 [52%] of 25 patients) than in those with PD-L1-negative tumours (15 [38%] of 40; appendix).
Table 2.
Best overall response by PD-L1 expression status
| Partial response | Stable disease | Progressive disease | |
|---|---|---|---|
| <5% (n=51) | 7 (14%) | 10 (20%) | 25 (49%) |
| ≥5% (n=25) | 6 (24%) | 6 (24%) | 11 (44%) |
| Unevaluable (n=10) | 3 (30%) | 4 (40%) | 2 (20%) |
Data are n (%). Patients with indeterminate best overall response (n=7), and best overall response not reported by the IRC (n=5) are not included. PD-L1 expression was evaluable in all these patients, except one with best overall response not reported.
32 (27%) of 117 patients had a dose delay, most often because of an adverse event, with 21 (66%) of 32 having only one delay. Most dose delays lasted less than 15 days. Accordingly, 99 (85%) of 117 patients received at least 90% of their planned dose intensity.
Almost three-quarters of patients reported a treatment-related adverse event of any grade; most commonly, fatigue, decreased appetite, and nausea (table 3). Grade 3–4 treatment-related adverse events occurred in about a sixth of patients, most commonly fatigue, pneumonitis, and diarrhoea (table 3). Most treatment-related immune-mediated adverse events were of low grade, with skin disorders and gastrointestinal events most prevalent (appendix). Three patients had treatment-related grade 3 diarrhoea, which resolved with either corticosteroid treatment (one patient) or supportive care. Six patients had treatment-related pneumonitis (none grade 4 or 5); one additional grade 3 pneumonitis was reported between 30 and 100 days after the last dose of nivolumab. All patients with pneumonitis were treated with corticosteroids, with a median time to resolution of 3·4 weeks (range 1·6–13·4). Four low-grade, treatment-related, renal adverse events were reported, none of which were grade 3–4. Treatment-related adverse events led to discontinuation for 14 (12%) of 117 patients: five (4%) for pneumonitis, two (2%) for fatigue, and one (1%) for each of anaphylactic reaction, hypersensitivity, adrenal insufficiency, diarrhoea, polyneuropathy, rash, and sensory neuropathy in both hands. At time of analysis, 15 (13%) of 117 patients were on treatment.
Table 3.
Treatment-related adverse events that occurred in at least 5% of all treated patients
| Any grade | Grade 3–4 | |
|---|---|---|
| Any | 87 (74%) | 20 (17%) |
|
| ||
| General disorders | ||
| Fatigue | 38 (33%) | 5 (4%)* |
| Asthenia | 14 (12%) | 0 |
|
| ||
| Gastrointestinal disorders | ||
| Nausea | 18 (15%) | 0 |
| Diarrhoea | 12 (10%) | 3 (3%)* |
| Dry mouth | 7 (6%) | 0 |
| Vomiting | 7 (6%) | 0 |
| Constipation | 6 (5%) | 0 |
|
| ||
| Metabolism and nutrition disorders | ||
| Decreased appetite | 22 (19%) | 0 |
|
| ||
| Skin and subcutaneous tissue disorders | ||
| Rash | 13 (11%) | 1 (1%)* |
| Pruritus | 7 (6%) | 1 (1%)* |
|
| ||
| Musculoskeletal disorders | ||
| Myalgia | 6 (5%) | 1 (1%)* |
|
| ||
| Respiratory disorders | ||
| Dyspnoea | 6 (5%) | 0 |
| Pneumonitis | 6 (5%) | 4 (3%)* |
|
| ||
| Blood and lymphatic system disorders | ||
| Anaemia | 7 (6%) | 1 (1%)* |
Data are number of events (%). Includes events reported between the first dose and 30 days after the last dose of nivolumab. Grade 3–4 adverse events reported by less than 5% of patients included: hyponatraemia (two [2%]); and polyneuropathy, decreased lymphocyte count, herpes zoster, adrenal insufficiency, vasculitis, hypersensitivity, anaphylactic reaction, and unassigned, each reported by one patient (one [1%]). One patient (1%) died from pneumonia, and one patient (1%) from ischaemic stroke.
All grade 3.
Two deaths were attributed, by the investigator, to nivolumab. One patient died of hypoxic pneumonia 28 days after the last dose of nivolumab. This patient had rapid tumour progression and bronchial obstruction with possible associated opportunistic infection. Although this condition was distinct from pneumonitis, the investigator reported the adverse event as possibly related to nivolumab as an inflammatory component could not be ruled out, and no bronchoscopy or autopsy was done. A second patient died of ischaemic stroke 41 days after the first and only nivolumab dose. Both patients had multiple comorbidities and progressive disease (appendix).
Discussion
Our findings show that nivolumab monotherapy provides clinically meaningful activity and an acceptable safety profile for patients with advanced refractory squamous non-small-cell lung cancer. The prognosis for patients who have progressed after treatment with two or more chemotherapy regimens is very poor. No standard treatments exist for such patients, other than best supportive care or experimental treatment in clinical trials, and new drugs are urgently needed.
To the best of our knowledge, CheckMate 063 is the first phase 2 study to report the activity of a PD-1 immune checkpoint inhibitor for advanced non-small-cell lung cancer (panel). Although systemic chemotherapy provides modest therapeutic gains for patients with advanced squamous non-small-cell lung cancer, patients progress after chemotherapy and have few treatment options beyond second-line treatment. Immunotherapy harnesses the ability of the immune system to recognise non-self tumour antigens, and to constantly adapt and detect new antigens, and thus holds promise as a novel therapeutic approach for several cancers including non-small-cell lung cancer.
The results of our study corroborate data from a phase 1 study.6,7 An IRC-assessed objective response in 14·5% of patients treated with nivolumab is a clinically meaningful improvement over previously reported objective response rates (2–8% of patients),12–14 within the context of a single arm study; furthermore, responses were durable. Subgroup analyses showed objective responses within each subpopulation. Although a smaller proportion of women than men had an objective response, the small sample size and lack of published reports to address potential differences between sexes for this class of drugs for other tumour types precludes further conclusions.
Our patient population was refractory to treatment: most patients had received at least three previous regimens, had progressive disease as the best response to their most recent treatment, and entered the study within 3 months of completing their most recent treatment. Despite this fact, eight responses occurred in patients who had progressed on their most recent previous treatment therapy, and entered within 3 months of completing the most recent treatment, showing the activity of nivolumab for patients with aggressive disease. Although the time from initial diagnosis of advanced disease to nivolumab treatment was not recorded, it would be unusual for a patient with stage IV disease to have a substantial lag between diagnosis and treatment, and we do not expect it to substantially alter the results. Additional evidence of a therapeutic benefit was noted in the form of durable stable disease in many patients, clinical activity for CNS disease, and non-conventional responses in patients who continued nivolumab after disease progression, suggesting an immune-related pattern of antitumour activity.
1-year overall survival exceeded expectations based on previous reports for this population, although cross-trial comparisons should be made with caution in patients with squamous non-small-cell lung cancer.12–14 17% of participants had stage IIIB disease and the prognosis for such patients who have malignant effusion is much the same as for stage IV disease, making it unlikely that this subgroup accounts for the longer survival. Furthermore, because all patients entered this study with advanced, chemotherapy-refractory disease, there is no distinction between these subsets in terms of prognosis. Survival at 1 year and beyond is a useful measure of benefit of nivolumab and other immune-oncological treatments.21
Several studies have assessed PD-L1 expression as a potential biomarker of response to drugs targeting the PD-1 pathway,5,22–26 although the best cutoff is yet to be defined. Use of a 10% cutoff for PD-L1 positivity resulted in the same classification of patients as with a 5% cutoff.
Some PD-L1 expression-negative patients had an objective response, suggesting that nivolumab has activity in this subgroup. Although objective responses were numerically higher in patients with positive PD-L1 expression, interpretation of these results is limited because of the small sample size and wide confidence intervals. Although the samples were archived and intervening treatment might have affected PD-L1 status at the time of nivolumab initiation, activity of PD-1 blockade for PD-L1-negative patients has also been reported from contemporary biopsies.27 Although overexpression of PD-L1 might serve as a hallmark of tumours that have successfully evaded an immune response (and therefore might be more susceptible to therapeutic intervention with an anti-PD-L1 antibody), nivolumab could alleviate inhibitory T-cell signalling independently of tumoral PD-L1 expression, for example by blocking interactions with other PD-1 ligands such as PD-L2. In view of similar observations of clinical activity of anti-PD-1/PD-L1 therapeutic antibodies in PD-L1 negative patients,22,25,26 PD-L1 expression might not be necessary for achieving objective response with this class of agents, or the clinical effect of PD-L1 expression might be better represented by other endpoints, such as survival.
Panel: Research in context.
Systematic review
We searched PubMed and congress abstracts from the yearly meetings in the past 5 years of the American Society of Clinical Oncology, European Cancer Congress, and World Conference on Lung Cancer for the terms “PD-1”, “PD-L1”, “nivolumab”, “pembrolizumab”, “lambrolizumab”, “MPDL3280A”, and “MEDI4736”. No language restriction was used. We identified several phase 1 trials showing that PD-1 and PD-L1 inhibitors have antitumour activity in advanced non-small-cell lung cancer and other ongoing phase 2 trials assessing the activity of PD-1 and PD-L1 inhibitors. However, results of these phase 2 trials have not yet been reported. Data from a phase 1 study5 of heavily pretreated patients with advanced non-small-cell lung cancer showed clinical activity with nivolumab that was much the same for non-squamous and squamous subtypes.
Interpretation
Nivolumab showed activity in patients with advanced, refractory, squamous non-small-cell lung cancer and was associated with a manageable safety profile, consistent with previous studies. Our results suggest that nivolumab may be a promising future treatment for patients with advanced, refractory squamous non-small-cell lung cancer, and should be explored in randomised, controlled, phase 3 trials.
The tumour microenvironment is highly complicated. Colocalisation of PD-L1 and tumour infiltrating lymphocytes is predictive of response,28 and additional studies of melanoma29 have shown that pre-existing CD8 T cells located at the invasive tumour margin are associated with expression of the PD-1 immune inhibitory axis and might predict response to treatment. PD-L1 expression by other tumour-infiltrating immune cells seems to correlate with response.30 Results from ongoing randomised studies will help to further define the predictive or prognostic relationship between PD-L1 expression and nivolumab activity.
The safety profile of nivolumab was consistent with the phase 1 trial. Treatment-related immune-mediated adverse events were generally infrequent and not severe, and could be managed with established guidelines. Pneumonitis is a clinically important treatment-related adverse event associated with treatment of non-small-cell lung cancer, including with nivolumab.31–34 In our study, nivolumab-related pneumonitis was as common as in the phase 1 trial.6,7 By contrast with cytotoxic chemotherapy,35 haematological toxic effects were infrequently reported with nivolumab. Moreover, a third or fewer of patients in our study had low-grade treatment-related fatigue, decreased appetite, nausea, asthenia, rash, or diarrhoea.
Few treatment options exist for advanced, refractory squamous non-small-cell lung cancer, with no clear standard of care. Our study shows clinically meaningful activity and a manageable safety profile of nivolumab for this patient population and supports assessment of nivolumab in phase 3 studies of first-line and second-line treatment.
Supplementary Material
Acknowledgments
This study was funded by Bristol-Myers Squibb. We thank all patients and families, as well as the study teams involved in the trial, for making this trial possible. We also thank Suresh Alaparthy and Haolan Lu for their substantial contributions to the study conduct and analyses and Dako for collaborative development of the automated PD-L1 immunohistochemistry assay. Medical writing and editorial assistance was provided by Susan Leinbach, Clinical Solutions Group, and Elyse Mallimo and Karin McGlynn, StemScientific, funded by Bristol-Myers Squibb.
Footnotes
Contributors
SJA, AL-C, AD, RMH, HL, GAO, DP, NAR, RS, and TES collected, analysed, and interpreted data. FC, CC, GKD, JM, P-JS, and JW collected and interpreted data. BPL collected data and was the primary investigator at Beth Israel Comprehensive Cancer Center. EB collected data and was the primary investigator at Ospedale Campo Di Marte. LTC, LH, BM, EM, SGN, and GZ collected data. AD and BJL searched the published work. AL-C, CG, JM, GAO, RS, and TES conducted the study. SJA, RMH, NAR, and JW designed and conducted the study. CB designed the study, analysed and interpreted data, and designed the figures. BJL designed the study, collected, analysed, and interpreted data, designed the figures, and wrote the first draft. CTH collected, analysed, and interpreted data. SSR designed the study, collected, analysed, and interpreted data, and wrote the report. NAR wrote the first draft. DRG contributed to patient recruitment and study conduct. All authors wrote the report, and approved the final draft for submission. ACR Image Metrix (Philadelphia, PA USA) and RadMD, LLC (Doylestown PA) were involved in the independent radiology review.
Declaration of interests
NAR has received personal fees from Bristol-Myers Squibb, Genentech, Roche, MedImmune, AstraZeneca, and Merck. TES has received research grant support from Bristol-Myers Squibb and personal fees from Genentech, Eli Lilly, Celgene, and Boehringer Ingelheim. SJA has received research grant support from MedImmune, and personal fees from Bristol-Myers Squibb, MedImmune, and AstraZeneca. LH has received research grant support from Astellas, has served as a non-paid consultant to Bayer and Xcovery, and has received personal fees from Bristol-Myers Squibb, Merck, Clovis, Helix Bio, and Genentech. HL has received personal fees from Bristol-Myers Squibb and Merck, and non-financial support from Bristol-Myers Squibb and Roche. BM has served as a consultant to Bristol-Myers Squibb and on advisory boards for Merck Sharp & Dohme. GAO has received research grant support from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, New Link Genetics, Genentech, and Boehringer Ingelheim, and has received personal fees from Genentech and Boehringer Ingelheim. DRG has received research grant support from Bristol-Myers Squibb. BPL has received personal fees from Eli Lilly, Genentech, Pfizer, Biodesix, and Boehringer Ingelheim. GZ has received personal fees from Bristol-Myers Squibb. JW has received research grants from Boehringer Ingelheim, Novartis, Pfizer, Roche, and Bayer, and has served on advisory boards for and received lecture fees from Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Clovis, Novartis, Pfizer, Roche, and Merck Sharp and Dohme. PJS has received personal fees, non-financial support, and support for clinical studies from Roche. FC has received personal fees from Bristol-Myers Squibb. CC has served as a consultant to Bristol-Myers Squibb, Merck, and Roche. RS has received research grant support and honoraria from and has served on advisory boards for Bristol-Myers Squibb. RMH has served on advisory boards for Bristol-Myers Squibb. CTH, CB, and BJL are employed by and own stock in Bristol-Myers Squibb. SSR has received research grant support and personal fees from Bristol-Myers Squibb and has received personal fees from Amgen, AstraZeneca, Aveo, Boehringer Ingelheim, Celgene, Genentech, Eli Lilly, and Novartis. SJA, DRG, AD, and SSR have received funding from the National Institutes of Health (USA). The other authors declare no competing interests.
References
- 1.Domingues D, Turner A, Dilla Silva M. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy. 2014;6:1221–35. doi: 10.2217/imt.14.82. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Horn L, Antonia S, et al. Clinical activity and safety of anti-PD1 (BMS-936558, MDX-1106) in patients with advanced non-small-cell lung cancer (NSCLC) Proc Am Soc Clin Oncol. 2012;30:7509. abstr. [Google Scholar]
- 7.Gettinger SN, Horn L, Gandhi L, et al. Long-term survival, clinical activity, and safety of nivolumab (anti-PD-1; BMS-936558, ONO-4538) in patients (Pts) with advanced non-small cell lung cancer (NSCLC) Int J Radiat Oncol. 2014;90:3428. abstr. [Google Scholar]
- 8.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–92. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004;59:828–36. doi: 10.1136/thx.2003.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–25. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penrod JR, Korytowsky B, Petrilla A, et al. Survival of U.S. Medicare patients with advanced non-small cell lung cancer by line of therapy. Proc Am Soc Clin Oncol. 2014;32:6582. abstr. [Google Scholar]
- 13.Scartozzi M, Mazzanti P, Giampieri R, et al. Clinical predictive factors for advanced non-small cell lung cancer (NSCLC) patients receiving third-line therapy: selecting the unselectable? Lung Cancer. 2010;68:433–37. doi: 10.1016/j.lungcan.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Massarelli E, Andre F, Liu DD, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. 2003;39:55–61. doi: 10.1016/s0169-5002(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 4.0. Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Department of Health and Human Services; May 28, 2009. [accessed Dec 18, 2014]. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 19.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 20.Greenwood M. Reports on Public Health and Medical Subjects. Vol. 33. London: HM Stationery Office; 1926. The natural duration of cancer; pp. 1–26. [Google Scholar]
- 21.Hodi S, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) Proc Am Soc Clin Oncol. 2013;31:3016. abstr. [Google Scholar]
- 23.Topalian SL, Sznol M, Brahmer JR, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: survival and long-term safety in a phase I trial. J Clin Oncol. 2013;31:3002. abstr. [Google Scholar]
- 24.Powderly JD, Koeppen H, Hodi FS, et al. Biomarkers and associations with the clinical activity of PD-L1 blockade in a MPDL3280A study. Proc Am Soc Clin Oncol. 2013;31:3001. abstr. [Google Scholar]
- 25.Taube JM, Klein AP, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garon EB, Gandhi L, Rizvi N, et al. Antitumor activity of pembrolizumab (Pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients (pts) with advanced non-small cell lung carcinoma (NSCLC) Ann Oncol. 2014;25:LBA43 (abstr). [Google Scholar]
- 27.Rizvi NA, Garon EB, Patnaik A, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC) Proc Am Soc Clin Oncol. 2014;32:8007. abstr. [Google Scholar]
- 28.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–67. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [accessed Dec 17, 2014];Iressa (gefitinib) US prescribing information. NDA 21-399/S-008,2004. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001016/WC500036358.pdf.
- 32. [accessed Dec 17, 2014];Tarceva (erlotinib) US prescribing information. 2014 Apr; http://www.gene.com/download/pdf/tarceva_prescribing.pdf.
- 33. [accessed Dec 17, 2014];Gemcitabine (Gemzar) US prescribing information. 2011 Feb; http://pi.lilly.com/us/gemzar.pdf.
- 34.Grande C, Villanueva MJ, Huidobro G, Casal J. Docetaxel-induced interstitial pneumonitis following non-small-cell lung cancer treatment. Clin Transl Oncol. 2007;9:578–81. doi: 10.1007/s12094-007-0106-4. [DOI] [PubMed] [Google Scholar]
- 35.Schuette WHW, Gröschel A, Sebastian M, et al. A randomized phase II study of pemetrexed in combination with cisplatin or carboplatin as first-line therapy for patients with locally advanced or metastatic non–small-cell lung cancer. Clin Lung Cancer. 2013;14:215–33. doi: 10.1016/j.cllc.2012.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.