Abstract
Connexin 43 (Cx43) hemichannels and gap junctions in osteocytes are responsive to mechanical loading, which is important for bone formation and remodeling. However, the mechanism of these Cx43-forming channels in the process of mechanical unloading is still not very clear. In this study, unloading caused by weightlessness was simulated by using a random position machine (RPM). Osteocytic MLO-Y4 cells were subjected to 2 h of RPM treatment, and levels of Cx43 mRNA and total and cell surface expressed protein were determined by quantitative real-time PCR, western blotting, and biotinylation analysis. Although mRNA was elevated by RPM, total protein level of Cx43 was not altered; however, surface biotinylated Cx43 was significantly reduced. Interestingly, RPM promoted the retention of Cx43 in the Golgi apparatus detected by co-immunofluorescence with antibodies against Cx43 and 58 K Golgi marker protein. Dye uptake assay showed that hemichannels were induced open after RPM for 2 h. Consistently, prostaglandin E2 release was increased and this increase was completely attenuated with the treatment of a Cx43 hemichannel blocking antibody. Together, this study demonstrates increased activity of Cx43 hemichannels to RPM, and active Cx43 hemichannels with prostaglandin E2 release are likely to module biological function under simulated weightless conditions.
Keywords: connexin43, hemichannel, osteocyte, random position machine
Bone tissue modeling and remodeling are greatly impacted by mechanical loading. Bone cells, especially osteocytes are highly responsive to mechanical stimulation. Osteocytes, the most abundant cell type embedded in bone mineral matrix, are well known as a major coordinator to orchestrate the responses of bone cells to mechanical loading. In osteocytes, one of the important mechanical sensing molecules is connexin 43 (Cx43), which forms gap junctions and hemichannels1,2 and is involved in the transduction of mechanical signals between adjacent cells or between the cell and extracellular environment, respectively.3,4 Fluid flow shear stress promotes the opening of mechanosensitive hemichannels in osteocytic MLO-Y4 cells,5 leading to the release of regulatory factors, such as prostaglandin E2 (PGE2)6 and ATP.7 These factors act in an autocrine/paracrine fashion to regulate the functions of other bone cells, including osteoblasts and osteoclasts.8,9,10
Bone loss is a serious physiological problem for astronauts. Weightlessness causes 1–2% decrease of bone mass during space flight. Fourteen-day space flight leads to increased numbers of empty osteocytic lacunae and destruction of osteocytes in bone tissue.11 Also 12.5-day space mission reduces the osteocytes in rat cortical bone periosteum.12 The involvement of Cx43 in response to the unloading regimes in vivo has been reported using Cx43 knockout mouse models. Lloyd et al. investigated the response of osteoblast/osteocyte-specific deletion of Cx43 mice to three weeks of mechanical unloading using hindlimb suspension (HLS), a model used to simulate the effects of weightlessness on the ground.13 They found that Cx43 deficiency desensitized bone to the effects of mechanical unloading.14 Another study by Grimston et al. showed similar observation in osteoblast/osteocyte-specific Cx43 knockout mice using a muscle paralysis unloading model with the treatment of botulinum toxin A (BtxA).15 However, the cellular mechanism with regards of the role of Cx43 in weightless conditions is largely unknown. We showed earlier that gravity changes decreased the expression of Cx43 in MLO-Y4 cells after 24 h of parabolic flight.16 In this study, we investigated the effects of mechanical unloading induced by random position machine (RPM) on the expression and cellular distribution of Cx43, the activity of hemichannels and secretion of PGE2 in MLO-Y4 cells. RPM was used to simulate the effects of weightlessness, in which the direction of gravity vector varied continuously so that cells could not respond to gravity. RPM has been reported to generate similar biological responses as weightlessness conditions.17,18
MATERIALS AND METHODS
Cell Culture
The MLO-Y4 cell line was generously provided by Dr. Lynda Bonewald (University of Missouri-Kansas City).1 The cells were cultured in a-MEM (Life Technologies, CA) with 2.5% fetal bovine serum (FBS) (Life Technologies) and 2.5% calf serum (CS) (Life Technologies) including 1% penicillin/streptomycin.
Random Position Machine (RPM)
RPM was manufactured by the Center for Space Science and Applied Research of the Chinese Academy of Sciences.19 This device is composed of an inner and an outer frame, and the rotation speed and direction of each frame are automatically regulated by a program (Fig. 1). Cells were cultured on glass slides (2.0×6.0 cm2) at the density of 1.0×104/cm2 for 24 h. The slides were then placed in the flasks specially designed for RPM. The dimension of the flask is 4 cm (L)×2.5 cm (W)×1.8 cm (H), with a total volume of 20 ml. The flasks were filled completely with medium and tightly capped to avoid bubbles and subsequent fluid shear stress (without air or CO2 permeation), and then randomly divided into control and RPM-treated groups. For RPM-treated group, the flasks were placed in inner frame of the RPM. The random mode of speed (0.1–10 rpm) and direction (three dimensions) were chosen for the inner and outer frames.17,20 The time averaged gravitational vector acting on these samples was reduced to 2×10−3 g at the center of the frame.21 The static control cells were cultured in the same incubator with RPM-treated groups at 37°C. After 2 h, the cells or the conditioned medium were collected for the next experiments.
Figure 1.
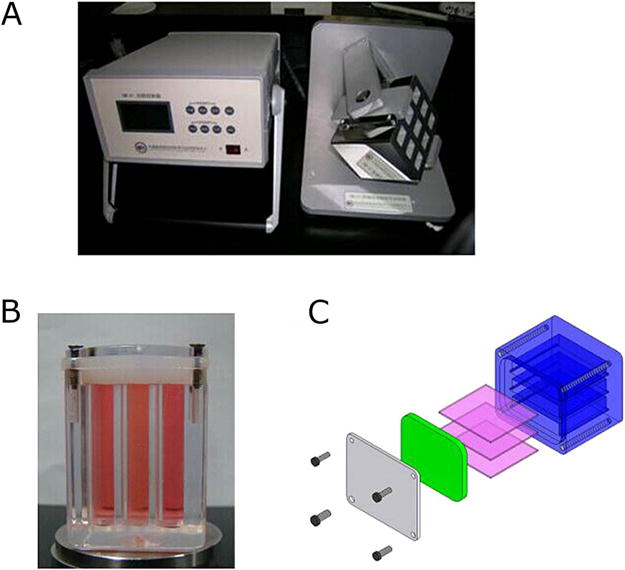
Random position machine (RPM) was used to simulate the effects of weightlessness. (A) RPM set up. Left panel is the controller to control the rotation speed and direction; right panel shows the inner and outer frames. (B) The flask specially designed for RPM. (C) The internal structure of the flask, which is tightly capped by silicone pad and screws, and three glass slides are placed into the flask at a time.
Haematoxylin and Eosin (H&E) Staining
Cells on slides were washed twice with phosphate-buffered saline (PBS), pH 7.4 and fixed in 0.5% glutaraldehyde solution in PBS for 10 min. After incubated in 0.5% hematoxylin for 10 min and 0.5% eosin for 7 min, respectively, the cells were dehydrated by a series of ethanol solutions with increasing concentrations, and then made transparent with dimethylbenzene. The slides were sealed and observed under phase microscope.
PGE2 Measurement
MLO-Y4 cells were preincubated with Cx43(E2) antibodies (1:50 dilutions) specific to block the hemichannels22 for 30 min and were then subjected to RPM for 2 h. The flasks were removed from RPM and the conditioned medium was collected. The extracellular PGE2 released into the medium was measured using PGE2 EIA kit (Cayman, Ann Arbor, MI) according to the manufacturer’s instruction.
Quantitative Real-Time PCR Analysis
Total RNA was extracted using Trizol (Invitrogen, CA). Reverse transcription experiment was performed based on the protocol provided with the kit (TaKaRa, Dalian, China). Primers were designed and synthesized (Sangon, Shanghai, China) based on the sequence information available in GenBank. These primer sequences are shown in Table 1. SYBR Green real-time relative quantitative RT-PCR analysis was performed with SYBR Premix Ex TaqTM based on the manufacturer’s protocol (TaKaRa). Each sample was measured in triplicate and normalized to 18S rRNA. The Livak method was used to estimate the relative change in gene expression.23
Table 1.
PCR Primer Sequence
| Gene Name | Primer Sequences | Annealing Temperature (°C) |
|---|---|---|
| cox-2 | F5GGTCTGGTGCCTGGTCTGATGAT3 R5CTGCTGGTTTGGAATAGTTGCTC3 |
55 |
| 18S | F5AATCAGGGTTCGATTCCGGA3 R5 CCAAGATCCAACTACGAGCT3 |
55 |
Immunofluorescence Labeling
The cells were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature (RT), rinsed three times with PBS for 5 min each time and permeabilized with 0.25% TX-100 (in PBS) for 30 min. After rinsed twice with PBS, cells were blocked with 3% BSA in PBS for 30 min at RT. The cells were then incubated overnight at 4°C with affinity-purified antibodies against Cx43 (E2, 1:300 dilution) and Golgi 58 K protein (1:50) (Abcam, Cambridge, MA), followed by rinsing four times with PBS and incubating 1.5 h with fluorescein isothiocyanate (FITC) or rhodamine labeled secondary antibody (1:50) (EarthOx, CA). Hoechst dye (5 μg/ml) (Beyotime, Nantong, China) was used for nucleus staining. The slides were sealed and observed under confocal laser fluorescence microscope (Leica TCS SP5, Germany). The extent of colocalization of Cx43 and Golgi bodies was quantified using the Pearson’s correlation coefficient (Rr) and Overlap coefficient (R) with NIH Image J software.
Dye Uptake Assay
Dye uptake assay was performed according to previous protocol.24 MLO-Y4 cells were cultured at density of 7.0×103/cm2 for 24 h (most of cells were not in contact with each other), then subjected to 2 h of RPM or Cx43(E2) antibodies (1:300 dilution) treatment, and followed by washing three times, 5 min each with recording solution (154 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4). The cells were exposed to ethidium bromide (Etd+) (TaKaRa) for 5 min and followed by rinsing three times with PBS. Cells were fixed with 2% paraformaldehyde in PBS for 30 min and observed under fluorescence microscope. Image J software was used to analyze dye uptake. In each visual field, integrated optical density was calculated, and the extent of dye uptake was determined as a ratio of integrated optical density (IOD) to total cell number.
Biotinylation Analysis
Biotinylation analysis was performed according to previous protocol.6 MLO-Y4 cells were labeled with or without 1 mg/ml EZ-link Sulfo-NHS-LC Biotin (Thermo Scientific, NH) in PBS at 4°C for 20 min and the biotinylation analysis was performed according to the manufacturer’s instructions (Pierce Chemical, IL). The cell lysates were immunoblotted with Cx43(E2) (1:300 dilution) or GAPDH antibody. The cell surface expression of Cx43 was determined as a ratio of biotinylated Cx43 to total Cx43 protein.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 statistics software (San Diego, CA). All data were obtained from at least three replicates and presented as mean value standard deviation (SD). One-way analysis of variance (ANOVA) and Student Newman–Keuls test were used to compare groups. Asterisks indicate the degree of significant differences compared with the controls (*p < 0.05; **p < 0.01; ***p < 0.001).
RESULTS
RPM Decreased the Surface Expression of Cx43, But Had No Effect on Total Cx43 Protein
Osteocytic MLO-Y4 cells were subjected to RPM treatment for 2 h and no detectable change of cellular morphology was observed (Fig. 2). Quantitative real-time PCR and western blotting were used to determine levels of Cx43 mRNA and protein. Cx43 mRNA expression was significantly increased (Fig. 3A), while total protein level of Cx43 was not affected by RPM treatment (Fig. 3B). To assess the level of Cx43 on the cell surface, biotinylation assay was performed, and biotinylated and total control samples were analyzed by western blotting. Compared to total Cx43, the biotinylated Cx43 protein was significantly reduced by RPM (Fig. 3C and D). Therefore, RPM reduced the cell surface expression of Cx43, but not the total Cx43.
Figure 2.
Cell morphology of MLO-Y4 cells was not altered after 2 h of RPM treatment. Cells were stained with hematoxylin and eosin (upper panels) or Hoechst dye (5 μg/ml) (lower panels) and observed under phase or fluorescence microscope. Bar, 50 μm.
Figure 3.
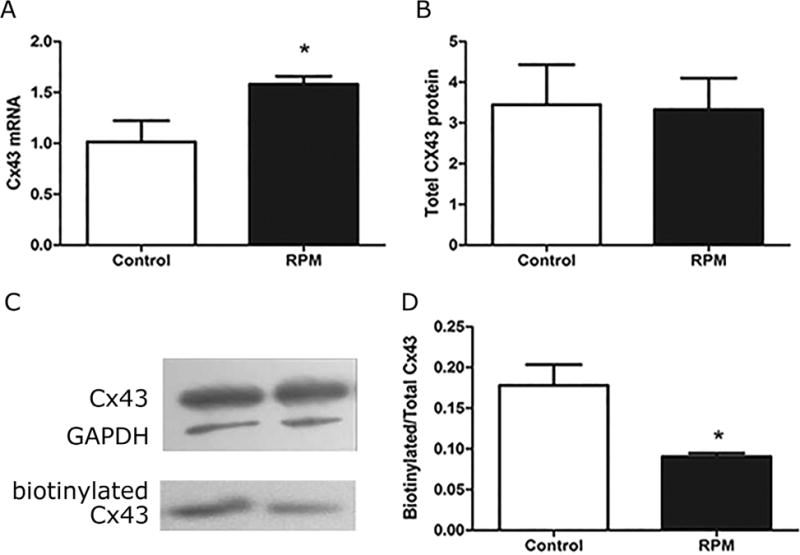
Two hours of RPM treatment had no effect on total protein level of Cx43, but decreased the amount of Cx43 on cell surface. Quantitative real time PCR and western blotting were performed to determine the expression levels of Cx43 mRNA (A) and protein (B), respectively. The surface expression of Cx43 was determined by biotinylation assay. Cell extracts bound to avidin beads were immunoblotted with Cx43(E2) (1:300 dilution) or GAPDH (1:2,000) antibody. (C) The intensity of protein bands was quantified by densitomery (D). *p < 0.05.
RPM Reduced the Delivering of Cx43 From the Golgi to Plasma Membrane
Cx43 hemichannels (connexons) are reported to be assembled in trans-Golgi network before migrating to the plasma membrane.25 Confocal microscopy detection showed that after the treatment with RPM for 2 h, more Cx43 protein was accumulated in the Golgi bodies and less on the cell surface compared to control, as shown by co-localization of Cx43 and Golgi 58 K marker protein (Fig. 4A). Quantification analysis using Pearson’s correlation co-efficient (Rr-value) (Fig. 4B, left panel) or overlap coefficient (R-value) (Fig. 4B, right panel) further confirmed the significant increase of Cx43 protein accumulated in the Golgi.
Figure 4.
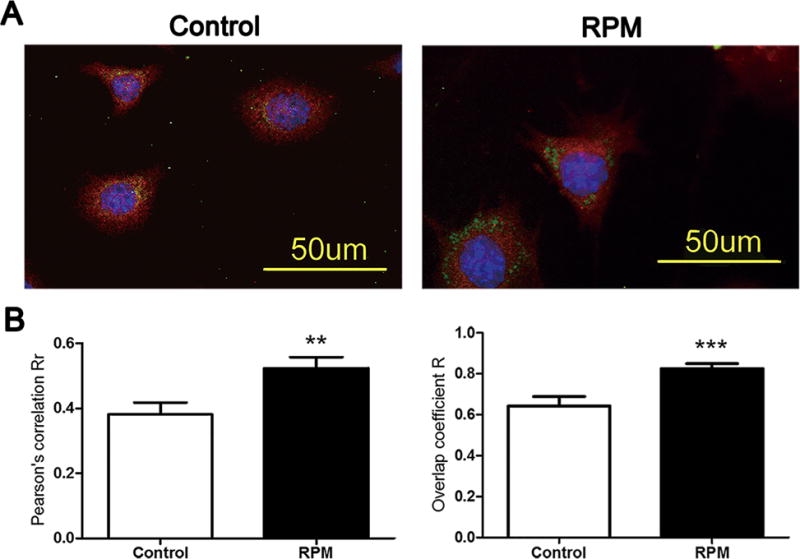
Cx43 was retained in the Golgi bodies after 2 h of RPM. MLO-Y4 cells were double labeled with antibodies against Cx43 (red, 1:300) and 58 K Golgi marker (Green, 1:50) antibodies and, followed by rhodamine and FITC-conjugated secondary antibodies, respectively (A). The degree of colocalization of Cx43 and 58 K was analyzed using the Pearson’s correlation coefficient (Rr) (left panel) and Overlap coefficient (R) (right panel) using the Image J software (B). **p < 0.01; ***p < 0.001.
RPM Promoted the Opening of Hemichannels and PGE2 Release
The activities of Cx43 hemichannels in response to RPM were studied using Etd+ dye uptake assay. The quantification analysis using integrated optical density showed that opening of hemichannels detected by the uptake of Etd+ dye was significantly enhanced by RPM as compared to non-treated control in MLO-Y4 cells (Fig. 5). The uptake assay showed that Cx43 (E2) antibody significantly blocked the opening of hemichannels (Fig. 6A and B). RPM also significantly increased the release of PGE2 and this release was abrogated by Cx43(E2) antibodies (Fig. 6C). However, the mRNA expression of cyclooxygenase 2 (Cox-2), an inducible enzyme required for PGE2 synthesis, was not influenced by RPM treatment (Fig. 6D).
Figure 5.
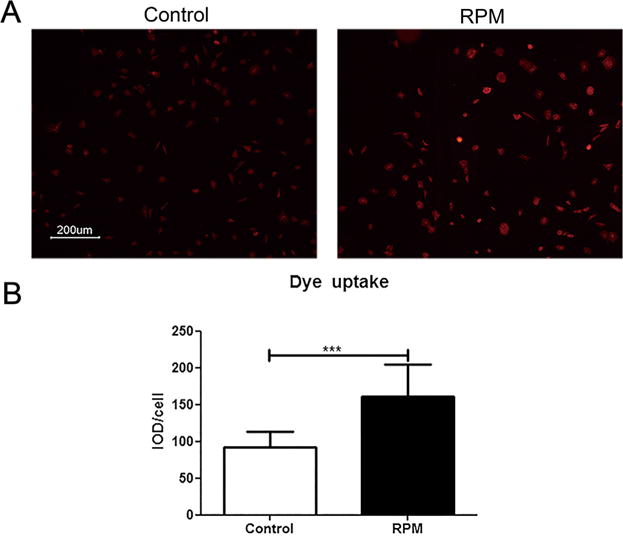
The opening of hemichannels was promoted by 2 h of RPM treatment. Etd+ dye uptake was performed after cells were subjected to RPM for 2 h. Etd+ dye uptake was observed under fluorescence microscope (A) and quantified and presented as a ratio of integrated optical density (IOD) to total cell number using Image J (B). ***p < 0.001.
Figure 6.
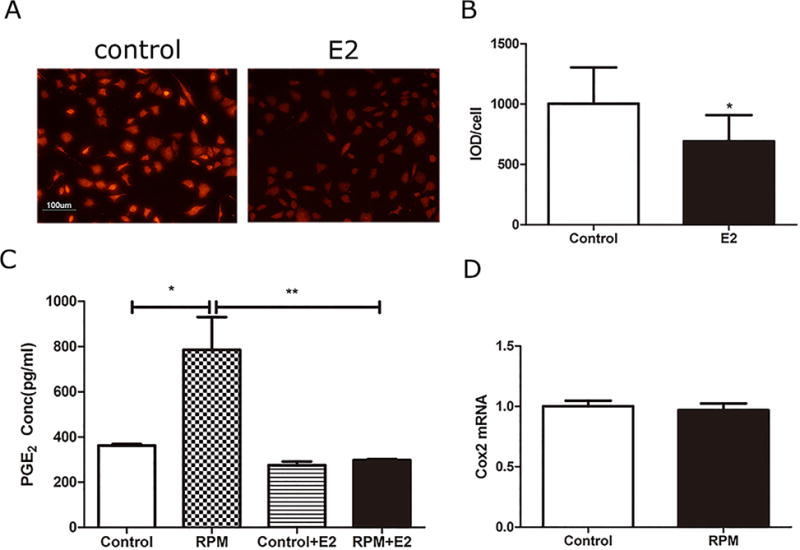
The increase of PGE2 release in response to RPM treatment was mediated by active Cx43 hemichannels, but not by the increase of Cox-2 expression. Two hour-RPM treatment increased Etd+ dye uptake in MLO-Y4 cells and this uptake was significantly inhibited by Cx43(E2) antibody (E2) (A and B). RPM treatment promoted the release of PGE2, which was inhibited by Cx43(E2) (E2) antibody (C). Cox-2 mRNA expression was not altered by 2 h of RPM treatment in MLO-Y4 cells (D). *p < 0.05; **p < 0.01.
DISCUSSION
Mechanical loading plays a critical role in skeletal tissues to maintain normal bone structure and function. In last decade or so, osteocytes are being recognized as a major coordinator in modulating biological responses of other bone cells to mechanical loading.26,27 Osteocytes sense and translate the mechanical stimulation to chemical signals, and transmit to other bone cells through gap junctions between adjacent cells, or hemichannels between cells and extracellular environment.28,29 In osteocytes, Cx43 is the most abundant connexins responsible for the formation of gap junctions and hemichannels, therefore plays a key role in mediating mechanotransduction. Mechanical stimuli are known to affect the expression and cellular distribution of Cx43 in osteocytes. Cheng et al. reported that 2 h of FFSS caused a redistribution of Cx43 from a location around the nucleus to punctate spots in the cytoplasm and in the dendritic processes in MLO-Y4 cells.30 Fluid flow shear stress (FFSS) enhances the cell surface expression of Cx43,6,22 and this enhancement sustains up to 24-h after FFSS.31 In contrast to all other studies, Thi et al. have shown that in MLO-Y4 cells membrane-bound Cx43 downregulates at both 5 and 20 dyn/cm2 of flow shear stress, whereas cytosolic Cx43 noticeably increases in response to both levels of shear stress.32 Several papers have reported the graviresponses of Cx43 expression in osteocytes. The study from our laboratory shows a reduction of Cx43 expression after hypo- and hyper-gravity treatment provided by parabolic flight.16 Matsuda et al. have shown that gap junction communication is significantly decreased after 24 h of multi-dimensional gravity in MLO-Y4 cells.33 Cx43 deficiency mouse model shows desensitization of the bone to simulated weightlessness modeled by HLS.14 Grimston et al. also showed similar results after BtxA injection to simulate mechanical unloading.15 These two studies have shown that conditional deletion of Cx43 in osteoblasts and osteocytes are partially resistant to the cortical bone loss induced by mechanical unloading, indicating that Cx43 is involved in the bone responses to weightlessness conditions. Here, we showed that the total Cx43 protein expression was not changed by 2 h of RPM treatment, though a slight increase of Cx43 mRNA was observed. It is unlikely that RPM causes a general effect on protein expression, because in our previous studies the changes of protein expression pattern were not homogenous, some proteins increased, while the others decreased.19,34 Moreover, we showed that RPM had no effect on cell morphology, cell viability and apoptosis. The increased Cx43 mRNA without changes in protein level can be explained by delayed protein translation or/and increased degradation. Further studies will be required to sort out these possibilities.
Cx43 is reported to be localized in the trans-Golgi network where it assembled from monomers to hexameric connexons and then transported to plasma membrane to form hemichannels or gap junctions.35 Mechanical simulation promotes the migration of Cx43 from Golgi to the cell surface to form mechanosensitive hemichannels.36 Contrary to mechanical stimulation, we showed here that lesser amount of Cx43 protein was present on the cell surface after RPM treatment, while the total amount of Cx43 was not changed compared with the control. The results suggest that RPM treatment increases the retention of Cx43 in Golgi bodies, and reduces the transport of Cx43 from Golgi to plasma membrane. A previous study reports that the interaction with 14-3-3 assists the delivery of Cx43 to plasma membrane to form hemichannels, and knocking down 14-3-3 expression reduces the surface expression of Cx43 by over 50%.36 It is plausible that the decrease of membrane transport of Cx43 by RPM could be caused by the downregulation of 14-3-3.
In general, hemichannel activity is correlated with cell surface expression level of connexins. However, we observed inverse responses to RPM by which surface expressed Cx43 was reduced while hemichannel opening was enhanced. A previous study in HeLa cells also showed that enhanced activity of hemichannels was inversely correlated with cell surface levels of Cx46 protein.24 The results indicated that increased Cx43 hemichannels is not directly regulated through the enhancement of Cx43 expression. Firstly, only small percentage of Cx43 on cell surface forms hemichannels.37 In addition to protein level, connexin channels are regulated through multiple factors including protein phosphorylation, intracellular trafficking, assembly and direct protein– protein interaction.38,39 Second, increased activity may actually reflect altered permeability (gating) of functioning hemichannels.24
We showed that RPM treatment increased the release of PGE2 and this release was attenuated with the inhibition of Cx43 hemichannels. This result suggests that hemichannels mediate PGE2 release in response to RPM. Our previous work also shows that PGE2 production increases after subjecting MLO-Y4 cells to 48 h of two-dimensional (2D) clinostat treatment.40 Simulated weightlessness by RPM and 2D clinostat both promote the release of PGE2. Moreover, previous studies have shown that osteocytes release PGE2 by Cx43 hemichannels in response to mechanical stress.6,28,31,41,42 Both mechanical loading and simulated weightlessness cause PGE2 release, which may indicate a common response of osteocytes to changed physical environment,. As a key regulatory factor of bone metabolism, PGE2 could modulate bone remodeling via the regulation of both bone-forming osteoblasts and bone-resorbing osteoclasts.8,43–45 Furthermore, intermittent treatment with PGE2 is anabolic, while continuous PGE2 treatment is catabolic with a negative balance on bone.46 Cox-2 mediates the biosynthesis of PGE2 and its mRNA expression is increased in response to mechanical stimulation.47 But in our study, RPM had no effect on the level of Cox-2 mRNA. Therefore, the increased release of PGE2 is unlikely caused by its biosynthesis.
Together, our results suggest that short-term RPM treatment reduces surface expression of Cx43, which may be caused by the inefficient transport of Cx43 from Golgi bodies to plasma membrane. RPM promotes the opening of hemichannels with increased release of PGE2. This study suggests that Cx43 hemichannel is likely to be a key factor in mediating biological function of bone cells in response to weightlessness.
Acknowledgments
The authors thank Mingzhi Luo for the photos of Figure 1.
Grant sponsor: National Natural Science Foundation of China; Grant numbers: 31328016, 31170812, 81472090; Grant sponsor: Natural Science Basic Research Plan in Shaanxi Province; Grant number: 2015JM8443; Grant sponsor: Fundamental Research Funds for the Central Universities; Grant number: 3102014JKY15012; Grant sponsor: National Institute of Health; Grant number: EY012085; Grant sponsor: Welch Foundation; Grant number: AQ-1507.
Footnotes
Conflicts of interest: All authors state that they have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Research design: HX, JXJ, and PS; Study conduct: RL, DN, JZ, RY, and JL; Data acquisition and analysis: HX, JXJ, RL, DN, JZ, and MAR; Drafting and revising manuscript: HX, JXJ, and PS. All authors have read and approved the final submitted manuscript.
References
- 1.Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J Bone Miner Metab. 1999;17:61–65. doi: 10.1007/s007740050066. [DOI] [PubMed] [Google Scholar]
- 2.Gu G, Nars M, Hentunen TA, et al. Isolated primary osteocytes express functional gap junctions in vitro. Cell Tissue Res. 2006;323:263–271. doi: 10.1007/s00441-005-0066-3. [DOI] [PubMed] [Google Scholar]
- 3.Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473:188–192. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yellowley CE, Li Z, Zhou Z, et al. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 5.Burra S, Nicolella DP, Francis WL, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci USA. 2010;107:13648–13653. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherian PP, Siller-Jackson AJ, Gu S, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genetos DC, Kephart CJ, Zhang Y, et al. Oscillating fluid flow activation of gap junction hemichannels induces atp release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhary S, Blackwell K, Voznesensky O, et al. Prostaglandin E2 acts via bone marrow macrophages to block PTH-stimulated osteoblast differentiation in vitro. Bone. 2013;56:31–41. doi: 10.1016/j.bone.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genetos DC, Yellowley CE, Loots GG. Prostaglandin E2 signals through PTGER2 to regulate sclerostin expression. PLoS ONE. 2011;6:e17772. doi: 10.1371/journal.pone.0017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batra N, Kar R, Jiang JX. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim Biophys Acta. 2012;1818:1909–1918. doi: 10.1016/j.bbamem.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodionova NV, Oganov VS, Zolotova NV. Ultrastructural changes in osteocytes in microgravity conditions. Adv Space Res. 2002;30:765–770. doi: 10.1016/s0273-1177(02)00393-9. [DOI] [PubMed] [Google Scholar]
- 12.Doty SB. Space flight and bone formation. Materwiss Werksttech. 2004;35:951–961. doi: 10.1002/mawe.200400840. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd SA, Lewis GS, Zhang Y, et al. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27:2359–2372. doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd SA, Loiselle AE, Zhang Y, et al. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57:76–83. doi: 10.1016/j.bone.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimston SK, Goldberg DB, Watkins M, et al. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res. 2011;26:2151–2160. doi: 10.1002/jbmr.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di SM, Qian AR, Qu LN, et al. Graviresponses of osteocytes under altered gravity. Adv Space Res. 2011;48:1161–1166. [Google Scholar]
- 17.v Loon JJWA. Some history and use of the random positioning machine, RPM, in gravity related research. Adv Space Res. 2007;39:1161–1165. [Google Scholar]
- 18.Dedolph RR, Dipert MH. The physical basis of gravity stimulus nullification by clinostat rotation. Plant Physiol. 1971;47:756–764. doi: 10.1104/pp.47.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo M, Yang Z, Li J, et al. Calcium influx through stretch-activated channels mediates microfilament reorganization in osteoblasts under simulated weightlessness. Adv Space Res. 2013;51:2058–2068. [Google Scholar]
- 20.Infanger M, Kossmehl P, Shakibaei M, et al. Simulated weightlessness changes the cytoskeleton and extracellular matrix proteins in papillary thyroid carcinoma cells. Cell Tissue Res. 2006;324:267–277. doi: 10.1007/s00441-005-0142-8. [DOI] [PubMed] [Google Scholar]
- 21.Maccarrone M, Battista N, Meloni M, et al. Creating conditions similar to those that occur during exposure of cells to microgravity induces apoptosis in human lymphocytes by 5-lipoxygenase-mediated mitochondrial uncoupling and cytochrome c release. J Leukocyte Biol. 2003;73:472–481. doi: 10.1189/jlb.0602295. [DOI] [PubMed] [Google Scholar]
- 22.Siller-Jackson AJ, Burra S, Gu S, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Ren Q, Riquelme MA, J Xu, et al. Cataract-causing mutation of human connexin 46 impairs gap junction, but increases hemichannel function and cell death. PLoS ONE. 2013;8:e74732. doi: 10.1371/journal.pone.0074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 26.Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10:259–264. doi: 10.1080/cac.10.4-6.259.264. [DOI] [PubMed] [Google Scholar]
- 29.Yellowley CE, Li Z, Zhou Z, et al. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 30.Cheng B, Zhao S, Luo J, et al. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 31.Siller-Jackson AJ, Burra S, Gu S, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thi MM, Kojima T, Cowin SC, et al. Fluid shear stress remodels expression and function of junctional proteins in cultured bone cells. Am J Physiol Cell Physiol. 2003;284:C389–C403. doi: 10.1152/ajpcell.00052.2002. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda J, Kurata K, Fukunaga T, et al. Bone marrow cell differentiation regulated by gel-embedded osteocyte under multi-dimensional gravity. J Biomech. 2006;39:S469–S469. [Google Scholar]
- 34.Hu LF, Qian AR, Wang Y, et al. Inhibitory effect of simulated microgravity on differentiating preosteoblasts. Adv Space Res. 2013;51:107–114. [Google Scholar]
- 35.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 36.Batra N, Riquelme MA, Burra S, et al. 14-3-3theta Facilitates plasma membrane delivery and function of mechanosensitive connexin 43 hemichannels. J Cell Sci. 2013;127:137–146. doi: 10.1242/jcs.133553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batra N, Riquelme MA, Burra S, et al. Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation. J Biol Chem. 2014;289:10582–10591. doi: 10.1074/jbc.M114.550608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao X, Lee SC, Reuss L, et al. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc Natl Acad Sci USA. 2007;104:4919–4924. doi: 10.1073/pnas.0603154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Wu J, Weng Y, et al. Two-dimensional clinorotation influences cellular morphology, cytoskeleton and secretion of MLO-Y4 osteocyte-like cells. Biologia. 2012;67:255–262. [Google Scholar]
- 41.Burra S, Jiang JX. Connexin 43 hemichannel opening associated with Prostaglandin E(2) release is adaptively regulated by mechanical stimulation. Commun Integr Biol. 2009;2:239–240. doi: 10.4161/cib.2.3.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein-Nulend J, Burger EH, Semeins CM, et al. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12:45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez-Yanez GO, Symons AL. Prostaglandin E2 affects osteoblast biology in a dose-dependent manner: an in vitro study. Arch Oral Biol. 2012;57:1274–1281. doi: 10.1016/j.archoralbio.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Matemba SF, Lie A, Ransjö M. Regulation of osteoclastogenesis by gap junction communication. J Cell Biol. 2006;99:528–537. doi: 10.1002/jcb.20866. [DOI] [PubMed] [Google Scholar]
- 45.Kaji H, Sugimoto T, Kanatani M, et al. Dexamethasone stimulates osteoclast-like cell formation by directly acting on hemopoietic blast cells and enhances osteoclast-like cell formation stimulated by parathyroid hormone and prostaglandin E2. J Bone Miner Res. 1997;12:734–741. doi: 10.1359/jbmr.1997.12.5.734. [DOI] [PubMed] [Google Scholar]
- 46.Tian XY, Zhang Q, Zhao R, et al. Continuous infusion of PGE2 is catabolic with a negative bone balance on both cancellous and cortical bone in rats. J Musculoskelet Neuronal Interact. 2007;7:372–381. [PubMed] [Google Scholar]
- 47.Norvell SM, Ponik SM, Bowen DK, et al. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004;96:957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]


