Abstract
BACKGROUND & AIMS
Sustained virological response (SVR) to antiviral therapy for hepatitis C virus (HCV) correlates with changes in biochemical measures of liver function. However, little is known about the long-term effects of SVR on liver fibrosis. We investigated the effects of HCV therapy on fibrosis, based on the Fibrosis-4 (FIB4) score, over a 10-year period.
METHODS
We collected data from participants in the Chronic Hepatitis Cohort Study—a large observational multicenter study of patients with hepatitis at 4 US health systems—from January 1, 2006, through December 31, 2013. We calculated patients’ FIB4 score and the aminotransferase-to-platelet ratio index (APRI) score over a 10-year period. Of 4731 patients with HCV infection, 1657 (35%) were treated and 755 (46%) of these patients achieved SVR.
RESULTS
In propensity score–adjusted analyses, we observed significant longitudinal changes in FIB4 score that varied with treatment and response to treatment. In patients achieving SVR, FIB4 scores decreased sharply, remaining significantly lower over the 10-year period than in untreated patients or patients with treatment failure (P < .001). In independent analyses, men and patients with HCV genotype 1 or 3 infections had higher FIB4 scores than women or patients with HCV genotype 2 infections (P < .01 for both). Findings were similar in a sensitivity analysis that substituted the APRI as the marker of fibrosis instead of the FIB4 score.
CONCLUSIONS
SVR to HCV treatment appears to induce long-term regression of fibrosis based on FIB4 scores collected over 10 years from a large observational study of US hepatitis patients. Patients receiving no treatment or with treatment failure had progressive increases in FIB4 scores.
Keywords: CHeCS, Fibrosis Progression, Growth Curve Analysis of Trajectory, Outcome
The extent to which antiviral therapy for hepatitis C virus (HCV) alters the course of fibrosis progression is not well understood. Various studies have described fibrosis progression in untreated patients, and several histologic studies have shown improvements in fibrosis after sustained virological response (SVR) to therapy.1–7
Although liver biopsy may be used to assess fibrosis, its use is decreasing.8 A noninvasive, easily obtained, and inexpensive marker of liver fibrosis may address challenges in longitudinal monitoring of HCV patients, especially in large cohorts or when more expensive or complex tests—such as liver biopsy and transient elastography—are not routinely available. Serum-based markers of fibrosis, including the Fibrosis-4 (FIB4) score and the aminotransferase-to-platelet ratio index (APRI), have been validated in controlled environments; these measures also are used in clinical trials of antiviral treatments. We have further validated these markers in the Chronic Hepatitis Cohort Study (CHeCS) HCV population; the validated area under the receiver operator characteristic curve was 0.83 to 0.85 for FIB4 and 0.80 to 0.81 for APRI.9,22 Changes in FIB4 have been shown to correlate with changes in liver fibrosis stage over time in HCV patients,10,16 and have been used to compare progression of liver fibrosis between HCV-positive and HCV-negative patients in the Veterans’ Affairs health care system.11,12 Likewise, FIB4 and APRI have been used as end points for post-treatment and post-SVR outcomes in HCV and human immunodeficiency virus (HIV)/HCV co-infected patients.
We investigated the impact of HCV treatment therapy on longitudinal change of FIB4 values collected across 10 years of follow-up evaluation in a subset of HCV-infected patients from CHeCS.13
Materials and Methods
Chronic Hepatitis Cohort Study
CHeCS is a retrospective/prospective, observational multicenter study that includes hepatitis patients from 4 large US health systems; the study design and execution have been described previously.14,15 CHeCS follows US Department of Health and Human Services guidelines for the protection of human subjects; protocols were approved by the institutional review boards at each site. Briefly, electronic administrative claims data and electronic health records (EHRs) for HCV patients age 18 years and older who received health services at any study site between January 1, 2006, and December 31, 2013, were used to identify study candidates; eligibility was confirmed during medical chart abstraction. To reflect a real-world setting, patients were excluded if they had participated in a clinical trial of HCV antiviral therapy or were co-infected with hepatitis B; HIV co-infected patients remained eligible for inclusion.
For each patient, observation commenced at the index date. For treated patients, this was defined as the date of first treatment initiation. For untreated patients, an automated computer algorithm using a frequency-matched approach was used to select index dates at or after the HCV diagnosis date (the earliest date of an HCV-associated diagnosis code and/or a positive laboratory test in the patient’s medical records). We also performed a sensitivity analysis by substituting the last treatment initiation date for the index date to include prior treatment experience at baseline. Patients receiving ongoing HCV treatment without sufficient follow-up evaluation or those who received a liver transplant before their index date were excluded.
Outcome
We used the FIB4 score as the outcome of interest and APRI for the sensitivity analysis, calculated using the following formulas:
We used laboratory tests collected within 7 days of one another and patient age at the time of laboratory assessment. Aminotransferase levels and platelet counts measured during antiviral therapy were excluded because therapy can influence these parameters.16 The FIB4 score and APRI were summarized using a median smoother for every 90-day interval. Because of non-normality, data were log-transformed. Patients with at least 2 FIB4 intervals after the index date were included in the analysis.
Control Variables at Index Date
Demographic information included age, sex, race/ ethnicity, estimated median annual household income, and insurance status at index date. Racial/ethnic classifications included Native American, Asian, black, white, or Pacific Islander/Hawai’ian. For analytic purposes, Asian and Pacific Islander/Hawai’ian was considered a single group. Insurance status (insured/not insured) was classified by the encounter nearest the index date. Consistent with our published results,14 income was categorized using US Census variable P053001.17 Index year was used as a baseline control variable.
A subset of commonly known risk factors (age, sex, race, HCV genotype [GT], Charlson/Deyo index [calculated from inpatient, outpatient, and claims data for 12 months before the index date], diabetes diagnosis, and recent drug/alcohol abuse) were selected as covariates to study the effect of HCV treatment on progression of liver fibrosis. Additional variables were included in the analysis to adjust for possible confounding owing to treatment selection bias. Clinical data captured before and at index included comorbid conditions,18 HIV co-infection, and laboratory testing. We also used International Classification of Diseases, 9th revision diagnostic and procedure codes as well as Current Procedural Terminology-version 4 procedure codes to assess possible contraindications to antiviral therapy (Supplementary Table 1).
Anti–Hepatitis C Virus Therapy and Response
Detailed antiviral medication data (drug name, start/ stop dates) were collected via chart abstraction at or after the index date. Combination therapy was identified when multiple hepatitis drugs were administered concomitantly. Data on routine HCV RNA quantification tests were obtained via the EHR. Patients were classified as having achieved SVR if laboratory tests collected 12 weeks or more after therapy showed undetectable viral RNA loads; otherwise they were classified as having treatment failure (TF). Treatment status (SVR, TF, or untreated) was considered a time-varying covariate.
Statistical Analysis
We hypothesized that liver fibrosis would change over time, and that changes would be associated with antiviral HCV therapy as well as known risk factors. Before our analysis, however, we assessed possible selection bias for receipt of antiviral therapy. If bias was present, we used a weighted propensity-score method to adjust for differences between patients who had or had not received antiviral therapy. This method included logistic regression modeling of the controlling baseline covariates. Patient data were weighted based on the inverse propensity to receive/not receive antiviral therapy (the IPTW approach).19 Covariate balance was checked after adjustment.
We then examined the effects of treatment and time on FIB4 progression (in a natural log scale) using mixed models with specific covariance patterns for longitudinal data, adjusted by IPTW. Specifically, the trajectory of FIB4 for each individual was modeled as a function of time and of time-varying patient treatment group variables. Time was treated as a nominal class variable. We considered a variety of covariance structures, including directly-specified compound symmetry, autoregressive with/without heterogeneous variances, and indirectly specified structures, based on random coefficients in time. Any variable with individual (univariate) effects on the change in FIB4 was a candidate for the initial model. We used the Bayesian Information Criterion (BIC) to assess the models’ goodness-of-fit. The final model retained variables that significantly contributed to differentiation of FIB4 trajectories with the lowest (ie, best-fitting) BIC scores.
We also tested treatment-by-risk factor (covariate) interactions, followed by further evaluation of quantitative interactions (magnitude but not direction of response varies by the presence/absence of the risk factor) or qualitative interaction (change in direction of response; eg, a treatment benefits the young but harms the old).20 All interactions were quantitative; because they did not contribute to goodness-of-fit and could potentially complicate the models, they were dropped from the final analysis.
Four sensitivity analyses were conducted, as follows: (1) APRI as the outcome of interest, owing to the inclusion of age in the formula for calculating FIB4 and thus high correlation between these; (2) substitution of last treatment initiation date for index date, owing to patients receiving more than one course of treatment; (3) a 1-to-1 (treated and untreated) matched cohort to match controls at baseline; and (4) omission of several important treatment selection or prognostic variables (eg, baseline fibrosis, covariates related to treatment contraindications) for propensity score calculation. In the last sensitivity analysis, we tested the treatment group effect to determine whether it had changed; a robust treatment effect indicates that unobserved confounding can be disregarded.21
Results
We identified 6975 CHeCS patients with confirmed HCV infection and a baseline FIB4 measurement. We excluded 2244 of these patients: 144 were enrolled in a clinical trial; 74 had HCV treatment/SVR outside of the CHeCS systems; 254 had ongoing therapy; 75 had hepatitis B virus co-infection; and 1709 had insufficient follow-up evaluation (<2 time points). In total, 4731 patients were included in the analysis. Of these, 1657 (35%) were treated; 1367 (82%) received 1 course of therapy, and 290 received more than 1 course. The median course duration was 11 months. Of the 1657 treated patients, 755 (46%) achieved SVR. Laboratory follow-up periods ranged from less than 180 days to more than 10 years.
Patient Characteristics at Baseline
Table 1 describes our sample. Asian patients were over-represented (6%) because of the participation of a large health care system in Hawai’i. Notably, at baseline roughly half (2290; 48%) of all patients had advanced fibrosis; patients with and without advanced fibrosis received antiviral treatment at the same rate (35%). The median time from initial indication of HCV infection/ diagnosis to initiation of antiviral treatment was approximately 2 years; the interquartile range (25%–75%) was 1 to 7 years.
Table 1.
Exposures and Treatment Differences at Index Date, Before and After Weighting, Using Propensity Scores
| Before weighting
|
After weighting
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Label | All (N = 4731) | Untreated (N = 3074) | Treated (N = 1657) | P value (raw) | Untreated (N = 3074) | Treated (N = 1657) | P value |
| Site | KPNW | 1084 (23%) | 647 (21%) | 437 (26%) | <.001 | 13% | 13% | .709 |
| KPHI | 469 (10%) | 304 (10%) | 165 (10%) | 12% | 12% | |||
| HFHS | 2098 (44%) | 1471 (48%) | 627 (38%) | 41% | 41% | |||
| GHS | 1080 (23%) | 652 (21%) | 428 (26%) | 35% | 34% | |||
| Index age, y | <40 | 640 (14%) | 437 (14%) | 203 (12%) | <.001 | 16% | 15% | .917 |
| 40 <50 | 1479 (31%) | 898 (29%) | 581 (35%) | 30% | 31% | |||
| 50 <60 | 1967 (42%) | 1250 (41%) | 717 (43%) | 42% | 42% | |||
| ≥60 | 645 (14%) | 489 (16%) | 156 (9%) | 12% | 12% | |||
| Sex | Female | 1889 (40%) | 1245 (41%) | 644 (39%) | .273 | 42% | 41% | .347 |
| Male | 2842 (60%) | 1829 (59%) | 1013 (61%) | 58% | 59% | |||
| Race | Asian/other | 293 (6%) | 175 (6%) | 118 (7%) | <.001 | 6% | 6% | .913 |
| Black | 1344 (28%) | 1038 (34%) | 306 (18%) | 22% | 22% | |||
| White | 2918 (62%) | 1734 (56%) | 1184 (71%) | 68% | 69% | |||
| Unknown | 176 (4%) | 127 (4%) | 49 (3%) | 3% | 3% | |||
| Index year | <2000 | 406 (9%) | 271 (9%) | 135 (8%) | .253 | 5% | 5% | .552 |
| 2000 <2005 | 1622 (34%) | 1025 (33%) | 597 (36%) | 25% | 26% | |||
| 2005 <2010 | 2142 (45%) | 1416 (46%) | 726 (44%) | 49% | 50% | |||
| ≥2010 | 561 (12%) | 362 (12%) | 199 (12%) | 21% | 19% | |||
| Insurance type | Medicaid | 704 (15%) | 538 (18%) | 166 (10%) | <.001 | 16% | 16% | .987 |
| Medicare | 1178 (25%) | 875 (28%) | 303 (18%) | 22% | 21% | |||
| Private | 2558 (54%) | 1443 (47%) | 1115 (67%) | 56% | 57% | |||
| None | 189 (4%) | 144 (5%) | 45 (3%) | 4% | 4% | |||
| Unknown | 102 (2%) | 74 (2%) | 28 (2%) | 1% | 1% | |||
| Median household income | Missing | 106 (2%) | 86 (3%) | 20 (1%) | <.001 | 2% | 3% | .721 |
| <$15K | 153 (3%) | 119 (4%) | 34 (2%) | 3% | 3% | |||
| $15 <30K | 1055 (22%) | 782 (25%) | 273 (16%) | 21% | 20% | |||
| $30 <50K | 2164 (46%) | 1366 (44%) | 798 (48%) | 48% | 47% | |||
| $50 <75K | 1002 (21%) | 584 (19%) | 418 (25%) | 20% | 20% | |||
| ≥$75K | 251 (5%) | 137 (4%) | 114 (7%) | 7% | 7% | |||
| Alanine aminotransferase | <LLN and/or normal | 1882 (40%) | 1276 (42%) | 606 (37%) | .004 | 41% | 40% | .775 |
| ULN ≤2 ×ULN | 1634 (35%) | 1038 (34%) | 596 (36%) | 33% | 34% | |||
| >2 ×ULN | 1215 (26%) | 760 (25%) | 455 (27%) | 26% | 26% | |||
| Weighted Deyo Charlson score (includes liver comorbidities) | 0 | 2493 (53%) | 1627 (53%) | 866 (52%) | <.001 | 52% | 52% | .957 |
| 1 | 1151 (24%) | 705 (23%) | 446 (27%) | 26% | 26% | |||
| 2 | 388 (8%) | 247 (8%) | 141 (9%) | 8% | 8% | |||
| 3 | 699 (15%) | 495 (16%) | 204 (12%) | 14% | 13% | |||
| HCV RNA, IU/mL | Undetectable (normal) | 104 (5%) | 18 (2%) | 86 (7%) | <.001 | 4% | 5% | .271 |
| Detectable ≤ 100,000 | 251 (12%) | 112 (13%) | 139 (11%) | 11% | 11% | |||
| Detectable >100,000 | 1581 (75%) | 665 (78%) | 916 (73%) | 76% | 75% | |||
| Indeterminate | 172 (8%) | 61 (7%) | 111 (9%) | 9% | 9% | |||
| HCV genotype | 1 | 2689 (57%) | 1675 (54%) | 1014 (61%) | <.001 | 65% | 65% | .788 |
| 2 | 456 (10%) | 208 (7%) | 248 (15%) | 10% | 10% | |||
| 3 | 371 (8%) | 190 (6%) | 181 (11%) | 9% | 9% | |||
| Other/unknown | 1215 (26%) | 1001 (33%) | 214 (13%) | 16% | 15% | |||
| Baseline HIV status indicator | No HIV | 4629 (98%) | 3004 (98%) | 1625 (98%) | .434 | 98% | 98% | .945 |
| HIV | 102 (2%) | 70 (2%) | 32 (2%) | 2% | 2% | |||
| Diabetes | No | 4120 (87%) | 2639 (86%) | 1481 (89%) | <.001 | 89% | 89% | .822 |
| Yes | 611 (13%) | 435 (14%) | 176 (11%) | 11% | 11% | |||
| Substance abuse | No | 4082 (86%) | 2541 (83%) | 1541 (93%) | <.001 | 91% | 92% | .398 |
| Yes | 649 (14%) | 533 (17%) | 116 (7%) | 9% | 8% | |||
| Decompensated cirrhosis | No | 4538 (96%) | 2945 (96%) | 1593 (96%) | .579 | 94% | 95% | .576 |
| Yes | 193 (4%) | 129 (4%) | 64 (4%) | 6% | 5% | |||
| Absolute contraindication | No | 3389 (72%) | 2210 (72%) | 1179 (71%) | .590 | 75% | 74% | .338 |
| Yes | 1342 (28%) | 864 (28%) | 478 (29%) | 25% | 26% | |||
| Relative contraindication | No | 3386 (72%) | 2093 (68%) | 1293 (78%) | <.001 | 76% | 77% | .764 |
| Yes | 1345 (28%) | 981 (32%) | 364 (22%) | 24% | 23% | |||
| Nonadvanced fibrosis | FIB4 < 1.81 | 2441 (52%) | 1585 (52%) | 856 (52%) | .949 | 55% | 54% | .506 |
| Advanced fibrosis | FIB4 ≥ 1.81 | 2290 (48%) | 1489 (48%) | 801 (48%) | 45% | 46% | ||
| Natural log of FIB4 score | 0.7± 0.9 | 0.7 ± 0.9 | 0.6 ± 0.8 | .057 | 0.6 ± 1.47 | 0.6 ± 1.06 | .667 | |
| Natural log of APRI score | −0.2 ± 1.0 | −0.2 ± 1.1 | −0.2 ± 0.9 | .664 | −0.3 ± 1.7 | −0.2 ± 1.21 | .643 | |
ALT, alanine aminotransferase; GHS, Geisinger Health System; HFHS, Henry Ford Health System; KPHI, Kaiser Permanente, Hawaii; KPNW, Kaiser Permanente Northwest; LLN, lower limit of normal; ULN, upper limit of normal.
Antiviral HCV Treatment Allocation
In unadjusted comparisons, untreated patients were older, more likely to have a history of substance abuse, and more likely to be black than untreated patients (Table 1). Untreated patients also had higher comorbidity scores, were less likely to have insurance, and had lower median household incomes. A larger proportion of treated patients had an abnormal alanine aminotransferase result at baseline. After propensity-score adjustment, demographic and clinical characteristics were balanced between treatment groups (Table 1).
Final Model: Factors Impacting Fibrosis Trajectory
The final model with an autoregressive covariance pattern and time as a nominal variable showed the best data fit. Descriptive analyses indicated the following: (1) change in FIB4 was nonlinear and varied by individual; and (2) treatment impact was time-dependent (treatment-by-time interaction with P value < .0001) (Table 2). The basic model with an autoregressive covariance pattern was selected for further examination, given evidence of superior fit. Race, sex, baseline FIB4 score, and hepatitis genotype were retained in the final model, resulting in improved fit (BIC 18,442) compared with the unadjusted model (BIC 21,125). Sensitivity analysis showed a similar trend in treatment effects and post-treatment group-by-time interaction (detailed later).
Table 2.
Parameter Estimates and Model Fitting Results for logFIB4 Over Time
| Unadjusted model
|
Adjusted model
|
|||
|---|---|---|---|---|
| Parameter | Estimate | P value | Estimate | P value |
| Intercept | 0.61 | <.001 | 0.35 | <.001 |
| Interval (time) | a | <.001 | a | <.001 |
| Treatment group effect | <.001 | <.001 | ||
| Treatment-by-time interaction | <.001 | <.001 | ||
| FIB4 at baseline: | 1.07 | <.001 | ||
| >1.81 vs ≤1.81 | ||||
| Genotype | ||||
| 1 vs 2 | 0.26 | <.001 | ||
| 3 vs 2 | 0.33 | <.001 | ||
| Other/unknown vs 1 | 0.20 | <.001 | ||
| Race | ||||
| Asian/other vs white | −0.03 | .375 | ||
| Black vs white | −0.11 | <.001 | ||
| Age, y | ||||
| <40 vs ≥60 | −0.69 | .034 | ||
| 40 to <50 vs ≥60 | −0.19 | .029 | ||
| 50 to <60 vs ≥60 | −0.06 | .027 | ||
| Sex, male vs female | 0.05 | <.001 | ||
| Akaike information criterion | 24,894.4 | 21,134.3 | ||
| BIC or Schwarz criterion | 24,907.3 | 21,147.2 | ||
Impact of Hepatitis C Antiviral Therapy and Sustained Virological Response on Liver Fibrosis Progression
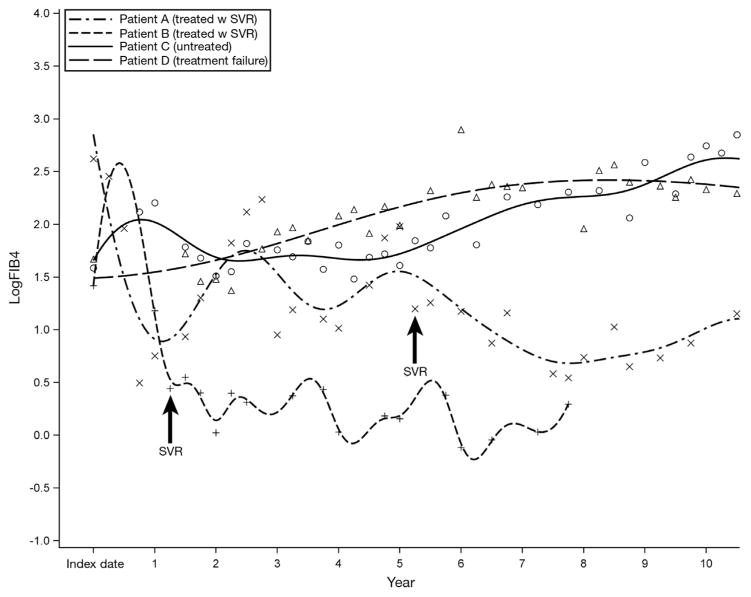
Figure 1 shows 4 representative trajectories from our sample. Patient A did not achieve SVR until completion of a third course of therapy, approximately 5 years after the first treatment initiation. Patient B achieved SVR after the first course of treatment. Notably, FIB4 decreased after SVR in both patients, consistent with the findings of the French national prospective cohort of patients co-infected with HIV and HCV (ANRS CO13 HEPAVIH) Cohort investigators.12 Furthermore, this reduction continued long term, suggesting possible regression of fibrosis. Conversely, patients C (untreated) and D (treatment failure) showed upward trends in FIB4 score.
Figure 1.
Four individual trajectories of logFIB4 across 10 years of follow-up evaluation. Data were smoothed by the B-spline method (thick lines).
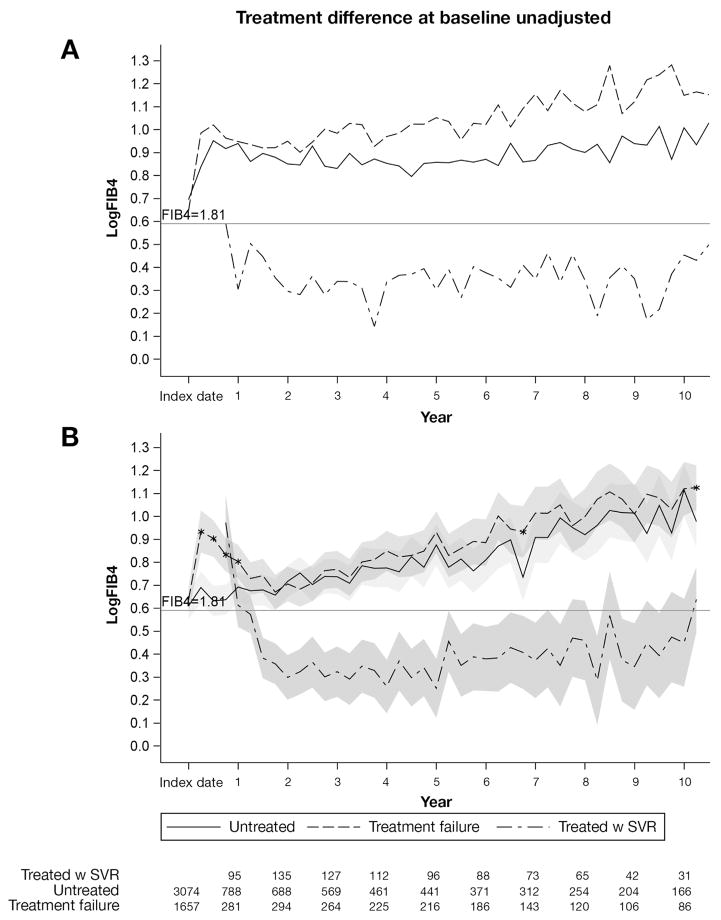
Figure 2A presents the observed mean trajectories of FIB4 (in log scale) by treatment group. A cut-off value of 1.81 (0.59 after log transformation) is presented as a reference; this cut-off value was validated previously in this cohort to predict advanced fibrosis.22 At baseline, 1657 patients had received treatment and 3074 patients were untreated. The first SVR occurred at interval 3 (24 weeks after treatment initiation) in 68 patients. At the end of the follow-up period, 755 treated patients had achieved SVR.
Figure 2.
Average observed (top) and predicted (bottom) logFIB4 from an unadjusted model with 95% confidence bands (shaded) over 10 years by treatment group. (A) Treatment failure and (B) untreated patients were compared at each time interval. *Significant difference between treatment failure and untreated patients at a specific time interval (P < .05). The number of patients at each time point is noted at the bottom.
Figure 2B presents the final model estimates of FIB4 trajectories by treatment group (Table 2), which showed patterns similar to those of the observed data. As shown in Figure 2B, predicted FIB4 in patients who achieved SVR (Figure 2B, uneven dashed line) can be broken up into 3 phases: (1) decrease; (2) plateau; and (3) increase. FIB4 started higher than 1.81 and sharply decreased, remaining lower than 1.81 from years 2 to 5, when a gradual increase began. At 77 weeks after treatment initiation, this decrease became significantly different from baseline (0.25; 22% on original scale; P < .0001). We noted that a few patients showed a late increase, likely because missing FIB4 values resulted in a wide confidence interval for the predicted results. FIB4 in the SVR group was consistently lower over time than in the untreated or TF groups (P < .05). In untreated patients, FIB4 gradually increased; in year 2, the increase became statistically significant (increased by 11%; P = .013). By year 5, FIB4 had increased by 44% (P < .0001) compared with baseline.
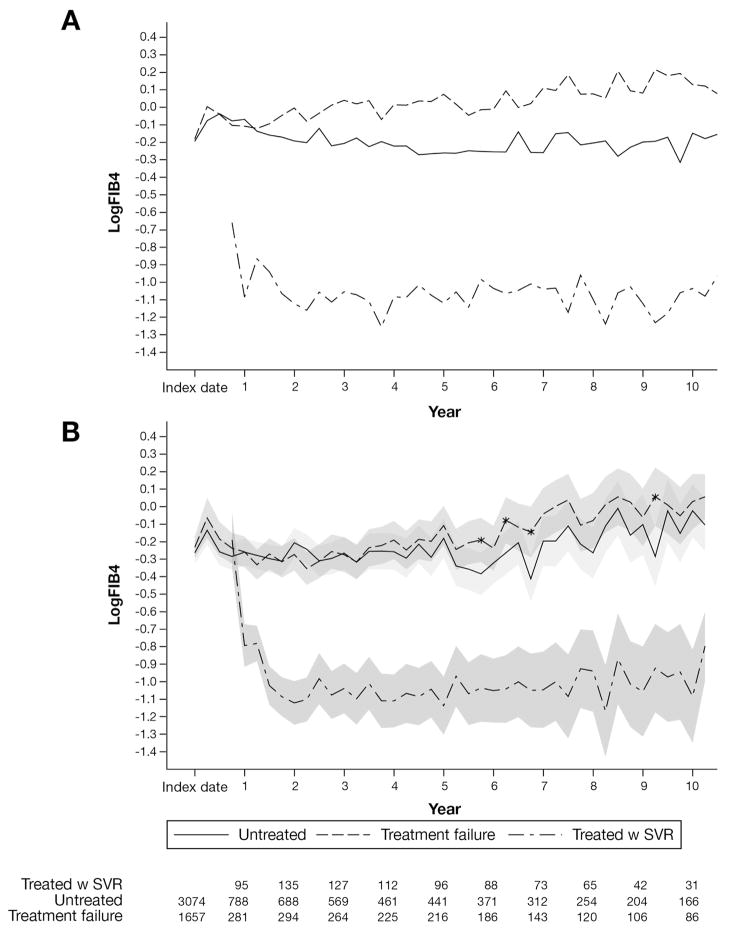
Sensitivity analyses showed similar effects of treatment on APRI trajectories (Figure 3). APRI remained significantly lower in SVR patients than in untreated or TF patients. Similar treatment trajectories were observed using the last treatment initiation date as the index date, with follow-up evaluation limited to 6 years (data not shown). We also observed a consistent treatment effect in our 1:1 matched cohort (Supplementary Figure 1), as well as in analyses that omitted several key covariates for the calculation of propensity scores (Supplementary Figure 2).
Figure 3.
Average observed (top) and predicted (bottom) logAPRI with 95% confidence bands (shaded) over 41 intervals of 90 days by group. Treatment failure and untreated patients were compared at each time interval. *Significant difference between treatment failure and untreated patients at a specific time interval (P < .05). The number of patients at each time point is noted at the bottom.
Additional Risk Factors for Liver Fibrosis Progression
Across time (Table 2), men showed consistently higher FIB4 levels than women (coefficient = 0.05; P < .001), and black patients presented with lower FIB4 than white patients (coefficient = −0.11; P < .001). GT 1 and 3 patients had higher FIB4 scores than GT 2 patients; although GT 3 patients showed lower FIB4 than GT 1 patients, the difference was not significant (coefficient = 0.04; P = .19). We noted that patients with lower baseline FIB4 scores (<1.81) tended to remain low over time (P < .001). Age remained an important risk factor; older age at baseline was associated with a higher FIB4 over time. No qualitative risk factor-by-treatment interaction was detected, indicating that risk factor effects were consistent among the treatment groups.
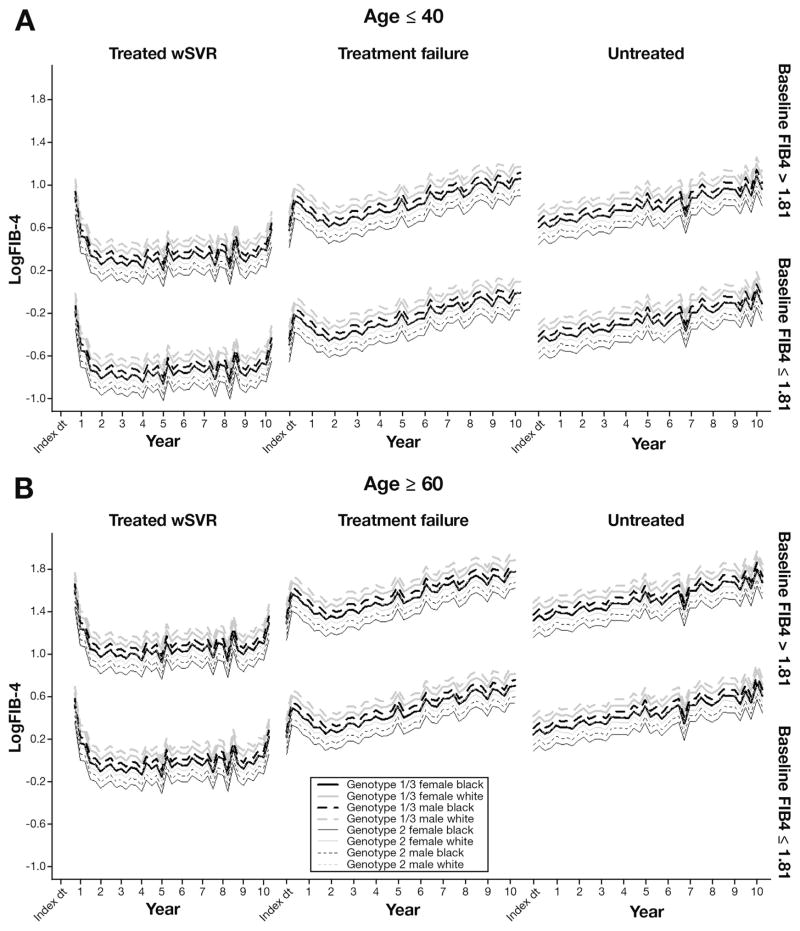
Overall Risk Profile for Liver Fibrosis Progression
In Table 2, patients’ FIB4 trajectories were classified into subgroups based on combinations of risk factors and treatment status, which is shown in Figure 4. FIB4 growth patterns among treatment groups were similar to those described in Figure 2B. Older patients (>60 y) showed consistently higher FIB4 than younger patients (≤40 y). Interestingly, white male patients with HCV GTs 1 or 3 showed the highest FIB4 levels in each of the 3 treatment groups, whereas black female patients with HCV GT 2 had the lowest FIB4 levels.
Figure 4.
Predicted average trajectories of logFIB4 over 10 years by group (baseline FIB4, HCV GT, sex, and race) for patients 40 years or younger vs older than 60 years. Thick lines, GT 1 or 3 patients; thin lines, GT 2 patients; solid lines, women; dashed lines, men; black lines, black patients; gray lines, white patients. Lines are stratified by baseline FIB4 levels (top, >1.81; bottom, ≤1.81). The expected trajectory for black female GT 1 patients overlaps with the trajectory for white male GT 2 patients.
Discussion
This study investigated the longitudinal progression of FIB4 in a real-world sample of more than 4000 HCV patients. In propensity score–adjusted analyses, we observed significant longitudinal changes in FIB4 that varied by treatment status. In successfully treated patients, SVR appears to induce long-term regression of fibrosis; in contrast, the absence of treatment and treatment failure were characterized by progressively increasing FIB4 levels. These findings provide further evidence for the applicability of repeated measures of FIB4 in HCV-infected patients.
Initial FIB4 values were lower among patients who achieved SVR; these values continued to decrease and then remained stable through the years. Although FIB4 increased slightly after year 5, these changes were likely due to fewer data points and inclusion of patient age in the formula; this was confirmed using APRI, which is calculated without age (Figure 3B). Untreated patients and patients with treatment failure showed increasing FIB4 over time, and values for both groups remained higher than 1.81.
Our finding that FIB4 trajectory decreases after SVR is consistent with smaller histologic studies.1–4 A significant regression of fibrosis has been observed previously among hepatitis B patients after long-term virologic suppression with tenofovir disoproxil fumate.23 Although the exact mechanism is unclear, suppression of viral replication may reduce hepatic inflammation and consequent fibrosis, allowing regeneration of healthy tissue. Notably, SVR patients showed flat FIB4 trajectories despite increasing age—suggesting stability of fibrosis or possibly fibrotic regression. The consistent results from our sensitivity analyses further validate these findings. Although APRI changes appeared to be more stable, FIB4 has significantly higher predictive ability for liver fibrosis (P < .0001)9; future analyses may use hidden Markov modeling to permit consideration of a range of clinical conditions, or include markers developed from laboratory results and liver-related diagnosis codes (International Classification of Diseases, 9th revision codes).
Our results showed that FIB4 trajectories varied based on factors such as sex, race, genotype, baseline FIB4, age, as well as treatment/response groups. Patients infected with HCV GTs 1 or 3 (Figure 4, thick lines) showed significantly higher FIB4 levels and steeper FIB4 trajectories across all time points and in each of the 3 treatment groups than patients with GT 2. Although there were no significant differences between GTs 1 and 3 trajectories, GT 3 patients showed a relatively lower FIB4 (coefficient = 0.04; P = .19).
In addition, patients in any of the 3 treatment groups with a FIB4 less than 1.81 at the index date remained below this cut-off value throughout follow-up evaluation (Figure 4). White patients also presented with a higher FIB4 than black patients; likewise, older age at baseline was a risk factor for higher FIB4 over time. Men consistently showed higher FIB4 over time than women. Although HIV co-infection, diabetes mellitus, and substance abuse (including alcohol) were individual risk factors for FIB4 progression, the effects diminished after adjustment for other covariates. Notably, risk factor effects were consistent among the treatment groups, indicating that treatment status remained the most important influence on FIB4 trajectories.
Our study had some limitations. The calculation of FIB4 uses patient age at the time of laboratory assessment, thus longitudinal FIB4 measures tend to increase over time, even with unchanged laboratory parameters. Also, because of our reliance on electronically collected observational data as well as inclusion of patients from the early 1990s forward, our ability to calculate the index date body mass index and to include detailed alcohol consumption data were limited. Another limitation of this study was that the duration of HCV infection in this population was estimated. However, we used several levels of data to confirm the date of diagnosis or first indication of infection, prioritized in the following order: (1) patient self-report; (2) medical chart abstraction; (3) HCV medication prescriptions or fills extracted from the EHR; and (4) diagnosis and procedure codes from the EHR. Patients with acute HCV were excluded from the cohort. We also found that unadjusted clinical profiles and propensity scores differed between the treated and untreated groups, although adjustment using IPTW (based on 43 covariates collected at baseline) resulted in excellent balance between the 2 groups. Likewise, our sensitivity analysis, which omitted several treatment-selection and prognostic covariates, showed consistent treatment group effects. We are confident in our estimated treatment effects and that unobserved confounding has not influenced our results.21
Despite the considerable progress in the development of noninvasive methods to assess liver fibrosis, none is widely accepted yet as an equivalent to liver biopsy. The decrease observed in serum biomarker values in the present study may not always represent a reversal of histologic fibrosis. However, many recent studies indeed have shown that improvement in serum markers correlates with decreasing fibrosis stage.10,12,16
Both our own and other large cohort studies have shown the clinical benefits of SVR after HCV antiviral therapy, including a reduction in all-cause mortality. Such findings suggest a regression of fibrosis. By using a biomarker for liver fibrosis previously validated in a static setting, we now expand on smaller studies and show that this marker can be used successfully for longitudinal analyses. Based on the present analysis of FIB4 trajectories across 10 years in a large, racially diverse sample of HCV patients receiving routine care, our findings strongly suggest that patients who achieve SVR likely show a sustained regression of hepatic fibrosis.
Supplementary Material
Acknowledgments
The Chronic Hepatitis Cohort Study investigators and sites are as follows: Scott D. Holmberg, Eyasu H. Teshale, Philip R. Spradling, Anne C. Moorman, Fujie Xu, Jim Xing, and Cindy Tong, Division of Viral Hepatitis, National Centers for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA; Stuart C. Gordon, David R. Nerenz, Mei Lu, Lois Lamerato, Jia Li, Loralee B. Rupp, Nonna Akkerman, Nancy Oja-Tebbe, and Talan Zhang, Henry Ford Health System, Detroit, MI; Joseph A. Boscarino, Zahra S. Daar, and Robert E. Smith, Center for Health Research, Geisinger Health System, Danville, PA; Vinutha Vijayadeva and John V. Parker, The Center for Health Research, Kaiser Permanente-Hawaii, Honolulu, HI; Mark A. Schmidt, Judy L. Donald, and Erin M. Keast, The Center for Health Research, Kaiser Permanente-Northwest, Portland, OR.
Funding: The Chronic Hepatitis Cohort Study is funded by the CDC Foundation, which currently receives grants from AbbVie, Gilead Sciences, and Janssen Pharmaceuticals, Inc. Past funders include Genentech (a Member of the Roche Group) and Vertex Pharmaceuticals. Past partial funders include Bristol-Myers Squibb. Granting corporations do not have access to Chronic Hepatitis Cohort Study data and do not contribute to data analysis or manuscript writing.
Abbreviations used in this paper
- APRI
aminotransferase-to-platelet ratio index
- BIC
Bayesian Information Criterion
- CHeCS
Chronic Hepatitis Cohort Study
- EHR
electronic health record
- FIB4
Fibrosis-4
- GT
genotype
- HCV
hepatitis C virus
- SVR
sustained viral response
- TF
treatment failure
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2016.01.009.
Conflicts of interest
This author discloses the following: Stuart C. Gordon receives grant/research support from AbbVie Pharmaceuticals, Bristol-Myers Squibb, Gilead Pharmaceuticals, Intercept Pharmaceuticals, and Merck, and is a consultant/advisor for AbbVie Pharmaceuticals, Amgen, Bristol-Myers Squibb, CVS Caremark, Gilead Pharmaceuticals, and Merck, and is on the Data Monitoring Board for Tibotec/ Janssen Pharmaceuticals. The remaining authors disclose no conflicts.
References
- 1.D'Ambrosio R, Aghemo A, Rumi MG, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56:532–543. doi: 10.1002/hep.25606. [DOI] [PubMed] [Google Scholar]
- 2.George SL, Bacon BR, Brunt EM, et al. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729–738. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallet V, Gilgenkrantz H, Serpaggi J, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403. doi: 10.7326/0003-4819-149-6-200809160-00006. [DOI] [PubMed] [Google Scholar]
- 4.Pockros PJ, Hamzeh FM, Martin P, et al. Histologic outcomes in hepatitis C-infected patients with varying degrees of virologic response to interferon-based treatments. Hepatology. 2010;52:1193–1200. doi: 10.1002/hep.23809. [DOI] [PubMed] [Google Scholar]
- 5.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 6.Pol S, Carnot F, Nalpas B, et al. Reversibility of hepatitis C virus-related cirrhosis. Hum Pathol. 2004;35:107–112. doi: 10.1016/j.humpath.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 8.American Association for the Study of Liver Diseases IDSoA. [Accessed: January 13, 2015];Recommendations for testing, managing, and treating hepatitis C. 2014 Available from: www.hcvguidelines.org.
- 9.Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930–937. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki N, Kurosaki M, Tanaka K, et al. Noninvasive estimation of fibrosis progression overtime using the FIB-4 index in chronic hepatitis C. J Viral Hepat. 2013;20:72–76. doi: 10.1111/j.1365-2893.2012.01635.x. [DOI] [PubMed] [Google Scholar]
- 11.Butt AA, Yan P, Lo Re V, 3rd, et al. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern Med. 2015;175:178–185. doi: 10.1001/jamainternmed.2014.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohort TACH. Regression of liver stiffness after sustained HCV virological responses among HIV/HCV- coinfected patients. AIDS. 2015;29:1–10. doi: 10.1097/QAD.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 13.North CS, Hong BA, Adewuyi SA, et al. Hepatitis C treatment and SVR: the gap between clinical trials and real-world treatment aspirations. Gen Hosp Psychiatry. 2013;35:122–128. doi: 10.1016/j.genhosppsych.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56:40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Rupp LB, Moorman AC, et al. Comparative effectiveness research of chronic hepatitis B and C cohort study (CHeCS): improving data collection and cohort identification. Dig Dis Sci. 2014;59:3053–3061. doi: 10.1007/s10620-014-3272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haseltine EL, Penney MS, George S, et al. Successful treatment with telaprevir-based regimens for chronic hepatitis C results in significant improvements to serum markers of liver fibrosis. J Viral Hepat. 2015;22:701–707. doi: 10.1111/jvh.12382. [DOI] [PubMed] [Google Scholar]
- 17.Bureau UC. Data and documentation for the American Community Survey. Available from: www.census.gov/acs/www/data_documentation/documentation_main.
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu M, Lyden PD, Brott TG, et al. Beyond subgroup analysis: improving the clinical interpretation of treatment effects in stroke research. J Neurosci Methods. 2005;143:209–216. doi: 10.1016/j.jneumeth.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Steventon A, Grieve R, Sekhon J. A comparison of alternative strategies for choosing control populations in observational studies. Health Serv Outcomes Res Methodol. 2015;15:1–25. doi: 10.1007/s10742-014-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmberg SD, Lu M, Rupp LB, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis. 2013;57:240–246. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.