Abstract
Carbapenem antibiotics are used as a last resort to treat serious Gram-negative bacteria (GNB) infections, however carbapenemase-producing strains of GNB have emerged as a major source of resistance. Owing to the highly transmissible nature of plasmid-borne carbapenemases, numerous reports have warned about the likely spread into the community from healthcare settings. Since the prevalence of carbapenem-resistant Enterobacteriaceae (CRE) in the community is largely unknown, we conducted a scoping review of the literature to assess the percentage of CRE isolates that could be associated with the community. Initially, 361 studies were assessed and 15 met the inclusion criteria. Although 5 studies (33.3%) found no community-associated CRE, the remaining 10 studies identified percentages ranging from 0.04% to 29.5% of either community-associated or community-onset CRE among their samples, with US-based studies alone ranging from 5.6–10.8%. The presence of CRE in the community poses an urgent public health threat.
Keywords: Carbapenem-resistant Enterobacteriaceae, Antimicrobial resistance, Community-associated infections
1. Introduction
Gram-negative bacteria (GNB) are becoming increasingly resistant to most antibiotics [1]. The rise of carbapenem-resistant (CR) bacteria is considered one of the most urgent current public health concerns [1,2], since carbapenems are often used as a last resort to treat GNB infections [3]. A lack of alternative treatment options has led to a mortality rate of up to 50% for infections with CR strains [4]. Carbapenem-resistant Enterobacteriaceae (CRE), most notably Escherichia coli and Klebsiella pneumoniae, were relatively uncommon before 2000 but have doubled in prevalence over the following decade among healthcare-associated infections (HAIs) [5]. The US Centers for Disease Control and Prevention (CDC) warn of the potential for CRE to spread into the community given that drug-susceptible Enterobacteriaceae are a common cause of community-associated infections (CAIs) [6], and this progression into the community has already been demonstrated by extended-spectrum β-lactamase (ESBL)-producing GNB [1,7–9]. Previous reviews on CRE have focused on HAIs, but most have warned of the impending risk for community transmission [4,5,10–14]. Transmission of CRE into long-term care facilities (LTCFs) has already been demonstrated [10,15,16], but there is a lack of data and consensus on the presence of CRE among individuals in the community with no known connection to the healthcare setting.
2. Epidemiology of carbapenem resistance
Much of the rise in carbapenem resistance has been attributed to the rapid spread of plasmid-borne genetic determinants encoding β-lactamases [6]. Although not all carbapenemase enzymes confer carbapenem resistance, many are capable of hydrolysing all β-lactam antibiotics. Increasing numbers of Enterobacteriaceae are reported as frequent carriers of genes encoding two of the most concerning subclasses of carbapenemases: K. pneumoniae carbapenemase (KPC), which has become endemic in parts of the Americas, southern Europe, Israel and China; and the New Delhi metallo-β-lactamase (NDM), which has become endemic in northern Europe and the Asia Pacific region, most notably the UK and India [7,17,18]. Although KPC is the most common carbapenemase worldwide, NDMs have been shown to be more promiscuous [19].
OXA-48-type oxacillinase is the third most prevalent carbapenemase globally, found most often in North Africa and Europe. Besides NDM, two other forms of metallo-β-lactamase carbapenemases, namely Verona integron-encoded metallo-β-lactamase (VIM) and imipenemase metallo-β-lactamase (IMP), are less common but are equally concerning due to the similar transmission mechanisms. In some regions, the epidemic is overlapping, such as the rise of NDM and OXA-48 behind KPC seen across Europe, and poses a real threat to infection control. As of April 2016, 48 states in the USA had reported the presence of CRE arising from KPC, 25 reporting NDM, 19 reporting OXA-48 and 6 reporting VIM [20]. Colistin is one of the few treatment options available for CRE, yet increasing accounts of colistin resistance are also being documented [21,22]. The first report of plasmid-borne colistin resistance (mcr-1) has been identified among multidrug-resistant E. coli in the USA, leading to concerns of pandrug-resistant bacteria [23].
The molecular epidemiology of CRE from healthcare settings has been well described in the USA and elsewhere [5,10,14]. Both clonal expansion within healthcare settings and independent carbapenem resistance-conferring plasmid acquisition within patients have been reported. In studies where molecular methods have been employed, multilocus sequence typing (MLST) sequence type 258 (ST258) was found to be most prevalent overall, with ST11 predominating in Asia [24]. Various molecular methods to characterise CRE, including identifying the resistance gene on mobile elements and whole-genome sequencing, have enabled both strain and resistance-harbouring plasmid tracking. Of note, carbapenemase-producing Enterobacteriaceae are not synonymous with CRE, as there are other methods of conferring carbapenem resistance, but carbapenemases are the most common route and the most concerning.
In the USA, the CDC initiated surveillance of CRE in 2013 [25], and hospital-based data revealed 11 % of K. pneumoniae and 2% of E. coli to be CR [1]. Initially, CRE had been primarily reported from high-income countries [26,27], but regional patterns of resistance vary, with the highest proportion of carbapenem resistance found in India at 57% among K. pneumoniae isolates [3]. Similar increases were found in southern Europe, with Greece reporting an incidence of carbapenem resistance upwards of 60% in K. pneumoniae isolates for 2014 [28]. Recent studies have identified a high prevalence (16–19%) of ESBL-producing GNB in community settings [29]. With carbapenemases following similar plasmid-mediated genetic exchange as ESBLs, there has been a call for further research on the spread of CRE into community settings owing to the highly transmissible nature of carbapenemase-producing genetic elements [25].
3. The role of asymptomatic carriage in community-associated infections
Much of the dissemination of resistant clones is through carriage in commensal microflora, which often goes undetected unless carriage (or colonisation) leads to infection [30]. Colonisation with CRE is most often defined by gastrointestinal tract carriage identified through the use of a rectal swab or faecal sampling [31]. Gut microbiota carriage can be identified through clinical culture even when the patient remains asymptomatic. Although colonisation is generally a prerequisite for infection, it is largely unknown what percentage of colonised patients will progress to active infection. A recent systematic review pooled 1806 hospital-based patients identified as colonised with CRE at the time of admission and found that 299 (16.5%) progressed to clinical infection [31]. Isolation of hospital patients with known CRE colonisation at the time of admission, whether symptomatic or not, has led to a significant reduction of nosocomial transmission of CRE infection [18]. Since much of the focus on CRE has been in acute and chronic care settings, we conducted a scoping review of the literature to assess the proportion of CRE isolates that could be associated with the community.
4. Methods
4.1. Search strategy
We chose to conduct a scoping review of the literature because our goal was to provide an overview of the breadth of information available regarding the presence of CAI or community-onset (CO) CRE. Scoping reviews are often selected over systematic reviews when the topic of interest is heterogeneous in nature and has not been well reviewed [32]. Nevertheless, this review followed the guidelines developed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) group [33]. Since the first published case of CRE was in 1996 [34], we conducted a literature search through PubMed and Embase electronic databases for articles published from 1996 through March 2016. Keywords searched included: (carbapenem-resistant OR carbapenemase OR carbapenem-resistant enterobacteriaceae) AND (community OR outpatient OR community-associated OR community-acquired OR community-onset). MeSH and Emtree subject headings were used to expand CRE and community-acquired infection. See the Appendix for detailed search strategies for the two databases. Results were filtered by English language. Reference lists of review articles were also examined. A health sciences librarian assisted with the search strategy, and two independent reviewers (AMK and ELL) selected the final articles for inclusion, with a third author reviewing the final selections (BM).
4.2. Inclusion and exclusion criteria
Observational studies that (i) tested for CRE among a larger sample or (ii) only included CRE in their sample were included. All studies either had to be conducted in a community setting or had to delineate between HAI and CAI or CO. Both studies that provided data on previous healthcare exposure (community-associated) and those that did not explicitly test for these risk factors (community-onset) were included. Both CRE cases with active infection and asymptomatic colonisation were included. Exclusion criteria included studies only documenting HAI, Gram-positive bacteria, case studies and review articles. Studies that only tested for ESBL were not included, nor were studies evaluating animal models of infection. Studies that made no mention of community or outpatients in the abstract were also excluded. In the final iteration, the two studies that only included Pseudomonas aeruginosa were removed to focus solely on CRE.
5. Results
The initial database searches yielded 361 unique papers, and 4 additional studies were found in reference lists of review articles (Fig. 1). After refining the search based on the exclusion criteria, 43 studies were reviewed in full. Fifteen studies conducted across all continents, with the majority in Asia (5; 33.3%), North America (4; 26.7%) and Europe (3; 20.0%), met inclusion criteria (Fig. 2).
Fig. 1.
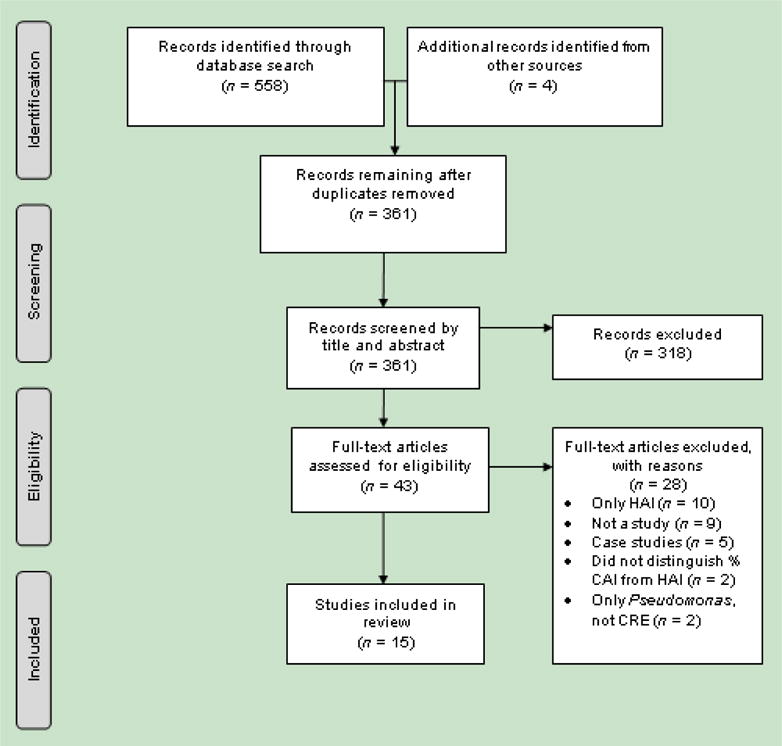
Study selection process. HAI, healthcare-associated infection; CAI, community-associated infection; CRE, carbapenem-resistant Enterobacteriaceae.
Fig. 2.
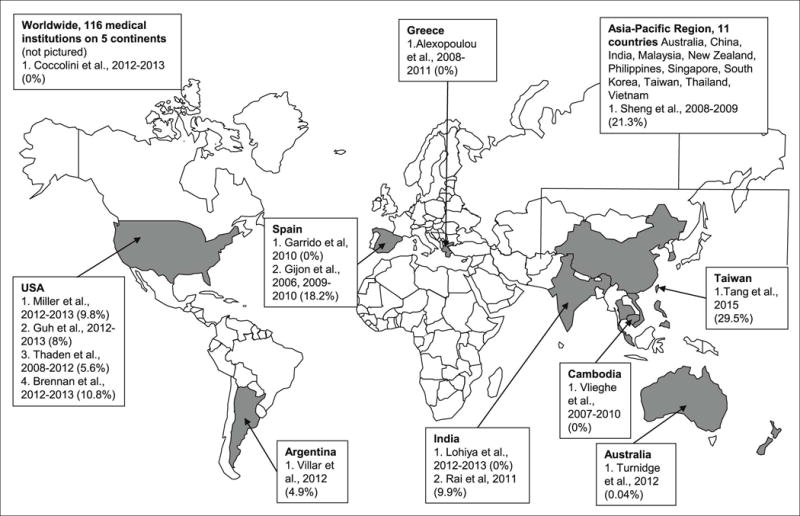
Geographical distribution of studies included in the review (percentage of community-associated or community-onset carbapenem-resistant Enterobacteriaceae). Dates represent the study period.
5.1. Heterogeneity of study design
The proportion of CAI- or CO-CRE in the 15 studies ranged from 0% to 29.5%, with sample sizes ranging from 11 to 2802 (Table 1). The 15 studies fell into two broad categories by study design: (i) 5 studies only included cases of CRE and provided the proportion of CAI within the sample [16,25,35–37]; and (ii) the other 10 studies searched for CRE among a larger sample from either a hospital or community setting. Those conducted in a hospital setting provided the proportion of CAI- or CO-CRE.
Table 1.
Studies included in the scoping review
| Study, year | Country (region) | Study period | Study design | Sample | Results (resistance among all bacterial isolates in sample) | Definition of CAI | Conclusions (CRE in community) |
|---|---|---|---|---|---|---|---|
| Tang et al., 2016 [36] | Taiwan (Tainan City) | Jan.– July 2015 | Single-centre, retrospective | N = 78 CRE cases | CR Klebsiella pneumoniae, 42/78 (53.8%) CR Enterobacter cloacae, 24/78 (30.8%) CR Escherichia coli, 11/78 (14.1%) |
HAI if hospitalised >48 h in previous 2 weeks or residence in LTCF; all others CAI | Of the 78 cases of CRE, 23 (29.5%) were CAI; included colonisation |
| Miller and Johnson, 2015 [35] | USA (North Carolina) | Jan. 2012–Dec. 2013 | Single-centre, retrospective case–control | N = 41 CRE cases | CR Enterobacter spp., 20/41 (49%) CR K. pneumoniae, 18/41 (44%) |
‘likely community-acquired’ with no definition given, but variables on medical history collected | Of 41 cases of CRE, 4 (9.8%) were likely CAI; excluded asymptomatic colonisation |
| Guh et al., 2015 [25] | USA (7 metro areas) | Jan. 2012–Dec. 2013 | Population-and laboratory-based active surveillance | N = 599 CRE cases in 481 individuals; 371/569 (65.2%) cases with data available were hospitalised at time of culture or within 30 days of having positive culture | CR K. pneumoniae, 351/599 (58.6%) CR E. coli, 89/599 (14.9%) CR E. cloacae, 75/599 (12.5%) |
‘community-associated’ if no documented relevant healthcare exposure prior to positive culture | Of the 481 individuals with CRE, ca. 8% had no documented preceding healthcare exposure |
| Coccolini et al., 2015 [38] | Worldwide (116 medical institution s across 5 continents) | Jan.– June 2012 Oct. 2012–Mar. 2013 |
Two multicentre prospective cohorts | N = 306 patients with IAIs secondary to acute cholecystitis with microbiological studies performed and 267 bacteria isolated | ESBL E. coli, 12/267 (4.5%) ESBL K. pneumoniae, 4/267 (1.5%) CR K. pneumoniae: 1/267 (0.4%) |
‘community-acquired’ with no definition given | The single case of CRE was not CAI |
| Vlieghe et al., 2015 [42] | Cambodia (Phnom Penh) | 2007–2010 | Single-centre, prospective cohort | BSIs in hospital, 91 Enterobacteriaceae isolates with cefotaxime resistance | ESBL Enterobacteriaceae, 85/91 (93.4%) Colistin-resistant Enterobacter spp., 3/91 (3.3%) |
‘community-acquired’ if infection started before or during first 2 days of hospitalisation | No CRE found in either HAI or CAI sample |
| Lohiya et al., 2015 [40] | India (Haryana) | Nov. 2012–Dec. 2013 | Community surveillance programme | N = 433 healthy individuals providing urine samples; 58/433 (13.4%) positive for Enterobacteriaceae | ESBL E. coli, 20% ESBL K. pneumoniae, 8.7% | No definition given, but entire sample considered ‘community’ because sampled healthy individuals | No CRE found in sample |
| Thaden et al., 2014 [16] | USA (25 community hospitals in North & South Carolina, Virginia and Georgia) | Jan. 2008–Dec. 2012 | Multicentre, prospective cohorts | N = 305 CRE cases; 180 (59%) with symptomatic infection, 125 (41%) asymptomatic colonisation | CR K. pneumoniae, 277/305 (90.8%) | ‘Community acquired’ if infection or colonisation occurring <48 h of patient’s admission and none of the risk factors: previous hospitalisation, surgery, dialysis, or LTCF in past 12 months or presence of invasive device | Of the 305 cases of CRE, 17 (5.6%) were defined as CAI |
| Garrido et al., 2014 [41] | Spain (Zaragoza) | Jan.– June 2010 | Single-centre, prospective surveillance programme | N = 3695 faecal samples from 2508 patients presenting with GI complaint as outpatient or inpatient at hospital; 167 (4.5%) positive for Enterobacteriaceae | ESBL E. coli, 142/167 (85.0%) AmpC E. coli, 16/167 (9.6%) ESBL K. pneumoniae, 12/167 (7.2%) |
Infections of the ‘community’ were from all outpatients | No CRE found in HAI or CO sample |
| Rai et al., 2014 [45] | India (East Delhi) | 2011 | Single-centre, prospective surveillance programme | N = 242 stool samples positive for Enterobacteriaceae from 123 outpatients undergoing GI surgery | CRE, 24/242 (9.9%) | No definition given, but entire sample considered ‘community’ because attending an outpatient clinic | Since entire sample was from community, all 9.9% of isolates were CO-CRE carriage |
| Brennan et al., 2014 [37] | USA (Michigan) | Sept. 2012–Feb. 2013 | Multicentre surveillance programme | N = 102 cases of CRE | CR K. pneumoniae, 89/102 (87%) CR E. coli, 13/102 (13%) |
‘community onset’ if specimen was collected ≤3 days after admission, but still considered HAI if exposure to healthcare in past 90 days | Of the 102 cases of CRE, 66 (64.7%) were deemed CO, but only 11 (10.8%) had no documented exposure to healthcare in past 90 days and could be classified as CAI |
| Turnidge et al., 2013 [43] | Australia (all six states) | 2012 | Multicentre surveillance programme | N = 2802 UTI isolates from patients presenting to outpatient clinic, emergency departments or community practitioners | ESBL E. coli, 91/2802 (3.2%) ESBL K. pneumoniae, 21/2802 (0.7%) CR E. cloacae, 1/2802 (0.04%) |
No definition given, but entire sample considered ‘community-onset’ because presenting as outpatients | Since entire sample was from community, the single CRE case (0.04%) was considered CO |
| Villar et al., 2013 [29] | Argentina (Buenos Aires) | Mar.– July 2012 | Single-centre surveillance programme | N = 164 non-hospitalised patients presenting faecal samples at clinic with GI complaints | ESBL-producing Enterobacteriaceae, 31/164 (18.9%) CRE, 8/164 (4.9%) |
‘community-acquired’ defined by no hospitalisation in past 2 months or antibiotic use in past 7 days | Since entire sample was from community, all 4.9% were community CRE carriage |
| Sheng et al., 2013 [44] | Asia-Pacific region (11 countries) | 2008–2009 | Multicentre prospective cohort | N = 5585 IAIs positive for Enterobacteriaceae; 699 (12.5%) positive for β-lactamase gene | CRE, 197/699 (28.2%) | Presumed ‘community-acquired’ if organisms isolated <48 h of hospitalisation | Of the 197 CRE cases, 42 (21.3%) were CAI |
| Alexopoulou et al., 2013 [39] | Greece (Athens) | 2008–May 2011 | Single-centre retrospective | N = 156 cirrhotic patients with spontaneous bacterial peritonitis, 47 with positive ascetic fluid culture included in sample | ESBL E. coli, 3/47 (6.4%) CR K. pneumoniae KPC, 4/47 (8.5%) |
Community-acquired if present on admission or developed within first 48 h after hospitalisation | Of the 4 cases of CRE, none were CO |
| Gijón et al., 2012 [46] | Spain (Madrid) | Jan.– Apr. 2006 July 2009–Jan. 2010 |
Two prospective cohorts | N = 1100 faecal samples randomly selected from laboratory from 1043 patients | CRE VIM, 11/1043 (1.1%) | Patients from community setting defined by samples from non-hospitalised patients, with no hospitalisation in past 3 months | Of the 11 CRE cases, 2 (18.2%) were among non-hospitalised patients with no previous admission and considered community CRE carriage |
CAI, community-associated infection; CRE, carbapenem-resistant Enterobacteriaceae; CR, carbapenem-resistant; HAI, healthcare-associated infection; LTCF, long-term care facility; IAI, intra-abdominal infection; ESBL, extended-spectrum β-lactamase; BSI, bloodstream infection; GI, gastrointestinal; CO, community-onset; UTI, urinary tract infection.
Five studies (33.3%) found no CAI/CO-CRE. Of these, two studies identified a small number of CRE among inpatients presenting with abdominal infections, but none were CAI/CO [38,39]. In the other three studies, no CRE was found among urinary samples of healthy individuals [40], faecal samples of outpatients presenting with gastrointestinal symptoms [41] or inpatients with bloodstream infections [42]. Of note, these five studies also tested for ESBL and all found cases of ESBL-CAI. For the five studies only including cases of CRE, 5.6–29.5% of the samples were defined as likely CAI because all five studies collected data on previous exposure to the healthcare system [16,25,35–37]. For the final five studies that tested for the presence of CRE in the hospital or community, the percentage of CAI/CO-CRE ranged from 0.04% to 21.3%. The 0.04% arose from Australian surveillance for CRE among outpatient urinary tract infections (UTIs) [43] and the 21.3% arose from 197 cases of CRE identified in intra-abdominal infections in the Asia-Pacific region in which 42 were isolated <48 h from admission [44].
5.2. Defining community-associated or community-onset infections
Overall there was no standard definition of CAI among the included studies. Although six studies (40.0%) provided no formal definition for CAI versus HAI, four of these considered their entire sample ‘community’ because all isolates were obtained from outpatients [40,41,43,45]. Two separate definitions of CAI were used in the remaining nine studies. Four studies defined CAI as the presence of infection <48–72 h following admission [36,39,42,44]. The other five studies defined CAI as cases where there was no known prior exposure to the healthcare system, but this varied substantially. The definition of exposure to a healthcare setting varied and included one or a combination of the following: previous antibiotic use with varying time frames; presence of invasive medical devices; exposure to LTCFs; and previous hospitalisation with varying time frames ranging from the past 90 days to the past year. Only one study combined all elements in their definition, which matches with the formal definition for CAI provided by the CDC [16]. Owing to this inconsistency among the definition of community, the results are provided as CAI- or CO-CRE. Community-onset infections may or may not have been acquired from previous healthcare exposure.
5.3. Bacterial isolate source and species
Most studies did not distinguish between CAI or HAI isolates in terms of body site. Urine was the primary source of isolates [16,25,35,37,40,43], followed by faeces [29,41,45,46], intra-abdominal fluid [38,39,44], blood [42] and respiratory culture [36]. For the five studies only including CRE, K. pneumoniae was the most common organism [16,25,36,37], except a US-based study that had a slightly higher percentage of Enterobacter spp. [35]. For the other 10 studies testing for the presence of CRE among hospital and community samples, E. coli was the most common organism overall, followed by K. pneumoniae or Enterobacter cloacae. The most common mechanism of antibiotic resistance among these studies was the presence of ESBLs [29,38–44].
A variety of automated systems were used to identify CRE among the isolates, with all but one study [38] documenting the use of the Clinical and Laboratory Standards Institute (CLSI) breakpoints for the minimum inhibitory concentration (MIC) for carbapenem susceptibility testing. In addition, eight studies used the modified Hodge test to detect the presence of carbapenemase activity among Enterobacteriaceae [16,25,29,37,39–41,46]. Further genotypic analyses were uncommon. Only four studies identifying CRE conducted PCR to test for the genes encoding the various carbapenemases [25,43,44,46]. Of these four, the US-based study identified KPC as the most common carbapenemase [25], the study conducted primarily in India found the greatest frequency of NDM [44], VIM was most common in the study conducted in Spain [46] and a single case of IMP was identified in an Australian cohort [43].
5.4. Colonisation
For the two studies that identified the presence of asymptomatic colonisation or carriage as a proportion of their CRE sample, frequency ranged from 11.5% to 41 % of the total sample [16,36]. One study excluded asymptomatic colonisation [35], whilst most of the studies did not mention colonisation or carriage as their entire sample had some type of active infection. Five studies tested specifically for colonisation, with three finding faecal carriage of CRE [29,45,46] ranging from 1.1% to 9.9% of the sample, and two not finding any CRE carriage [40,41]. One study reported a prevalence of 29% CRE colonisation or previous CRE infection prior to admission as a risk factor for subsequent CRE infection [37].
5.5. Co-morbidities
Six studies documented the most common co-morbidities in their samples. These included cancer [36], diabetes [25,35], human immunodeficiency virus (HIV) [42] and cardiovascular disease [37,38]. For the single study that did test for differences in CRE by co-morbidity between HAI and CAI, no statistically significant difference was found [36].
6. Discussion
Although CRE is still uncommon in community settings, a high degree of variability was found among studies. Among those studies that detected CAI/CO-CRE, the numbers were concerning, with the majority reporting a proportion ranging from 7.7–29.5% globally and from 5.6–10.8% in the USA. The reported percentage of CAI/CO-CRE was generally higher in Asia, most notably Taiwan and India. Kumarasamy et al., for example, tested for the presence of CRE conferred by NDM among Enterobacteriaceae and found that the majority of samples in India arose from the community [30]. Although only four studies in this review included a genomic analysis of carbapenemases, their findings support previous findings of geographic distribution, namely KPC most common in a US sample, NDM in an Asian sample and VIM in southern European samples [10,11,14]. Of note, we did not find any studies that met our selection criteria that were conducted in Africa.
These results demonstrate the difficulty of separating HAI and CAI, even with the use of standardised definitions. The CDC defines CAI as any infection that is not associated with the hospital [47], but there is an important distinction between CAIs and community-onset infections. In both cases, infections develop in the community, but community-onset infections may be either healthcare-associated or not. To be defined as a CAI, the patient must lack specific risk factors for healthcare exposure included in the CDC definition, which was originally used to define community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infection [48]. To be considered a CAI, patients should have none of the following healthcare risk factors: (i) recent hospitalisation, surgery, dialysis or residence in a LTCF <1 year before the onset of illness; and (ii) no permanent indwelling catheter or percutaneous medical device. The CDC defines community-onset infections as having a positive culture from a sample collected ≤3 days following admission to a facility [49]. Owing to the number of studies in this review that provided no or partial testing for previous healthcare exposure, we had to expand our description of community cases to CAI/CO-CRE.
The findings demonstrate that CRE among CAIs appears to mirror HAIs. Both for CAIs and HAIs, the majority of CRE are isolated from urine, with higher rates of faecal carriage as well [4]. This follows the same pattern demonstrated by ESBL, in which community-associated UTIs began to develop increased resistance [9] and resistant strains were found in healthy faecal samples [8]. Just as with ESBLs, there are likely community accelerators for spread. Hence, risk factors for colonisation and/or infection are the same as other drug-resistant organisms [12,13,35]. Long-term care settings, for example, are important reservoirs for community-onset, healthcare-associated transmission [37,50,51]. In the population-based surveillance of Guh et al., 55.9% of patients positive for CRE were sent to LTCFs following hospital discharge, demonstrating the potential for interfacility spread [25].
In February 2016, active surveillance using perirectal swabs in a US hospital revealed six asymptomatic patients who were colonised with VIM-producing CRE [52]. This is an example of resistant strains spreading silently through asymptomatic colonisation and posing a threat to timely identification and containment of these organisms.
Other studies are also identifying the presence of CRE outside of human sources. A study in India demonstrated the importance of environmental sampling after discovering carbapenemases in drinking water [53]. The study also noted the importance of seasonality, with increased risk for contamination of water sources during the rainy season. Animals present another source of community transmission. Researchers in China discovered colistin resistance encoded on a plasmid among livestock [54]. With plasmid-borne colistin resistance recently found in humans [23], there is increased concern that these additional reservoirs for CRE may drive the community-based epidemic as resistant organisms continue to move beyond healthcare settings.
As with all reviews, this study had limitations. First, we did not assess the quality of the studies reviewed, as our aim in this scoping review was to assess the breadth of studies that have been conducted to date. We only included studies published in English in which evidence of community-associated versus healthcare-associated determination was reflected in the abstract, and it is likely that some studies were missed. Further, we present the proportion of CAI/CO-CRE in order to provide a comparable percentage among the various studies, but study design and sample size variation may have impacted the precision of the true proportion. This heterogeneity in study design and sample size is a major limitation of this review as it is difficult to state and compare percentages from small collection samples with potential sampling bias.
CRE are already present in the community. The high degree of variability in terms of geographical prevalence, isolate type and source, patterns of resistance and methods of study indicate a need for increased epidemiological surveillance. Many countries still lack nationwide surveillance [12]. Persons asymptomatically colonised with CRE present a potential reservoir for CAI and since the goal is to stop transmission, screening to identify high-risk individuals is important. To enhance our understanding of transmission dynamics, molecular epidemiology should be used to examine HAI-CRE genotypes and connect them to CAI-CRE genotypes. These methods can greatly enhance our understanding of CRE transmission within populations and boost surveillance associated with interspecies and intraspecies lateral gene transfer [55]. With carbapenemases following a similar genetic mode of transmission as ESBLs, it behoves us to look at the endemicity of ESBLs across many countries as an early warning sign for the likely increased spread of CRE into the community.
Highlights.
Reviewed 15 studies to assess proportion of carbapenem-resistant Enterobacteriaceae (CRE) in community settings.
Percentage of community-associated or community-onset CRE ranged from 0–29.5%.
Percentage of community-based CRE was highest in parts of Asia.
Risk for increased spread of CRE in community due to resistance transmission on plasmids.
Acknowledgments
Funding: AMK is supported by a National Institutes of Health T32 ‘Training in interdisciplinary research to prevent infections (TIRI)’ [5T32NR013454-04]. The funding source had no role in the study design, collection, analysis and interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Appendix
-
Search strategy for PubMed
((carbapenem-resistant OR carbapenemase OR ‘carbapenem-resistant enterobacteriaceae’) AND ((community-acquired infection[MeSH Major Topic]) OR community-associated OR community-acquired OR community-onset OR communit* OR outpatient))
-
Search strategy for Embase
‘carbapenem resistant enterobacteriaceae’/exp OR ‘carbapenem resistant enterobacteriaceae’ OR ‘carbapenem-resistant’ OR ‘carbapenemase’
AND
‘community acquired infection’/exp OR ‘community acquired infection’ OR ‘community associated’ OR ‘community onset’ OR ‘communit*’ OR ‘outpatient’
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.US Centers for Disease Control and Prevention (CDC) Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/ [accessed 4 June 2016]
- 2.The White House. Report to the president on combating antibiotic resistance: Executive order of the president. President’s Council of Advisors on Science and Technology; 2014. https://www.whitehouse.gov/the-press-office/2015/03/27/fact-sheet-obama-administration-releases-national-action-plan-combat-ant [accessed 4 June 2016] [Google Scholar]
- 3.The Center for Disease Dynamics, Economics & Policy (CCDEP) The state of the world’s antibiotics, 2015. http://cddep.org/publications/state_worlds_antibiotics_2015 [accessed 4 June 2016]
- 4.Van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115–20. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–7. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 6.US Centers for Disease Control and Prevention (CDC) Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE)—November 2015 update CRE toolkit. http://www.cdc.gov/hai/organisms/cre/cre-toolkit/ [accessed 4 June 2016]
- 7.National Institute of Allergy and Infectious Diseases (NIAID) NIAID’s Antibacterial Resistance Program: current status and future directions 2014. https://www.niaid.nih.gov/sites/default/files/arstrategicplan2014.pdf [accessed 19 June 2017]
- 8.Ebrahimi F, Mózes J, Mészáros J, Juhász Á, Kardos G. Carriage rates and characteristics of Enterobacteriaceae producing extended-spectrum β-lactamases in healthy individuals: comparison of applicants for long-term care and individuals screened for employment purposes. Chemotherapy. 2014;60:239–49. doi: 10.1159/000375407. [DOI] [PubMed] [Google Scholar]
- 9.Salles MJC, Zurita J, Mejía C, Villegas MV. Resistant Gram-negative infections in the outpatient setting in Latin America. Epidemiol Infect. 2013;141:2459–72. doi: 10.1017/S095026881300191X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–96. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18:413–31. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 12.Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20:854–61. doi: 10.1111/1469-0691.12748. [DOI] [PubMed] [Google Scholar]
- 13.Tängdén T, Giske CG. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med. 2015;277:501–12. doi: 10.1111/joim.12342. [DOI] [PubMed] [Google Scholar]
- 14.Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20:821–30. doi: 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2015;60:1153–61. doi: 10.1093/cid/ciu1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaden JT, Lewis SS, Hazen KC, Huslage K, Fowler VG, Jr, Moehring RW, et al. Rising rates of carbapenem-resistant Enterobacteriaceae in community hospitals: a mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect Control Hosp Epidemiol. 2014;35:978–83. doi: 10.1086/677157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bush K. Proliferation and significance of clinically relevant β-lactamases. Ann N Y Acad Sci. 2013;1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 18.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis. 2011;52:1–8. doi: 10.1093/cid/cir025. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Cunningham MA, Mire J, Tesar C, Sacchettini J, Joachimiak A. NDM-1, the ultimate promiscuous enzyme: substrate recognition and catalytic mechanism. FASEB J. 2013;27:1917–27. doi: 10.1096/fj.12-224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Centers for Disease Control and Prevention (CDC) Healthcare-associated infections (HAIs) Tracking CRE infections. http://www.cdc.gov/hai/organisms/cre/TrackingCRE.html#CREmap [accessed 4 June 2016]
- 21.Rossi Gonçalves I, Ferreira ML, Araujo BF, Campos PA, Royer S, Batistão DW, et al. Outbreaks of colistin-resistant and colistin susceptible KPC-producing Klebsiella pneumoniae in a Brazilian intensive care unit. J Hosp Infect. 2016;94:322–9. doi: 10.1016/j.jhin.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Ergönül Ö, Aydin M, Azap A, Başaran S, Tekin S, Kaya Ş, et al. Healthcare-associated Gram-negative bloodstream infections: antibiotic resistance and predictors of mortality. J Hosp Infect. 2016;94:381–5. doi: 10.1016/j.jhin.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 23.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, et al. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the USA. Antimicrob Agents Chemother. 2016;60:4420–1. doi: 10.1128/AAC.01103-16. Erratum in: Antimicrob Agents Chemother 2016;60:5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22:686–96. doi: 10.1016/j.tim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guh AY, Bulens SN, Mu Y, Jacob JT, Reno J, Scott J, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA. 2015;314:1479–87. doi: 10.1001/jama.2015.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temkin E, Adler A, Lerner A, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci. 2014;1323:22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- 27.Lerner A, Adler A, Abu-Hanna J, Percia SC, Matalon MK, Carmeli Y. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin Microbiol Infect. 2014;21:470.e1–7. doi: 10.1016/j.cmi.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 28.European Centre for Disease Prevention and Control (ECDC) Antimicrobial resistance surveillance in Europe Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2014. http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2014.pdf [accessed 4 June 2016]
- 29.Villar HE, Baserni MN, Jugo MB. Faecal carriage of ESBL-producing Enterobacteriaceae and carbapenem-resistant Gram-negative bacilli in community settings. J Infect Dev Ctries. 2013;7:630–4. doi: 10.3855/jidc.2900. [DOI] [PubMed] [Google Scholar]
- 30.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobacteriaceae: a systematic review. Am J Infect Control. 2016;44:539–43. doi: 10.1016/j.ajic.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5:371–85. doi: 10.1002/jrsm.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 34.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–61. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller BM, Johnson SW. Demographic and infection characteristics of patients with carbapenem-resistant Enterobacteriaceae in a community hospital: development of a bedside clinical score for risk assessment. Am J Infect Control. 2015;44:134–7. doi: 10.1016/j.ajic.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Tang HJ, Hsieh CF, Chang PC, Chen JJ, Lin YH, Lai CC, et al. Clinical significance of community- and healthcare-acquired carbapenem-resistant Enterobacteriaceae isolates. PLoS One. 2016;11:e0151897. doi: 10.1371/journal.pone.0151897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brennan BM, Coyle JR, Marchaim D, Pogue JM, Boehme M, Finks J, et al. Statewide surveillance of carbapenem-resistant Enterobacteriaceae in Michigan. Infect Control Hosp Epidemiol. 2014;35:342–9. doi: 10.1086/675611. [DOI] [PubMed] [Google Scholar]
- 38.Coccolini F, Sartelli M, Catena F, Montori G, Di Saverio S, Sugrue M, et al. Antibiotic resistance pattern and clinical outcomes in acute cholecystitis: 567 consecutive worldwide patients in a prospective cohort study. Int J Surg. 2015;21:32–7. doi: 10.1016/j.ijsu.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Alexopoulou A, Papadopoulos N, Eliopoulos DG, Alexaki A, Tsiriga A, Toutouza M, et al. Increasing frequency of Gram-positive cocci and Gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013;33:975–81. doi: 10.1111/liv.12152. [DOI] [PubMed] [Google Scholar]
- 40.Lohiya A, Kant S, Kapil A, Gupta SK, Misra P, Rai SK. Pattern of antibiotic resistance among community derived isolates of Enterobacteriaceae using urine sample: a study from northern India. J Clin Diagn Res. 2015;9:LC15–9. doi: 10.7860/JCDR/2015/14230.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrido A, Seral C, Gude MJ, Casado C, González-Domínguez M, Sáenz Y, et al. Characterization of plasmid-mediated β-lactamases in fecal colonizing patients in the hospital and community setting in Spain. Microb Drug Resist. 2014;20:301–4. doi: 10.1089/mdr.2013.0109. [DOI] [PubMed] [Google Scholar]
- 42.Vlieghe ER, Huang TD, Phe T, Bogaerts P, Berhin C, De Smet B, et al. Prevalence and distribution of β-lactamase coding genes in third-generation cephalosporin-resistant Enterobacteriaceae from bloodstream infections in Cambodia. Eur J Clin Microbiol Infect Dis. 2015;34:1223–9. doi: 10.1007/s10096-015-2350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turnidge JD, Gottlieb T, Mitchell DH, Coombs GW, Pearson JC, Bell JM. Australian Group on Antimicrobial Resistance Community-onset Gram-negative Surveillance Program: annual report, 2010. Commun Dis Intell Q Rep. 2013;37:E219–23. doi: 10.33321/cdi.2013.37.33. [DOI] [PubMed] [Google Scholar]
- 44.Sheng WH, Badal RE, Hseuh P-R. Distribution of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in Asia-Pacific: the Study for Monitoring Antimicrobial Resistance Trends (SMART) Antimicrob Agents Chemother. 2013;57:2981–8. doi: 10.1128/AAC.00971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rai S, Das D, Niranjan DK, Singh NP, Kaur IR. Carriage prevalence of carbapenem-resistant Enterobacteriaceae in stool samples: a surveillance study. Australas Med J. 2014;7:64–7. doi: 10.4066/AMJ.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gijón D, Curiao T, Baquero F, Coque TM, Cantón R. Fecal carriage of carbapenemase-producing Enterobacteriaceae: a hidden reservoir in hospitalized and nonhospitalized patients. J Clin Microbiol. 2012;50:1558–63. doi: 10.1128/JCM.00020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 48.David MZ, Glikman D, Crawford SE, Peng J, King KJ, Hostetler MA, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197:1235–43. doi: 10.1086/533502. [DOI] [PubMed] [Google Scholar]
- 49.US Centers for Disease Control and Prevention (CDC) Multidrug-resistant organism & Clostridium difficile infection (MDRO/CDI) Module. CDC; 2016. http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf [accessed 4 June 2016] [Google Scholar]
- 50.Cascio GL, Soldani F, Mazzariol A, Lleo MM. The high incidence of carbapenem-resistant Klebsiella pneumoniae in urine from elderly hospital patients may facilitate the spread of resistant strains to the community. Microb Drug Resist. 2014;20:67–72. doi: 10.1089/mdr.2013.0036. [DOI] [PubMed] [Google Scholar]
- 51.Prabaker K, Lin MY, McNally M, Cherabuddi K, Ahmed S, Norris A, et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control. 2012;33:1193–9. doi: 10.1086/668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaffee AQ, Roser L, Daniels K, Humbaugh K, Brawley R, Thoroughman D, et al. Notes from the field: Verona integron-encoded metallo-β-lactamase-producing carbapenem-resistant Enterobacteriaceae in a neonatal and adult intensive care unit—Kentucky, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:190. doi: 10.15585/mmwr.mm6507a5. [DOI] [PubMed] [Google Scholar]
- 53.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–62. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 54.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2015;16:161–8. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 55.Pecora ND, Li N, Allard M, Li C, Albano E, Delaney M, et al. Genomically informed surveillance for carbapenem-resistant Enterobacteriaceae in a health care system. mBio. 2015;6:e01030–15. doi: 10.1128/mBio.01030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


