Abstract
Pericytes are periendothelial mesenchymal cells residing within the microvasculature. Skeletal muscle and cardiac pericytes are now recognized to fulfill an increasing number of functions in normal tissue homeostasis, including contributing to microvascular function by maintaining vessel stability and regulating capillary flow. In the setting of muscle injury, pericytes contribute to a regenerative microenvironment through release of trophic factors and by modulating local immune responses. In skeletal muscle, pericytes also directly enhance tissue healing by differentiating into myofibers. Conversely, pericytes have also been implicated in the development of disease states, including fibrosis, heterotopic ossication and calcification, atherosclerosis, and tumor angiogenesis. Despite increased recognition of pericyte heterogeneity, it is not yet clear whether specific subsets of pericytes are responsible for individual functions in skeletal and cardiac muscle homeostasis and disease.
Keywords: Perivascular stem cell, Mesenchymal stem cell, PSC, MSC, Heart, Muscle
1. Introduction
Pericytes are perivascular cells that are found in abundance in all vascularized organs where they regulate numerous functions, including vessel growth, permeability, and contractility (Cappellari & Cossu, 2013). In skeletal muscle, pericytes appear to play additional roles in tissue regeneration, including differentiation into myofibers (Dellavalle et al., 2007). Pericytes are however also implicated in the development of fibrosis, heterotopic ossification, atherosclerosis, and tumor angiogenesis, diseases that represent some of the most frequent causes of morbidity and mortality in the western world (Collett & Canfield, 2005; Fang & Salven, 2011; Henderson et al., 2013; Matthews et al., 2016). Despite these critical roles in tissue physiology and disease, relatively little is known about skeletal muscle and cardiac pericytes (Armulik et al., 2011). The key barrier to our understanding of pericytes is the lack of truly specific markers and thus a lack of consensus on pericyte identity. With increasing recognition of pericyte heterogeneity, it is not yet clear whether subsets of pericytes are responsible for individual pericyte functions. Approaches that combine genetic lineage tracing, anatomical location, and expression of surface markers have facilitated an improved understanding of pericyte roles in health and disease. In this review, we outline current concepts in anatomy, molecular markers, and developmental origins of skeletal and cardiac muscle pericytes. We report proposed roles of skeletal and cardiac muscle pericytes in organ homeostasis and in the response to muscle injury and disease. Finally, we discuss the potential of pericytes from these organs as therapeutic agents of regeneration and repair.
2. Pericyte anatomy
Pericytes are periendothelial mesenchymal cells that reside within the microvasculature, sharing a basement membrane with underlying endothelial cells (Armulik et al., 2011) (Fig. 1). Classically described to be present on capillaries, there is considerable evidence to suggest that pericytes are ubiquitous in higher order vessels such as pre-capillary arterioles, post-capillary venules, and veins while conspicuously absent in the lymphatic vasculature (Campagnolo et al., 2010; Norrmen et al., 2011). Given their periendothelial distribution, pericytes are frequently confused with vascular smooth muscle cells (vSMCs), which reside in this location on arterioles. In contrast to arteriolar vSMCs, pericytes have a nearly rounded cell body with numerous finger-like projections that extend longitudinally spanning the abluminal surface of several endothelial cells. These primary processes extending along the length of the capillary give rise to secondary processes that run perpendicular to the primary processes, partially encircling the capillary with tips of secondary processes making connections with endothelial cells (Armulik et al., 2005, 2011). In addition to forming connections with underlying capillary endothelial cells, pericytes connect with endothelial cells in neighboring capillaries with fine processes that traverse the intercapillary space cells (Armulik et al., 2011). In vitro studies of isolated cardiac pericytes have demonstrated that these cells are also capable of forming connections with other pericytes likely via gap junctional proteins such as connexins (Nees et al., 2012). Dye transfer studies have demonstrated rapid transfer of dye from pericytes to endothelial cells as well as between adjacent pericytes suggesting that pericytes along with endothelial cells likely form a functional intercommunicating unit in the vasculature (Larson et al., 1987). In comparison, pericytes do not form robust connections with vascular smooth muscle cells. Although the physiologic significance of pericyte–pericyte and pericyte–endothelial connections are not clear, the functional coupling of pericytes to endothelial cells and not vascular smooth muscle cells likely represents a mechanism for pericyte-mediated regulation of the vasculature independent of vascular smooth muscle cells.
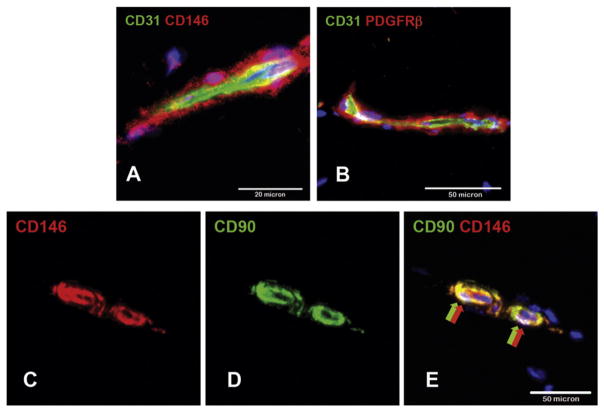
Fig. 1.
Immunohistochemistry demonstrating the intimate relationship of pericytes to endothelial cells. (A) Adult mouse skeletal muscle pericytes expressing CD146 and PDGFRβ surround CD31+ microvascular endothelial cells. (B) Adult human skeletal muscle pericytes co-expressing CD90 and CD146 surround CD146+ microvascular ECs.
Pericyte density varies between different organs as does the area of the abluminal endothelial surface that they cover (Armulik et al., 2011). Pericyte density and coverage appears to correlate with endothelial barrier properties (brain > lungs > muscle) (Armulik et al., 2011), endothelial cell turnover (large turnover equates to less coverage), and orthostatic blood pressure (larger coverage in lower body parts) (Diaz-Flores et al., 2009; Armulik et al., 2011). The brain is thought to be organ with the greatest density of pericytes with an endothelial cell–pericyte ratio between 1:1 and 3:1 (Sims, 1986; Mathiisen et al., 2010). By contrast, skeletal muscle vasculature has substantially fewer pericytes covering endothelial cells with an endothelial–pericyte ratio of approximately 100:1 (Diaz-Flores et al., 2009). The pericyte content of the cardiac micro-vasculature is thought to be closer to that of the cerebral vasculature with endothelial–pericyte ratios of 2:1–3:1 (Nees et al., 2012). It is estimated that there are approximately 3.6 × 107 pericytes/cm3 of left ventricular tissue (Nees et al., 2012) and the number of pericytes exceeds the number of myocytes in unit volume of left ventricular tissue. The cardiac pre-capillary arteriole, capillary, and post-capillary venule, constituting the core microcirculatory unit is thus richly inundated with pericytes with less than 1% of the length of this microcirculatory unit being free of pericytes (Nees et al., 2012).
3. Molecular markers
Anatomical and ultrastructural definitions are not useful for isolating pericytes from tissues such as skeletal muscle or heart, and consequently a host of molecular markers have been suggested for identifying these cells (Table 1) (Armulik et al., 2011; Murray et al., 2013). Widely recognized pericyte markers include platelet-derived growth factor receptor beta (PDGFRβ), NG2 (chondroitin sulfate proteoglycan 4), CD13, alpha smooth muscle actin (αSMA), desmin, and CD146. In skeletal muscle, the expression of alkaline phosphatase by pericytes has been used to distinguish them from Pax7 (paired box protein 7) or MyoD expressing satellite cells, which are frequently in close anatomic apposition (Dellavalle et al., 2011). In addition, Pax7-positive skeletal muscle satellite cells can be further distinguished from pericytes due to their expression of Nestin and lack of NG2 expression (Birbrair et al., 2011). Finally, CD34 expression has also been used in the identification and isolation of cardiac pericytes by some groups (Campagnolo et al., 2010; Avolio et al., 2015). Pericytes are increasingly recognized to share differentiation potential and an immunophenotype with classical culture-derived mesenchymal stem cells (MSCs). Crisan et al. isolated human perivascular cells from multiple organs, including heart, depleted the cells of myogenic, endothelial, or hematopoietic cells and demonstrated multi-lineage long term differentiation capacity into myogenic, adipogenic, chrondrogenic, and osteogenic lineages (Crisan et al., 2008). These perivascular cells identified by high expression of CD146 and lack of CD34, CD45, and CD56 expression exhibited characteristic markers of pericytes as well as the canonical markers of MSCs such as CD29, CD44, CD73, CD90, CD105, and alkaline phosphatase.
Table 1.
Markers used for the positive identification of pericytes in skeletal muscle and heart.
| Marker | Also known as | References (skeletal muscle) | References (heart) |
|---|---|---|---|
| CD10 | Neural endopeptidase | 1 | 1 |
| CD13 | Alanine aminopeptidase | 1 | 1 |
| CD29 | Integrin beta 1 | 2 | |
| CD34 | 3 | ||
| CD44 | Receptor for hyaluronic acid | 1,2,3 | 1,4 |
| CD73 | 5′nucleotidase, ecto | 1,2,3 | 1,4 |
| CD90 | Thy-1 | 1,2,3 | 1,4 |
| CD105 | Endoglin | 1,2,3 | 1,4 |
| CD108 | Sema L | 1 | 1,4 |
| CD109 | Platelet activation factor | 1 | 1 |
| CD140b | Platelet-derived growth factor beta (PDGFRβ) | 1,2,4 | 1,4 |
| CD140a | Platelet-derived growth factor beta (PDGFRα) | 5 | |
| CD146 | Melanoma cell adhesion molecule | 1,2,3,4 | 1,4 |
| CD164 | Sialomucin core protein 24 | 1 | 1 |
| CD166 | ALCAM | 1 | 1 |
| CD318 | CUB domain-containing protein 1 | 1 | 1 |
| CD340 | Human epidermal growth factor receptor 2 | 1 | 1 |
| CD349 | Frizzled-9 | 1 | 1 |
| NG2 | Neurol/glial antigen 2 | 1,2,4 | 1,4 |
| SM-MHC | Smooth muscle myosin heavy chain | 4 | |
| SSEA-4 | Stage-specific embryonic antigen-4 | 1 | 1 |
| STRO-1 | 5 | 5 | |
| HLA-CLI | Human leukocyte antigen class 1 | 1 | 1 |
| α-SMA | Alpha smooth muscle actin | 1,4 | 1,4 |
[References for table].
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Dar, A. et al. Multipotent Vasculogenic Pericytes From Human Pluripotent Stem Cells Promote Recovery of Murine Ischemic Limb. Circulation 125, 87–99 (2012).
Avolio, E., Meloni, M., Spencer, H. L., Riu, F., Katare, R., Mangialardi, G., Oikawa, A., Rodriguez-Arabaolaza, I., Dang, Z., Mitchell, K., Reni, C., Alvino, V. V., Rowlinson, J., Livi, U., Cesselli, D., Angelini, G., Emanueli, C., Beltrami, A. P., & Madeddu, P. (2015). Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res, 116, e81–94.
Chen, C.-W., Corselli, M., Péault, B. & Huard, J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J. Biomed. Biotechnol. 2012, 597,439–9 (2012).
Chen, W. C. W. et al. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. STEM CELLS 33, 557–573 (2015).
Psaltis, P. J., Harbuzariu, A., Delacroix, S., Holroyd, E. W. & Simari, R. D. Resident Vascular Progenitor Cells—Diverse Origins, Phenotype, and Function. J. of Cardiovasc. Trans. Res. 4, 161–176 (2010).
It is important to emphasize that no single molecular marker can be used to unequivocally identify all pericytes and as a result multiple markers are commonly used. Marker expression however may be labile. For instance, in addition to pericytes, αSMA may be robustly expressed in both skeletal muscle and heart myofibroblasts, which may reside in a perivascular distribution, particularly after injury (Uezumi et al., 2011; Christia et al., 2013; Deb & Ubil, 2014). Thus, solely relying on the expression of αSMA would not yield a pure population of pericytes from these tissues. Similarly, PDGFRβ can be expressed by myofibroblasts in skeletal muscle and a subset of cardiac fibroblasts, while desmin is expressed by cardiac muscle cells (Paulin & Li, 2004). Therefore, a combinatorial approach, using carefully selected markers, must be adopted for obtaining pure populations of pericytes from a particular organ.
It is increasingly clear that pericytes represent a heterogeneous population with identifiable subsets when characterized beyond a population level (Birbrair et al., 2013a). For instance, pericytes localized on venules express desmin and αSMA, whereas those on capillaries express desmin but are usually negative for αSMA (Birbrair et al., 2015). Within skeletal muscle, Nestin-GFP+/NG2− DsRed− (type 2) pericytes are thought to be myogenic, while nestin-GFP−/NG2-DsRed+ (type 1) pericytes are not (Birbrair et al., 2014c). Although both populations can form smooth muscle cells in culture, only type 2 pericytes are neurogenic (Birbrair et al., 2013a, 2013c). Our understanding of pericyte subsets and their functional roles however remains limited and as more cell surface markers are identified more pericyte subsets with divergent functional capabilities can be expected to be found.
4. Developmental origins
The developmental origins of pericytes are not entirely clear. In embryogenesis, pericytes derive from the lateral (splanchopleura) and the paraxial mesoderm (somites) (Armulik et al., 2011; Cappellari & Cossu, 2013). Pericytes originating from several developmental origins may be contained within a single mosaic vessel (Cheung et al., 2012). Pericytes of the head, thymus, and aortic outflow tract likely derive from the neural crest (Bergwerff et al., 1998), whereas the origins of pericytes from the gut and viscera have been mapped to the mesothelium (Wilm et al., 2005; Que et al., 2008; Asahina et al., 2011).
Several developmental sources of skeletal muscle pericytes have been proposed. Vessel associated mesodermal myogenic ancestors identified in the dorsal aorta of murine embryos co-express pericyte (αSMA), endothelial (CD31, VE-Cadherin, CD31), and early myogenic markers (M-cadherin, MyoD, Myf5, c-Met, and desmin) (De Angelis et al., 1999). Somite-derived precursors in the dermomyotome are induced to a myogenic or vasculogenic fate by reciprocal suppression of Pax3 and Foxc2 in a Notch-dependent manner (Lagha et al., 2009; Mayeuf-Louchart et al., 2014). In addition, endothelial cells may undergo so-called endothelial-to-mesenchymal transition, repopulating skeletal muscle with perivascular mesodermal precursors (Medici et al., 2010). Finally, neonatal skeletal muscle myoblasts have been shown to re-adopt pericyte features when pre-treated with PDGF-BB and Delta like ligand 4 (Dll4) (Cappellari et al., 2013).
Cardiac pericytes are thought to derive from the epicardium, a single layer of flattened epithelial cells that surrounds the outer layer of the myocardium (Mikawa & Gourdie, 1996). During cardiac development, epicardial cells undergo epithelial to mesenchymal transition (EMT) and generate mesenchymal cells that subsequently invade the developing myocardium and give rise to cardiac fibroblasts, pericytes, and coronary vascular smooth muscle cells. Quail chick chimera experiments, where labeled epicardial cells were implanted into the pericardial space of chicks, demonstrated the ability of epicardial cells to give rise to pericytes (Mikawa & Gourdie, 1996; Dettman et al., 1998; Wessels & Perez-Pomares, 2004). Subsequent fate-mapping experiments utilizing genetically labeled epicardial cells have also confirmed epicardial cells as precursors of pericytes (Cai et al., 2008). However, much remains to be elucidated on the mechanism by which epicardial progenitors are programmed to give rise to pericytes as opposed to fibroblasts or coronary smooth muscle cells.
Irrespective of anatomical location, endothelial cells are thought to be the critical regulators of pericyte recruitment, primarily through PDGF-BB/PDGFRβ signaling (Gaengel et al., 2009). Mice deficient in PDGF or PDGFRβ have perinatal lethality secondary to lack of mural cells and vascular instability (Leveen et al., 1994; Soriano, 1994). The angiopoietin 1 (Ang1)/Tie2 signaling system has also been implicated in pericyte recruitment and vessel stability. The ligand Ang1 is expressed by pericytes and binds to the Tie2 receptor on the endothelial cell augmenting pericyte recruitment and vessel stabilization (Sundberg et al., 2002). Ang-1 or Tie 2-deficient animals exhibit cardiovascular defects and die in utero secondary to mural cell deficiencies (Suri et al., 1996; Patan, 1998). Although pericytes do not directly communicate with smooth muscle cells, pericyte precursors have recently been shown to be capable of differentiating into vascular smooth muscle cells in response to Notch signaling (Volz et al., 2015). Taken together, these observations demonstrate that although cardiac pericytes are derived from epicardial EMT, a complex set of ligand-receptor interactions between the endothelial cell and pericytes/pericyte precursors orchestrate pericyte recruitment and stabilization of the microvasculature. Finally, pericytes from both skeletal and cardiac muscle are able to proliferate, and therefore, new pericytes can be derived from in vivo expansion of pre-existing pericytes.
5. Function in muscle development and homeostasis
Pericytes play multiple roles in the homeostasis of skeletal and cardiac muscle, including regulation of microvascular function and angiogenesis. In addition, emerging evidence suggests a central role for pericytes in skeletal muscle formation (Birbrair & Delbono, 2015). Schematica outlining proposed functions/roles of skeletal and cardiac muscle pericytes in homeostasis and injury/disease states are outlined in Figs. 2 and 3.
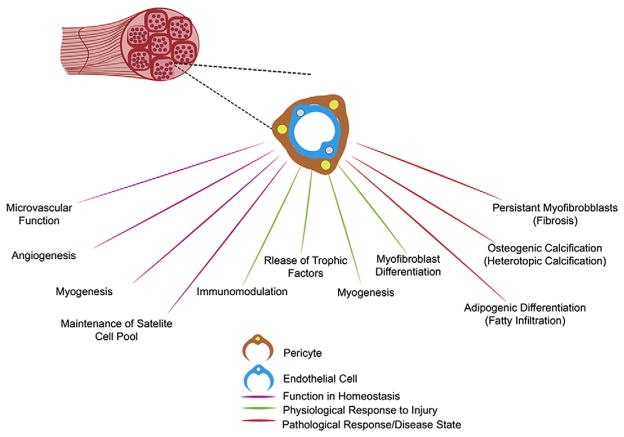
Fig. 2.
Skeletal muscle pericytes in health and disease. Schematic outlining proposed functions/roles of pericytes in skeletal muscle homeostasis, in the physiological response to injury, and in pathology/disease states.
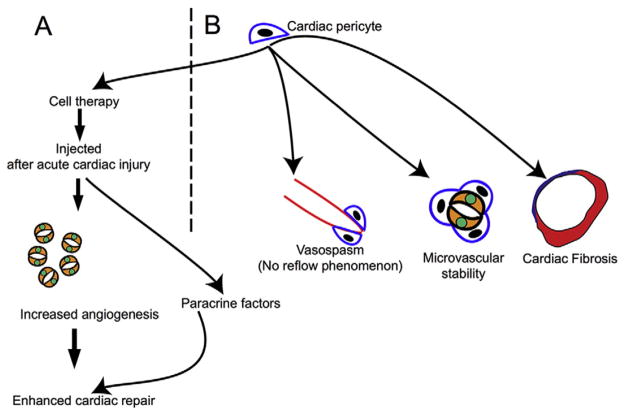
Fig. 3.
Role of the cardiac pericytes in cardiac physiology and disease. (A) Cardiac pericytes as agents of cardiac cell therapy. When injected into the infarcted heart, they enhance angiogenesis and exert other beneficial effects by paracrine mechanisms. (B) Pericytes contribute directly to cardiac fibrosis after cardiac injury by adopting fibroblast fates. They regulate microvascular stability and potentially contribute to “no reflow” after ischemia–reperfusion injury.
One of the key functions of pericytes in both skeletal and cardiac muscle is in the modulation of angiogenesis (Amselgruber et al., 1999) through the promotion of endothelial cell survival and migration (Raza et al., 2010). During the development of new vessels, activated endothelial cells invade the extracellular matrix following a vascular endothelial growth factor (VEGF) gradient and form sprouting vessel tips (Ozerdem & Stallcup, 2003). Endothelial cells lining these newly established tubes produce PDGF-BB, which recruits pericytes through the binding of their surface PDGFβ receptors (Hellstrom et al., 1999; Bjarnegard et al., 2004). Recruited pericytes act to stabilize the vessel wall via direct contact or paracrine signaling with endothelial cells inducing their differentiation and growth arrest (Gerhardt & Betsholtz, 2003). As previously discussed, the interaction of pericyte secreted Angiotensin I on the Tie2 receptor on endothelial cells is critical in the quiescence and maturation of the activated endothelium in newly formed vessels (Uemura et al., 2002). Nestin+/NG2+ type 2 pericytes, but not Nestin−/NG2+ type 1 pericytes, have been shown to form vascular networks with endothelial cells in vitro and participate in tumor angiogenesis (Birbrair et al., 2014b).
In the central nervous system, pericytes are thought to play a role in regulating permeability across the blood–brain barrier (Armulik et al., 2010; Daneman et al., 2010). Mice deficient in pericytes or exhibiting decreased pericyte function have increased vascular permeability, presumably secondary to absent interactions of pericytes with endothelial junctions. Pericyte-deficient blood vessels upregulate expression of genes associated with increased vascular permeability such as VEGFA, Ang2, and Adrenomedullin (Armulik et al., 2010; Daneman et al., 2010). In the heart, it is also likely that pericytes contribute to the maintenance of microvascular function given that loss of pericytes following treatment with the anti-cancer drug sunitinib is associated with microvascular dysfunction. Pericyte loss with sunitinib leads to decreased basal coronary blood flow and impaired coronary flow reserve upon vasodilator challenge (Chintalgattu et al., 2013). In addition to maintaining microvascular flow, cardiac pericytes may be involved in regulating the shape of blood vessels as loss of pericytes in the cardiac microvasculature was associated with increased tortuosity of blood vessels (Chintalgattu et al., 2013). Consistent with the role of pericytes in maintaining vascular permeability in the brain, loss of cardiac pericytes resulted in increased interstitial permeability (Chintalgattu et al., 2013). Little is known about mechanisms maintaining or renewing pericytes in vivo however the PDGF-BB/PDGFRβ pathway likely plays an important role in pericyte maintenance as the toxic effects of the drug sunitinib on cardiac pericyte loss were ameliorated with use of a PDGFR inhibitor (Chintalgattu et al., 2013).
As pericytes sit in a periendothelial location and can express contractile proteins such as αSMA, it has been long thought that pericytes can regulate blood flow to tissue beds by altering capillary resistance. In the central nervous system, pericytes appear to be capable of altering capillary diameter and blood flow in response to electrical and chemical stimuli (Diaz-Flores et al., 2009). One study utilizing in vivo two photon microscopy observed that, while pericytes could induce capillary constriction, neural activity mediated enhanced blood flow occurred predominantly at the level of arterioles and not via pericytes (Fernandez-Klett et al., 2010). However, the ability of pericytes to constrict capillaries and reduce capillary diameter and flow has been implicated in the “no reflow” phenomenon following cerebral ischemia where pericyte-induced capillary constriction prevents blood flow across capillary beds even following resumption of blood flow in the culprit vessel (O’Farrell & Attwell, 2014). The ability of pericytes to alter microvascular flow in the skeletal muscle and heart is less clear. Changes in vasomotor tone in the heart are thought to occur in arteries and arterioles enriched in smooth muscle cells that are responsive to neural or circulating vasoactive hormones. The “no reflow” phenomenon is well known to occur in the heart following resumption of flow in the epicardial coronary artery and has been attributed to microvascular dysfunction (O’Farrell & Attwell, 2014). It is thought that such mechanisms of pericyte contraction and constriction of capillary flow that have been demonstrated in the brain could well play a role in the “no–reflow” phenomenon following cardiac ischemic-reperfusion injury as well.
Recent evidence suggests that pericytes in human skeletal muscle proliferate/mobilize in response to exercise-induced angiogenesis with a significant increase in pericyte density, thickness, and endothelial coverage and a concurrent decrease in basement membrane density and thickness in ultrastructural analyses of capillaries in human muscle biopsies (Baum et al., 2015). Furthermore skeletal muscle pericytes are also considered to be myogenic precursors distinct from satellite cells, themselves thought to be the primary source of postnatal myoblasts (Zammit et al., 2006). Farup et al. reported that native human muscle pericytes respond to contraction mode-specific resistance exercise and notably decline in number (Farup et al., 2015). This correlates with concomitant increases of CD90+ and PDGFRα+ mesenchymal stem/stromal and Pax7+ satellite cell pools, suggesting the contribution of pericytes to muscle progenitor pool expansion following prolonged resistance training.
In addition to the described direct contribution of skeletal muscle pericytes to myogenesis, these cells may also actually stabilize the satellite cell pool within skeletal muscle. Satellite cells are localized under the basal lamina as non-activated cells, adjacent to small capillaries. Skeletal muscle pericytes promote satellite cell quiescence through the secretion of Ang1 and IGF1-dependent activation (Kostallari et al., 2015). The induced ablation of NG2+ perivascular cells in an experimental mouse model significantly impaired satellite cell quiescence and resulted in a considerable increase in proliferating Pax7+ myogenic progenitor cells (Kostallari et al., 2015). The capacity for pericytes to regulate the niche of other stem cells both in the skeletal muscle and in the heart, as it has been described and characterized in the bone marrow should be further explored in future studies (Birbrair & Frenette, 2016).
6. Role in muscle injury and disease
6.1. Response to injury
In young healthy subjects, skeletal muscle is well recognized for its remarkably robust endogenous capacity for repair (Huard et al., 2002). In addition to their repertoire of functions in homeostasis, pericytes adopt further roles in respect to skeletal muscle repair processes, including in neovascularization, release of trophic factors, immunomodulation, and increased myogenic differentiation (Caplan & Correa, 2011).
Human pericytes in culture have been shown to produce a unique secretome of pro-reparative growth factors, cytokines, chemokines, and microRNAs, which may enhance endogenous repair mechanisms. These factors include VEGF, basic fibroblast growth factor, heparin-binding epidermal growth factor, keratinocyte growth factor, transforming growth factor-β1 (TGFβ1), PDGF, thrombopoietin, Ang1, Ang2, hepatocyte growth factor, stem cell factor, stromal cell-derived factor-1 alpha, and microRNA-132 (Katare et al., 2011; C.W. Chen et al., 2013; Avolio et al., 2015).
In response to injury, pericytes have also been shown to modulate local tissue immune responses via several independent pathways (Armulik et al., 2005). Human brain pericytes are thought to regulate T-lymphocyte diapedesis through the expression of the vascular endothelial adhesion molecule-1, which interacts with very late antigen-4, its T-cell expressed ligand (Verbeek et al., 1995). Neural crest-derived pericytes control the release of thymocytes into the circulation through the expression of sphingosine-1-phosphate and its interaction with sphingosine-1-phosphate receptor-1 on newly formed thymocytes (Zachariah & Cyster, 2010). Moreover, it has been suggested that inter-cellular adhesion molecule-1 (ICAM1) expression by NG2+ brain pericytes plays an important part in coordinating leukocyte emigration from the vasculature in response to local inflammatory mediators via the upregulation and release of macrophage migration inhibitory factor (MIF) (Stark et al., 2013). Extravasated leukocytes bound to pericytes are instructed with pattern recognition and motility programs, a process that is prevented through inhibition of MIF. CCAAT/enhancer binding protein delta (C/EBPδ) is also upregulated in brain inflammation and is induced in brain pericytes by interleukin-1β (IL1β). The knockdown of C/EBPδ in cultured brain pericytes enhances the IL1β-induced production of the pro-inflammatory factors ICAM1, IL8, monocyte chemoattractant protein-1 (MCP1), and IL1β and reduced superoxide dismutase-2 and cyclooxygenase-2 (COX2) production (Rustenhoven et al., 2015). It has therefore been speculated that pericytes, through the induction of C/EBPδ, can play a role in limiting the infiltration of peripheral immune cells in the brain. Muscle pericytes also have high expression of immunoregulatory cytokines and chemokines, including COX2, heme oxygenase 1 (HMOX1), leukemia inhibitory factor (LIF), hypoxia-inducible factor 1 alpha, monocyte chemoattractant protein-1 (MCP1), and IL6 (C.W. Chen et al., 2013). In an in vitro muscle injury model where pericytes are co-cultured with scratch injured C1C12 mouse myoblast cells in transwells, the activation of the immunomodulatory transcription factor nuclear-factor kappa-B (NF-κB) in pericytes significantly increased over time and correlated with elevated MCP1 secretion (LaBarbera et al., 2015). Importantly, skeletal muscle pericytes express little-to-no pro-inflammatory cytokines, such as IL1α, tumor necrosis factor alpha (TNFα), and (interferon gamma) IFNγ, even under hypoxic conditions (C.W. Chen et al., 2013).
Lineage tracing studies have indicated that pericytes contribute directly to regenerating myofibers following injury in models of acute injury and in the setting of muscular dystrophies (Dellavalle et al., 2007, 2011). Dellavalle et al. identified a subset of myogenic cells expressing alkaline phosphatase (AP) as well as pericyte markers, including NG2 (Dellavalle et al., 2007). These pericytes can be sorted from human skeletal muscle and readily differentiate into myotubes both in culture and following transplantation into the skeletal muscle of scid-mdx muscular dystrophy mouse models. Furthermore, by using alpha-sarcoglycan null mouse models and by crossing these with AP-CreERT2 mice, Dellavalle et al. were able to show that endogenous AP+ skeletal muscle pericytes significantly contribute to the numbers of regenerating fibers in muscular dystrophy (Dellavalle et al., 2011). The contribution to myogenesis is enhanced in the setting of acute muscle injury. Interestingly, the pericyte contribution to myofibers varies among different muscles, ranging from <1% (tibialis anterior muscle) to 7% of the fibers (diaphragm) (Dellavalle et al., 2007). To investigate whether specific pericyte subsets are responsible for myogenic differentiation in muscle injury, Birbrair et al. transplanted type 1 nestin+/NG2+ pericytes and type 2 nestin+/NG2− pericytes into injured skeletal muscle and found that only the latter contribute to new muscle formation (Birbrair et al., 2013a). In addition, Birbrair et al. have demonstrated that type 2 pericytes have the capacity to form a specific type of neural progenitors (NG2+-glia). These cells have several similarities with Schwann cells, raising the possibility that these pericytes may participate in the muscle reinnervation process (Birbrair et al., 2013b). Greater investigation of the contribution of pericyte subsets to myogenic regeneration following skeletal muscle injury is warranted to further determine the role of specific pericyte subsets to this process.
6.2. Fibrosis
In addition to exhibiting a pro-repair phenotype in skeletal muscle, pericytes have also been implicated in the development of fibrosis, adipogenic differentiation, and heterotopic ossification.
Muscle fibrosis following severe or iterative injury or in the setting of the muscular dystrophies is a major cause of morbidity worldwide (Mann et al., 2011). Skeletal muscle fibrosis is characterized by the excessive production and deposition of collagenous extracellular matrix by myofibroblasts, compromising myofiber contractility, tissue architecture, and ultimately organ function (Wynn & Ramalingam, 2012; Friedman et al., 2013; Rockey et al., 2015). Fibrosis secondary to acute skeletal muscle injury results in significant functional impairment and predisposes to further injury (Mann et al., 2011; Uezumi et al., 2014). At present, the cellular and molecular mechanisms regulating fibrosis in skeletal muscle remain poorly understood and consequently treatment options are severely limited (Leask, 2015). A number of putative myofibroblast progenitor populations have been implicated in the development of muscle fibrosis, including fibro-adipogenic progenitors (FAPs) and cells expressing Gli1, ADAM12 (Dulauroy et al., 2012), and PDGFRα (Uezumi et al., 2011). Intriguingly some of these markers, including ADAM12 and Gli1, are expressed by pericytes raising the prospect of pericyte subpopulations contributing to the myofibroblast progenitor pool (Dulauroy et al., 2012; Greenhalgh et al., 2013; Kramann et al., 2015).
Dulauroy et al. demonstrated that transient expression of ADAM12 identifies a distinct pro-inflammatory subset of stromal cells that become activated following muscle injury (Dulauroy et al., 2012). The authors fate-mapped these cells using an inducible, tetracycline transactivator-based system. This involved the generation of triple transgenic mice that expressed tetracycline transactivator under control of the ADAM12 locus, Cre under control of the tetracycline transactivator, and the conditional reporter Rosa26floxSTOP-YFP locus. In these mice, yellow fluorescent protein (YFP) labeling of the progeny of ADAM12+ cells was temporally controlled by the administration of doxycycline to prevent Cre expression. This allowed the separate fate mapping of fetal and adult ADAM12+ cells following cardiotoxin-induced muscle injury. The genetic strategies employed by the authors, combined with a parabiosis experiment, allowed them to demonstrate that the majority of collagen-producing, αSMA+ myofibroblasts that developed following acute dermal or muscle injury were generated from tissue-resident ADAM12+ cells that reside in a perivascular locus and co-express the pericyte marker PDGFRβ. Furthermore, the ablation of ADAM12+ cells in skeletal muscle (using mice that also expressed the human diphtheria toxin receptor under control of the ADAM12 locus) markedly reduced the generation of pro-fibrotic cells and interstitial collagen accumulation.
Chronic activation of PDGFRα has also been associated with widespread organ fibrosis, suggesting that PDGFRα+ cells may also have a role in skeletal muscle fibrosis (Olson & Soriano, 2009). Type 1 pericytes and FAPs express this receptor, and like pericytes, FAPS line the skeletal muscle vasculature, suggesting that their roles might overlap (Joe et al., 2010). The extent of perivascular PDGFRα+ cells’ contribution to skeletal muscle fibrosis has not been determined as yet. Interestingly, age may alter the pro-fibrotic potential of specific skeletal muscle pericyte subsets. Birbrair et al. demonstrated that in young mice, type 2 pericytes have myogenic potential while type 1 pericytes appear quiescent. In aged animals, however, type 2 pericytes exhibit markedly diminished myogenic capacity while type 1 pericytes produce collagen (Birbrair et al., 2013d).
Unlike skeletal muscle the heart possesses a poor ability to regenerate after injury and following acute myocardial infarction heals predominantly via a fibrotic repair response. Cardiac fibroblasts are activated, form myofibroblasts, and secrete collagen that forms scar tissue to replace dead cardiac muscle (Deb & Ubil, 2014). Increasingly, it is being recognized that cells in the injured region exhibit plasticity and non-fibroblast cell sources have been shown to generate myofibroblasts in the injured area (Krenning et al., 2010; Ubil et al., 2014). As pericytes are mesenchymal cells that share some markers with both fibroblasts and MSCs, they have been investigated in the heart for their ability to generate myofibroblasts and contribute to fibrosis. Fate map studies in the kidney and liver suggest that PDGFRβ-expressing cells can contribute to fibrosis (Fabris & Strazzabosco, 2011; Schrimpf & Duffield, 2011). Birbrair et al. also detected type 1 and type 2 pericytes in the heart and demonstrated that in areas of infarcted myocardium type 1 cells but not type 2 cells increase in numbers after 14 days. Although type 1 cell numbers increased, these cells did not express collagen 1, which was expressed by an unidentified cells type (Birbrair et al., 2014a, 2014c). A more recent study demonstrated that a subset of perivascular cells labeled by the expression of Gli1 (a transcription factor of the sonic hedgehog pathway) served as precursors of myofibroblasts and contributed to fibrosis after injury in multiple organs, the heart included (Kramann et al., 2015). The authors demonstrated that Gli1 marked a population of perivascular cells that exhibited MSC like properties, were able to undergo tri-lineage differentiation and were a subpopulation of PDGFRβ expressing interstitial cells. In a model of cardiac fibrosis induced by the infusion of angiotensin II, the authors noted that Gli1 labeled perivascular cells expressed αSMA and adopted myofibroblast fates. Collagen-rich areas were full of Gli1 labeled cells, and after acute myocardial injury, 60% of myofibroblasts in the injured region were shown to be derived from Gli1 cells (Kramann et al., 2015). Using a cell-specific ablative approach where Gli1 cells were targeted with Diphtheria toxin, the authors observed that ablation of these cells was associated with substantial reductions in fibrosis, emphasizing the physiologic role of Gli1 perivascular cells in mediating fibrosis. It is to be noted however that the authors observed that Gli1-expressing cells constituted only a small fraction of PDGFRβ cells and why depletion of a small population of cells led to such marked reduction in fibrosis remains unclear (Kramann et al., 2015). It is possible that Gli1+ perivascular cells also regulate pro-fibrotic properties of native cardiac fibroblasts and depletion of this subpopulation of pericytes could have affected fibroblast proliferation and repair. The physiologic role of pericytes versus resident cardiac fibroblasts in orchestrating acute and chronic cardiac fibrosis represents an open area of investigation.
6.3. Skeletal muscle pericytes and adipogenesis
Pericytes isolated from various tissues, including skeletal muscle, have demonstrated adipogenic differentiation potential in vitro (Crisan et al., 2008). In the aforementioned study, Birbrair et al. demonstrated that in skeletal muscle adipogenic differentiation potential was restricted to type 1 pericytes, only these pericytes expressed the adipogenic marker PDGFRα, and that unlike type 2 pericytes, type 1 pericytes cannot form muscle cells (Birbrair et al., 2013a). This pericyte subset may contribute to fat accumulation and infiltration in diseased skeletal muscle in such disorders as obesity, dystrophies, and aging.
6.4. Skeletal muscle pericytes and heterotopic ossification
Up to one-third of all patients undergoing hip arthroplasty or who have had a severe long bone fracture develop heterotopic ossification that may result in pain, swelling, and a restricted range of motion (Tippets et al., 2014). In myositis ossificans progressiva (also known as fibrodysplasia ossificans progressiva), an extremely rare inherited condition, ossification in muscle and connective tissues occurs spontaneously or following injury (Ramirez et al., 2014). Although the mutation for fibrodysplasia ossificans progressiva is known, this is merely a proximate genetic cause—the cells that respond by forming bone in both acquired and genetic forms of heterotopic ossification are not known. As pre-MSC residents in skeletal muscle with robust osteogenic potential, pericytes have emerged as key suspects (Kan et al., 2013). Understanding why these progenitors pathologically may manifest their osteogenic potential in this setting could provide valuable insights into future therapies.
7. Pericytes as potential cellular therapeutic agents
The existence of pericytes in nearly all vascularized organs and their pro-repair potential make them an attractive potential donor source for cell therapy (Chen et al., 2015b).
Given the observation that pericytes can differentiate readily in vitro into myoblasts in appropriate myogenic conditions, a number of investigators have sought to harness their potential as myogenic precursors for the treatment of skeletal muscle injury. Indeed intramuscular injection of freshly sorted or cultured pericytes derived from human adipose or skeletal muscle regenerated human myofibers efficiently in dystrophic or injured mouse muscle (Xu et al., 2005). In a study by Dellavalle et al. cells of human origin participated in host muscle regeneration, revealed by detection of human dystrophin-positive and/or human spectrin-positive myofibers (Dellavalle et al., 2007). Many of these human myofibers co-expressed human lamin A/C, indicating their sole human origin and not intermediate products of cell fusion. Surprisingly, human myofibers were located at regions distant (up to 2 cm) to the implantation site, suggesting active migration of outgrown human myogenic precursors over long distances (Dellavalle et al., 2007).
The ability of pericytes to enhance myocardial repair has been demonstrated; however, the underlying mechanisms are less clear than those in skeletal muscle. Following the transplantation of CD34+ adventitial pericytes, obtained from human saphenous veins, into infarcted mouse hearts, an improvement in cardiac function was observed alongside a reduction in scar size and an increase in myocardial neovascularization. This response was largely attributed to microRNA-132 secretion by pericytes (Katare et al., 2011). Studying a different population of CD146+/CD34−/CD45−/CD56− pericytes obtained from the microvasculature of human skeletal muscle Chen et al. similarly demonstrated significant improvement in cardiac function following injection into acutely infarcted mouse hearts (C.W. Chen et al., 2013). Injured hearts that received pericytes exhibited significant amelioration in post-injury decline of cardiac pump function, had decreased extent of scarring, decreased inflammation, and augmented angiogenesis. The authors observed that transplanted pericytes homed to perivascular regions in the infarcted heart but gave rise to only minimal new myocytes or endothelial cells. These successful cell therapy studies utilizing pericytes mirror the success and cellular mechanisms of benefit using bone marrow-derived mesenchymal stem cells for cardiac repair (Laflamme & Murry, 2011). Rather than generating cardiac muscle, bone marrow-derived MSCs exerted salutary effects via paracrine mechanisms (Hodgkinson et al., 2016). The bio-active factors released by MSC are capable of supporting muscle regeneration through angiogenic and anti-apoptotic effects while their immunomodulatory properties inhibit immunosurveillance of the injured tissues preventing autoimmunity (Nauta & Fibbe, 2007). Similarly, it is thought that the release of trophic factors is the primary contribution of transplanted pericytes to tissue regeneration, rather than differentiation and engraftment (Chen et al., 2015a; Tawonsawatruk et al., 2016). In the study by Chen et al., the ability of pericytes to secrete anti-inflammatory cytokines such as IL6 and heme oxygenase 1 could have played a role in decreasing post-injury cardiac inflammation. The capillary density of injured hearts that received pericytes increased by 45% compared to non-pericyte injected injured control hearts and pericytes under hypoxic conditions upregulated expression of VEGF and VEGFR2 and downregulated expression of the anti-angiogenic factor Ang2 (C.W. Chen et al., 2013). Most recently, CD34+/CD146−/CD31− pericytes obtained from human neonatal cardiac tissue have been shown to enhance in vitro vascular network formation and stimulate the migration of c-Kit+ cardiac stem cells (Avolio et al., 2015). The results of these studies highlight the ability of pericytes to regulate neovascularization and repair in the injured heart via modulation of the angiogenic/anti-angiogenic secretome (C.W. Chen et al., 2013)
Several strategies to enhance skeletal muscle regeneration through pericyte-mediated angiogenesis have also been proposed (Beckman et al., 2013). Human muscle pericytes form tight associations and interactions with endothelial cells in three-dimensional microvascular models in vitro, suggesting that they may be able to directly assist restoration of microvascular networks (C.W. Chen et al., 2013). Furthermore, purified human pericytes form dual-layered microvascular tubes when co-cultured with endothelial cells in Matrigel plugs (Fig. 4). CD34+ pericytes from the adventitia also markedly enhance the network-forming capacity of endothelial cells in co-culture, suggesting physical interactions between these two cell types favoring microvascular remodeling and stabilization (Campagnolo et al., 2010). CD34+ adventitial pericytes and endothelial cells also express complementary components of the Ang-1/Tie-2 and PDGFRβ/PDGF-BB signaling systems consistent with pericyte–endothelial cell cross-talk (Campagnolo et al., 2010). Interestingly, muscle pericytes not only promote neoangiogenesis in vitro but also contribute to the pruning of excessive/immature microvessels via CXCR3-induced involution of endothelial cells resulting in the inhibition of microvessel formation and induction of microvessel dissociation (Bodnar et al., 2013). In addition, pericyte–endothelial cellular interactions in pathological conditions may regulate the recruitment and contribution of pericytes beyond angiogenesis, for example, in mesenchymal activation (W.C. Chen et al., 2013). Together these results suggest that transplanted pericytes may have therapeutic potential in skeletal muscle, via direct pericyte–endothelial cell interactions, in the promotion of mature revascularization at the microvascular level.
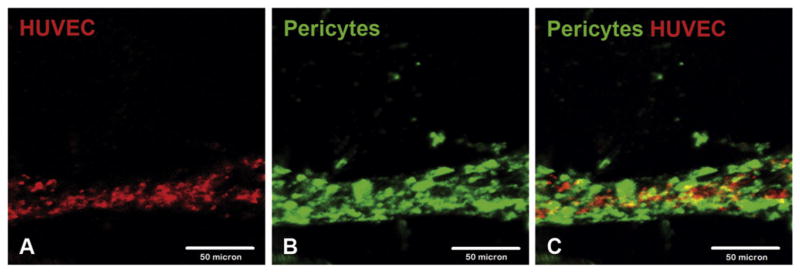
Fig. 4.
Purified human pericytes and HUVECs co-form a dual-layered microvascular tube in 3D Matrigel plugs. Confocal microscopy revealed the formation of a dual-layer microvascular structure by fluorescent activated cell sorting-purified human pericytes (green) and HUVECs (red) in a 3D Matrigel plug where HUVECs formed an endothelial tube tightly surrounded by pericytes at 72 h after encapsulation: (A) HUVECs, (B) human pericytes, and (C) merge (scale bars: 50 μm).
In summary, recent evidence indicates robust skeletal myogenic, angiogenic, and paracrine properties of pericytes ex vivo that contribute to the repair and regeneration of experimental muscle injury. As such, pericytes should be considered one of the prime adult precursor cell candidates for therapeutic muscle repair. It should be noted however that pericytes are a heterogeneous population and questions about the organ of choice for harvesting pericytes as well as selection strategies for harvesting optimal subsets of pericytes will first need to be addressed to determine the future of pericytes as safe and effective agents for cardiac cell therapy (Caplice & Deb, 2004).
8. Concluding remarks
Pericytes play critical roles in the homeostasis and response to injury of both skeletal and cardiac muscle. Recent studies support unique functions for pericyte subsets that may enable new therapeutic strategies. Further efforts must be made to characterize specific markers for pericyte subpopulations to better establish their roles in health and disease. Pericytes exhibit multiple characteristics and functions that make them attractive potential agents for skeletal and cardiac tissue regeneration. However, they have also been shown to contribute to fibrosis, heterotopic ossification, or fat accumulation and thus their application must be based on sound scientific understanding.
Acknowledgments
This work was supported by a Wellcome Trust funded Edinburgh Clinical Academic Track (ECAT) Lectureship (ref. 097483) and Royal College of Surgeons of Edinburgh small research grant to I.R.M., and a Wellcome Trust Senior Research Fellowship in Clinical Science (ref. 103749) to N.C.H and by grants HL 129178 and HL102190 from the National Institutes of Health and a grant from the James Eason Cardiovascular Discovery Fund to AD.
Abbreviations
- αSMA
alpha smooth muscle actin
- ADAM12
disintegrin and metalloproteinase domain-containing protein 12
- Ang
angiopoietin
- AP
alkaline phosphatase
- CD
cluster of differentiation
- C/EBPδ
CCAAT/enhancer binding protein delta
- Cox2
cyclooxygenase 2
- CXCR
CXC chemokine receptors
- EMT
epithelial to mesenchymal transition
- GFP
green fluorescent protein
- GLI1
glioblastoma 1
- FAP
fribro-adipogenic progenitor
- HMOX1
heme oxygenase 1
- ICAM1
intercellular adhesion molecule 1
- IFNδ
interferon gamma
- IL
interleukin
- IP10
inducible protein 10
- LIF
leukemia inhibitory factor
- MCP1
monocyte chemoattractant protein 1
- MIF
migration inhibitory factor
- MSC
mesenchymal stem cell
- NG2
neural/glial antigen 2
- NF-κB
nuclear-factor kappa-B
- PDGF
platelet-derived growth factor
- PDGFRβ
platelet-derived growth factor beta
- TGFβ
transforming growth factor beta
- Tie2
tyrosine kinase with immunoglobulin-like and EGF-like domains 1
- TNFα
tumor necrosis factor alpha
- VE-cadherin
vascular endothelial cadherin
- VEGF
vascular endothelial growth factor
- vSMCs
vascular smooth muscle cells
- YFP
yellow fuuorescent protein
Footnotes
Conflict of interest
Frank Petrigliano has received consultant/speaker fees from Biomet and has grant support from the musculoskeletal transplant foundation.
The remaining authors declare that there are no conflicts of interest.
References
- Amselgruber WM, Schafer M, Sinowatz F. Angiogenesis in the bovine corpus luteum: An immunocytochemical and ultrastructural study. Anat Histol Embryol. 1999;28:157–166. doi: 10.1046/j.1439-0264.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, … Betsholtz C. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, … Madeddu P. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116:e81–e94. doi: 10.1161/CIRCRESAHA.115.306146. [DOI] [PubMed] [Google Scholar]
- Baum O, Gubeli J, Frese S, Torchetti E, Malik C, Odriozola A, … Tschanz SA. Angiogenesis-related ultrastructural changes to capillaries in human skeletal muscle in response to endurance exercise. J Appl Physiol. 2015;119:1118–1126. doi: 10.1152/japplphysiol.00594.2015. [DOI] [PubMed] [Google Scholar]
- Beckman SA, Chen WC, Tang Y, Proto JD, Mlakar L, Wang B, Huard J. Beneficial effect of mechanical stimulation on the regenerative potential of muscle-derived stem cells is lost by inhibiting vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2013;33:2004–2012. doi: 10.1161/ATVBAHA.112.301166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: Implications for vascular morphology? Circ Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Delbono O. Pericytes are essential for skeletal muscle formation. Stem Cell Rev. 2015;11:547–548. doi: 10.1007/s12015-015-9588-6. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370:82–96. doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One. 2011;6:e16816. doi: 10.1371/journal.pone.0016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, … Delbono O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther. 2014a;5:122. doi: 10.1186/scrt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013a;22:2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res. 2013b;319:45–63. doi: 10.1016/j.yexcr.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013c;10:67–84. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013d;305:C1098–C1113. doi: 10.1152/ajpcell.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes: Multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 2014c;6:245. doi: 10.3389/fnagi.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015;128:81–93. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014b;307:C25–C38. doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, … Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Rodgers ME, Chen WC, Wells A. Pericyte regulation of vascular remodeling through the CXC receptor 3. Arterioscler Thromb Vasc Biol. 2013;33:2818–2829. doi: 10.1161/ATVBAHA.113.302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, … Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, … Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplice NM, Deb A. Myocardial-cell replacement: The science, the clinic and the future. Nat Clin Pract Cardiovasc Med. 2004;1:90–95. doi: 10.1038/ncpcardio0051. [DOI] [PubMed] [Google Scholar]
- Cappellari O, Cossu G. Pericytes in development and pathology of skeletal muscle. Circ Res. 2013;113:341–347. doi: 10.1161/CIRCRESAHA.113.300203. [DOI] [PubMed] [Google Scholar]
- Cappellari O, Benedetti S, Innocenzi A, Tedesco FS, Moreno-Fortuny A, Ugarte G, … Cossu G. Dll4 and PDGF-BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev Cell. 2013;24:586–599. doi: 10.1016/j.devcel.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA, … Huard J. Human pericytes for ischemic heart repair. Stem Cells. 2013a;31:305–316. doi: 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Park TS, Murray IR, Zimmerlin L, Lazzari L, Huard J, Peault B. Cellular kinetics of perivascular MSC precursors. Stem Cells Int. 2013b;2013:983059. doi: 10.1155/2013/983059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Baily JE, Corselli M, Diaz ME, Sun B, Xiang G, … Peault B. Human myocardial pericytes: Multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells. 2015a;33:557–573. doi: 10.1002/stem.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Peault B, Huard J. Regenerative translation of human blood-vessel-derived MSC precursors. Stem Cells Int. 2015b;2015:375187. doi: 10.1155/2015/375187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintalgattu V, Rees ML, Culver JC, Goel A, Jiffar T, Zhang J, … Khakoo AY. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med. 2013;5:187ra169. doi: 10.1126/scitranslmed.3005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013;61:555–570. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, … Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, … Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb A, Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol. 2014;70:47–55. doi: 10.1016/j.yjmcc.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, … Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, … Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, … Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis. 2011;31:11–32. doi: 10.1055/s-0031-1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Salven P. Stem cells in tumor angiogenesis. J Mol Cell Cardiol. 2011;50:290–295. doi: 10.1016/j.yjmcc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Farup J, De Lisio M, Rahbek SK, Bjerre J, Vendelbo MH, Boppart MD, Vissing K. Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle. J Appl Physiol. 2015;119:1053–1063. doi: 10.1152/japplphysiol.01108.2014. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: Nearing the starting line. Sci Transl Med. 2013;5:167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial–mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial–pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Greenhalgh SN, Iredale JP, Henderson NC. Origins of fibrosis: Pericytes take centre stage. F1000 Prime Rep. 2013;5:37. doi: 10.12703/P5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, … Sheppard D. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016;118:95–107. doi: 10.1161/CIRCRESAHA.115.305373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. Muscle injuries and repair: Current trends in research. J Bone Joint Surg Am. 2002;84-a:822–832. [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, … Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L, Peng CY, McGuire TL, Kessler JA. Glast-expressing progenitor cells contribute to heterotopic ossification. Bone. 2013;53:194–203. doi: 10.1016/j.bone.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, … Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostallari E, Baba-Amer Y, Alonso-Martin S, Ngoh P, Relaix F, Lafuste P, Gherardi RK. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development. 2015;142:1242–1253. doi: 10.1242/dev.115386. [DOI] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, … Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarbera KE, Hyldahl RD, O’Fallon KS, Clarkson PM, Witkowski S. Pericyte NF-kappaB activation enhances endothelial cell proliferation and proangiogenic cytokine secretion in vitro. Physiol Rep. 2015:3. doi: 10.14814/phy2.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagha M, Brunelli S, Messina G, Cumano A, Kume T, Relaix F, Buckingham ME. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell. 2009;17:892–899. doi: 10.1016/j.devcel.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Larson DM, Carson MP, Haudenschild CC. Junctional transfer of small molecules in cultured bovine brain microvascular endothelial cells and pericytes. Microvasc Res. 1987;34:184–199. doi: 10.1016/0026-2862(87)90052-5. [DOI] [PubMed] [Google Scholar]
- Leask A. Getting to the heart of the matter: New insights into cardiac fibrosis. Circ Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Matthews BG, Torreggiani E, Roeder E, Matic I, Grcevic D, Kalajzic I. Osteogenic potential of alpha smooth muscle actin expressing muscle resident progenitor cells. Bone. 2016;84:69–77. doi: 10.1016/j.bone.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeuf-Louchart A, Lagha M, Danckaert A, Rocancourt D, Relaix F, Vincent SD, Buckingham M. Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proc Natl Acad Sci U S A. 2014;111:8844–8849. doi: 10.1073/pnas.1407606111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, … Peault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2013;71(8):1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, … Juchem G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: The second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol. 2012;302:H69–H84. doi: 10.1152/ajpheart.00359.2011. [DOI] [PubMed] [Google Scholar]
- Norrmen C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation. 2011;123:1335–1351. doi: 10.1161/CIRCULATIONAHA.107.704098. [DOI] [PubMed] [Google Scholar]
- O’Farrell FM, Attwell D. A role for pericytes in coronary no-reflow. Nat Rev Cardiol. 2014;11:427–432. doi: 10.1038/nrcardio.2014.58. [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- Paulin D, Li Z. Desmin: A major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DM, Ramirez MR, Reginato AM, Medici D. Molecular and cellular mechanisms of heterotopic ossification. Histol Histopathol. 2014;29(10):1281–1285. doi: 10.14670/HH-29.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593–598. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- Rockey DC, Bell PD, Hill JA. Fibrosis—A common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- Rustenhoven J, Scotter EL, Jansson D, Kho DT, Oldfield RL, Bergin PS, … Dragunow M. An anti-inflammatory role for C/EBPdelta in human brain pericytes. Sci Rep. 2015;5:12132. doi: 10.1038/srep12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimpf C, Duffield JS. Mechanisms of fibrosis: The role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- Sims DE. The pericyte—A review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, … Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Kowanetz M, Brwon LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: Phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82(4):387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, … Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tawonsawatruk T, West CC, Murray IR, Soo C, Peault B, Simpson AH. Adipose derived pericytes rescue fractures from a failure of healing—Non-union. Sci Rep. 2016;6:22779. doi: 10.1038/srep22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippets DM, Zaryanov AV, Vincent Burke W, Patel PD, Suarez JC, Ely EE, Figueroa NM. Incidence of heterotopic ossification in direct anterior total hip arthroplasty: A retrospective radiographic review. J Arthroplasty. 2014;29(9):1835–1838. doi: 10.1016/j.arth.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, … Deb A. Mesenchymal–endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, … Nishikawa S. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Ikemoto-Uezumi M, Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol. 2014;5:68. doi: 10.3389/fphys.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, … Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Westphal JR, Ruiter DJ, de Waal RM. T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J Immunol. 1995;154:5876–5884. [PubMed] [Google Scholar]
- Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, … Red-Horse K. Pericytes are progenitors for coronary artery smooth muscle. Elife. 2015:4. doi: 10.7554/eLife.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Malladi P, Wagner DR, Longaker MT. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr Opin Mol Ther. 2005;7:300–305. [PubMed] [Google Scholar]
- Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]