Abstract
Consumption of broccoli mediates numerous chemo-protective benefits through the intake of phytochemicals, some of which modulate aryl hydrocarbon receptor (AHR) activity. Whether AHR activation is a critical aspect of the therapeutic potential of dietary broccoli is not known. Here we administered isocaloric diets, with or without supplementation of whole broccoli (15% w/w), to congenic mice expressing the high-affinity Ahrb/b or low-affinity Ahrd/d alleles, for 24 days and examined the effects on AHR activity, intestinal microbial community structure, inflammatory status, and response to chemically induced colitis. Cecal microbial community structure and metabolic potential were segregated according to host dietary and AHR status. Dietary broccoli associated with heightened intestinal AHR activity, decreased microbial abundance of the family Erysipelotrichaceae, and attenuation of colitis. In summary, broccoli consumption elicited an enhanced response in ligand-sensitive Ahrb/b mice, demonstrating that in part the beneficial aspects of dietary broccoli upon intestinal health are associated with heightened AHR activity.
Keywords: AHR, ICZ, indole-3-carbinol, broccoli, intestinal homeostasis, Ah receptor
1. Introduction
Plants of the genus Brassica, often referred to as cruciferous vegetables (e.g. broccoli, cabbage, Brussels sprouts, etc.), provide critical components of a healthy diet, such as vitamins, minerals, and fiber. Consumption of these plants correlates with decreased incidence of cancer (Latte, Appel et al. 2011). While individual chemical constituents found in broccoli have been reported in isolation to exhibit beneficial properties, the relative importance of each chemical within the context of dietary broccoli in conferring these beneficial effects is poorly understood (Zhang, Talalay et al. 1992; Bradlow, Sepkovic et al. 1999; Higdon, Delage et al. 2007).
Broccoli (Brassica oleracea) has been extensively studied for its content of various nutritional phytochemicals, such as glucosinolates (Kushad, Brown et al. 1999). Enzymatic hydrolysis of glucosinolates by plant myrosinases generates numerous metabolites including isothiocyanates, thiocyanates, and epithionitriles (Fenwick, Heaney et al. 1983) which exhibit diverse biological activities, Perhaps one of the most studied components of this class of glucosides are indole-3-carbinol (I3C), indole-3-acetonitrile (I3ACN) and 3,3′diindolylmethane (DIM), breakdown products of the glucosinolate, glucobrassicin. I3C and its oligomeric products exhibit multiple biological activities, including induction of apoptosis, decreased DNA-adduct formation, and reduced estrogen signaling, but have been characterized and extensively investigated as ligands and modulators of aryl hydrocarbon receptor (AHR) activity (Aggarwal and Ichikawa 2005). Additionally, gastric acid-mediated condensation of I3C in the stomach yields 2-(indol-3-ylmethyl)-3,3′-diindolylmethane (Ltr-1) and indolo[3,2-b]carbazole (ICZ), a potent ligand and activator of the AHR (Bjeldanes, Kim et al. 1991). As such, broccoli represents a rich source of dietary AHR ligands (Hubbard, Murray et al. 2015b).
The AHR is a ligand activated transcription factor, of the basic region helix-loop-helix-PER/ARNT/SIM homology super-family, first identified as the mediator of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity (Poland and Knutson 1982). The receptor possesses a promiscuous ligand binding domain that is responsive to an array of xenobiotic compounds, such as polycyclic aromatic hydrocarbons (PAH’s) and polychlorinated biphenyls (PCBs) (Poland and Knutson 1982). The AHR has been historically characterized for its capacity to induce metabolism of exogenous compounds through transcriptional regulation of the cytochrome P450 gene battery (Denison, Fisher et al. 1988; Rowlands and Gustafsson 1997). The physiological roles of the AHR have expanded beyond xenobiotic metabolism, to include an array of endogenous functions. This concept is supported by the generation and phenotypic characterization of AHR null mice, which exhibit numerous physiological anomalies, including immune dysfunction, decreased hepatic vasculature development, and reduced fertility (Baba, Mimura et al. 2005; McMillan and Bradfield 2007).
Additional studies provide evidence that ligand-activated AHR participates in the maintenance of intestinal homeostasis. AHR null mice, relative to their wild-type counterparts, are more susceptible to various modes of intestinal challenge, such as chemically-induced colitis or C. rodentium infection (Li, Innocentin et al. 2011; Qiu, Heller et al. 2012). Protection is also conferred in ligand-responsive mice by administration of AHR agonists, such as TCDD and I3C (Takamura, Harama et al. 2010; Furumatsu, Nishiumi et al. 2011). In the absence of exogenous agonists, AHR activity is likely mediated by ligands produced endogenously or provided via dietary sources, such as cruciferous vegetables. Administration of whole broccoli has been previously shown to alter the resident microflora in mice and to decrease colonic inflammation (Paturi, Mandimika et al. 2012). The mechanism(s) by which broccoli confers these activities and their dependency upon AHR-activation have not been fully investigated.
Here, the impact of broccoli consumption upon AHR activation, intestinal microbial community structure, and intestinal homeostasis is examined in vivo. To delineate the physiological impacts of broccoli consumption that associate with AHR ligand-responsiveness we utilized congenic mice expressing either the Ahrb/b (high-affinity) or Ahrd/d alleles (low-affinity) (Nebert and Bausserman 1970). Mice harboring the Ahrd/d allele exhibit decreased sensitivity to many prototypical AHR ligands (Poland 1975). Data presented here demonstrate that a significant component of the beneficial effects of broccoli can be attributed to activation of the AHR signaling pathway, resulting in an altered microbiome and protection from chemically induced colitis.
2. Materials and Methods (additional methodology can be found in the supplement)
2.1 Animals and husbandry
All animal studies were performed with approval and under the support of the Institutional Animal Care and Use Committee (IACUC Protocol #45967, The Pennsylvania State University). C57BL6/J mice were originally purchased from Jackson Laboratories (Bar Harbor, ME). C57BL6/J-Ahrb/b and derived C57BL6/J-Ahrd/d were subsequently bred in-house. Animals were housed in autoclaved polypropylene cages with corncob bedding in a specific pathogen-free environment with ad libitum access to indicated diets and water. Germ-free 129S6/SvEv-Il10−/− mice were purchased from the National Gnotobiotic Resource Center at the University of North Carolina School of Medicine. Germ-free C57BL6/J mice were bred in-house and maintained by The Pennsylvania State University Gnotobiotic Animal Research Facility.
2.2 Preparation of broccoli diet
Broccoli of the Lieutenant cultivar was used for generation of a custom broccoli diet. Broccoli was thoroughly rinsed, chopped finely, freeze-dried, and stored at −80 °C prior to manufacturing of custom diet. Purified AIN-93G and the custom broccoli rodent diets were manufactured by Dyets Inc. (Bethlehem, PA). The nutritional components of the 15% (w/w) broccoli diet were adjusted to ensure similar nutritional and caloric content profiles relative to that of defined AIN-93G diet. The diet composition was adjusted based upon previously established nutritional component concentrations in broccoli (Salunkhe 1998). The compositions of both the control AIN-93G diet and customized 15% broccoli diet are listed in Supplementary Table S1.
2.3 Feeding studies
C57BL6/J-Ahrb/b and Ahrd/d mice were weaned onto a standard animal chow diet. Mice (8–10-week-old) were acclimatized to AIN-93G purified rodent chow for seven days. This was followed by continuation of the AIN-93G diet in control groups or administration of the 15% broccoli diet for 24 days, unless otherwise indicated.
2.4 16S rDNA gene Illumina MiSeq analyses
Bacterial DNA was amplified across the V4/V4 region of the 16S rRNA gene (Primers available in Supplementary Table S2) with FastStart high-fidelity amplification kit (Roche, Indianapolis, IN) and the following cycling conditions (94 °C, 3 min; 94 °C, 15 secs, 55 °C, 45 secs; 72 °C, 1 min for 30 cycles; 72°C, 8 min). Product amplification was confirmed by agarose gel electrophoresis and the observation of a single product of 359 bp. The amplified V4 16S rRNA gene products were transferred to the Genomics Core Facility (The Pennsylvania State University) for 16S sequencing using the Illumina MiSeq platform (150 × 150 paired end). Sequence files were then obtained and analyzed using the Mothur software pipeline (Kozich, Westcott et al. 2013). Reads were trimmed at 320 bp and aligned to the SILVA database. Chimaeras were removed using UChime (Edgar, Haas et al. 2011) and reads were classified at a 75% cutoff using the ribosomal database project’s (RDP) training set. The summary file was used for further taxonomic analysis. Further sequence analysis involved converting the trimmed fasta file into a distance file and aligning it to a phylogenic tree. This tree file was compared to an operational taxonomic unit (OTU) file for Generalized Unifrac (GUnifrac) analysis (Chen, Bittinger et al. 2012).
2.5 Metagenomic analysis of the microbiome
A repeated analysis of the raw sequence data was completed again using the Mothur software pipeline with the reads aligned to the GreenGenes database (Kozich, Westcott et al. 2013). This alignment is needed to create a biom file for Phylogenic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis (Langille, Zaneveld et al. 2013). Significantly different predicted pathways were discovered using LDA effect size (LEfSe). LEfSe combines the Kruskal-Wallis and the Willcoxon statistical tests to show biologically relevant and statistically significant pathways.
2.6 RNA isolation and quantitative PCR expression analyses
Mice were euthanized by carbon dioxide asphyxiation and tissues were excised and immediately frozen in liquid nitrogen and stored at −80°C. Tissues were homogenized in 1 mL Tri Reagent (Sigma, St. Louis, MO) together with 10–20 zirconia/silica beads (1 mm diameter) (BioSpec Products, Bartletsville, OK) using a Bertin Precellys 24 homogenizer (VWR, Radnor, PA). Total RNA was isolated according to manufacturer’s protocol. A total of 1.5 μg RNA per sample was utilized as template for cDNA synthesis using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) according to manufacturer’s protocol. cDNA was diluted 1:10 in nuclease-free water. Quantitative PCR reactions totaling 20 μL were comprised of 6 μl dilute cDNA template, 10 μl PerfeCTa SYBR mastermix (Quanta Biosciences, Gaithersburg, MD), 2 μl forward primer (3 nM), and 2 μl reverse primer (3 nM). Reactions were performed using the cycling conditions (95°C, 3 min; 95°C, 30 sec, target-specific annealing °C, 72°C, 45 sec for 40 cycles; 72°C, 5 min) on a CFX Connect platform (Biorad). Primers can be found in Supplementary Table S2.
2.7 Illumina Hiseq colonic RNA expression profiles
C57BL6/J Ahrb/b male mice were fed control (n=3) or broccoli (n=3) supplemented diet as described above. Animals were euthanized by carbon dioxide asphyxiation and placed into an anaerobic chamber (Coy Lab Products, Grass Lake, MI) containing an atmosphere comprising 3.3% hydrogen and <15 ppm oxygen. Tissues were excised under anaerobic conditions, frozen in liquid nitrogen, and stored at −80°C. RNA was extracted as described above and subjected to further purification using the RNeasy purification kit (Qiagen, Hilden, Germany). The RNA samples were transferred to the Genomics Core Facility (The Pennsylvania State University) for Poly-A selection and sequencing on the Illumina HiSeq 2500 (150 bp single end reads). RNAseq reads were aligned to the Mus musculus genome (mm10, RefSeq genes) using TopHat version 2.0.13 with default parameters (Trapnell, Pachter et al. 2009). Alignment results are given in Supplementary Table S3. Reads mapping to genes were counted using HTseq-count version 0.5.4p3 with parameters ‘-s no -a 10’(Anders and Huber 2010).
2.8 Ingenuity pathway analysis
To eliminate read bias, mapped reads were normalized to transcript length to generate normalized RPKM reads (Reads per kilobase per million) for further analyses. Reads were filtered by those whose expression varied significantly between groups, according to a Student’s two-tailed t-test. Significantly altered genes that exhibited at least a 1.5-fold change in expression were analyzed using Ingenuity Pathway Analysis software (Qiagen, Hilden, Germany) according to manufacturer’s instructions.
2.9 Statistical analysis
Where indicated, two-tailed unpaired parametric Students’ t-test was performed. Correlation analyses were performed using Pearson’s correlation coefficient (r) with a confidence interval of 95% and a two-tailed significance test. Significance thresholds of *p< 0.05, **p< 0.01 and ***p< 0.001 were applied. Statistical analyses and graphing was performed using Graphpad Prism v5.
3. Results
3.1 Dietary Broccoli Induces the Prototypic AHR Target Gene, Cyp1a1
Activation of the AHR in response to dietary administration of whole broccoli was initially assessed in C57BL6/J mice that express the high affinity Ahrb/b allele. Mice were acclimatized to a defined pellet diet of AIN-93G (Low in AHR ligands) for seven days, and then administered finely ground diets containing concentrations of 0% or 10% (w/w) freeze-dried broccoli for an additional seven days. Broccoli at 10% in the diet promoted a significant 3-fold elevation in duodenal Cyp1a1 expression (Supplementary Fig. S1). In our judgment, this represented a modest level of AHR mediated activity and thus 15% broccoli was tested and a significant 15-fold induction of Cyp1a1 was observed (Fig. 1A). Thus, all further studies utilized a 15% (w/w) broccoli diet on an AIN-93G background in which the nutritional composition has been controlled to ensure similar caloric content relative to the AIN-93G control diet. Importantly, these data indicate that AHR-mediated intestinal Cyp1a1 expression is dose-dependently sensitive to dietary broccoli. Glucosinolate levels vary between different broccoli cultivars and storage conditions. Extractions of the Lieutenant cultivar we used and subsequent quantification via HPLC-MS revealed glucobrassicin content to be 1.6 μmoles/g (dry weight). This value is within previously established ranges of glucobrassicin levels detected in different varieties of broccoli cultivars (Kushad, Brown et al. 1999). Analysis of 15% broccoli diet consumption rates revealed a mean intake of 3.34 g per day, which equates to 354 μg of glucobrassicin per day, which is equivalent to a mean intake of 14 mg/kg/day.
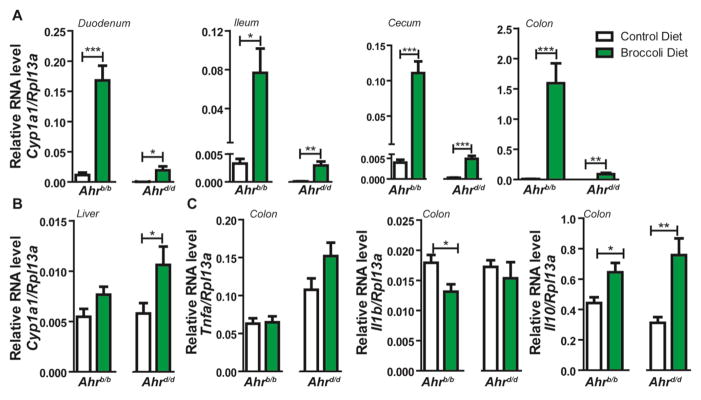
Fig. 1.
Ahr allele status dictates responsiveness and systemic penetration of broccoli-derived agonists. (A) Real time PCR quantification of (A) intestinal Cyp1a1 expression, (B) Hepatic Cyp1a1 expression, and (C) Colonic inflammatory gene expression (Tnfa, Il1b, Il10) normalized to eukaryotic Rpl13a from Ahrb/b and Ahrd/d mice fed AIN-93G (control) or isocaloric broccoli diet (15%) for 24 days. Data represent the mean gene expression (n=8 per genotype/diet group) ± standard error of mean (SEM).
3.2 Broccoli supplemented diet facilitates differential activation of the AHR in congenic Ahrd/d and Ahrb/b mice
The physiological impacts of broccoli consumption associated with activation of the AHR were evaluated using congenic male C57BL6/J mice possessing either the ligand-sensitive Ahrb/b or less-sensitive Ahrd/d alleles. Mice were acclimatized to AIN-93G diet for seven days, followed by a 24-day dietary intervention of modified AIN-93G rodent diet supplemented with 15% broccoli (n=16 per genotype, 8 per diet). Examination of the percentage increase in body weight over 24 days revealed no significant contribution of either diet or genotype. (Supplementary Fig. S2). Epididymal adipose tissue weight was found to be unaltered by dietary broccoli in Ahrb/b mice, however, a decrease in mean epididymal adipose mass of 22.5% occurred in broccoli fed Ahrd/d mice relative to control-fed mice (Supplementary Fig. S2). Gastrointestinal activation of AHR by dietary broccoli was assessed by quantification of Cyp1a1 expression in the duodenum, ileum, cecum, and colon (Fig. 1A). Duodenal Cyp1a1 expression was enhanced significantly relative to controls in both Ahrb/b and Ahrd/d animals, 15-fold and 85-fold, respectively. However, upon comparison of genotypes, Ahrb/b mice were found to express 9-fold higher levels of Cyp1a1 in the duodenum than Ahrd/d mice, in response to dietary broccoli. Similar dietary and genotypic trends of AHR transcriptional activity were observed throughout the intestinal tract, i.e. the ileum, cecum, and colon (Fig. 1A). Systemic penetrance of broccoli derived AHR ligands and resultant AHR activation, were evaluated by quantification of hepatic Cyp1a1 expression (Fig. 1B). Contrary to observations in intestinal tissues, broccoli diet did not mediate a significant increase in AHR target gene expression within the Ahrb/b genotype. However, within the Ahrd/d mice, a significant two-fold increase in hepatic Cyp1a1 expression was observed. In addition, it was previously observed that administration of AHR ligands can alter intestinal inflammatory status (Furumatsu, Nishiumi et al. 2011). Colonic expression of the cytokines Tnfa, Il1b, and Il10 associated differentially with dietary or genotypic status (Fig. 1C). Expression of Tnfa was elevated in Ahrd/d relative to Ahrb/b mice and increased further following broccoli consumption. Expression of proinflammatory Il1b was reduced by 25% by consumption of broccoli, but this effect was only observed in Ahrb/b mice. Broccoli consumption also mediated a 1.5–2-fold increase in anti-inflammatory cytokine Il10 expression, irrespective of Ahr genotype.
3.3 Dietary Broccoli and Genotype alter microbial community structure
AHR status is reported to influence intestinal microbial community structure (Murray, Nichols et al. 2016). To assess the combinatorial impact of AHR status and consumption of broccoli upon microbial community structure, we performed bacterial 16S rRNA gene profiling of DNA isolated from cecal luminal contents of Ahrb/b and Ahrd/d mice following 24 days of broccoli consumption. Cecal microbial population profiles were obtained from 16S rRNA gene sequencing (mean 155,000 reads/sample, n=8 per group). Comparative analyses of phylogenetic differences between genotypes and dietary broccoli supplementation were conducted using g-unifrac. Differences in bacterial populations are calculated as distances based upon the presence of abundant (weighted unifrac) and rare (unweighted unifrac) bacterial populations, where a shorter distance between points indicates similarities in overall microbial composition. Analyses of microbial composition by g-unifrac indicate that individuals segregate into distinct groups dependent upon genotype and diet (Supplementary Fig. S3).
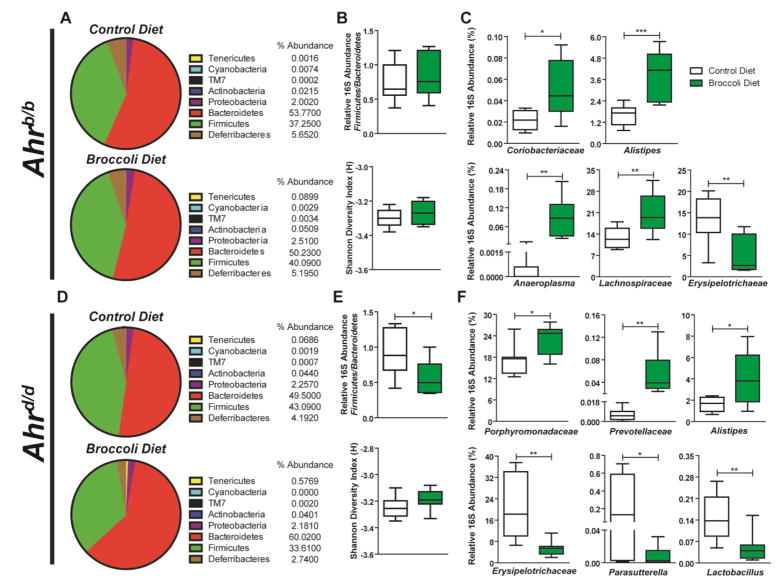
Phyla level analyses of 16S rRNA gene sequencing derived from Ahrb/b mice fed a control or broccoli diet identify significant differences in the percentage abundance of 16S reads associated with Tenericutes (57-fold increase), Cyanobacteria (3-fold decrease), TM7 (15-fold increase), and Actinobacteria (2-fold increase) (Fig. 2A). Consumption of broccoli diet by Ahrb/b mice did not significantly influence 16S reads associated with Proteobacteria, Bacteroidetes, Firmicutes, or Deferibacteria, which together constitute over 90% of the total reads for all samples analyzed. As such, there was no significant change in the Firmicutes/Bacteroidetes ratio or the overall microbial community diversity as measured by the Shannon Diversity Index score associated with dietary broccoli (Fig. 2B). In total, 16S reads aligned with approximately 224 different taxonomic classifications per individual (Supplementary Fig. S4–S8). Significant variations in the relative abundancies of 35 taxa associated with broccoli consumption in Ahrb/b mice (Fig. 2C). The increased abundance of 16S reads associated with Actinobacteria in broccoli fed Ahrb/b mice correlated with a 2-fold enrichment of the family Coriobacteriaceae (r=0.9999, p< 0.0001). Within the Bacteroidetes phylum, a significant increase (2-fold) within the family Rikenellaceae was observed in broccoli fed Ahrb/b mice, correlating with an increase of the genus Alistipes (r=1.0, p< 0.0001), which accounted for a 2% increase relative to total 16S reads. Significant enrichment of 16S reads associated with the class Mollicutes (57-fold) correlated with a corresponding increase in the genus Anaeroplasma (r=1.0, p< 0.0001). Increased abundance of 16S reads associated with the class Clostridia was found to strongly correlate with the family member Lachnospiraceae (r=0.9485, p< 0.0001). Increased 16S reads associated with Clostridia accounted for an average 11% increase relative to total 16S reads. Conversely, a trend towards depletion of 16S reads associated with the class Erysipelotrichia, which accounted for an average decrease of 8% relative to total reads, was observed in broccoli fed Ahrb/b mice. Decreased Erysipelotrichia was found to strongly correlate with a decrease in the family member Erysipelotrichaceae (r=1.0, p< 0.0001). Notably, 16S reads associated with four genera of microbes, Asaccharobacter, Syntrophococcus, Anaerostipes, and Ruminococcus, are solely present in Ahrb/b mice fed a broccoli diet.
Fig. 2.
Dietary broccoli mediates divergence of cecal microbial community structure in Ahrb/b and Ahrd/d mice. Pie chart representation and relative abundance of bacterial phyla from control or broccoli-fed (A) Ahrb/b and (D) Ahrd/d mice. Broccoli impacts Firmicutes/Bacteroidetes ratio and overall microbial diversity as determined by the Shannon Diversity Index found in (B) Ahrb/b and (E) Ahrd/d mice. Relative 16S rDNA abundances of microbial taxa that are significantly altered by administration of broccoli diet in (C) Ahrb/b and (F) Ahrd/d mice. Data represent the mean relative 16S rDNA abundance (%) (n=8 per genotype/diet group) ± SEM.
Phyla level analyses of 16S rRNA gene sequencing between Ahrd/d mice fed a control or broccoli diet identify significant differences in the percentage abundance of 16S reads associated with Bacteroidetes (1.2-fold increase) and TM7 (3-fold increase) (Fig. 2D). Consumption of broccoli diet by Ahrd/d mice did not coincide with significant phyla variations in 16S reads associated with Proteobacteria, Tenericutes, Actinobacteria, Cyanobacteria, Firmicutes, or Deferibacteria. The increase in reads associated with the phyla Bacteroidetes accounted for an average 11% increase relative to total 16S reads and facilitated a significant decrease in the relative Firmicutes/Bacteroidetes ratio in broccoli-fed Ahrd/d mice (Fig. 2E). Analysis of microbial diversity in Ahrd/d mice identified no significant change in Shannon Diversity Index associated with broccoli consumption (Fig. 2E). In total, relative 16S reads associated with 224 different taxonomic classifications were quantified per individual (Supplementary Fig. S4–S8). Expanded 16S sequence analyses identified 29 significant changes in relative taxa abundance associated with dietary broccoli in Ahrd/d mice (Fig. 2F). The increased abundance of 16S reads associated with Bacteroidetes in broccoli-fed Ahrd/d mice correlated with an enrichment of three families Porphyromonadaceae (r=0.6108, p< 0.05), Prevotellaceae (r=0.6479, p< 0.01), and Rikenellaceae (r=0.5768, p< 0.05). The significant increase in 16S reads associated with the family Rikenellaceae correlated with an increase of the genus Alistipes (r=1.0, p< 0.0001). This increase in 16S reads associated with Alistipes accounted for a 2% increase relative to total 16S reads. A decrease in 16S reads associated with the order Burkholderiales (27-fold) correlated with a corresponding decrease in the genus Parasutterella (r=0.9576, p< 0.0001). A reduction in 16S reads associated with the class Bacilli (3-fold) was found to strongly correlate with the family member Lactobacillus (r=0.9858, p< 0.001). A trend towards depletion of 16S reads associated with the class Erysipelotrichia, which accounted for an average decrease of 15% relative to total reads, was observed in broccoli fed Ahrd/d mice. Decreased Erysipelotrichia strongly correlated with a decrease in the family member Erysipelotrichaceae (r=1.0, p< 0.0001). Within Ahrd/d mice, 16S reads associated with the family Staphylococcaceae are only observed in broccoli-fed Ahrd/d mice. Enrichment of 16S reads associated with the increase in Staphylococcaceae correlate with a corresponding increase in the genus Staphylococcus (r=0.7660, p< 0.001).
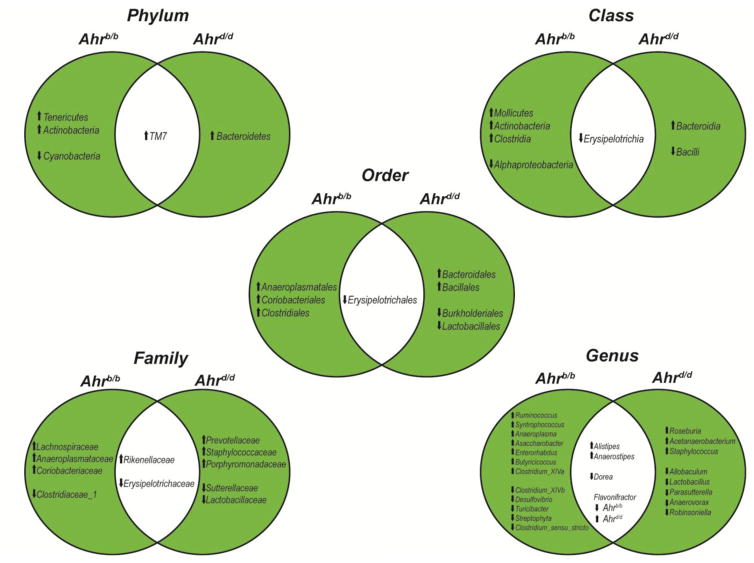
Alterations in bacterial community composition between Ahrb/b and Ahrd/d mediated by broccoli were compared to identify changes associated with increased sensitivity to AHR ligands (Fig. 3). Unique changes in 16S read abundance of three phyla, four classes, three orders, four families, and 12 genera were observed in broccoli-fed Ahrb/b mice, relative to controls. In contrast, 16S reads associated with one phylum, two classes, four orders, five families, and eight genera were found to be uniquely altered in Ahrd/d mice fed broccoli. Shared changes within the bacterial community compositions, independent of genotype and AHR activation status, identified significant variations within one phylum, one class, one order, two families, and four genera. More specifically, broccoli consumption was associated with increased 16S read abundance corresponding to the phylum TM7, family Rikenellaceae, and genera Alistipes and Anaerostipes. Decreased 16S read abundance associated with the class Erisipelotrichia, order Erysipelotrichales, family Erysipelotrichaceae, and the genus Dorea was observed following broccoli administration regardless of Ahr genotype. Notably, the relative abundance of bacteria within the genus Flavonifractor was significantly decreased in Ahrb/b mice, but significantly elevated in Ahrd/d mice upon broccoli consumption. Overall these data demonstrate significant differences in intestinal microbe community structure that segregate according to the host’s diet and Ahr status.
Fig. 3.
Ahr status contributes to alteration of cecal microbe populations by broccoli. Venn diagram plots depict significant (p<0.05) changes in microbial taxa that occur dependently (green) or independently (white) of Ahr status, based upon relative 16S rDNA abundances.
3.4 Microbial status does not associate with an enhanced capacity to produce AHR ligand from indole-3-carbinol
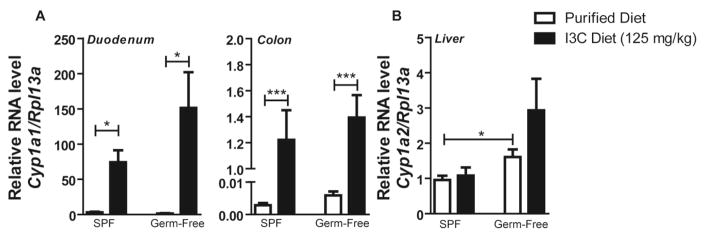
It is well documented that acid condensation reactions of I3C yield potent AHR ligands (Bradfield and Bjeldanes 1987). Microbial metabolism of I3C may contribute to endogenous formation of AHR ligands, and could impact the observed physiological effects of dietary broccoli by increasing AHR ligand bioavailability in the distal colon (Kwon 1994). To address this hypothesis, we performed parallel dietary feeding studies in which conventional and germ-free C57BL6/J mice were fed a purified diet with or without supplementation of I3C (125 mg/kg) for seven days. Intestinal AHR activation was compared between animals by quantitative PCR analysis of Cyp1a1 expression (Fig. 4A). Similar duodenal expression profiles of Cyp1a1 were observed in conventional and germ-free mice. In contrast to previous findings, we observed no significant increase in colonic AHR activity following I3C administration attributed to alteration of microbial status. Equivalent levels of basal AHR activity were observed in conventional and germ-free animals, suggesting minimal microbial contribution to intestinal generation of I3C-derived AHR ligands. Hepatic AHR activity, as measured by Cyp1a2 induction, was not elevated in conventional mice by consumption of I3C (Fig. 4B). However, basal hepatic AHR activity increased in germ-free relative to conventional mice.
Fig. 4.
Bacterial status does not influence formation of broccoli-derived AHR ligands. Specific-pathogen free (SPF) and Germ-free (GF) C57BL6/J-Ahrb/b mice were fed control or indole-3-carbinol (I3C) (125mg/kg) diets for a duration of 7 days, followed by real time PCR quantification of (A) Duodenal/colonic expression of prototypic target gene Cyp1a1 and (B) Hepatic expression of Cyp1a2, both normalized to eukaryotic Rpl13a. Data represent the mean gene expression (SPF+control diet n=5, SPF+I3C diet n=6, GF+control diet n=4, GF+I3C diet n=6) ± SEM.
3.5 Metagenomic analyses of microbial communities identify differential metabolic potential associated with dietary broccoli and AHR status
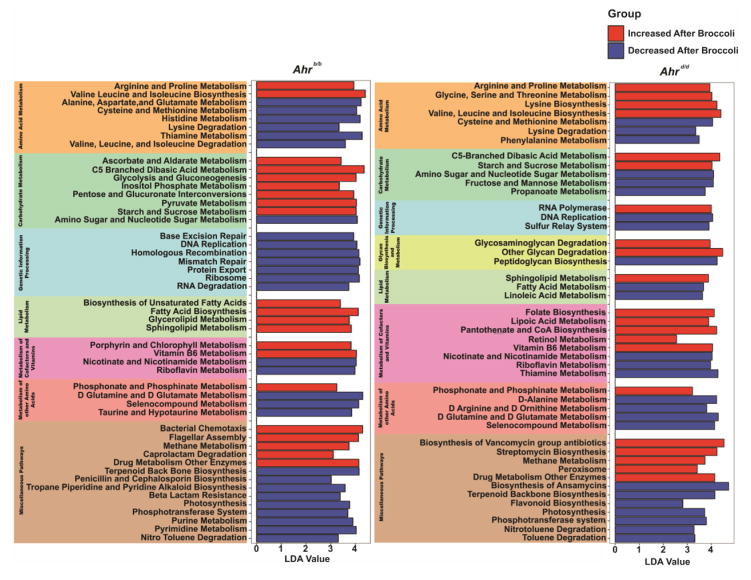
Having established that dietary broccoli and Ahr status can mediate divergence of overall cecal microbial community composition in mice, we utilized whole genome metagenomic pathway analyses to assess differences in microbial metabolic potential. Numerous alterations in microbial metabolic capacity were found to be mediated by host consumption of broccoli (Fig. 5). In total, 49 and 46 microbial metabolic pathways were significantly altered by broccoli ingestion in Ahrb/b or Ahrd/d mice, respectively. Broccoli consumption associated with a decreased representation of ten metabolic pathways: riboflavin metabolism, lysine degradation, nicotinate and nicotinamide metabolism, D-glutamine/D-glutamate metabolism, terpenoid backbone biosynthesis, nitrotoluene degradation, photosynthesis, amino-sugar/nucleotide-sugar metabolism, thiamine metabolism, and seleno-compound metabolism, independent of Ahr status. In Ahrb/b mice, dietary broccoli associated with decreased abundance of 18 pathways involved in amino acid metabolism (22%), genetic information processing (44%), metabolism of other amino acids (6%), and miscellaneous pathways (28%). In Ahrd/d mice, broccoli associated with decreased abundance of 15 pathways involved in amino acid metabolism (13%), carbohydrate metabolism (13%), genetic information processing (13%), glycan biosynthesis and metabolism (7%), lipid metabolism (13%), metabolism of other amino acids (13%), and miscellaneous pathways (27%).
Fig. 5.
Metagenomic analysis of differential microbial metabolic potential. Significantly different predicted pathways mediated by broccoli were discovered using LDA effect size (LEfSe). LEfSe combines the Kruskal-Wallis and the Willcoxon statistical tests to show biologically relevant and statistically significant pathways. Metabolic pathways were grouped by established KEGG classifications. Data represent linear discriminate analysis (LDA) score for indicated pathways that significantly increase (red) or decrease (blue) due to broccoli consumption in Ahrb/b and Ahrd/d mice.
Increased representation of nine metabolic pathways: methane metabolism, phosphonate/phosphinate metabolism, valine/leucine/isoleucine biosynthesis, starch/sucrose metabolism, drug metabolism, arginine/proline metabolism, C5-branched dibasic amino acid metabolism, sphingolipid metabolism, and vitamin B6 metabolism all were observed in both Ahrb/b and Ahrd/d broccoli-fed mice. In Ahrb/b mice, broccoli associated with increased abundance of 12 pathways involved in carbohydrate metabolism (42%), lipid metabolism (25%), metabolism of cofactors and vitamins (8%), and miscellaneous pathways (25%). In Ahrd/d mice, broccoli associated with increased abundance of 12 pathways involved in amino acid metabolism (17%), genetic information processing (8%), glycan biosynthesis and metabolism (17%), lipid metabolism (13%), metabolism of cofactors and vitamins (33%), and miscellaneous pathways (25%). This data provides further support to the concept that Ahr status and consumption of broccoli can influence microbial community structure and their metabolic contribution to overall host physiology.
3.6 1H-NMR analysis of cecal metabolite profiles from broccoli-fed Ahrb/b and Ahrd/d mice
The observed variance in the metabolic potential of intestinal microbes associated with broccoli consumption and Ahr status suggest possible changes in microbial metabolic output and subsequent alteration of metabolite profiles in the cecal luminal contents. Relative quantification of short chain fatty acids (SCFAs), glucose, lactate, bile acids, and amino acids levels within cecal content extracts were measured by 1H-NMR targeted peak integration analyses (Supplementary Fig. S9). Of the metabolites measured, only the SCFA propionate and lactate were found to be significantly decreased in Ahrb/b mice following administration of broccoli. In contrast, broccoli consumption associated with significant changes in the luminal concentration of numerous metabolites in Ahrd/d mice. Concentrations of acetate, a SCFA that is a principle energy source for host epithelial cells, and bile acids, host-derived emulsifiers and bioactive signaling factors, were elevated in the cecal contents of Ahrd/d broccoli-fed mice. In contrast, glucose, tyrosine, and phenylalanine metabolite concentrations were significantly decreased upon broccoli administration. These data provide support that the host genotype, dietary status, and resultant alteration of microbial populations can combinatorially influence intestinal metabolite availability, which may further influence host signaling and gene expression.
3.7 Dietary broccoli attenuates symptoms of chemically induced colitis
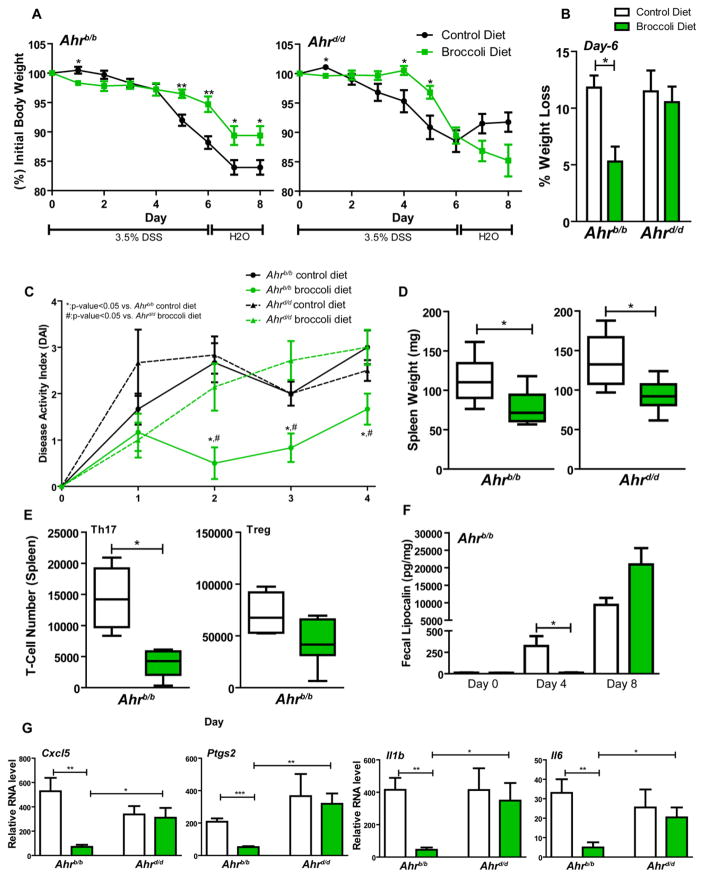
The AHR has been previously investigated for its role in reducing disease severity associated with chemically induced inflammatory models such as dextran sodium sulfate (DSS) colitis (Takamura, Harama et al. 2010). Utilizing a DSS-colitis model, we investigated the capacity for dietary broccoli to remediate intestinal disease severity in conjunction with differential AHR activation potential through use of Ahrb/b and Ahrd/d mice (Fig. 6A). Mice were given access to control or broccoli diets for 14 days prior to administration of 3.5% DSS in drinking water. Mice were maintained on these diets for the remainder of the study. DSS-supplemented water was administered for six consecutive days, at which point the mice were switched to tap water for 48 h prior to euthanasia. Animals were monitored daily for weight loss, and clinical symptoms of disease severity, i.e. stool consistency, blood in stool, rectal bleeding, and lethargy. As stated previously, broccoli consumption alone does not cause significant change in weight relative to control mice (Supplementary Fig. S2). Ahrb/b mice exposed to DSS and fed a control diet exhibited marked decreases in body weight following day 4 of administration. The onset of weight-loss in Ahrb/b mice was delayed by administration of broccoli diet, which led to significant differences in the percent of weight lost that was maintained throughout the duration of the study time course. Ahrd/d broccoli-fed mice also exhibited attenuated weight loss, however this effect was only observed during days four and five of DSS administration. By day six, the last day of DSS exposure, significant attenuation of overall weight-loss is singularly observed in Ahrb/b broccoli-fed mice, which exhibit only a 5% decrease in total body weight, compared to an average 12% decrease in all other experimental groups (Fig. 6B). Colitis disease activity was quantified for the initial four days of the study when fecal pellets were produced by all observed individuals. Disease activity scores indicate significant attenuation of assessed clinical parameters associated with broccoli-fed Ahrb/b mice relative to Ahrb/b control diet and Ahrd/d broccoli diet groups (Fig. 6C). An enlarged spleen is indicative of a heightened inflammatory status or infection and is known to occur in both acute and chronic models of DSS-colitis. Both Ahrb/b and Ahrd/d mice displayed significant attenuation of the clinical manifestation of splenomegaly as evidenced by a respective 31% and 33% lowered mean spleen weight relative to control fed mice (Fig. 6D).
Fig. 6.
Sensitivity to broccoli derived AHR ligands contributes to attenuation of DSS-colitis disease severity. Attenuation of DSS-mediated body weight loss in response to consumption of broccoli in (A) Ahrb/b and Ahrd/d mice. (B) Influence of broccoli consumption on day 6 of DSS exposure. (C) Disease activity index (DAI) and (D) spleen weight were assessed in treated mice. (E) Box and whisker plot depicting Ahrb/b splenic TH17 and Treg numbers following broccoli administration and DSS challenge. (F) ELISA quantification of fecal lipocalin (pg/mg) at days 0, 4, and 8 of DSS challenge in Ahrb/b fed control or broccoli diet. Data are shown as means ± SEM (n=6 per genotype/diet). (G) Nanostring hybridization analysis of colonic inflammatory cytokines expression (Cxcl5, Ptgs2, Il1b, Il6) in broccoli-fed Ahrb/b and Ahrd/d mice.
Our previous data indicates broccoli is protective against DSS-chemical challenge. However, this phenotype may be a consequence of broccoli mediated host adaptation prior to or an active response during intestinal challenge. Histological analyses of mucosal damage by assessment of severity/extent of inflammatory cell infiltration and loss of intestinal crypt morphology displayed no difference between control and broccoli-fed Ahrb/b mice (Supplementary Fig. S10). Previous studies found that ligand-activation of the AHR facilitates decreased symptom severity in chemically-induced inflammatory models by enhancement of anti-inflammatory regulatory T-cell (Treg) differentiation (Quintana, Basso et al. 2008). In the absence of an inflammatory challenge broccoli had no effect upon Treg differentiation in Ahrb/b or Ahrd/d mice. Although increased splenic T-Helper 17 (TH17) cell numbers (1.6-fold) were observed in Ahrd/d mice (Supplementary Fig. S11). Upon challenge with DSS, broccoli mediated a significant reduction (3-fold) in splenic pro-inflammatory TH17 cells, but no increase in Treg cell populations in Ahrb/b mice (Fig. 6E). To examine possible timeline differences in the onset of intestinal inflammation, a non-invasive measurement of fecal lipocalin 2 (Lcn2) was utilized. The lack of fecal Lcn2 production at day 4 in broccoli-fed Ahrb/b mice indicate delayed onset of DSS-induced intestinal stress (Fig. 6F). Taken together, these data suggest that broccoli consumption may prime the host to better adapt to instances of intestinal challenge.
To assess whether broccoli fed mice exhibited an attenuated constitutive inflammatory status Nanostring™ hybridization was performed. Quantification of colonic inflammatory gene expression identified four genes [Cxcl5 (4-fold), Ptgs2 (6-fold), Il1b (8-fold), and Il6 (4-fold)] that exhibited significantly decreased expression in Ahrb/b mice fed broccoli, in contrast to broccoli-fed Ahrd/d mice (Fig. 6G).
3.8 Microbial reconstitution of germ-free Il10−/− mice
Administration of DSS induces colitis by disruption of intestinal epithelial barrier integrity, which increases microbial translocation out of the intestinal lumen. Microbial composition, the presence of opportunistic pathogens, and host genetic risk factors all concomitantly contribute to susceptibility to intestinal inflammatory disease. A lack of AHR signaling promotes colonization of segmented filamentous bacteria and an increased intestinal inflammatory tone (Murray, Nichols et al. 2016). This finding suggests that AHR activity and dietary broccoli may promote differential colonization of microbial communities that vary in their intrinsic capacity to induce intestinal inflammation. To compare the contribution of microbial communities upon intestinal inflammatory status we utilized a germ-free Il10−/− mouse reconstitution model, in which cecal microbes from broccoli and control fed donors were gavaged into Il10−/− mice. Their innate lack of Il10 expression predisposes them to onset of spontaneous colitis following reconstitution of enteric bacteria (Sellon, Tonkonogy et al. 1998). Microbial reconstitutions from Ahrb/b mice fed control or broccoli diets mediated similar colonic expression of pro-inflammatory cytokines (Tnfa, Il1b, Il6, Il17a, and Lcn2), suggesting that broccoli-dependent microbial differences are unable to induce a heightened intestinal inflammatory status (Supplementary Fig. S12). Therefore, the observed benefit of broccoli upon Ahrb/b mice in DSS-colitis models is likely attributed to effects upon host gene expression. In contrast, microbial communities derived from broccoli-fed Ahrd/d mice mediate a heightened colonic inflammatory tone in recipient mice, characterized by significantly increased expression of Tnfa (3-fold), Il1b (4-fold), Il6 (2-fold), and Il17a (2-fold) (Supplementary Fig. S13).
3.9 RNA-Seq analysis of dietary broccoli impact upon colonic gene expression
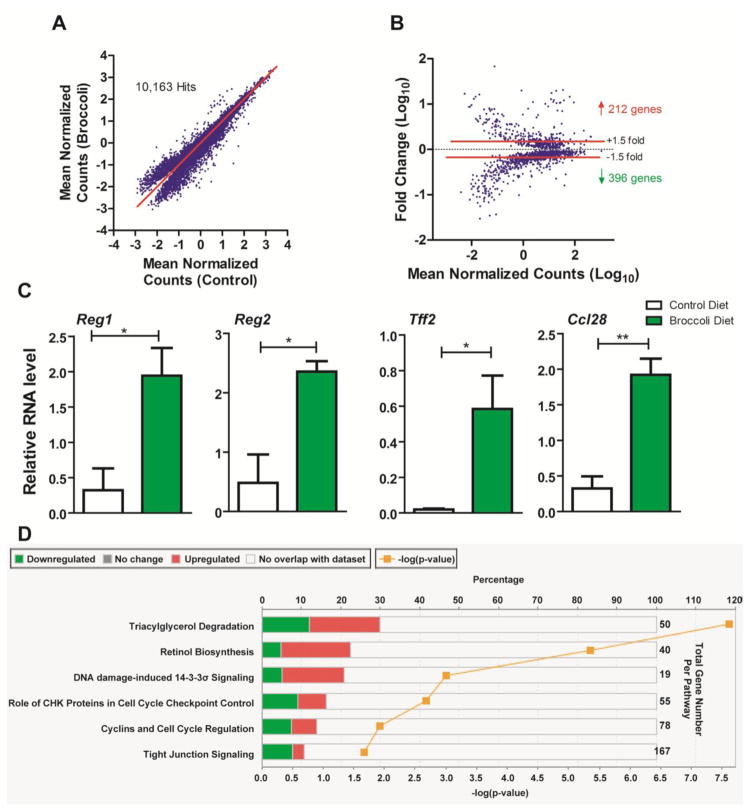
RNA-sequencing was utilized as a non-targeted high-throughput method to further investigate the impact of dietary broccoli upon colonic gene expression in Ahrb/b mice on day 8 of the DSS exposure study. Reads were mapped to 10,163 known transcripts (Fig. 7A). Transcripts that displayed significantly altered expression (p<0.05, by student’s t-test) between control and broccoli diet groups were compared via Bland-Altman plot. In total 608 targets were found to exhibit at least a 1.5-fold change in expression (212 upregulated, 396 down-regulated) (Fig. 7B). The top 20 increased and decreased transcripts are listed in the Supplementary Table S4. Additionally, a number of genes were found to be induced by dietary broccoli that have roles in the maintenance of intestinal epithelial homeostasis Reg1, Reg2, Tff2. In addition, dietary broccoli enhanced Ccl28 expression, which regulates mucosal homing of T and B lymphocytes and possesses potent antimicrobial activity against Candida albicans, Gram-negative bacteria, and Gram-positive bacteria (Hieshima, Ohtani et al. 2003; Kunkel and Butcher 2003). To confirm the inducibility of these genes by broccoli consumption, real-time PCR was performed and the data obtained confirmed enhanced colonic expression in Ahrb/b mice of Reg1 (6-fold), Reg2 (5-fold), Tff2 (30-fold), and Ccl28 (6-fold) (Fig. 7C). Ingenuity pathway analysis (IPA) predicted alteration of six canonical pathways associated with consumption of dietary broccoli; triacylglyerol degradation, retinol biosynthesis, DNA damage-induced 14-3-3σ signaling, role of CHK proteins in cell cycle checkpoint control, cyclins and cell cyclin regulation, and tight junction signaling (Fig. 7D). Of these pathways, cyclins and cell cycle regulation was predicted to be significantly elevated by broccoli consumption, in contrast, the role of CHK proteins in cell cycle checkpoint control were suggested to be significantly decreased. Overall these data suggest that dietary broccoli can significantly alter host colonic gene expression and such alterations may enhance the proliferative capacity of the intestinal mucosa.
Fig. 7.
RNA-sequencing identified differential colonic gene expression profiles mediated by dietary broccoli in Ahrb/b mice after DSS exposure on day 8. (A) Dot Plot displays distribution of reads that were mapped to known transcripts, deviation from the diagonal indicate differential expression due to broccoli consumption, relative to controls. (B) Bland-Altman plot of Log10 mean normalized (RPKM) counts vs. fold-change in expression. Ingenuity Pathway Analysis identified elevation of 4 genes associated with intestinal health altered by broccoli consumption (Reg1, Reg2, Tff2, and Ccl28). (C) Real time PCR quantification of Reg1, Reg2, Tff2, and Ccl28 normalized to eukaryotic Rpl13a. Data represent the mean gene expression (n=3 per diet group) ± standard error of mean (SEM). (D) Ingenuity Pathway Analysis identified top canonical pathways altered by broccoli consumption in the colon of Ahrb/b mice. For a given pathway, green bars indicate the percentage of down-regulated genes, and red bars indicate the percentage of upregulated genes.
4. Discussion
In the present study, we observed that broccoli mediated a greater level of intestinal AHR activation in Ahrb/b relative to Ahrd/d mice. This is likely an indication of a reduced capacity for the Ahrd/d isoform to bind and respond to broccoli-derived ligands. Previous studies suggest administration of broccoli will activate the AHR; however, these studies fail to consider the array of chemical components present in broccoli that could impact uptake and metabolism of I3C or antagonize the AHR. We found that the administration of the chemical mixture within broccoli does not mitigate AHR activation. Notably we observe the occurrence of broccoli mediated benefits and AHR activation at relatively low daily doses of I3C (4.64 mg/kg), which is 15–60 fold lower than that used in previous studies (Stoner, Casto et al. 2002; Julliard, De Wolfe et al. 2016). While heightened doses of I3C may promote intestinal health, they will lead to systemic circulation of AHR ligands that are associated with development of hepatocarcinoma (Stoner, Casto et al. 2002; (NTP) 2014). We found that broccoli consumption did not mediate upregulation of hepatic AHR activity in Ahrb/b mice. This was in direct contrast to significantly increased AHR activity in the liver of Ahrd/d mice. This elevation of hepatic AHR activity is an indicator of systemic circulation of broccoli derived AHR ligands. The strain difference observed is likely a consequence of differential induction of drug metabolism enzymes by the intestinal epithelium, which has recently been identified as the “gate keeper” to systemic circulation of AHR ligands (Schiering, Wincent et al. 2017). Elevated hepatic AHR activity, as a result of xenobiotic treatment, is associated with increased risk of hepatic steatosis and is likely mediated in part by AHR induced expression of the fatty acid transporter, CD36 (Kawano, Nishiumi et al. 2010; Angrish, Mets et al. 2012). These results suggest that the effective dose of AHR ligands present within broccoli can promote local intestinal AHR activation and its associated benefits, while avoiding systemic exposure.
The microbiome is now widely considered an additional organ within an organism through its influence upon host immune system development, displacement of pathogenic organisms, energy utilization and metabolism. Broccoli consumption is associated with altered cecal microbe community composition and metabolism, as well as decreased prevalence of bacterial species associated with Crohn’s Disease (Paturi, Mandimika et al. 2012). We find that consumption of broccoli and AHR responsiveness combinatorially contributes to the divergence of numerous taxa. Broccoli consumption was found to associate with a marked expansion of the genus Alistipes and decreased prevalence of the Erysipelotrichaceae family, independent of Ahr status. Alistipes bacteria that have been found to be significantly more abundant in the gut microbiota of healthy subjects, compared to patients diagnosed with non-alcoholic fatty liver disease (Jiang, Wu et al. 2015). Several reports have documented potential roles for Erysipelotrichaceae in host physiology and disease. Enriched abundance of Erysipelotrichaceae is associated with the prevalence of colorectal cancer in human epidemiological studies (Chen, Liu et al. 2012) and mouse models of 1,2-dimethylhydrazine-induced colon cancer (Zhu, Jin et al. 2014). Additionally, increases in Erysipelotrichaceae correlate with the development of TNF-driven Crohn’s disease-like transmural inflammation (Schaubeck, Clavel et al. 2015). Decreased abundance of Erysipelotrichaceae has been found to associate with increased consumption of flavonoids, such as quercetin (Etxeberria, Arias et al. 2015), and may suggest a mechanism for broccoli to elicit a similar effect. Therefore, broccoli mediated depletion of Erysipelotrichaceae may contribute to an improved host intestinal inflammatory status. Relative 16S rRNA gene abundance of Erysipelotrichaceae represent on average 14–20% of total assigned reads in both Ahrb/b and Ahrd/d control fed mice, which are depleted 8–15% following broccoli consumption. However, differential displacement of Erysipelotrichaceae, a member of the Firmicutes phylum, occurs in Ahrb/b relative to Ahrd/d mice. Such that in Ahrb/b mice, 16S read abundance associated with Firmicutes is maintained following the loss of Erysipelotrichia due to a corresponding increase in Clostridia associated 16S reads. In contrast, Erysipelotrichia are displaced by the microbiota taxonomically classified within the Bacteroidetes phylum in Ahrd/d mice, facilitating a significant decrease in the Firmicutes:Bacteroidetes ratio. The relative proportion of Firmicutes:Bacteroidetes has been demonstrated to correlate to host adiposity. Specifically, a lower ratio, or increased abundance of Bacteroidetes, corresponds to decreased incidence of obesity in ob/ob genetic mouse models through altered caloric utilization by the host conferred by the resident microflora (Ley, Backhed et al. 2005; Turnbaugh, Ley et al. 2006). We find that alteration of the Firmicutes:Bacteroidetes ratio by broccoli in Ahrd/d mice corresponded to decreased epididymal adiposity, not observed in Ahrb/b mice. In summary, the data indicates that broccoli is able to significantly alter cecal microbial composition and metabolism through mechanisms that associate with or are independent of host AHR status.
Our studies utilizing congenic mice under specific pathogen free or germ-free conditions fed purified diets supplemented with I3C indicate that intestinal microbes are unlikely to contribute to increased production of AHR ligands from cruciferous vegetables. In contrast, the enhanced sensitivity of germ-free mice, relative to conventional controls, to I3C with regard to hepatic Cyp1a2 expression suggests that the intestinal microbiota may metabolically eliminate I3C within the intestine, thus limiting systemic AHR activation. Upon further examination, control fed animals were found to display no significant differences in intestinal AHR activation as measured by Cyp1a1 expression. This suggests that intestinal AHR activity in mice is not increased by the metabolic activity of resident microflora. In contrast, human AHR is known to respond to numerous microbial-derived molecules, such as indole derivatives (Hubbard, Murray et al. 2015a; Hubbard, Murray et al. 2015b). Therefore, microbial derived ligands may heighten human intestinal AHR activity prior to consumption of dietary ligands. This effect could lower the amount of dietary agonist required to mediate a physiological impact upon the intestinal tract. Also the gastric pH of humans is lower than that of mice (1.5 to 3.5 versus 3.1 to 4.5) (Kararli 1995), which could further enhance the efficiency of I3C conversion to potent AHR agonists and decrease the amount of broccoli consumption required to be beneficial.
The capacity for AHR ligands to mitigate disease severity in models of autoimmune (EAE), pathogenic (C. rodentium), and chemical challenge (i.e. chemically-induced colitis) are well documented (Rouse, Singh et al. 2013; Schiering, Wincent et al. 2017). Administration of the broccoli-derived phytochemical I3C and its principle gastric-condensation products (DIM, and ICZ) has also been shown to promote similar effects (Rouse, Singh et al. 2013). In our study, we find that DSS-induced colitis severity is significantly attenuated by prior and concurrent administration of dietary broccoli. Notably, mitigation of DSS-mediated weight-loss was found to initially occur in both Ahrb/b and Ahrd/d phenotypes, but maintained in Ahrb/b mice following day six of the experimental time course. It is likely that the benefit of broccoli consumption is not wholly dependent upon glucobrassicin, but other bioactive constituent phytochemicals such as sulfurophane and phenylethyl isothiocyanate (Stoewsand 1995). However, the concomitant decreases in severity of weight loss, disease activity, and inflammatory cytokine expression (Cxcl5, Ptgs2, Il1b, Il6) observed in Ahrb/b mice fed broccoli, but not Ahrd/d mice, suggest that AHR responsiveness to dietary agonists may be a significant contributor to broccoli-associated maintenance of intestinal homeostasis. Previous studies suggest that AHR activation promotes a decreased inflammatory tone by enhanced differentiation of naïve CD4+ T-cells to favor formation of anti-inflammatory Treg cells (Funatake, Marshall et al. 2008; Rouse, Singh et al. 2013). In contrast, we find that broccoli consumption and associated activation of the receptor does not promote elevated differentiation of Treg cells, but does reduce the number of resident TH17 cells. Also, reconstitution experiments using colitis-prone Il10−/− mice indicate that the therapeutic effect of broccoli in Ahrb/b mice is not likely a consequence of altered intestinal inflammatory signaling as a result of increased virulence or pathogenicity of resident microbes. Therefore, the broccoli-associated benefit observed in the models of intestinal challenge likely originates form alterations of host gene expression.
To investigate additional mechanisms of the therapeutic effect of broccoli consumption upon the host, we utilized high throughput RNA sequencing to evaluate colonic gene expression profiles in mice after dietary intervention. These data suggest that broccoli consumption is able to significantly modulate expression of hundreds of genes. Ingenuity pathway analyses suggest that broccoli is able to significantly upregulate expression of cyclin and cell cycle regulation genes and down-regulate others involved in cell cycle checkpoint control. Elevated colonic expression of Reg1, Reg2, and Tff2 genes was observed in broccoli-fed Ahrb/b mice. Both Reg1 and Reg2 have been found to be upregulated in cases of intestinal amebiasis (Peterson, Guo et al. 2012) or irritable bowel disease (IBD) (Dieckgraefe, Crimmins et al. 2002) and thought to maintain intestinal epithelial barrier function by acting as a mitogenic or anti-apoptotic signaling factor. Also Tff2 signaling is known to promote gastric mucosal healing through actions that likely involve both inhibition of acid secretion and stimulation of mucosal proliferation (Farrell, Taupin et al. 2002). In summary these data would suggest that enhanced intestinal proliferative potential could be an effect of broccoli consumption and likely would enhance epithelial barrier function and protection against intestinal challenge.
Currently, over 1–2 million people in the United States and Canada have been diagnosed with irritable bowel disease (IBD), including ulcerative colitis and Crohn’s disease (Loftus 2004). The pathogenesis of IBD is characterized by a dysregulation of host proinflammatory signaling pathways in response to luminal microbes (Kaser, Zeissig et al. 2010). The Crohn’s and Colitis Foundation of America suggest that patients avoid cruciferous vegetables due to their fibrous nature and capacity to increase local gas production and irritation. However, our results would suggest that broccoli may be of preventative benefit in cases of heightened intestinal inflammation, such as Crohn’s disease and colitis. Consumption of puréed broccoli or dietary supplementation of low doses of I3C may provide a heightened therapeutic effect, while minimizing risks for patients. Notably, the scale of anti-carcinogenic and anti-inflammatory effect of cruciferous vegetable consumption has been found to directly associate with glucosinolate levels (Lippmann, Lehmann et al. 2014). Allometric calculations indicate humans (65 kg) would need to consume 900 g (5 cups) of raw broccoli daily to receive the scaled effective dose used in this study (Hu and Hayton 2001). Selection of broccoli cultivars that yield higher levels of glucosinolates may further enhance observed chemo-protective effects and enhanced maintenance of intestinal homeostasis, while requiring lower levels of consumption. Alternatively, other subspecies of Brassica oleracea could be substituted in place of broccoli, such as Brussels sprouts, which can contain up to 4.3-fold higher glucobrassicin levels than the utilized broccoli lieutenant cultivar (Kushad, Brown et al. 1999).
5. Conclusions
Broccoli consumption alters the host microbiome and improves intestinal resistance to chemical challenge, which suggests there is a therapeutic effect on the maintenance of intestinal homeostasis. Most importantly, this latter effect of broccoli consumption is mediated through AHR activation. Therefore, the selection of broccoli cultivars with increased levels of glucosinolates, especially those that lead to the formation of AHR ligands, may be of increased health benefit. Furthermore, numerous other foodstuffs contain AHR ligands that may also exhibit similar health effects (Jeuken, Keser et al. 2003).
Supplementary Material
Highlights.
Broccoli consumption activates the colonic Ah receptor (AHR) in mice that express the high ligand affinity AHRb, but not the low affinity AHRd.
Broccoli consumption influences the composition of the mouse cecal microbiome in part dependent on the form of the AHR expressed.
Broccoli consumption decreases inflammation in the gastrointestinal tract of AHRb, but not AHRd mice.
Broccoli consumption protects the gastrointestinal tract of AHRb, but not AHRd mice from dextran sodium sulfate toxic insult.
Acknowledgments
We thank the Penn State Genomics Core Facility, the Penn State Metabolomics Core Facility, Animal diagnostic Laboratory, and Gnotobiotic Animal Research Facility- University Park, PA for their many contributions to this project. We also express our gratitude to John Esslinger and Brian Campbell for providing the broccoli cultivar used for the study. We also thank Marcia H. Perdew for excellent editorial assistance.
Funding Sources
This project was supported by Agriculture and Food Research Initiative Competitive Grant # 2014-06624 from the USDA National Institute of Food and Agriculture and by grants from the National Institutes of Health Grants ES004869 and ES019964.
Abbreviations
- AHR
aryl hydrocarbon receptor
- I3C
indole-3-carbinol
- DIM
3,3′-diindolylmethane
- ICZ
indolo[3,2-b]carbazole
- I3ACN
indole-3-acetonitrile
- Ltr-1
2-(indol-3-ylmethyl)-3,3′-diindolylmethane
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- PAHs
polycyclic aromatic hydrocarbons
- PCBs
poly-chlorinated biphenyls
- OTU
operational taxonomic unit
- DSS
dextran sodium sulphate
- EAE
experimental auto-immune encephalitis
- Treg
Regulatory T-cell
- TH17
T-Helper-17 cell
- IBD
irritable bowel disease
Footnotes
Chemical Compounds
Glucobrasscin (PubChem CID: 6602378); Indole-3-carbinol (PubChem CID: 3712); 3,3′-Diindolylmethane (PubChem CID: 3071); Indolo[3,2-b]carbazole (PubChem CID: 114764).
Disclosure/Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (NTP), N. T. P. Toxicology Studies of Indole-3-Carbinol in F344/N Rats and B6C3F1/N Mice and Toxicology and Carcinogenesis Studies Of Indole-3-Carbinol in Harlan Sprague Dawley Rats and B6C3F1/N Mice (Gavage Studies) National Institute of Health; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4(9):1201–15. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrish MM, Mets BD, et al. Dietary fat is a lipid source in 2,3,7,8-tetrachlorodibenzo-rho-dioxin (TCDD)-elicited hepatic steatosis in C57BL/6 mice. Toxicol Sci. 2012;128(2):377–86. doi: 10.1093/toxsci/kfs155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Mimura J, et al. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25(22):10040–51. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjeldanes LF, Kim JY, et al. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88(21):9543–7. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield CA, Bjeldanes LF. Structure-activity relationships of dietary indoles: a proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health. 1987;21(3):311–23. doi: 10.1080/15287398709531021. [DOI] [PubMed] [Google Scholar]
- Bradlow HL, Sepkovic DW, et al. Multifunctional aspects of the action of indole-3-carbinol as an antitumor agent. Ann N Y Acad Sci. 1999;889:204–13. doi: 10.1111/j.1749-6632.1999.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Bittinger K, et al. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics. 2012;28:2106–2113. doi: 10.1093/bioinformatics/bts342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu F, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7(6):e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Fisher JM, et al. Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988;85(8):2528–32. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckgraefe BK, Crimmins DL, et al. Expression of the regenerating gene family in inflammatory bowel disease mucosa: Reg Ialpha upregulation, processing, and antiapoptotic activity. J Investig Med. 2002;50(6):421–34. doi: 10.1136/jim-50-06-02. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria U, Arias N, et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem. 2015;26(6):651–60. doi: 10.1016/j.jnutbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Farrell JJ, Taupin D, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109(2):193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick GR, Heaney RK, et al. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18(2):123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- Funatake CJ, Marshall NB, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation of alloreactive CD8+ T cells toward a regulatory T cell phenotype by a mechanism that is dependent on aryl hydrocarbon receptor in CD4+ T cells. J Immunotoxicol. 2008;5(1):81–91. doi: 10.1080/15476910802019037. [DOI] [PubMed] [Google Scholar]
- Furumatsu K, Nishiumi S, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56(9):2532–44. doi: 10.1007/s10620-011-1643-9. [DOI] [PubMed] [Google Scholar]
- Hieshima K, Ohtani H, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170(3):1452–61. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Delage B, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–36. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu TM, Hayton WL. Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS PharmSci. 2001;3(4):E29. doi: 10.1208/ps030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015a;5:12689. doi: 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, I, Murray A, et al. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015b;43(10):1522–35. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeuken A, Keser BJ, et al. Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J Agric Food Chem. 2003;51(18):5478–87. doi: 10.1021/jf030252u. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wu N, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliard W, De Wolfe TJ, et al. Amelioration of Clostridium difficile Infection in Mice by Dietary Supplementation With Indole-3-carbinol. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–80. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, et al. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Nishiumi S, et al. Activation of the aryl hydrocarbon receptor induces hepatic steatosis via the upregulation of fatty acid transport. Arch Biochem Biophys. 2010;504(2):221–7. doi: 10.1016/j.abb.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Applied and Environmental Microbiology. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–9. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- Kushad MM, Brown AF, et al. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47(4):1541–8. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Grose KR, Riby J, Chen YH, Bjeldanes LF. In vivo production and enzyme-inducing activity of indolo[3,2-b]carbazole. Journal of Agricultural and Food Chemistry. 1994;42(11):2536–2540. [Google Scholar]
- Langille M, Zaneveld J, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature biotechnology. 2013;31:814–21. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latte KP, Appel KE, et al. Health benefits and possible risks of broccoli - an overview. Food Chem Toxicol. 2011;49(12):3287–309. doi: 10.1016/j.fct.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Innocentin S, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–40. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Lippmann D, Lehmann C, et al. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014;5(6):1073–81. doi: 10.1039/c3fo60676g. [DOI] [PubMed] [Google Scholar]
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor sans xenobiotics: endogenous function in genetic model systems. Mol Pharmacol. 2007;72(3):487–98. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- Murray IA, Nichols RG, et al. Expression of the aryl hydrocarbon receptor contributes to the establishment of intestinal microbial community structure in mice. Sci Rep. 2016;6:33969. doi: 10.1038/srep33969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Bausserman LL. Genetic differences in the extent of aryl hydrocarbon hydroxylase induction in mouse fetal cell cultures. J Biol Chem. 1970;245(23):6373–82. [PubMed] [Google Scholar]
- Paturi G, Mandimika T, et al. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a(−/−) mice, a model of inflammatory bowel diseases. Nutrition. 2012;28(3):324–30. doi: 10.1016/j.nut.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Peterson KM, Guo X, et al. The expression of REG 1A and REG 1B is increased during acute amebic colitis. Parasitol Int. 2012;60(3):296–300. doi: 10.1016/j.parint.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Poland AaGE. Genetic expression of aryl hydrocarbon hydroxylase by 2,3,7,8-tetrachlorodibenzo-p-dioxin: evidence for a receptor mutation in genetically non-responsive mice. Mol Pharm. 1975;11:389–398. [Google Scholar]
- Qiu J, Heller JJ, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Rouse M, Singh NP, et al. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol. 2013;169(6):1305–21. doi: 10.1111/bph.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27(2):109–34. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- Salunkhe DK, Kadam SS. Handbook of vegetable science and technology : production, composition, storage, and processing. New York: Marcel Dekker; 1998. [Google Scholar]
- Schaubeck M, Clavel T, et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2015;65(2):225–37. doi: 10.1136/gutjnl-2015-309333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Wincent E, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542(7640):242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66(11):5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoewsand GS. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables--a review. Food Chem Toxicol. 1995;33(6):537–43. doi: 10.1016/0278-6915(95)00017-v. [DOI] [PubMed] [Google Scholar]
- Stoner G, Casto B, et al. Development of a multi-organ rat model for evaluating chemopreventive agents: efficacy of indole-3-carbinol. Carcinogenesis. 2002;23(2):265–72. doi: 10.1093/carcin/23.2.265. [DOI] [PubMed] [Google Scholar]
- Takamura T, Harama D, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88(6):685–9. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, et al. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, et al. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89(6):2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Jin Z, et al. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS One. 2014;9(6):e90849. doi: 10.1371/journal.pone.0090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.