Abstract
Individuals who have experienced high levels of childhood stress are at increased risk for a wide range of behavioral problems that persist into adulthood, yet the neurobiological and molecular mechanisms underlying these associations remain poorly understood. Many of the difficulties observed in stress-exposed children involve problems with learning and inhibitory control. This experiment was designed to test individual’s ability to learn to inhibit responding during a laboratory task. To do so, we measured stress-exposure among a community sample of school-aged children, and then followed these children for a decade. Those from the highest and lowest quintiles of childhood stress exposure were invited to return to our laboratory as young adults. At that time, we reassessed their life stress exposure, acquired fMRI data during an inhibitory control task, and assayed these individuals’ levels of methylation in the FKBP5 gene. We found that individuals who experienced high levels of stress in childhood showed less differentiation in the dorso-lateral prefrontal cortex between error and correct trials during inhibition. This effect was associated only with childhood stress exposure and not by current levels of stress in adulthood. In addition, FKBP5 methylation mediated the association between early life stress and inhibition-related prefrontal activity. These findings are discussed in terms of using multiple levels of analyses to understand the ways in which adversity in early development may affect adult behavioral adaptation.
Keywords: Executive Function, Early Life Stress, Inhibitory Control, FKBP5 Methylation, Prefrontal Cortex
Individuals exposed to chronic high levels of early life stress are at risk for a broad range of behavioral problems that begin in childhood and persist into adulthood. For example, high levels of childhood adversity have been associated with increased risk for impulsivity and emotion regulation difficulties, alcohol and substance abuse, externalizing problems, as well as depression and anxiety disorders (Norman et al., 2012; Pechtel & Pizzagalli, 2011; Shonkoff & Garner, 2011). Although the associations between early adversity and these negative outcomes later in development are well established, there remain gaps in our understanding of the mechanisms through which early adversity leads to adult dysfunction. In this study, we examined the role of early adversity on the efficient recruitment of brain circuitry underlying executive function, or control of behavior.
There is strong evidence that early stress exposure hinders executive function in children and adolescents. For example, children who experienced maltreatment or were exposed to familial trauma have been observed to show more impulsivity, and poorer performance on tasks involving working memory, inhibitory control, attention, planning, and processing speed compared to typically-developing controls (dePrince, Weinzierl, & Combs, 2009; Pollak, 2015). During an incentive-based executive function task, children who had experienced maltreatment displayed excessive risk-taking, insensitivity to different outcomes, and slower decision-making relative to their nonmaltreated peers (Weller & Fisher, 2012). Furthermore, earlier onset and longer chronicity of maltreatment appears to be associated with more profound deficits in inhibitory control and working memory (Cowell, Cicchetti, Rogosch, & Toth, 2015). Taken together, these findings raise the possibility that executive function deficits, broadly construed, reflect vulnerability of relevant brain circuitry to stress exposure early in development.
Addressing this question has been especially complicated by the fact that it has been difficult to establish the relative impact of adversity early in development versus the cumulative affects of stress throughout development. One possibility is that individuals exposed to very high levels of stress early in their development tend to continue to experience more stressful events throughout their lives (Evans, Li, & Whipple, 2013), and this continued stress exposure results in continued problems with impulsivity and emotion regulation. Another possibility is that exposure to extreme stress during early childhood exerts profound effects on brain and behavioral function that continue to manifest themselves in adulthood. Therefore, we attempted to determine the relative role of adversity early versus later in development, as described below.
Executive function is a term used to refer to cognitive abilities subserved by a number of regions within the prefrontal cortex (PFC; Menon et al., 2001). These brain regions include dorsolateral (dlPFC), medial (mPFC), and anterior cingulate (ACC), and appear to be profoundly affected by severe stress exposure early in childhood (for review, see Bick & Nelson, 2016). For example, both physical abuse (Hanson et al., 2010) and severe neglect due to institutional care (Hodel et al., 2015) have been linked to smaller PFC volumes, and child maltreatment is associated with reduced cortical thickness (indicative of less gray matter) in ACC, orbitofrontal cortex, and superior frontal gyrus (Kelly et al., 2013). Child maltreatment is also associated with smaller volumes in hippocampus and amygdala (Hanson et al., 2015), regions that are crucial for memory and for signaling the salience of information in the environment. And, in fact, developmental effects associated with these regions include difficulties in associative learning (Hanson, 2017; Harms et al., in press).
High levels of childhood stress exposure have been particularly noted to affect inhibitory control tasks. These types of tasks require participants to inhibit prepotent responses to stimuli. As an example, in a Go/No-go task, an individual responds on most trials, building up a prepotent response, but on rare trials a specific stimulus signals that no response should be made. In a Go/Change task--similar to a Go/No-go task, but requiring a switch to an alternative response, rather than no response, on rare trials--adolescents who were experienced early caregiver deprivation showed longer reaction times on Change trials relative to controls, as well as greater activation in regions such as dorsal ACC, inferior frontal cortex, and striatum for correct Change versus Go trials (Mueller et al., 2010). Similarly, trauma-exposed youth showed less activation in left medial frontal cortex than controls during successful response inhibition (Carrion et al., 2008). Another study (Bruce et al., 2013) found evidence for compensatory brain activity among maltreated children in foster care. These data indicated that during successful response inhibition, the children who had experienced maltreatment had less activation in insula, frontal gyrus, and ACC than non-maltreated youth who were also in foster care. But the maltreated children also had more activation in parietal lobule (Bruce et al., 2013). This compensatory parietal activity may have allowed maltreated children to perform as well as controls on behavioral aspects of the task. Indeed, several studies have found differences in brain activity, but not error rate, during response inhibition in stress-exposed adolescents (Mueller et al., 2010; Jankowski et al., 2016). These functional brain abnormalities likely reflect subtle differences in cognitive functioning that, while not affecting laboratory task performance, may influence children’s ability to handle more complex situations, such as social interactions (Bruce et al., 2013).
In light of this evidence for compensatory brain activity in youth who experienced early stress, one particular aspect of executive function may be informative for understanding cognitive vulnerability. That is how individuals are able to process and differentiate correct versus error trials during situations that require inhibitory control. Stress-exposed youth may have particular difficulty with this type of processing compared to typically-developing youth. Examining event-related potentials during a Go/No-go task among children who had endured prolonged institutionalization or foster care, McDermott et al. (2012) found the children exposed to adversity had low processing of no-go cues relative to controls. In addition, institutionalized children, who experienced the most severe stress, showed less differential reactivity between correct and error responses, reflected in the error-related negativity (ERN), as compared to foster care children. Consistent with these findings, maltreated adolescents displayed less inferior, middle, and medial frontal fMRI activity during correct inhibition, but more subcortical activity during inhibition errors, relative to nonmaltreated youth (Jankowski et al., 2016). As in Bruce et al. (2013), there were no differences in accuracy and reaction time between groups. Yet diminished frontal activation during inhibitory control, and less differentiation between correct and error trials, suggests that the individuals who experienced early adversity are having difficulty processing errors and updating their responses. Such a deficit would undermine efficient learning.
How might the experience of adversity affect the development of these cognitive processes? One mechanism through which early stress exposure could exert persistent effects on brain activity and behavior is through changes in gene expression (Zannas, Wiechmann, Gassen, & Binder, 2016). DNA methylation is an epigenetic mechanism used by cells to regulate gene expression (i.e., switching genes between “on” or “off” positions). Childhood abuse has been associated with demethylation in the FK506 binding protein 5 (FKBP5) gene, an important regulator of the stress hormone system and glucocorticoid receptor sensitivity (Klengel et al., 2013). This demethylation appears to contribute to glucocorticoid resistance, higher cortisol levels, and prolonged recovery following exposure to stress (Zannas & Binder, 2013). Exposure to high levels of glucocorticoids (via cortisol) has been linked to disruptions in cognitive processing (Belanoff et al., 2001; McEwen & Sapolsky, 1995). And in nonhuman animals, chronically high glucocorticoid exposure leads to disrupted structural and functional development of the prefrontal cortex (Arnsten, 2009; Mizoguchi et al., 2003; Radley et al., 2004). Thus, these systems have become a central part of understanding the affects of averse caregiving on children’s development (Gunnar, 2016). For this reason, we examined methylation of the FKBP5 gene, a key regulator of glucocorticoid activity, as an additional biological measure of stress exposure (Binder et al., 2008; White et al., 2012).
The current study tested two predictions. Our first hypothesis was that individuals who experienced high levels of childhood adversity would have less prefrontal activation during differentiation of error versus correct trials. Lower levels of brain activation to error trials would suggest that these individuals were engaging in less processing of their errors and not updating their subsequent responses following errors. At the same time, greater activation to correct trials would suggest these individuals needed greater cortical recruitment to inhibit prepotent responses. In this manner, both effects would undermine the efficiency of participants’ learning. Next, we explored whether FKBP5 methylation would mediate associations between child adversity and brain activity during inhibitory control. Finally, we were interested in the effects of childhood versus adult life stress. Across the above analyses, we examined whether the effects of participants’ level of stress exposure in childhood were maintained when controlling for participants’ current level of stress in their adult lives.
Method
Participants
Individuals in the present experiment were recruited from a larger study of 161 people who had participated in a previous study when they were children (Hanson et al., 2015). Participants were assessed with the Youth Life Stress Interview (YLSI; Rudolph & Flynn, 2007), when they were 9–13 years old (mean = 11.2 years). We re-contacted participants from the highest and lowest quintiles of childhood stress scores a decade later, when participants were entering early adulthood. These individuals had either high YLSI scores (4.0 or above) or relatively low scores reflecting normative levels of childhood stress exposure (2.5 or below). Fifty-four individuals ranging in age from 19.0 years to 23.7 years (mean=20.5 years) agreed to participate in the current study. Within this group of 54 participants, 29 individuals (17 female) were assessed as having had high levels of stress during early childhood, and 25 individuals (11 female) were assessed as having relatively low levels of childhood stress. In addition to those who agreed to participate, 12 individuals we contacted declined participation: 9 were currently living out of state and could not travel back to the lab, 1 declined because she was pregnant and could not undergo fMRI scanning, 1 did not wish to undergo the neuroimaging component, and 1 individual was currently in prison. Of those who declined, 7 were from the low stress group and 5 were from the high stress group; these individuals did not differ on any childhood or demographic measures from those who did participate.
This project was approved by the University of Wisconsin Institutional Review Board, and all participants provided informed consent. A number of participants had to be excluded from some aspect of the data analyses: 2 participants agreed to participate but could not undergo MRI scanning because of claustrophobia; 8 participants were excluded from fMRI analyses because of excessive head motion, but were included in the behavioral analysis; and 1 participant was excluded due to significant mental health issues (suspected active psychosis). Ten additional participants were excluded from the analyses due to a programming error in which button-box presses were not recorded. This resulted in a final group of 33 participants for fMRI analysis and 39 for behavioral analysis (18 female, M age = 20.6 years). Among the group of 39, 23 participants identified themselves as white, 11 as African-American, 2 as Hispanic, and 3 as Asian. High- and Low-stress participants did not differ on tests of cognitive and motor functions (Intra-Extra Dimensional Shift and Motor Screening Test from the CANTAB; ps>.1).
Procedures
Participants had their life stress measured in our laboratory when they were children (mean age 11.2 years), and were re-contacted approximately 10 years later. They returned to our laboratory as young adults, and underwent an MRI scan during which they performed a Go/No-go task. Following the MRI scan, participants had their current life stress re-evaluated. We also obtained DNA samples at this time. These measures are described in greater detail below.
Measures
Childhood Stress Exposure
The Youth Life Stress Interview (YLSI) assesses the child’s exposure to severe negative life events and circumstances. Trained interviewers used semi-structured questions to assess the context of the event (e.g., timing, duration, objective consequences). Data from these interviews were then evaluated by an independent team of three to seven raters who provided a consensual rating on a 10-point scale reflecting an overall level of cumulative life stress. The following examples illustrate the kinds of experiences children in this study described that were associated with each score. A life stress score of 1 was given to a child whose pet was hit by a car, but the pet was not seriously injured. A score of 5 was given to a child who was placed in foster care early in life and then experienced multiple placements between families; during this time the child’s biological parent, with whom the child maintained a relationship, died. A score of 7.5 was given to a child whose parent and sibling both had serious, chronic medical and mental health problems; long-term instability in parental employment; severe inter-parental marital conflict resulting in parental separation; and extensive incarceration of one of the child’s parents. A score of 10 was given to a child who was homeless; had several close family members die unexpectedly; and had physically violent parents, resulting in separation of the child from the family. This rating system has high reliability and validity (Rudolph & Flynn, 2007).
Current Life Stress
The UCLA Life Stress Interview (UCLA LSI; Hammen et al., 1987) measures current life stress in adult participants. This interview queries ten domains including close friendships, social life, romantic relationship, family relationships, relationship with child/children, academic experiences, work, finances, health, and other (i.e., bereavement, moves, natural disasters, victimization, and legal issues). A trained interviewer conducted all interviews. The interviews were coded by a trained team of three researchers using a scale of 1–10, with 10 being extreme stress. High inter-rater reliability on scoring of domains and types of events, and good validity has been reported (Rudolph et al., 2000).
fMRI Task
The Go No-go task (Kaufman, Ross, Stein, & Garavan, 2003) consisted of 2 runs, each 270 seconds in duration (135 volumes). The letters X and Y were presented serially in an altering pattern, once per second. Subjects were instructed to press a button for every letter (“Go”), but withhold pressing the button (“NoGo”) when a letter repeats (e.g. the 5th letter in the string X-Y-X-Y-Y; see Figure 1). Each run had 25 NoGo events and 245 Go events, with an average duration of time between NoGo events (inter-stimulus interval, ISI) of 9.7 seconds in the first run, 9.8 seconds in the second run. Both runs had a minimum ISI of 4s, and a maximum ISI of 16s.
Figure 1.
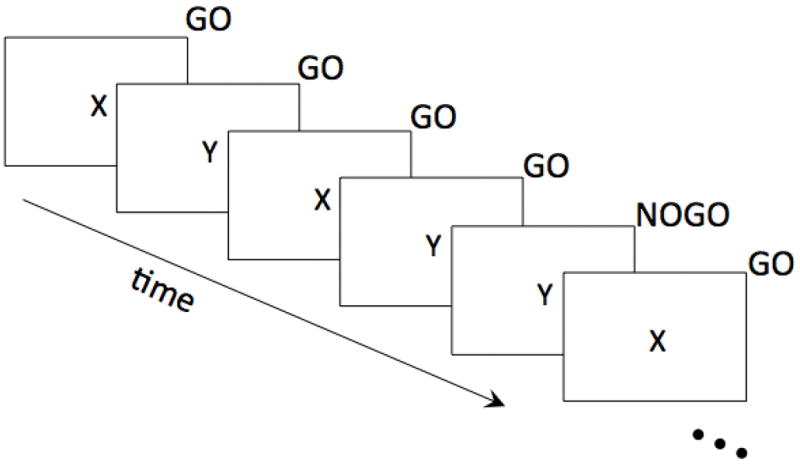
Schematic of the Go/NoGo task. In this example, participants were instructed to withhold a response when the same letter repeated twice.
MRI Data Acquisition
A series of structural and functional brain images were acquired on a 3T General Electric (GE) MR750 MRI scanner using an 8-channel receive-only RF head coil (General Electric Medical Systems, Waukesha, WI). Structural anatomical brain data was acquired using a T1-weighted BRAVO pulse sequence (TI:450ms, TR/TE/flip:8.16ms/3.18ms/12°, matrix:256×256×156, FOV:256mm, slice thick:1mm). Functional data was acquired using a series of sagittal T2*-weighted echo-planar images (2 runs, 135 image volumes per run, sagittal slices, resolution: 3.5mm × 3.5mm × 3.5mm, FOV: 22.4cm, TR: 2000ms, TE: 25ms, flip angle: 70 degrees).
fMRI Task Analyses
All MRI data analyses were performed using the Analysis of Functional NeuroImages (AFNI) analysis package (Cox, 1996), unless otherwise specified. Echo-planar MR images acquired during the task were first corrected for subject motion using a rigid body volumetric realignment (3dvolreg). The first 3 image volumes were discarded to allow magnetization to reach steady state. Data were then corrected for slice-timing differences (3dTshift), aligned to the T1-weighted anatomical image (align_epi_anat.py). The T1-weighted image was aligned to Talairach space using a 12-parameter affine transformation. This transformation was to the aligned echo-planar image (EPI) data as a single transformation from original to template space. The resultant fMRI data were then spatially smoothed by Gaussian kernel with a full-width at half maximum (FWHM) of 8mm (3dmerge), and converted to percent signal change.
Brain activation during the task was estimated using multiple linear regression (3dDeconvolve) with 2 regressors of interest, modeling the correct and incorrect NoGo responses. The 6 estimated motion realignment parameters, as well as constant and linear trend, were used as additional nuisance regressors. At the group level, differences in activation as a function of ELS were assessed on a voxel-wise level using a t-test (3dttest++) with the LSI-score as a covariate. More specifically, we ran 3 analyses, where we included either 1) the YLSI scores from the interview administered when the participants were children, 2) the LSI scores from the interview conducted in young adulthood on the same day as the scanning session, or 3) both early and current life stress scores. This latter analysis will show whether findings are specific to early life stress measures controlling for differences explained by current life stress. Voxel-wise t-tests were corrected for multiple comparisons by estimating the spatial autocorrelation function from the pre-processed fMRI data (3dFWHMx), and setting a minimum cluster size threshold based on a Monte Carlo simulation that incorporates this estimated autocorrelation function (3dClustSim; Cox et al., 2017).
FKBP5 DNA methylation analysis
DNA was extracted from saliva samples and methylation was analyzed for 26 sites located in regulatory regions of the FKBP5 gene using highly accurate methylation targeted bisulfite sequencing (HAM-TBS). 20 CpG sites are located in intronic glucocorticoid response elements (7 sites in intron 7, 9 sites in intron 5 and 4 sites in intron 2) and 6 sites in the promoter. Briefly, 500ng of DNA was used per sample and bisulfite treated using the EZ DNA Methylation Kit (Irvine, California). PCR of 7 amplicons was performed using Takara EpiTaq HS Polymerase (Saint-Germain-en-Laye, France) and following quantification with the Agilent 2200 TapeStation (Waldbronn, Germany) were pooled in equimolar quantities for each sample. Ampure XP beads (Krefeld, Germany) were used for a double size selection (200–500 bp) to remove primer dimers and high molecular DNA fragments. Libraries were generated using the Illumina TruSeq DNA PCR-Free HT Library Prep Kit (San Diego, California) according to the manufacturer’s instructions. Each library was quantified with the Qubit® 1.0 (Thermo Fisher scientific Inc., Schwerte, Germany), normalized to 4 nM and pooled. Paired-end sequencing was performed on an Illumina MiSeq Instrument (San Diego, California) with the MiSeq Reagent Kit v3 (2 × 300-cycles) with the addition of 30% of PhiX Library. The quality of the sequencing reads was checked with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and Illumina adapter sequences were removed using Cutadapt v.1.9.1. Bismark v.0.15.0 was used for the alignment to a restricted reference limited to our PCR targets. The methylation levels for all CpGs, CHGs and CHHs were quantified using the R package methylKit. All samples had sufficient bisulfite conversion rate (> 95%) and all CpG sites sequenced had coverage higher than 1000 reads.
Results
Hypothesis 1: Is early stress exposure related to neural activation during inhibitory learning
Activation of a cluster in dorsolateral prefrontal cortex (dlPFC) was associated with childhood stress exposure and the contrast of NoGo Error - NoGo Correct trials (p < 0.04, corrected; see Figure 2). This dlPFC cluster consisted of 221 voxels (2×2×2mm3) at an individual voxel-level significance of p<0.001, and a center of mass at MNI coordinate (28,29,52). As predicted, at this region of the dlPFC, individuals with normative levels of childhood stress exposure evinced higher levels of activation when they made an error (as compared to when they responded to a correct trial). In contrast, individuals with high childhood stress exposure responded similarly to error and correct trials. Although child and adult stress levels were positively correlated (r(31) = .37, p < .05), this relationship remained significant when we controlled for stress levels in adulthood (p < 0.04, corrected).
Figure 2.
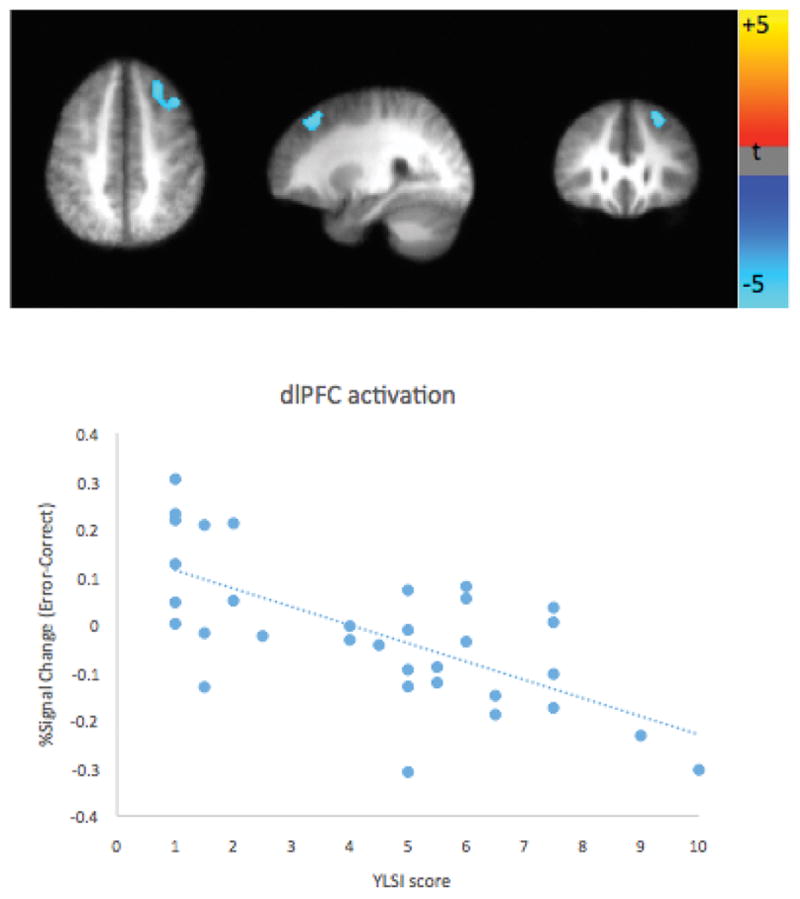
A region in dorsolateral prefrontal cortex shows significant correlation between early life stress (YLSI score) and the activation difference (Error-Correct NoGo trials) on the Go-NoGo task (p < .04, corrected).
No other brain regions were significantly associated with this interaction. No significant associations were found between childhood stress exposure and activation to only NoGo correct trials or only NoGo error trials. No significant associations were found between stress levels in adulthood and either the activation during the NoGo Error trials, NoGo Correct trials, or the difference in activation for NoGo Error – NoGo Correct trials (all p’s > 0.1).
Across all participants, the mean commission error rate was 6% (SD = .06), and the mean omission error rate was 11.3% (SD = .06). The mean reaction time to “Go” trials was 319.4 ms (SD = 65.07).
We also examined whether early adversity was correlated with reaction times for Go trials and accuracy (error rates). There was a positive correlation between early stress and reaction time, r(39) = .44, p = .005, indicating that participants exposed to early adversity responded more slowly than those who experienced low-stress childhoods. This correlation remained significant when controlling for stress levels in adulthood, r(36) = .44, p < .01. Error rates were not correlated with childhood or adult stress levels.
Hypothesis 2: Is FKBP5 methylation related to stress and brain activity during inhibition?
Among the 33 participants with good imaging data, we used Pearson correlations to determine methylation sites that were significantly correlated with measures of stress exposure in either childhood or adulthood. Correlation coefficients are shown in Table 1, mean methylation values in Table 2. The following methylation sites were significantly related to childhood stress, with higher stress corresponding to higher methylation (all p’s <0.05): intron 5_cg8, intron 5_cg2, intron 2_cg1, and intron 2_cg3. All of these effects correlated with childhood, but not adult, stress exposure. In contrast, intron 7 methylation significantly correlated with adult, but not childhood, stress exposure.
Table 1.
Correlation coefficients (Pearson R) for FKBP5 sites where methylation was significantly correlated with dlPFC activation, ELS, or adult stress.
| Int2 cg1 |
Int2 cg3 |
Int5 cg2 |
Int5 cg5 |
Int5 cg7 |
Int5 cg8 |
Int5 cg9 |
Int7 cg3 |
Int7 cg4 |
Int7 cg5 |
Int7 cg6 |
Int7 cg7 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Error-correct dlPFC activity | −.32 | −.33 | −.30 | −.36* | −.45* | −.49* | −.43* | .01 | −.16 | −.06 | .04 | −.17 |
| ELS | .36* | .37* | .40* | .30 | .34 | .37* | .35 | .10 | .28 | .27 | .15 | .23 |
| Adult Stress | .19 | .22 | .36* | .15 | .06 | .11 | .12 | .39* | .41* | −.42* | .40* | .37* |
Table 2.
Means and standard deviations of methylation levels for each site.
| Site | Mean | SD |
|---|---|---|
| Int 2 cg 1 | 68.77 | 10.30 |
| Int 2 cg 3 | 68.70 | 10.78 |
| Int 5 cg 2 | 87.78 | 3.96 |
| Int 5 cg 5 | 6.10 | 1.57 |
| Int 5 cg 7 | 4.28 | 1.01 |
| Int 5 cg 8 | 4.51 | 1.05 |
| Int 5 cg 9 | 4.33 | 0.98 |
| Int 7 cg 3 | 60.04 | 8.43 |
| Int 7 cg 4 | 85.54 | 5.98 |
| Int 7 cg 5 | 88.48 | 5.72 |
| Int 7 cg 6 | 66.97 | 7.17 |
| Int 7 cg 7 | 81.00 | 5.10 |
We next examined correlations between methylation and brain activity. Only one methylation site correlated with both child stress exposure and dlPFC differentiation between error versus correct responses: intron 5_cg8. This particular methylation also had the strongest correlation with error-related dlPFC activation (r(31) = −0.49, p = 0.005; Bonferroni-corrected p < .03). No methylation sites were correlated with adult stress exposure and error-related dlPFC activation.
Given the correlation of intron 5_cg8 with both childhood stress exposure and error-related dlPFC activity, we explored whether methylation at this site mediated this relationship. We used a standard multivariate analytic framework (MacKinnon et al., 2002) in R to test for statistical mediation, using a nonparametric bootstrap resampling with 5000 iterations. This analysis showed that the average causal mediated effect (ACME) of childhood stress exposure -> methylation -> dlPFC activation was marginally significant at p=0.08 (see Figure 3). To examine partial mediation, we also ran a model that included both childhood stress exposure and methylation to fit dlPFC activation. In this model, the pathway of methylation -> dlPFC activation nearly met our alpha threshold at p=0.056.
Figure 3.
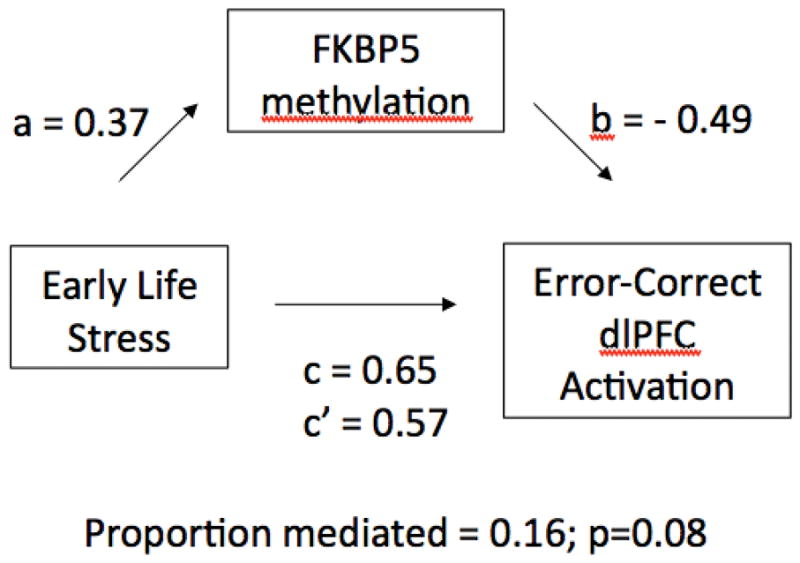
Statistical mediation model. We find a significant relationship between early life stress and Error – Correct dlPFC activation (β=0.65, p<.04; the c path). However, this relationship is reduced when FKBP5 methylation at intron 5, cg8 is added to the model (β=0.57, p=0.08; the c’ path).
Discussion
In an effort to begin to identify potential developmental mechanisms through which early adversity leads to behavioral problems, we examined the neural correlates of inhibitory control in adults who had experienced normative to very high levels of childhood stress. We found that stress exposure did not affect individual’s accuracy on an inhibitory control task. However, individuals who endured very high levels of child stress exposure had reduced prefrontal differentiation of error versus correct trials, and and had slower response times than those who did not have high stress childhoods. We observed the effects of childhood adversity during the inhibitory control task in the dlPFC brain region, a circuit known to be central for successful response inhibition (Simmonds, Pekar, & Mostofsky, 2007). The patterns of results reported here suggest less efficient recruitment of dlPFC among those individuals who had very stressful childhoods. Specifically, these individuals tended to require high levels of engagement to suppress prepotent responses. Because recruitment of the dlPFC during inhibitory control decreases with age (Bunge et al., 2002; Durston et al., 2002; Rubia, Smith, Taylor & Brammer, 2007), less dlPFC engagement should be needed as cognitive control ability increases. Thus, the individuals with high childhood stress exposure in this study may be construed as having less mature patterns of prefrontal activation during inhibitory control. Our exploratory analysis of FKBP5 methylation provides preliminary evidence that early adversity could result in changes in the epigenetic state of a gene that regulates the stress response system, which in turn contributes to altered prefrontal function.
Consistent with other reports in the literature (Bruce et al., 2013; Jankowski et al., 2016), we observed that exposure to childhood stress was associated with slower response times but not lower accuracy on the go-NoGo task. Paired with the neuroimaging data, this pattern of results is consistent with the view that those who had stressful childhoods were using compensatory cognitive processes in order to inhibit their prepotent responses. For example, slower reaction times on Go trials would allow more time to recruit inhibition-related circuitry on NoGo trials (Bogacz et al., 2010). Slowed responses have also been tied to stress exposure suggesting that stress may impair action production leading not only to slower responses, but inaction such as freezing during threat (de Berker et al., 2016).
The specific patterns of prefrontal activation observed here show both similarities and differences with previous studies examining inhibitory control in stress-exposed individuals. Our findings are similar to those of McDermott et al. (2012), which reported reduced differentiation of error versus correct NoGo trials in previously institutionalized children via the ERN, an ERP component that reflects frontal engagement and awareness of error (Nieuwenhuis et al., 2001). However, Bruce et al. (2013) and Jankowski et al. (2016) reported reduced frontal engagement among maltreated children and adolescents during correct inhibition trials, whereas we observed reduced frontal engagement during error versus correct trials, but not correct trials alone, in adults with high ELS. Several factors could account for this discrepancy. First, these prior studies examined adolescents, whereas our participants were adults; patterns of prefrontal recruitment and connectivity with other regions change dramatically between early adolescence and adulthood (Blakemore & Choudhury, 2006; Gee et al., 2013), and these changes may be moderated by stress (Rahdar & Galvan, 2014). Consistent with this interpretation, studies of adults with PTSD (Jovanovic et al., 2013; Stevens et al., 2016) also found reduced PFC engagement during correct inhibition trials.
We used a design that allowed us to prospectively follow individuals from childhood to young adulthood. This design allowed us to control for later life stress and avoid relying upon retrospective recall of childhood experiences from our participants. A prospective design has the advantage of not relying on accurate adult memories of childhood experiences, and may therefore yield more reliable associations between early stress and later outcomes than retrospective reports (Scott, Smith, & Ellis, 2010). Although, as expected, childhood stress and adulthood stress were positively correlated Evans et al., 2013), controlling for later stress did not change the associations between early stress and brain activity. The ability to distinguish here between early versus cumulative life stress helps to focus attention on the role of childhood adversity on the development of executive function systems, which are central to many aspects of adaptive behavior (Harms, Zayas, Meltzoff, & Carlson, 2014). Our results are consistent with previous literature implicating early childhood stress as especially detrimental to neurobehavioral development and life outcomes (for review, see Pechtel & Pizzagalli, 2011), and with animal research demonstrating that the consequences of stress exposure early in development are stronger and more persistent compared with stress exposure during adulthood (Hoffmann & Spengler, 2014; Russo et al. 2012).
Two other features of this study are worth noting. One is that the present sample has, by design, high variability in the degree of childhood stress individuals experienced. Our participants were drawn from a stratified community sample, rather than recruited to be a sample of severely maltreated individuals. There is evidence that higher severity of stress, such as that seen in chronic maltreatment, might result in greater abnormalities in prefrontal function than those we observed in this sample (Cisler et al., 2012). Relatedly, we measured childhood (and adulthood) stress continuously rather than binning participants into groups based on a specific type of life experience. Finally, though most previous fMRI work with Go/NoGo tasks focus on NoGo versus Go activation, we focused on the response to error versus correct NoGo trials to more specifically isolate the activation associated with making an error. Activation to correct (or incorrect) NoGo versus Go trials likely reflects several other cognitive processes such as attention, recognizing the NoGo, reaction to having recognized the NoGo event, and making a motor response (Criaud & Boulinguez, 2013). In sum, although there are some major differences among studies of stress and prefrontal function, there is consistency in implicating circuitry involving the prefrontal cortex as highly sensitive to the effects of early adversity (Hanson et al., 2010; Hodel et al., 2015), and the present data indicate that these effects of childhood adversity likely persist into adulthood.
A limitation of this study is that we did not measure inhibitory control and its neural correlates at the first time point in this study. It would be useful to know the extent to which childhood stress influenced the development of these processes in childhood, and to be able to measure changes in executive function over time as a function of stress exposure. In addition, our sample size was not large enough to systematically examine different polymorphisms of the FKBP5 gene, which have been linked to mental health problems and neural responses to threat through gene × environment interactions (Binder et al., 2008; White et al., 2012). Little is known about the mechanisms that link early stress, gene expression, glucocorticoid activity, brain development, and cognitive processes. Because most of our current understanding is derived from animal models (Arnsten, 2009), advances in these links between levels of analysis hold enormous promise.
This project used children’s reports of their experiences of childhood stress to examine aspects of their cognitive functioning in adulthood. To do so, we sampled across levels of analysis and methods, using subjective experience, behavioral measures of cognition, functional neuroimaging, and epigenetic analyses. We found that high levels of childhood adversity were associated with inefficiencies engaging inhibitory control in adulthood. This effect appears to be driven by early life experience rather than current levels of stress in adulthood. In addition, a promising hypothesis for future study to emerge from this project is that FKBP5, a gene already linked to stress and trauma, may account for the link between childhood stress and later cognitive effects, through the gene’s regulation of the stress-response system.
Acknowledgments
This work was supported by the National Institute of Mental Health (MH61285 to SDP) and a core grant to the Waisman Center Intellectual & Developmental Disabilities Research Center from the National Institute of Child Health and Human Development (P30-HD03352). M. Harms was supported by T32-MH018931. We acknowledge the assistance of M. Daniela Cornejo, Joanna Swinarska, Alex Rokni, and Anna Bechner, and also appreciate the generous participation of the individuals who agreed to partake in this study.
References
- Arnsten FT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanoff JK, Gross K, Yager A, Schatzberg AF. Corticosteroids and cognition. Journal of Psychiatric Research. 2001;35:127–145. doi: 10.1016/s0022-3956(01)00018-8. [DOI] [PubMed] [Google Scholar]
- Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology Reviews. 2016;41:177–196. doi: 10.1038/npp.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. JCAP. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Wagenmakers EJ, Forstmann BU, Niuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends in Neurosciences. 2009;33:10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Graham AM, Moore WE, Peake SJ, Mannering AM. Patterns of brain activation in foster childern and nonmaltreated children during an inhibitory control task. Development and Psychopathology. 2013;25:931–941. doi: 10.1017/S095457941300028X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garret A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety. 2008;25:514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Cisler JM, James GA, Tripathi S, Mletzko T. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptibleto the depressogenic effects of early life stress. Psychological Medicine. 2012;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, Toth SL. Malreatment and its effect on neurocognitive functioning: timing and chronicity matter. Development and Psychopathology. 2015;27:521–533. doi: 10.1017/S0954579415000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. fMRI clustering and false-positive rates. Proceedings of the National Academy of Sciences. 2017;114(17):E3370–E3371. doi: 10.1073/pnas.1614961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P. Have we been asking the right questions when assessingresponse inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience and Biobehavioral Reviews. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- De Berker AO, Tirole M, Rutledge RB, Cross GF, Dolan RJ, Bestmann S. Acute stress selectively impairs learning to act. Scientific Reports. 2016;6:29816. doi: 10.1038/srep29816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dePrince AP, Weinzierl KM, Combs MD. Executive function performance and trauma exposure in a community sample of children. Child Abuse and Neglect. 2009;33(6):353–61. doi: 10.1016/j.chiabu.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, et al. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–16. [Google Scholar]
- Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychological Bulletin. 2013;139:1342–96. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences U S A. 2013;110:15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Early Life Stress: What Is the Human Chapter of the Mammalian Story? Child Development Perspectives. 2016;10(3):178–183. [Google Scholar]
- Hammen C, et al. Children of depressed mothers: maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson JRT, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KL, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Bos W, Roeber BJ, Rudolph KD, Davidson RJ, Pollak SD. Early adversity and learning: implications for typical and atypical behavioral development. Journal of Child Psychology and Psychiatry. 2017 doi: 10.1111/jcpp.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Shannon-Bowen K, Hanson JL, Pollak SD. Instrumental Learning and Cognitive Flexibility Processes are Impaired in Children Exposed to Early Life Stress. Developmental Science. doi: 10.1111/desc.12596. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Zayas V, Meltzoff AN, Carlson SM. Stability of executive functionand predictions to adaptive behavior from middle childhood to pre-adolescence. Frontiers in Psychology. 2014;5:331. doi: 10.3389/fpsyg.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, van den Heuvel SE, Gunnar ME, Thomas KM. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Spengler D. DNA memories of early social life. Journal of Neuroscience. 2014;264:64–75. doi: 10.1016/j.neuroscience.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Jankowski KF, Bruce J, Beauchamp KG, Roos LE, Moore WE, Fisher PA. Preliminary evidence of the impact of early maltreatment and a preventive intervention on neural patterns of response inhibition in early adolescence. Developmental Science. 2016 doi: 10.1111/desc.12413.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, et al. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49(7):1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biological Psychiatry. 2013;74(11):845–852. doi: 10.1016/j.biopsych.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, … Binder EB, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets VA. Comparison of methods to test mediation and other intervening variable effects. Psychological methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, Fox NA. Early adversity and neural correlates of executive function: implications for academic adjustment. Developmental Cognitive Neuroscience. 2012;2:S59–66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5:205–16. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD. Multilevel developmental approaches to understanding the effects of child maltreatment: Recent advances and future challenges. Development and psychopathology. 2015;27(4pt2):1387–1397. doi: 10.1017/S0954579415000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Journal of Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebella networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: influence of gender and pubertal status. Development and Psychopathology. 2007;19:497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, et al. Toward an interpersonal life-stress model of depression: the developmental context of stress generation. Development and Psychopathology. 2000;12:215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nature Neuroscience. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Smith DR, Ellis PM. Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Archives of General Psychiatry. 2010;67:712–719. doi: 10.1001/archgenpsychiatry.2010.71. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2011;129:e232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasksdemonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JA, Fisher PA. Decision-making deficits among maltreated children. Child Maltreatment. 2012;18(3):184–194. doi: 10.1177/1077559512467846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes, Brain, & Behavior. 2012;11 doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms, and pleiotropism. Genes, Brain, & Behavior. 2013;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-stress-epigenetic regulation of FKBP5: Clinical and translational implications. Neuropsychopharmacology Reviews. 2016;41:261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]


