Abstract
Introduction
Precision medicine is only possible in oncology practice if targetable genes in fragmented DNA, such as DNA from formalin-fixed paraffin-embedded (FFPE) samples, can be sequenced using next generation sequencing (NGS). The aim of this study is to examine the quality and quantity of DNA from FFPE cancerous tissue samples from surgically resected and biopsy specimens.
Methods
DNA was extracted from unstained FFPE tissue sections prepared from surgically resected specimens of breast, colorectal and gastric cancer, and biopsy specimens of breast cancer. A total quantity of DNA ≥60 ng from a sample was considered adequate for NGS. The DNA quality was assessed by Q-ratios, with a Q-ratio >0.1 considered sufficient for NGS.
Results
The Q-ratio for DNA from FFPE tissue processed with neutral-buffered formalin was significantly better than that processed with unbuffered formalin. All Q-ratios for DNA from breast, colorectal and gastric cancer samples indicated DNA levels sufficient for NGS. DNA extracted from gastric cancer FFPE samples prepared within the last seven years is suitable for NGS analysis, whereas those older than seven years may not be suitable. Our data suggested that adequate amounts of DNA can be extracted from FFPE samples, not only of surgically resected tissue but also of biopsy specimens.
Conclusion
The type of formalin used for fixation and the time since FFPE sample preparation affect DNA quality. Sufficient amounts of DNA can be extracted from FFPE samples of both surgically resected and biopsy tissue, thus expanding the potential diagnostic uses of NGS in a clinical setting.
Keywords: Next generation sequencing, FFPE, DNA, surgically resected specimen, biopsy, precision medicine
INTRODUCTION
Over the past ten years, the Human Genome Project, followed by The Cancer Genome Atlas project, have revealed that cancer is a disease of the genome,1–8 and that genomic analysis may be used to identify mutations in tumor tissue that indicate appropriate treatments tailored for an individual patient.9, 10 In 2015, President Obama announced the Precision Medicine Initiative, in which individual variability and heterogeneity are considered in the prevention and treatment of cancer.11, 12 Precision medicine utilizing next generation sequencing (NGS) is expected to bring a paradigm shift from a pathological microscopic-based approach to a genetic signature-based diagnosis.13 The essence of precision medicine is to minimize the toxicity of cancer treatment while maximizing benefit by subgrouping patients according to genomic alterations that can be treated with specific molecular-targeted therapy.14 Given its concept, precision medicine is expected to provide cost-effectiveness by improving treatment efficacy while avoiding ineffective treatments.15
Although there are great expectations for the treatment of cancer patients with precision medicine, it is only possible in oncology practice if commonly available clinical samples can be analyzed by deep sequencing with NGS.16 It is only possible to apply NGS analysis for precision medicine if tumor DNA within stored samples is maintained in conditions that are appropriate for meaningful NGS analysis, and if only minimal artifacts are produced. In reality, however, the vast majority of clinical samples are stored as formalin-fixed, paraffin-embedded (FFPE) tissue in which DNA necessary for NGS is often fragmented.17 Recently, it has been reported that the quality of FFPE samples varies depending on how surgical specimens have been prepared and preserved.18 Therefore, it is extremely important that the next generation of surgeons who will be harvesting samples for NGS know how to appropriately handle samples for FFPE processing. Furthermore, it is important to clarify how much DNA can be harvested from a minimally sized sample, because often only a small amount of tissue is available in clinical settings such as from a biopsy or after neoadjuvant therapy.
The aim of this study is to examine the quality and quantity of FFPE cancer tissue samples obtained from surgically resected or biopsy specimens for deep sequencing with NGS. In this study, we validated the quality and quantity of DNA extracted from FFPE samples of breast, gastric and colorectal cancer.
MATERIALS AND METHODS
Patients
FFPE tissue blocks were processed using surgically resected specimens obtained from patients with breast cancer (BC) (n = 35), colorectal cancer (CRC) (n = 134), or gastric cancer (GC) (n = 138), after operations at Niigata University Medical and Dental Hospital or Niigata Cancer Center Hospital from 2009 through to 2016. The choice of the specific organ tissues was made based upon the prevalence in Japan. Indeed, top three most prevalent cancers in Japan are gastric, colon, and lung cancer in male, and breast, colon, and gastric cancer in female. FFPE tissue blocks were also prepared using biopsy specimens obtained from patients with BC (n = 20) at the same institutions from 2015 through to 2016. This study protocol was approved by the Institutional Review Board of Niigata University Medical and Dental Hospital. Informed consent was obtained from all patients.
FFPE processing
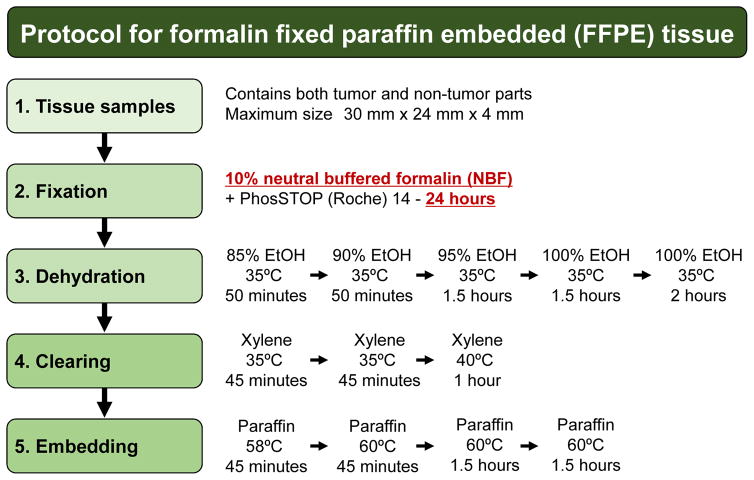
Surgically resected and biopsy specimens were fixed in neutral-buffered formalin (NBF) or unbuffered formalin (only for the experiments shown in Figure 2), and FFPE sample blocks were prepared by fixation, dehydration, clearing, and embedding steps, according to the standard protocol summarized in Figure 1. Briefly, tissue samples were fixed with NBF containing PhosSTOP (Roche) to preserve phosphorylated proteins. The fixation time was kept strictly to within 24 hours to avoid over-fixation which can result in extensive crosslinking. Five dehydration steps were performed in gradually increasing concentrations of ethanol, followed by three steps of clearing with xylene and four steps of embedding in paraffin wax.
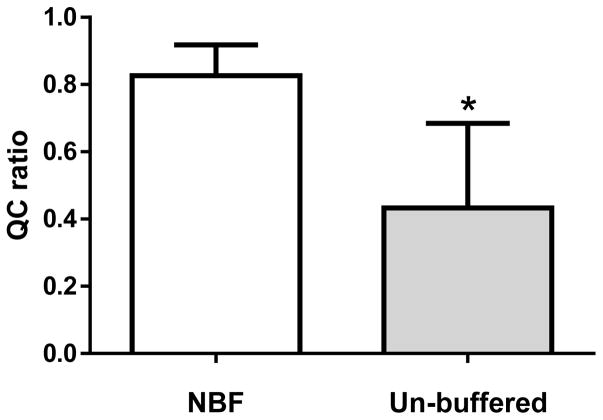
Figure 2. The Q-ratio for DNA extracted from formalin-fixed paraffin-embedded (FFPE) breast cancer tissues prepared with neutral-buffered formalin (NBF) or unbuffered formalin (Un-buffed).
The Q-ratio for DNA extracted from FFPE tissues prepared with NBF (left) and unbuffered formalin (right) were determined. Mean ± SD. *, P < 0.05.
Figure 1. Protocol for formalin-fixed paraffin-embedded (FFPE) tissue.
Tissue samples were obtained from surgical or biopsy specimens, which contained tumor and non-tumor tissue. Tissue processing for FFPE block preparation included fixation, dehydration, clearing, and embedding. The validated condition for each process is shown.
DNA extraction
Serial sections were prepared from each FFPE block: six unstained tissue sections (20 μm) for DNA extraction and one hematoxylin and eosin (H&E)-stained slide (4 μm) for use as a reference. Cancer cell-enriched areas were circled on the H&E-stained slides. Using the H&E-stained slides as a guide, the cancer cell-enriched area in the unstained tissue sections was scraped using the sharp edge of a sterilized razor. The tissue slices were collected into 1.5 ml DNA LoBind tube (Eppendorf). DNA was extracted using a BiOstic FFPE Tissue DNA Isolation Kit (MO Bio Laboratories).
DNA quantification
2–6 unstained sections per case were used for DNA extraction and the DNA quantity per section was calculated. The extracted DNA from FFPE samples was quantified using a Quant-iT™ dsDNA Assay Kit, High Sensitivity (Thermo Fisher Scientific), and Qubit 3.0 Fluorometer (Thermo Fisher Scientific). A total amount of DNA ≥ 60 ng (1.5 ng/μl) was considered sufficient for NGS analysis.
DNA quality check
The quality of an adequate quantity of DNA was assessed by determining the Q-ratio, in which 41 bp and 129 bp targets were amplified by qPCR and compared using a KAPA Human Genomic DNA Quantification and QC Kit (KAPA Biosystems). The 41 bp assay was used for absolute quantification of DNA samples. For an assessment of DNA quality, standard curves were generated and samples assayed with the 129 bp primer premix. Since poor DNA quality has a greater impact on the amplification of longer targets, the relative quality of a DNA sample can be inferred by normalizing the concentration obtained using the 129 bp assay against the concentration obtained from the 41 bp assay. This normalization generates a “Q-ratio” (with a value between 0 and 1) that is used to assess the fragmentation of DNA by comparing the amount of the long (129 bp) and short (41 bp) DNA fragments. The high Q-ratio represents less DNA fragmentation, while the low Q-ratio represents more DNA fragmentation. Therefore, the Q-ratio can be considered as a relative measure of DNA quality. High-quality human genomic DNA will have a Q129 bp/Q41 bp ratio of around 1. Damaged DNA will have Q129 bp/Q41 bp ratio < 1. In our study, DNA with a Q-ratio (129 bp/41 bp) > 0.1 was designated as being of high enough quality for NGS analysis based on our previous results.14, 19
Statistical analysis
Categorical variables were compared by Fisher’s exact test or the Pearson χ2 test, whereas continuous variables were compared by the Mann-Whitney U-test between two groups, and by the Kruskal-Wallis test among three groups. All statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc.). All tests were two-tailed and P < 0.05 was considered significant.
RESULTS
The quality of DNA is adequately preserved in FFPE tissues processed using NBF, but not unbuffered formalin
The Q-ratio for DNA extracted from FFPE tissues processed with NBF was significantly better than DNA extracted from FFPE tissues processed with unbuffered formalin (0.83 ± 0.09 vs. 0.43 ± 0.25; mean ± SD) (P < 0.01; Figure 2).
The quality of DNA is adequately preserved in different types of cancer tissue processed using NBF
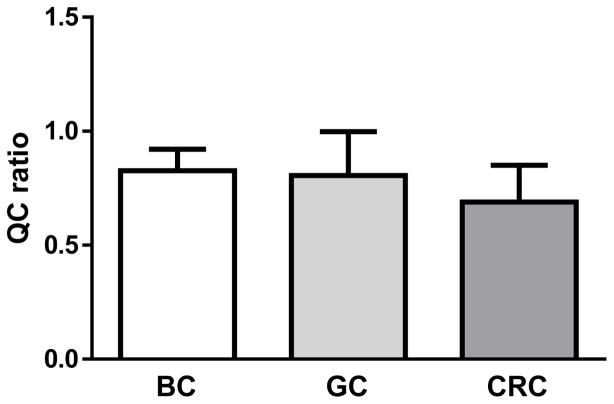
The Q-ratios for BC, GC, and CRC were more than sufficient for NGS analysis (0.83 ± 0.09 for BC, 0.81 ± 0.19 for GC, 0.69 ± 0.16; mean ± SD), although One-way ANOVA and Turkey Multiple Comparison test revealed that there was a slight, but significant difference between the Q-ratios for GC and CRC (P < 0.05; Figure 3).
Figure 3. Calculation of the Q-ratio for DNA extracted from formalin-fixed paraffin-embedded (FFPE) tissues from different organs.
The Q-ratio for DNA extracted from FFPE samples from breast cancer (BC), gastric cancer (GC) and colorectal cancer (CRC) tissues were determined. Mean ± SD. One-way ANOVA and Tukey Multiple Comparison test revealed that there was a significant difference between GC and CRC (P < 0.05).
The quality of DNA extracted from FFPE samples degrades over time
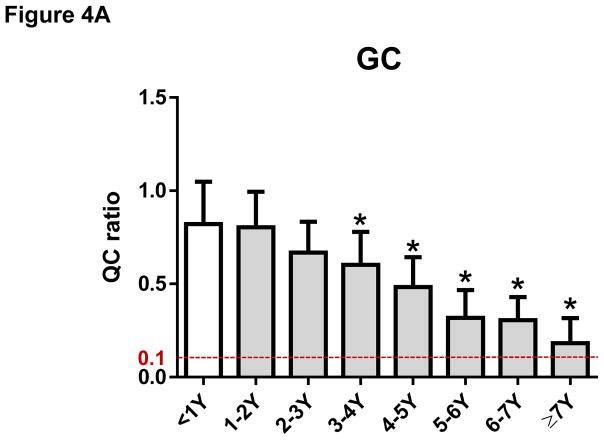
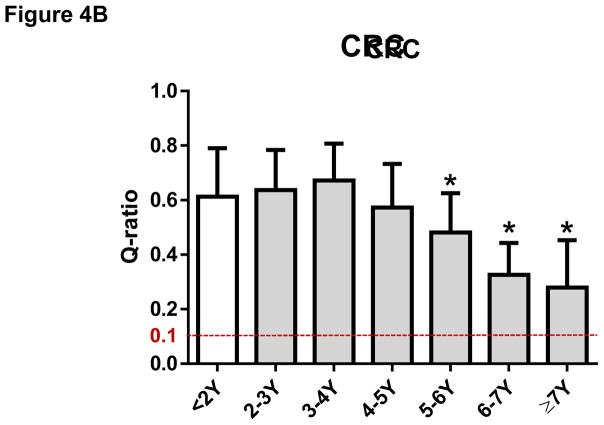
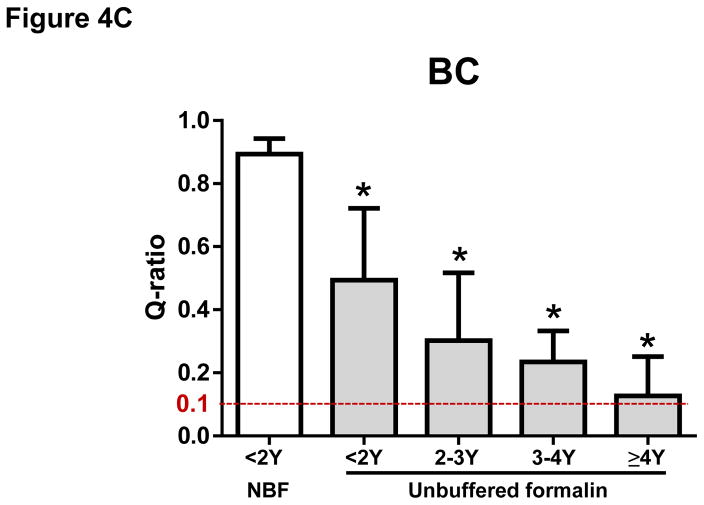
The mean ± SD of Q-ratios for GC samples were 0.82 ± 0.22 for <1 year, 0.80 ± 0.19 for 1–2 years, 0.66 ± 0.17 for 2–3 years, 0.60 ± 0.18 for 3–4 years, 0.48 ± 0.16 for 4–5 years, 0.32 ± 0.15 for 5–6 years, 0.31 ± 0.12 for 6–7 years, respectively. Our data indicate that DNA extracted from FFPE samples of GC prepared within the last seven years is suitable for NGS analysis whereas DNA from FFPE samples of GC older than seven years (0.18 ± 0.14) may not be suitable for NGS analysis (Figure 4A). The Q-ratios of GC samples for 3–4 years or older were significantly worse than those within one year (P < 0.05, respectively) (Figure 4A). Similar degradation over time was observed in FFPE samples of CRC. The mean ± SD of Q-ratios for CRC samples were 0.61 ± 0.18 for <2 years, 0.64 ± 0.15 for 2–3 years, 0.67 ± 0.13 for 3–4 years, 0.57 ± 0.16 for 4–5 years, 0.48 ± 0.14 for 5–6 years, 0.33 ± 0.13 for 6–7 years, 0.28 ± 0.17 for = 7 years, respectively (Figure 4B). The Q-ratios of CRC samples for 5–6 years or older were significantly worse than those within 2 years (P < 0.05, respectively) (Figure 4B). Moreover, Q-ratios of BC samples prepared with unbuffered formalin not only were worse than those with NBF, but also have degraded even earlier (the mean ± SD of Q-ratios were 0.89 ± 0.05 for < 2 years with NBF, 0.49 ± 0.23 for < 2 years with unbuffered formalin, 0.30 ± 0.22 for 2–3 years with unbuffered formalin, 0.23 ± 0.10 for 3–4 years with unbuffered formalin, 0.13 ± 0.12 for ≥ 4 years with unbuffered formalin) (Figure 4C). The Q-ratios of all BC samples with unbuffered formalin were significantly worse than Q-ratio of BC samples with NBF (P < 0.05; Figure 4C).
Figure 4. The Q-ratio for DNA from formalin-fixed paraffin-embedded (FFPE) tissue degraded with age.
(A) The Q-ratios for DNA from FFPE gastric cancer (GC) samples correlated with the age of the samples. Mean ± SD. *, P < 0.05 compared with <1Y. (B) The Q-ratios for DNA from FFPE colorectal cancer (CRC) samples correlated with the age of the samples. Mean ± SD. *, P < 0.05 compared with <2Y. (C) The Q-ratios for DNA from FFPE breast cancer (BC) samples correlated with the age of the samples. Mean ± SD. *, P < 0.05 compared with <2Y fixed with neutral buffered formalin (NBF).
Adequate amounts of DNA can be extracted from FFPE samples, not only from surgically resected specimens but also from biopsy specimens
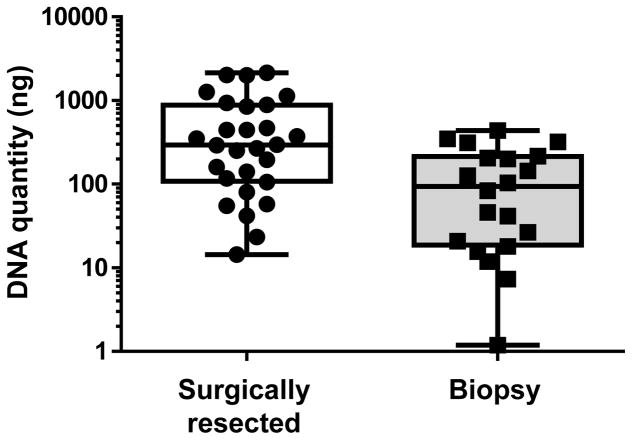
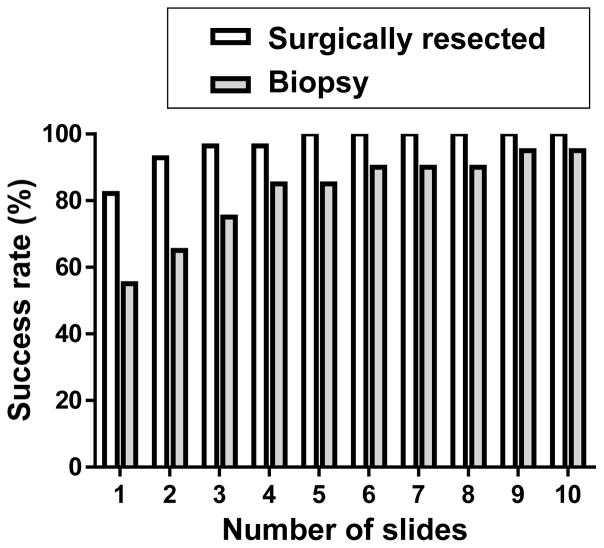
The median (range) of the DNA quantity from surgically resected BC samples was 294 (14-2141) ng, and that of from biopsy samples was 93 (1-439) ng (Figure 5). The success of obtaining adequate amounts of DNA (>60 ng) was determined as the number of unstained slides (20 μm), from either surgically resected or biopsy specimens from BC, required to yield sufficient amounts of DNA for analysis. For the resected specimens, only two unstained slides were necessary to obtain enough DNA for more than 90% of patients. For the biopsy specimens, four unstained slides were necessary to obtain enough DNA for more than 80% of patients, while the amount of DNA was insufficient from as many as ten unstained slides in 5% of patients (Figure 6).
Figure 5. Quantitation of DNA extracted from unstained slides of surgically resected and biopsy specimens for next generation sequencing.
DNA extracted from sectioned surgically resected (N = 28) and biopsy specimens (N = 20) of breast cancer tissue was quantitated. Each slide had sections 20 μm in thickness.
Figure 6. Estimation of the number of unstained slides of surgical and biopsy specimens required for extraction of DNA sufficient for next generation sequencing.
The estimated success rate is indicated for obtaining adequate amounts of DNA (>60 ng) from the required minimum number of unstained slides (each with tissue sections 20 μm in thickness) of breast cancer surgical and biopsy specimens.
DISCUSSION
Advances in NGS have enabled genome sequence data to be utilized in clinical practice,20 where the exact targetable cancer-causing mutations can be identified in each cancer patient. Given the ever-expanding expectation of cancer patients for precision medicine, we believe it is essential for the next generation of surgeons to know how to handle surgical samples in appropriate conditions for future NGS analysis. Further, we foresee that genomic data by NGS will be used not only in research but also in clinics in the near future. In the time when genomic analyses become part of a standard of care, surgeons who are not familiar with our results can be easily misled. For example, let’s assume there is a cancer patient who was diagnosed to have a recurrent tumor with core needle biopsy. We demonstrated that our technique allows extraction of DNA for NGS not only from surgical specimens, but also from core needle biopsy with very limited amount of tissue. When surgeons decide to analyze the sample with NGS, with our results, they know that they do not have to obtain further surgically resected specimen. Moreover, in this study, our data indicate that both the type of formalin used in processing and storage time affect DNA quality in FFPE samples, thus indicating the importance of sample preparation and preservation.
In the current study, we showed that DNA quality is adequately preserved in FFPE tissues processed with NBF, but not with unbuffered formalin, which agrees with a previous report.21 It has been suggested that FFPE tissues may sustain DNA damage such as (1) formaldehyde-induced crosslinks; (2) DNA fragmentation; (3) deamination of cytosine bases leading to C->T mutations; and (4) the generation of basic sites.22 The fixation time with formalin is also important for tissue preservation. Over-fixation has been reported to cause excessive crosslinking, which interferes with the extraction of nucleic acids and proteins for NGS analysis.18 On the other hand, under-fixation can lead to the degradation of nucleic acid and protein, or a change in gene expression in poorly perfused areas where the formalin has not reached.18 Furthermore, fixation with formalin needs to be performed immediately after removal of the specimen to avoid ischemic changes in the tissue.18 Here, we have shown that high-quality DNA can be obtained as long as an appropriate preservation procedure is performed following a standard protocol with NBF.
In the current study, we utilized manual microdissection without using laser-capture, for unstained sections. We avoided laser-capture microdissection because it causes thermal degradation to the surrounding area dissected by the laser.23 We extracted DNA from FFPE tissue samples, rather than frozen tissue samples. Although we could get better quality DNA from frozen tissue samples, they degraded sooner than FFPE tissue samples. In contrast, FFPE samples can be stored for a long time at room temperature, which is convenient and cost-effective. Furthermore, FFPE samples are routinely stored in many institutions, and large cohorts are readily available for retrospective analyses.24 It is also important for NGS analysis to confirm how much cancer tissue is included in the dissected tissue by morphological analyses using H&E staining of an adjacent section as a reference, which can be easily prepared from FFPE tissue samples, but not from frozen tissue sections.
FFPE tissue samples are considered as stable as frozen samples kept at very low temperatures, as described above. However, FFPE tissue samples can also degrade over time. Indeed, it has been reported that DNA, microRNAs and proteins in FFPE tissues are significantly reduced over time, especially when stored at room temperature on glass slides.25, 26 Our data indicated that GC samples older than seven years may not provide accurate data by NGS due to DNA degradation over time. CRC samples showed similar trends, while BC samples with bad preparation showed even earlier degradation. Therefore, clinicians should consider NGS analysis sooner rather than later for cancer patients who are at a high risk of recurrence.
There are infinite possibilities when it comes to utilizing NGS data in cancer care and research. In addition to detecting alterations in the genome, NGS provides gene expression data such as RNA-Seq, or epigenetic control data such as methylation status.27 Furthermore, it can also provide non-coding RNA data, such as information about microRNAs and long non-coding RNAs.27 Due to its comprehensive nature, large collections of NGS data such as at The Cancer Genome Atlas can be used as a representative database to validate whether previously reported laboratory research findings translate to actual patient survival outcomes.28 Moreover, the collection of a large number of samples enables analysis of tumor genome signature differences between various ethnicities.14 However, the NGS approach has limitations. First of all, one of the major limitations of NGS is the cost.29 At this time, whole-genome sequencing costs several to ten thousand US dollars per sample, depending on the vendor. When we begin to analyze every sample, the cost will be astronomical. The second limitation is the treatment.30 NGS can detect genome alterations; however, whether there is a targeted therapy for that alteration is a different story. Even when there is a clinical trial targeting a specific alteration, the patient may not have access to the trial, or the drug may not be approved in that country. The third limitation is the computational power required.31, 32 Future whole-genome sequences will need to be analyzed when NGS analysis is expanded into non-coding regions of the genome, and this will require “super computers”. Currently, these are the barriers, but it is also expected that these issues may be resolved with the advancement and widespread use of the technology, just like personal computers have become cheaper and faster.
To expand on the possibility of molecular diagnosis for cancer patients, NGS analysis using biopsy samples is undoubtedly critical. In the present study, we revealed that adequate quantities of DNA could be extracted from FFPE samples prepared not only from surgically resected specimens but also biopsy specimens. Thanks to technological advancement, the sample quality and quantity required for NGS analysis have recently shown rapid improvement. It is expected that NGS analysis of biopsy samples will be useful for patients receiving neoadjuvant chemotherapy, whose cancer tissue is dramatically altered or diminished, or patients with nonresectable advanced diseases.33 Repeated biopsies for NGS analysis from patients with tumor recurrence and drug resistance are expected to provide clues for overcoming resistant mechanisms. Further investigation will be needed to develop clinical sequencing using not only surgical specimens but also biopsy samples.
The main limitations of the current study are the nature of retrospective analysis, and the fact that this is not the first manuscript to describe the DNA extraction from FFPE slides. However, the following novelty of our study clearly distinguishes it from the others; 1) we demonstrated that our technique allows extraction of DNA for NGS not only from surgical specimens, but also from core needle biopsy with very limited amount of tissue, 2) we demonstrated the degradation of FFPE samples with largest number than any other studies. In addition, we believe that it is important for us surgeons to know how to preserve good quality of FFPE samples for reliable next generation sequencing because we are the ones to harvest the samples.
CONCLUSIONS
We examined the quality and quantity of DNA, for deep sequencing with NGS, from FFPE cancer tissue samples prepared from surgically resected and biopsy specimens. Our data indicate that DNA quality is affected by the type of formalin used for tissue fixation and the time since preservation. We also revealed that sufficient amounts of DNA can be extracted from FFPE samples of both surgically resected and biopsy specimens, thus expanding the potential diagnostic uses of NGS in a clinical setting. It is important for surgeons to know how to handle surgical specimens for meaningful NGS analysis.
Acknowledgments
We thank our colleagues Masato Nakajima, Mayuko Ikarashi, Chie Toshikawa, Junko Tsuchida, Kazuki Moro, Kizuki Yuza, Jun Sakata, Hitoshi Kameyama, Takashi Kobayashi, Chizuko Kanbayashi, and Hiroshi Izutsu. This project was supported by funding from Denka Co., Ltd. The authors would like to acknowledge funding from the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research, Grant Numbers 15H05676 and 15K15471 for M.N., and 15H04927 and 16K15610 for T.W. M.N. is also supported by Takeda Science Foundation. K.T. is supported by NIH/NCI grant R01CA160688 and Susan G. Komen Investigator Initiated Research Grant IIR12222224.
Footnotes
Author contributions: M.N. conceptualized the study, performed experiments, analyzed data and wrote the manuscript. Y.S., H.I., S.N., N.S., K.Kaneko, K.H., T.K., K.Kodama collected samples and performed experiments. K.T. and S.L. revised the article. T.W. provided supervision of experiments.
The author disclosure statement: K.Kodama is an employee of Denka Co., Ltd. S.L. is an employee of KEW Inc, who has been granted stock options by KEW Inc. The remaining authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varmus H. Genomic empowerment: the importance of public databases. Nat Genet. 2003;35(Suppl 1):3. doi: 10.1038/ng1186. [DOI] [PubMed] [Google Scholar]
- 2.Varmus H, Stillman B. Support for the Human Cancer Genome Project. Science. 2005;310:1615. doi: 10.1126/science.310.5754.1615b. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagemann IS, Cottrell CE, Lockwood CM. Design of targeted, capture-based, next generation sequencing tests for precision cancer therapy. Cancer Genet. 2013;206:420–431. doi: 10.1016/j.cancergen.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Thomas F, Desmedt C, Aftimos P, Awada A. Impact of tumor sequencing on the use of anticancer drugs. Curr Opin Oncol. 2014;26:347–356. doi: 10.1097/CCO.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 11.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowy DR, Collins FS. Aiming High--Changing the Trajectory for Cancer. N Engl J Med. 2016;374:1901–1904. doi: 10.1056/NEJMp1600894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S. Molecular Pathology Informatics. Surg Pathol Clin. 2015;8:187–194. doi: 10.1016/j.path.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Nagahashi M, Wakai T, Shimada Y, et al. Genomic landscape of colorectal cancer in Japan: clinical implications of comprehensive genomic sequencing for precision medicine. Genome Med. 2016;8:136. doi: 10.1186/s13073-016-0387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein DA, Shaib WL, Flowers CR. Costs and effectiveness of genomic testing in the management of colorectal cancer. Oncology (Williston Park) 2015;29:175–183. [PubMed] [Google Scholar]
- 16.Hagemann IS, Devarakonda S, Lockwood CM, et al. Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer. 2015;121:631–639. doi: 10.1002/cncr.29089. [DOI] [PubMed] [Google Scholar]
- 17.Endrullat C, Glokler J, Franke P, Frohme M. Standardization and quality management in next-generation sequencing. Appl Transl Genom. 2016;10:2–9. doi: 10.1016/j.atg.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arreaza G, Qiu P, Pang L, et al. Pre-Analytical Considerations for Successful Next-Generation Sequencing (NGS): Challenges and Opportunities for Formalin-Fixed and Paraffin-Embedded Tumor Tissue (FFPE) Samples. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada Y, Yagi R, Kameyama H, Nagahashi M, Ichikawa H, et al. Utility of comprehensive genomic sequencing for detecting HER2-positive colorectal cancer. Hum Pathol. 2017 doi: 10.1016/j.humpath.2017.02.004. (In Press) [DOI] [PubMed] [Google Scholar]
- 20.Nuovo GJ, Silverstein SJ. Comparison of formalin, buffered formalin, and Bouin’s fixation on the detection of human papillomavirus deoxyribonucleic acid from genital lesions. Lab Invest. 1988;59:720–724. [PubMed] [Google Scholar]
- 21.Friedman AA, Letai A, Fisher DE, Flaherty KT. Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer. 2015;15:747–756. doi: 10.1038/nrc4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do H, Dobrovic A. Sequence artifacts in DNA from formalin-fixed tissues: causes and strategies for minimization. Clin Chem. 2015;61:64–71. doi: 10.1373/clinchem.2014.223040. [DOI] [PubMed] [Google Scholar]
- 23.Legres LG, Janin A, Masselon C, Bertheau P. Beyond laser microdissection technology: follow the yellow brick road for cancer research. Am J Cancer Res. 2014;4:1–28. [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer DH, Sehn JK, Abel HJ, Watson MA, Pfeifer JD, Duncavage EJ. Comparison of clinical targeted next-generation sequence data from formalin-fixed and fresh-frozen tissue specimens. J Mol Diagn. 2013;15:623–633. doi: 10.1016/j.jmoldx.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuovo AJ, Garofalo M, Mikhail A, Nicol AF, Vianna-Andrade C, Nuovo GJ. The effect of aging of formalin-fixed paraffin-embedded tissues on the in situ hybridization and immunohistochemistry signals in cervical lesions. Diagn Mol Pathol. 2013;22:164–173. doi: 10.1097/PDM.0b013e3182823701. [DOI] [PubMed] [Google Scholar]
- 26.Carrick DM, Mehaffey MG, Sachs MC, et al. Robustness of Next Generation Sequencing on Older Formalin-Fixed Paraffin-Embedded Tissue. PLoS One. 2015;10:e0127353. doi: 10.1371/journal.pone.0127353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto R, De Summa S, Petriella D, Tudoran O, Danza K, Tommasi S. The value of new high-throughput technologies for diagnosis and prognosis in solid tumors. Cancer Biomark. 2014;14:103–117. doi: 10.3233/CBM-130328. [DOI] [PubMed] [Google Scholar]
- 28.Ramanathan R, Olex AL, Dozmorov M, Bear HD, Fernandez LJ, Takabe K. Angiopoietin pathway gene expression associated with poor breast cancer survival. Breast Cancer Res Treat. 2017 doi: 10.1007/s10549-017-4102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontanges Q, De Mendonca R, Salmon I, Le Mercier M, D’Haene N. Clinical Application of Targeted Next Generation Sequencing for Colorectal Cancers. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17122117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peixoto R, Sousa TT, Cruz M, Maluf F, Buzaid A. Next-generation sequencing (NGS) in metastatic breast cancer (mBC) patients: Translation from sequence data into clinical practice. J Clin Oncol. 2015;33:133. [Google Scholar]
- 31.Kari L, Hill KA, Sayem AS, et al. Mapping the space of genomic signatures. PLoS One. 2015;10:e0119815. doi: 10.1371/journal.pone.0119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karamichalis R, Kari L, Konstantinidis S, Kopecki S, Solis-Reyes S. Additive methods for genomic signatures. BMC Bioinformatics. 2016;17:313. doi: 10.1186/s12859-016-1157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos C, Stephens P, Hoke NN, et al. Association of PIK3CA mutation with response (ExRx) to cetuximab (C) in metastatic (met) triple-negative breast cancer (TNBC) J Clin Oncol. 2015;33:151. [Google Scholar]