Abstract
Early life stress (ELS) is a significant risk factor for the emergence of internalizing problems in adolescence. Beginning in adolescence, females are twice as likely as males to experience internalizing disorders. The present study was designed to examine sex differences in the association between ELS and internalizing problems in early pubertal adolescents, and whether and how corticolimbic function and connectivity may underlie these associations. Fifty-nine early-pubertal males and 78 early-pubertal females, ages 9–13 years (all Tanner Stage 3 or below) underwent fMRI as they performed an emotion label task that robustly interrogates corticolimbic function. Participants were also interviewed about their experience of ELS. Females exhibited a positive association between ELS and internalizing problems, whereas males exhibited no such association. Whole-brain and amygdala region-of-interest analyses indicated that whereas females exhibited a positive association between ELS and ventrolateral prefrontal cortex (vlPFC) during implicit emotion regulation, males showed no such association. Activation in these regions was positively associated with internalizing problems in females but not males; however, activation in these regions did not mediate the association between ELS and internalizing problems. Finally, both boys and girls exhibited an association between ELS and increased negative connectivity between right vlPFC and bilateral amygdala. Using a carefully characterized sample of early pubertal adolescents, the current study highlights important sex differences in the development of corticolimbic circuitry during a critical period of brain development. These sex differences may play a significant role in subsequent risk for internalizing problems.
Keywords: Internalizing problems, sex differences, puberty, amygdala, ventrolateral prefrontal cortex
Introduction
The experience of early life stress (ELS) is associated with mental health difficulties such as depression and anxiety (Andersen & Teicher, 2008; Heim & Nemeroff, 2001; Lupien, McEwen, Gunnar, & Heim, 2009; Teicher & Samson, 2016). In fact, exposure to ELS accounts for nearly one-third of all mood and anxiety disorders in the United States, underscoring the critical role of ELS as a risk factor for the onset of internalizing psychopathology (Green et al., 2010; McLaughlin et al., 2012). ELS has been theorized to confer risk for the emergence of psychopathology through several stress-related neurobiological pathways. Specifically, ELS may affect functioning of the hypothalamus-pituitary-adrenal (HPA) axis and consequently influence the development of neural structures with high densities of corticotropin-releasing factor (CRF) neurons, including portions of the prefrontal cortex (PFC) and amygdala (Heim et al., 2002; Lupien et al., 2009; Malter Cohen, Tottenham, & Casey, 2013; Nemeroff, 2004). ELS-induced alterations in the development of neural regions involved in this corticolimbic stress regulatory system may contribute to risk for psychopathology (for a review see Heim & Binder, 2012 and Lupien et al., 2009).
For instance, researchers have found that adults with a history of ELS exhibit both heightened amygdala response and positive functional connectivity, or temporal correlation, between right ventrolateral PFC (right vlPFC) and amygdala while labeling threatening (i.e., fearful and angry) faces, compared to adults without histories of adversity (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, 2006). Negative functional connectivity between PFC and amygdala during the processing of threat-related stimuli is typically found in adults and is posited to reflect adaptive emotion regulation by the PFC (Gee, Humphreys, et al., 2013). In contrast, the positive functional connectivity of PFC and amygdala in adults with ELS suggests that stress leads to atypical emotion regulation, presumably through ineffective PFC regulation of amygdala responses (Taylor et al., 2006). It is important to note, however, that most of the studies that have found altered corticolimbic function following exposure to ELS have been conducted with adult samples and have used retrospective reports of ELS obtained many years after exposure to stress (Burghy et al., 2012; Dannlowski et al., 2012; Fan et al., 2014; Heim & Binder, 2012; Herringa et al., 2013; Herringa et al., 2016; Taylor et al., 2006; van Harmelen et al., 2013, 2014). Thus, it is difficult to assess whether these neurobiological changes are a direct consequence of having experienced adversity early in development or, alternatively, are a signature of adult-onset psychopathologies.
Given the significant consequences of exposure to ELS, combined with the growing recognition that many of these effects are not observed until adolescence (Gee & Casey, 2015; Kessler et al., 2005; Lee et al., 2014), researchers have begun to focus on elucidating the psychological and neurobiological consequences of ELS during this developmental period (see Tottenham & Galván [2016] for a review). Investigators have posited that exposure to ELS alters the development of neural structures and connections that underlie emotion processing and regulation, including the PFC and amygdala, that, in turn, increases adolescents’ risk for developing internalizing disorders (Burghy et al., 2012; Eiland & Romeo, 2013; Maughan & Cicchetti, 2002; Pechtel & Pizzagalli, 2011; Tottenham et al., 2010). This is particularly salient in adolescence when these neural systems are undergoing dramatic reorganization (Casey, Jones, & Hare, 2008). Supporting this formulation, researchers have found that children and adolescents who have experienced high levels of ELS exhibit heightened activation to emotionally evocative faces and images in brain regions that are involved in processing salient stimuli (i.e., amygdala, anterior insula, dorsal anterior cingulate cortex (Garrett et al., 2012; Gee, Gabard-Durnam, et al., 2013; Marusak, Martin, Etkin, & Thomason, 2014; McCrory et al., 2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015; Suzuki et al., 2014; Tottenham, Hare, Millner, et al., 2011). Fewer investigators have examined how ELS may affect PFC regulation of the processing of emotional stimuli.
The PFC may be particularly sensitive to exposure to ELS given its high density of glucocorticoid receptors and its protracted development into adulthood (Giedd, 2004; McEwen & Morrison, 2013; Pechtel & Pizzagalli, 2011; Sanchez, Young, Plotsky, & Insel, 2000; Teicher et al., 2003). For example, McLaughlin et al. (2015) used a cognitive reappraisal task to examine fMRI responses of 13- to 19-year-old participants as they attempted to explicitly regulate their affective responses to emotionally salient images. Although previously maltreated adolescents showed the typical increased response in regions involved in processing salient stimuli to viewing negative images, they also showed increased activation in superior frontal gyrus and frontal pole when downregulating their negative affect to a negative image, relative to nonmaltreated adolescents. Similarly, Marusak et al. (2014) found that adolescents exposed to childhood trauma exhibited elevated dorsolateral PFC (dlPFC) activation when performing an emotional conflict task, suggesting early adverse experiences may have important consequences on the development of the PFC in adolescence.
In addition to the effects of ELS on activation to salient stimuli in the amygdala and the PFC, researchers have documented that ELS affects the functional connectivity between these two structures (Burghy et al., 2012; Gee, Gabard-Durnam, et al., 2013; Marusak et al., 2014; Wolf & Herringa, 2016). Using task-based connectivity analyses, researchers have found that adolescents exposed to ELS show stronger negative amygdala–PFC connectivity—a more mature, adult-like pattern of connectivity—while viewing negative stimuli (Gee, Gabard-Durnam, et al., 2013; Wolf & Herringa, 2016). These findings may reflect adaptive functioning in the face of adversity – children exposed to ELS may develop more adult-like patterns of connectivity at an earlier age in order to deal more effectively with environmental adversity. In fact, Gee et al. (2013) showed that among previously institutionalized children and adolescents, having a negative pattern of amygdala–medial PFC connectivity was associated with lower separation anxiety, indicating that this adult-like pattern may be protective following experiences of early adversity. However this pattern may also represent a premature end to a sensitive period for the development of this circuit. The long-term consequences of this earlier maturation are unclear, as are the developmental trajectories of the effects of ELS on this circuitry from childhood to adulthood.
Although researchers have now demonstrated that ELS affects the development of corticolimbic circuitry in childhood and adolescence, they have not examined possible sex differences in this association. ELS has, among females, been found to be a particularly important risk factor in the development of Major Depressive Disorder (MDD; Ge, Conger, & Elder, 2001; Ge, Lorenz, Conger, Elder, & Simons, 1994; Rudolph & Flynn, 2007; Weiss, Longhurt, & Mazure, 1999). Females also differ from males in their perceptions of stressful life events (Raffaelli et al., 2016), in their biological response to both acute and chronic stressors, and in their neural responses to negative stimuli (Bourke, Harrell, & Neigh, 2012; Kajantie & Phillips, 2006; for reviews see (Bangasser & Valentino, 2014; Novais, Monteiro, Roque, Correia-Neves, & Sousa, 2016; Ordaz & Luna, 2012; Stevens & Hamann, 2012). Given striking sex differences in the incidence of internalizing disorders—including evidence that females are twice as likely as males to develop MDD in adolescence and adulthood (Hankin & Abramson, 1999)—there may be sex-specific mechanisms, including sex-specific effects on corticolimbic circuitry, through which ELS contributes to vulnerability for internalizing problems in adolescence (Teicher et al., 2003).
In this context, the age at which sex differences in internalizing disorders become most pronounced corresponds to the complex developmental period of puberty; moreover, there is evidence that pubertal status is a stronger predictor of the onset of depression than is chronological age (Angold, Costello, & Worthman, 1998; Hayward, Gotlib, Schraedley, & Litt, 1999; Oldehinkel, Verhulst, & Ormel, 2011). Thus, puberty is a critically important period to study in order to understand neurobiological differences between males and females that may underlie sex differences in internalizing problems. It is important to note, however, that males and females differ significantly in pubertal timing; females typically experience the onset of puberty 1.5 years earlier than do males (Negriff & Susman, 2011). These sex differences in pubertal timing mean that age-matched samples of adolescent males and females are almost certain to be confounded by sex differences in pubertal stage. Similarly, given the difficulties inherent in recruiting and studying high-risk samples such as adolescents who have been exposed to maltreatment or who have experienced institutional care, many studies are simply not sufficiently powered to detect sex differences in the neurobiological effects of ELS during the narrow developmental window of early puberty during which sex differences in internalizing problems begin to emerge. Significant puberty-related changes occur in corticolimbic circuitry in adolescence (Peters, Jolles, Duijvenvoorde, Crone, & Peper, 2015; Spielberg, Forbes, et al., 2014; Spielberg, Olino, Forbes, & Dahl, 2014). Therefore, it is critical that we examine sex differences in corticolimbic development in carefully characterized samples of adolescent males and females, matched for pubertal development.
The present study was designed to address these issues by examining how ELS differentially affects male and female corticolimbic activation and connectivity during early puberty, a period in which sex differences in rates of internalizing problems begin to emerge. We also examined how corticolimbic circuitry is associated with internalizing disorders in adolescence. Given significant differences between males and females in pubertal timing (Negriff & Susman, 2011) and the documented impact of pubertal stage on corticolimbic circuitry (Peters et al., 2015; Spielberg, Forbes, et al., 2014; Spielberg, Olino, et al., 2014), in this study we used a puberty-matched sample of early adolescent boys and girls. We examined fMRI activation as boys and girls labeled emotion faces compared to matching emotion faces. Previous findings using this task suggest that labeling versus matching emotional face stimuli recruits implicit emotion regulation processes and elicits strong activation in the PFC, particularly in the ventrolateral PFC (Gee et al., 2012; Lieberman et al., 2007; Taylor et al., 2006). This design allows us to examine emerging differences in the effects of ELS on corticolimbic circuitry during a period of development in which sex differences in internalizing problems are beginning to emerge. Based on evidence of sex differences in rates of internalizing symptoms in this age group, we hypothesized that early-pubertal females will show a stronger association between exposure to ELS and internalizing problems than will their male counterparts. We predicted further that the association between ELS and internalizing problems in females would be mediated by increased amygdala activation and PFC activation during implicit emotion regulation. Finally, we expected to find greater negative connectivity between amygdala and PFC in individuals who were exposed to more severe ELS and, further, that this negative connectivity would mediate the association between ELS and internalizing problems. We also explored sex differences in patterns of PFC-amygdala connectivity, but made no directional hypotheses.
Methods
Participants and Procedure
A total of 137 participants met criteria for inclusion in this study and analyses: 59 early-pubertal males and 78 early-pubertal females, ages 9–13 years (M=11.42, SD=1.08). An additional thirty-nine participants were excluded from the analyses due to excessive motion (n=35) or incomplete scans (n=4). The participants were part of a larger study examining the effects of ELS on brain structure and function across early adolescence. After completing a brief phone interview to determine eligibility, participants were invited to the laboratory to complete parental consent and child assent forms. In this session, parents and children also completed interviews and questionnaires assessing their experience of ELS and measures of their cognitive and emotional functioning. Males and females were matched on pubertal development based on self-report Tanner Stage and exposure to ELS (see Table 1 for descriptive statistics by child sex). Participants were recruited from the San Francisco Bay area through a combination of print and online advertisements. Exclusion criteria included: 1) self-reported Tanner pubertal stage greater than 3 and the experience of menarche in female participants; 2) nonfluent English speakers; 3) contraindications to scan (e.g., metal implants, braces, etc.); 4) history of major neurological disorder or illness; and 5) intellectual delay or learning difficulties. Upon confirming eligibility, participants were invited to return within one month to complete the fMRI portion of the study. This study was approved by the Stanford University Institutional Review Board and all participants were compensated for their participation in the study.
Table 1.
Descriptive Statistics for Males and Females
| Males | Females | t or X2 | df | p | |
|---|---|---|---|---|---|
| Age | 12.018 (0.925) | 11.146 (1.033) | 5.115 | 135 | <.001 |
| Tanner Hair Development | 1.881 (0.672) | 1.872 (0.795) | 0.074 | 135 | 0.941 |
| Tanner Breast Development | 2.000 (0.615) | 2.083 (0.787) | −0.672 | 135 | 0.503 |
| Medication Use | 17% | 13% | 0.532 | 1 | 0.627 |
| Handedness (% R Dominant) | 88% | 81% | 0.865 | 1 | 0.510 |
| Race/Ethnicity | 3.859 | 1 | 0.570 | ||
| White/Caucasian | 44% | 40% | |||
| African American | 9% | 9% | |||
| Hispanic | 13% | 9% | |||
| Asian | 12% | 11% | |||
| Native American | 2% | 0% | |||
| Pacific Islander | 0% | 0% | |||
| Other | 18% | 31% | |||
| No response | 2% | 0% | |||
| Family Income | 9.787 | 1 | 0.550 | ||
| <$5000 | 0% | 1% | |||
| $5001–10,000 | 0% | 3% | |||
| $10,001–15,000 | 0% | 1% | |||
| $15,001--25,000 | 3% | 4% | |||
| $25,001--35,000 | 3% | 1% | |||
| $35,001--50,000 | 5% | 3% | |||
| $50,001--75,000 | 9% | 8% | |||
| $75,001--100,000 | 12% | 11% | |||
| $100,001--150,000 | 24% | 26% | |||
| $150.001+ | 34% | 33% | |||
| No response | 10% | 9% | |||
| ELS Severity | 6.864 (5.265) | 5.987 (4.768) | 1.019 | 135 | 0.310 |
| YSR Internalizing Problems | 11.220 (8.413) | 13.150 (10.276) | −1.168 | 134 | 0.245 |
M (SD) or %. ELS= early life stress.
Measures
Early Life Stress
We assessed levels of ELS severity and the impact of early life stressors using a modified version of Traumatic Events Screening Inventory for Children (TESI-C; (Ribbe, 1996)). In this interview we assessed 30+ types of stressful life experiences. For each type of ELS endorsed, interviewers followed up with general and specific probes in order to gather detailed information about the severity of the experience and the child’s perceived severity of the stressor (e.g., relationship of persons involved, duration of experience, consequences of experience). A panel of three coders, blind to the child’s subjective severity ratings and reactions and behaviors during the interview, rated the objective severity of each type of stressor based on a modified version of the UCLA Life Stress Interview coding system (Rudolph et al., 2000; Rudolph & Hammen, 1999). Coders made objective severity ratings on a 5-point scale (0 = non-event or no impact [e.g., witnessed debris from car crash]; 4 = extremely severe impact [e.g., experienced sexual abuse]; ICC=0.99). For each child, objective ratings for each type of ELS endorsed were summed to create an index of the cumulative severity of ELS.
Pubertal Status
Pubertal stage was determined using self-reported Tanner staging (Marshall & Tanner, 1969, 1970). Using schematic drawings of two secondary sex characteristics (pubic hair and breast/testes development), each participant reported on his/her developmental stage on a scale of 1–5. A Tanner staging of 1 signifies that no pubertal development has begun, and a staging of 5 signifies that adult levels of pubertal maturation have been achieved. To be included in this study, all participants rated themselves at stage 3 or below on measures of both pubic hair and breast/testes development. Tanner staging scores have been found to correlate with physicians’ physical examinations of pubertal development (Coleman & Coleman, 2002; Slora et al., 2009, see also Shirtcliff, Dahl, & Pollak, 2009).
Internalizing Problems
Participants completed the Youth Self Report (YSR; (Achenbach, 1991; Achenbach & Rescorla, 2001) measure, which assesses a broad array of behavioral problems in children and adolescents. For this study we examined total scores on the Internalizing Problems subscale of the YSR. One male participant did not complete this questionnaire.
Magnetic Resonance Imaging
Implicit Emotion Regulation fMRI Task
We used a modified emotion label task to examine the neural correlates of implicit emotion regulation (see (Hariri, Bookheimer, & Mazziotta, 2000; Lieberman et al., 2007). This task was designed based on the formulation that linguistic processing of an emotional expression (e.g., labeling an emotional face) requires greater downregulation of regions responsible for processing salient information than does perceptual processing of the same emotional expression (e.g., matching emotional faces), potentially serving as a marker of the neural correlates of implicit emotion regulation (Lieberman et al., 2007). This task requires participants to identify, or label, a target emotional expression (emotion label) or to match a target emotional expression to two other emotional expressions (emotion match). We also included a sensorimotor control condition in which participants were required to match a target shape to two other shapes (shape match). For the label conditions, participants were instructed to make a button press to indicate whether the appropriate label for the target figure was located on the bottom left or the bottom right of the screen. For all match conditions, participants were instructed to make a button press to indicate whether the target figure at the top of the screen was more similar to the figure below it on the left or on the right. Each face/label set was presented on the screen for 5000 ms, and each block of stimuli consisted of 10 trials. All task blocks were interspersed with rest blocks (lasting 15000 ms) in which participants were instructed to focus on a fixation cross in the middle of the screen. Each run consisted of one block of each task condition (Positive Label, Positive Match, Negative Label, Negative Match, Shape Match) as well as interspersed rest blocks. Presentation of conditions was randomized across participants, and all participants completed two complete runs of the task.
The task was presented using E-Prime software Version 2.0. A total of 50 trials were divided across two emotion conditions (positive, negative) and two response conditions (match and label) in addition to the control shape match condition. The positive and negative emotional expressions were facial displays from the NimStim picture set (Tottenham et al., 2009). Ten actors (5 female, 5 male) were displayed showing positive or negative emotional expressions. Positive emotional expression blocks included both high and low arousal positive displays including happy, excited, surprised, and calm facial expressions. Negative emotional expression blocks included both high and low arousal negative displays including sad, angry, and fearful facial expressions. To optimize power in testing our hypotheses, our analyses focused on the contrast of all emotion label relative to all emotion match conditions.
MRI acquisition
Participants who met eligibility criteria completed an fMRI scan. All scans were conducted on a 3 Tesla GE whole-body scanner (GE Healthcare Systems, Milwaukee, Wisconsin). Foam padding was used to minimize head movement. Two T2*-sensitive gradient echo-planar pulse sequences were used for functional imaging (TR = 2000 ms, TE = 30 ms, flip angle = 77°, matrix size = 70×70, 43 axial slices, FOV = 22.4 cm, 3 mm thick), each run lasting 5 minutes and 54 seconds. An automated high-order shimming procedure was used to reduce B0 inhomogeneity. Additional high-resolution structural images were acquired with an axial 3D FSPGR sequence with T1 contrast (TR = 6.0 ms, TE = 2 ms, flip angle = 12°, matrix size = 256×256, 186 axial slices, FOV = 23 cm, 0.9 mm) for spatial registration.
fMRI data analysis
Analyses were conducted in FSL Version 6.0.0 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), using FEAT (FMRI Expert Analysis Tool). The first four volumes of each participant’s functional scan were discarded to allow for stabilization of longitudinal magnetization. The remaining images were preprocessed using standardized procedures, including motion correction to the mean image using MCFLIRT (Motion Correction FMRIB’s Linear Image Registration Tool) (Jenkinson, Bannister, Brady, & Smith, 2002), slice-timing correction using Fourier-space time-series phase shifting, spatial smoothing using a Gaussian kernel of FWHM 5mm, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and high pass temporal filtering (Gaussian-weighted least squares straight line fitting, with sigma=50.0s). Functional data were linearly registered to a common stereotaxic space by first registering the in-plane T2 image to the T1-weighted structural image (6 degrees of freedom), transforming the T1-weighted structural image to the MNI152 T1 brain, and then applying that deformation matrix and resampling to 2 mm resolution onto the functional images using FLIRT (12 degrees of freedom; FMRIB’s Linear Image Registration Tool; Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001). All fMRI analyses were therefore performed in MNI space at 2 mm3 resolution.
Time series statistical analysis was conducted using FILM with local autocorrelation correction (Woolrich, Ripley, Brady, & Smith, 2001). The voxel-wise general linear model (GLM) included regressors for each block condition (positive/match, positive/label, negative/match, negative/label, and shape match) as well as their temporal derivatives. Twelve motion correction parameters, as well as an indicator function to identify volumes as having excessive motion according to framewise displacement of 0.9mm, were included as covariates of non-interest. Participants with absolute motion > 3mm or > 20% of volumes with framewise displacement > 0.9mm were excluded from the analyses (n=35). Both within-subject runs were combined in a fixed-effects model for each participant, which averaged the contrast estimates over runs within participant by setting the random effects variance to zero in FLAME (FMRIB’s Local Analysis of Mixed Effects) (Beckmann, Jenkinson, & Smith, 2003; Woolrich, 2008; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004). All participants were then combined in a higher-level mixed effects model to investigate within and between-group differences. Prior to thresholding, we used a binarized gray matter mask (obtained from https://canlabweb.colorado.edu/wiki/doku.php/help/core/brain_masks). Higher-level group analyses were conducted using FLAME (FMRIB’s Local Analysis of Mixed Effects State) stage 1 (Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). Given significant age differences between males and females, we included age as a covariate in all analyses.
Whole-Brain Analyses: Effects of Sex and ELS
We examined the effects of sex and ELS severity on BOLD response during contrasts of interest in whole-brain analyses. Statistical images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of α=.05 (Worsley, 2001).
Region of Interest Analysis of Amygdala
We also examined amygdala activation during implicit emotion regulation using a region-of-interest (ROI) analysis. A bilateral amygdala ROI was created using the Harvard-Oxford subcortical Atlas in FSL (25% threshold).
Psychophysiological Interaction (PPI) Analyses to Assess PFC-Amygdala Functional Connectivity
We used psychophysiological interaction (PPI) analyses to examine functional connectivity during implicit emotion regulation between the bilateral amygdala ROI and the two PFC regions that showed a significant interaction of ELS and sex (Friston et al., 1997; O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012). This analysis identifies regions in the brain that are correlated most strongly with the significant prefrontal ROIs during emotion label relative to emotion match conditions. For each PFC ROI, the GLM analysis was conducted in FSL and included regressors for each task condition, the ROI timeseries, and the interaction of the task contrast (emotion label vs. emotion match) and the ROI timeseries. Again, both within-subject runs were combined in a fixed-effects model for each participant, which averaged the contrast estimates over runs within participant by focusing the random effects variance to zero in FLAME (FMRIB’s Local Analysis of Mixed Effects; Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). All participants were then combined in a higher-level mixed effects model to investigate connectivity patterns across both groups. Higher-level group analyses were conducted using FLAME (FMRIB’s Local Analysis of Mixed Effects State) stage 1 (Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). Statistic images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of α=0.05 (Worsley, 2001). We then extracted parameter estimates from the bilateral amygdala ROI (as defined above) and used linear regression in SPSS (version 23) to examine the effect of sex, ELS, and the interaction between sex and ELS on connectivity patterns between bilateral amygdala and the two PFC regions that showed a significant interaction effect with sex and ELS severity, after covarying for chronological age. We used simple slope analyses to probe significant interactions (Aiken & West, 1991).
Demographics and Behavioral Analyses
All statistical analyses reported below were conducted with SPSS (version 23) using two-tailed tests (α=.05). Independent samples t-tests or chi-squared tests were used to compare males and females on demographic variables, exposure to ELS severity, and scores on the YSR Internalizing Problems subscale. We conducted analyses of covariance (ANCOVAs) to compare task performance between males and females, after covarying for chronological age. Finally, we conducted correlational analyses to test whether ELS severity was associated with task performance.
Mediation Analyses
Based on our findings (described below), we tested whether BOLD responses to emotion label relative to match in regions that showed a significant interaction between ELS severity and sex mediated the association between ELS severity and YSR Internalizing scores in females. To do this, we used a single-step nonparametric resampling procedure (1,000 samples with replacement) for testing indirect effects (Hayes, 2013). Mediation is supported when the indirect effect is statistically significant. To assess the indirect effect, we calculated 95% confidence intervals (CIs) for coefficients; if the CI does not include zero, the indirect effect is considered to be statistically significant.
Results
Demographic and Clinical Characteristics
Participant demographic and clinical characteristics are presented in Table 1. As shown in this Table, boys and girls did not differ significantly in Tanner stage, medication use, handedness, race/ethnicity, or family income. As expected, however, given that we matched boys and girls on pubertal status, the two sexes did differ in chronological age (t(135)=5.115, p<.001).
Sex Differences in the Effects of ELS on Internalizing Problems
There were no significant sex differences in ELS severity or in internalizing problems in our sample (see Table 1). In examining the contributions of sex and ELS severity to self-reported internalizing problems after controlling for chronological age, a linear regression indicated that sex moderated the association between ELS severity and internalizing problems (B=.903, SE=.306, t(131)=2.953, p=.004). Post-hoc simple slopes analyses within each sex controlling for age indicated that ELS was significantly associated with internalizing problems in females (r(75)=.476, p<.001), but not in males (r(55)=.079, p=.559).
fMRI Task Behavioral Performance
We conducted analyses of covariance (ANCOVAs) to compare task performance between males and females, controlling for age. Males and females did not differ on task accuracy or response time (RT; Table 2). ELS severity was associated with longer RT to match emotions (r(134) =.174, p=.042). ELS severity was not associated with RT to label emotions r(134)=.069, p=.425) or with task accuracy (|rs|<.048, ps>.576).
Table 2.
Task Performance
| Males | Females | ||
|---|---|---|---|
| Response Time (RT) | |||
| Emotion Label | 1847.172 (276.215) | 1857.447 (260.699) | F(1,134)=.092, p=.762 |
| Emotion Match | 1950.548 (315.084) | 2001.075 (295.768) | F(1,134)=.174, p=.678 |
| Accuracy (% Correct) | |||
| Emotion Label Accuracy | 72.966 (15.246) | 72.308 (12.714) | F(1,134)=.102, p=.750 |
| Emotion Match Accuracy | 83.517 (14.74) | 83.75 (14.83) | F(1,134)=.576, p=.449 |
Standard deviations are presented in parentheses.
Whole-Brain Analysis: Main Effect of ELS
We conducted whole-brain correlational analyses to examine the effect of ELS severity, controlling for the effects of chronological age, on brain regions involved in implicit emotion regulation (i.e., labeling emotion faces versus matching emotion faces). These analyses yielded a significant cluster that was negatively correlated with ELS severity: right lateral occipital cortex/precuneus cortex (k=1088; peak voxel: x=18, y=−72, z=42; Z=3.95).
We also conducted a linear regression to examine the effect of ELS severity, controlling for the effects of chronological age, on activation in a bilateral amygdala ROI during implicit emotion regulation. There was no association between ELS severity and bilateral amygdala activation (r(134)=−.049, p=.575).
Whole-Brain Analysis: Main Effect of Sex
We conducted whole-brain t-tests to examine sex differences, controlling for the effects of chronological age, in regions involved in labeling emotion faces versus matching emotion faces. Direct comparisons between males and females yielded one significant cluster in left lingual gyrus in which males showed significantly greater activation than did females (k=580; peak voxel: x=−12, y=−70, z=−6; Z=3.38). There were no clusters in which females showed greater activation than did males.
We also conducted an analysis of covariance to examine sex differences in activation in a bilateral amygdala ROI, controlling for the effects of chronological age. There were no significant differences between males and females in amygdala activation when labeling emotion faces relative to matching emotion faces (F(1,134)=.034, p=.855).
Whole Brain Analysis: Interaction of ELS and Sex
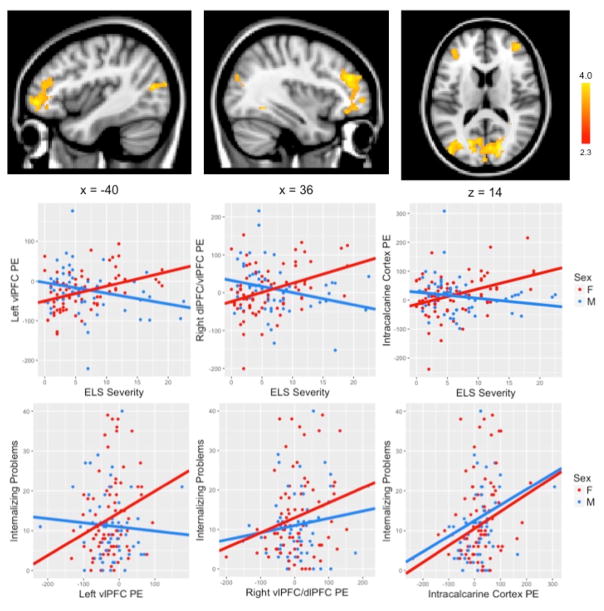
Finally, we conducted a whole brain interaction model to examine whether the linear association between ELS severity and brain regions involved in labeling emotion faces versus matching emotion faces differed by sex, controlling for the effects of chronological age. This interaction model yielded three significant clusters (Figure 1). Compared with males, females showed a significantly greater association between ELS and activation in left vlPFC (k=606; peak voxel: x=−36, y=48, z=−4; Z=3.8), right dlPFC/vlPFC (k=784; peak voxel: x=44, y=44, z=26; Z=4.1) and bilateral intracalcarine cortex (k=1999; peak voxel: x=−18, y=−72, z=12; Z=4.18).
Figure 1.
A whole brain interaction model examining whether the linear association between ELS severity and brain regions involved in labeling emotion faces versus matching emotion faces differed by sex yielded three significant clusters in left vlPFC, right dlPFC/vlPFC and bilateral intracalcarine cortex. Activation maps are thresholded at Z>2.3 and corrected for multiple comparisons using a cluster-based p<.05. MNI coordinates are indicated for slice distance (in mm). Parameter estimates (showing the amount of signal change measured in arbitrary units) of BOLD signal response were extracted from each significant cluster and plotted in the bar graph. Parameter estimates were also related to internalizing problems in males and females separately. vlPFC = ventrolateral prefrontal cortex; dlPFC = dorsolateral prefrontal cortex.
ROI Analysis of Amygdala: Interaction of ELS and Sex
We also conducted a linear regression to examine the interaction between ELS severity and sex in predicting bilateral amygdala ROI response to labeling emotion faces relative to matching emotion faces. This interaction was not significant (B=.488, SE=1.147, t(132)=.426, p=.671).
Associations between Brain Regions and Internalizing Symptoms
We next examined the association between self-reported internalizing problems and parameter estimates extracted from the ROIs defined by the interaction of ELS and sex, including left vlPFC, right dlPFC/vlPFC, and bilateral intracalcarine cortex, separately for males and females (Figure 1). Females showed a positive association between all three ROIs and YSR Internalizing Problems total score, such that greater activation in left vlPFC, right dlPFC/vlPFC, and intracalcarine cortex was associated with greater internalizing problems (left vlPFC: r(78)=.237, p=.037; right dlPFC/vlPFC: r(78)=.232, p=.041; intracalcarine cortex: r(78)=.260, p=.021). In contrast, males showed a positive association between internalizing problems and activation only in intracalcarine cortex (intracalcarine cortex: r(58)=.320, p=.014); there was no significant relation in males between internalizing problems and activation in left vlPFC or in right dlPFC/vlPFC (left vlPFC: r(58)=−.070, p=.601; right dlPFC/vlPFC: r(58)=.141, p=.293).
Mediation Analysis: Testing whether Brain Activation Mediates the Association between ELS Severity and Internalizing Symptoms in Females
Because there was a significant positive association between activation during implicit emotion regulation in left vlPFC, right dlPFC/vlPFC, and intracalcarine cortex and internalizing symptoms in females only, we tested whether activation in these regions mediated ELS severity and internalizing symptoms. Activation during implicit emotion regulation in these three regions did not mediate the association between ELS and internalizing problems in females; that is, the indirect effect did not differ significantly from zero (left vlPFC: point estimate=.010 [.018], 95% CI [−.011, .070]; right dlPFC/vlPFC: point estimate=.025 [.034], 95% CI [−.023, .117]; intracalcarine cortex: point estimate=.076 [.081], 95% CI [−.057, .270]).
PPI Analysis to Assess PFC-Amygdala Functional Connectivity
We also conducted PPI analyses to examine task-dependent functional connectivity between the two prefrontal regions that showed a significant interaction of ELS severity and sex and the bilateral amygdala ROI. We tested the main effects of ELS severity and sex and their interaction on the strength of these connections during emotion label relative to emotion match conditions. The main effects of ELS and sex, and the interaction of ELS and sex, on the strength of connectivity between left vlPFC and bilateral amygdala were not significant. There was a significant main effect of ELS severity on the strength of connectivity between right dlPFC/vlPFC and bilateral amygdala (B=−.002, SE=.001, t(132)=−2.352, p=.020), such that greater ELS severity is associated with more negative amygdala–PFC connectivity for emotion label relative to emotion match conditions (Figure 2).
Figure 2.
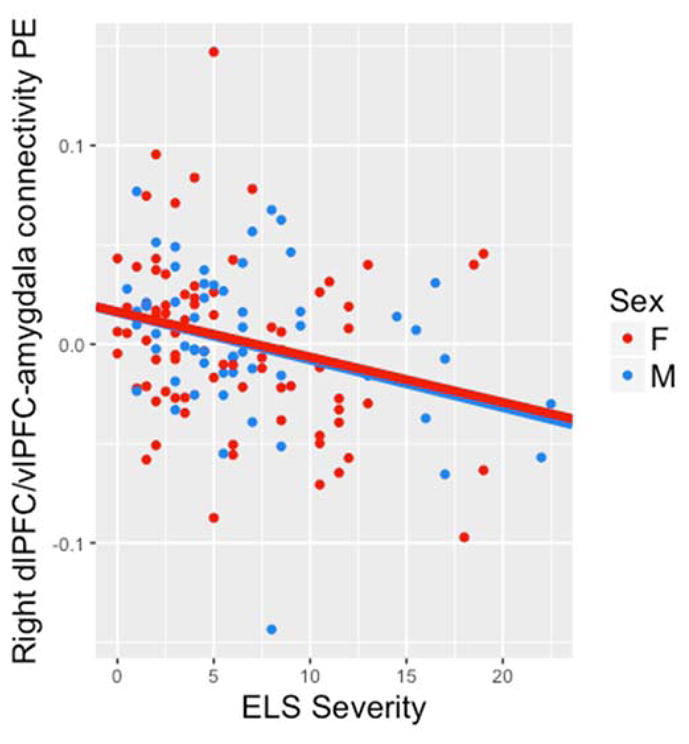
Strength of connectivity between right dlPFC/vlPFC and bilateral amygdala was negatively associated with ELS severity across males and females. vlPFC = ventrolateral prefrontal cortex; dlPFC = dorsolateral prefrontal cortex.
Associations between PFC-Amygdala Functional Connectivity and Internalizing Symptoms
Functional connectivity between bilateral amygdala and left vlPFC and right dlPFC/vlPFC was not associated with internalizing symptoms across all participants (left vlPFC: r(133)=−.138, p=.110; right dlPFC/vlPFC: r(133)=−.084, p=.334) or in each sex separately (males: left vlPFC: r(58), p=.776, right dlPFC/vlPFC: r(58)=.162, p=.225; females: left vlPFC: r(78)=−.181, p=.112, right dlPFC/vlPFC: r(78)=−.210, p=.065). Given null associations between functional connectivity and internalizing symptoms, we did not conduct follow-up mediation analyses.
Discussion
In a sample of puberty-matched early adolescent males and females, we documented differential associations between ELS severity and internalizing problems. Among girls, there was a significant association between ELS severity and internalizing problems, whereas among boys there was no such association. In attempting to understand the neurobiological bases of emerging sex differences in the effects of ELS on internalizing problems, we conducted whole-brain and a priori ROI analyses of brain activation while participants were performing an emotion label task designed to measure implicit emotion regulation. We found a significant interaction of ELS severity and sex in left vlPFC, right dlPFC/vlPFC, and bilateral intracalcarine cortex. Among girls, there was a positive association between ELS severity and activation in these regions during implicit emotion regulation, and as above, among boys there was no association between ELS severity and activation in these regions. Moreover, greater activation in these three regions was associated with higher levels of internalizing problems in girls. Nonetheless, this activation did not statistically mediate the association between ELS severity and internalizing problems. We also found a significant effect of ELS severity on right lateral occipital/precuneus cortex and a significant main effect of sex in left lingual gyrus during emotion label relative to emotion match conditions. Contrary to our hypothesis, ROI analyses yielded no significant main effects or interactions of ELS severity and sex on amygdala response during emotion label relative to emotion match conditions. When we examined patterns of PFC–amygdala functional connectivity, we found greater negative association between ELS severity and connectivity in both sexes combined between right dlPFC/vlPFC and bilateral amygdala during implicit emotion regulation; this did not mediate the relation between ELS and internalizing symptoms.
The vlPFC is largely involved in cognitive responses to negative emotions, including cognitive reappraisal and emotion regulation (Ochsner, Silvers, & Buhle, 2012) and thus plays a significant role in modulating negative affect (Forbes, Phillips, Silk, Ryan, & Dahl, 2011; Phan et al., 2005). Although there are no known direct anatomical connections between vlPFC and the amygdala, vlPFC is posited to modulate amygdala response through activation in the medial PFC (Pessoa, 2010; Pessoa, Kastner, & Ungerleider, 2002; Silvers et al., 2016). Cognitive reappraisal has been associated with a negative correlation between vlPFC and amygdala activation (Silvers et al., 2016). The vlPFC does, however, share direct anatomical connections with the dlPFC; research suggests that both these regions support executive function and inhibitory processes (Pessoa, 2010; Pessoa et al., 2002). Given our finding that greater vlPFC activation is associated with higher levels of ELS severity in early pubertal females, several possible interpretations can be generated. For example, heightened PFC activation in response to affective stimuli has been interpreted as reflecting immaturities in regulating an affective response (see Pfeifer & Blakemore (2012) for a review of this literature in adolescence). In this context, it is plausible that early pubertal females require additional neural engagement to label the facial emotion and, in turn, to downregulate amygdala response. Given the nonsignificant results of our mediation analyses, however, it is not clear how increased activation in these brain regions during this implicit emotion regulation task gives rise to heightened internalizing symptoms in early pubertal females. It is possible that increased neural recruitment at this point in early puberty contributes to maladaptive cognitive processes and risk for disorder in the future (i.e., late puberty when first depressive episodes commonly occur and sex differences in depressive symptoms peak); it is important that this possibility be examined longitudinally in future research.
One additional theoretical explanation for our finding that greater exposure to ELS is associated with greater recruitment of PFC in early pubertal females is the stress acceleration hypothesis. Integrating evidence from both animal and human studies of exposure to early adversity, the stress acceleration hypothesis posits that experiencing ELS leads to a faster maturation of neural circuits involved in emotional functioning, primarily the corticolimbic circuit. Callaghan and Tottenham (2016) argue that in the face of high levels of stress, accelerated development of neural circuitry involved in emotion functioning is adaptive in the short-term, as it facilitates earlier independence from a potentially unstable or harmful environment. Indeed, Gee et al. (2013) found a more mature pattern of amygdala–mPFC functional connectivity (i.e. more negative connectivity) in previously institutionalized children and adolescents relative to never-institutionalized children and adolescents, which was in turn associated with reduced separation anxiety in the previously institutionalized group only. However, this accelerated development could have later consequences and researchers have yet to explore this possibility longitudinally. Increasing age in adolescence has been associated with greater vlPFC activation both in an emotion regulation context and during passive viewing of fearful faces (McRae et al., 2012; Yurgelun-Todd & Killgore, 2006; see also Forbes, Phillips, Silk, Ryan, & Dahl, 2011). Given typical developmental increases in the recruitment of dorsal and lateral PFC (Cohen-Gilbert & Thomas, 2013; Somerville, Hare, & Casey, 2011; Tottenham, Hare, & Casey, 2011) in support of emotion regulation, the increased recruitment of left vlPFC and right dlPFC/vlPFC that we found in our sample of early adolescent females who were exposed to high levels of ELS may represent a more developmentally mature pattern of neural function. Indeed Gee et al. (2013) found heightened activation in the amygdala and in prefrontal and superior temporal gyrus when viewing fear faces in a sample of previously institutionalized children and adolescents, but no difference in mPFC activation between children with and without a history of adversity. When we examined functional connectivity of prefrontal ROIs that exhibited significant interactions of sex and ELS severity, we also found that ELS severity was associated with greater negative connectivity of right dlPFC/vlPFC and bilateral amygdala for emotion label relative to emotion match conditions. Although sex did not moderate this association, we believe that this is nonetheless an important demonstration of the stress acceleration hypothesis.
Contrary to our hypothesis, we did not find significant effects of stress, sex, or their interaction on amygdala activation during implicit emotion regulation. Activation in the amygdala increases in response to salient (both positive and negative) stimuli in the environment; amygdala activation is posited to serve as a motivating signal to guide learning and memory for emotional material. Our nonsignificant findings for the effect of ELS on amygdala activation during implicit emotion regulation stand in contrast to a large body of work suggesting that individuals exposed to ELS show heightened amygdala reactivity to emotion face stimuli (including angry, happy, fearful, sad and neutral faces; Garrett et al., 2012; Gee et al., 2013; Tottenham et al., 2011; Marusak et al., 2014; McCrory et al., 2013; Suzuki et al., 2014), as well as to negative images (McLaughlin et al., 2015). In understanding these discrepant findings, it is instructive to note that because of the block design of this task, we combined a range of emotional face stimuli, including happy, surprised, sad, angry, and fearful faces. The block design of the task prevents us from being able to assess reliably amygdala activation to each emotion. It is possible that the effects of ELS on amygdala activation are selective to fearful or angry faces or specific to task demands (i.e., passively attending to facial stimuli or performing a cognitive task involving emotional face stimuli). Future research should investigate the specificity of this effect across a range of emotional faces and task conditions.
Similarly, the majority of existing work examining the effects of ELS on amygdala activation to threat-related stimuli in adolescence uses an extreme-group approach to examine brain function in individuals exposed to severe and homogenous forms of ELS, including early institutional care and severe maltreatment, relative to healthy controls (McLaughlin et al., 2015; Mueller et al., 2010; Tottenham, Hare, Millner, et al., 2011). Less work has focused on the neurobioloical consequences of more commonly experienced forms of ELS such as those reported by the young participants in this study, including witnessing an injury or an accident and moving homes. It is possible that heightened amygdala reactivity as a consequence of ELS occurs only after exposure to extreme forms of early adversity or to specific types of ELS, such as exposure to severe threat or harmful input from caregivers early in development (Humphreys & Zeanah, 2015; McLaughlin, Sheridan, & Lambert, 2014; Teicher & Samson, 2016). The measure of ELS severity that we used in this study includes both more severe forms of ELS such as physical and sexual abuse, as well as less severe stressors. Thus, heightened amygdala reactivity may occur only after exposure to specific types of extreme ELS, a possibility that should be examined more explicitly in future research.
In summary, this is the first study to carefully assess sex differences in corticolimbic activation and connectivity during implicit emotion regulation in a unique sample of early pubertal youth as a function of exposure to ELS severity. In addition to the strengths of this investigation, there are three important limitations of this investigation. First, although we used careful coding systems to evaluate the cumulative effects of objectively rated ELS severity on corticolimbic development, we use the term ELS broadly to include multiple forms of adverse childhood experiences, ranging from maltreatment and neglect to residential moves and exposure to marital disagreements. As we noted above, researchers have suggested that different forms of early adverse experiences have different psychobiological consequences (Humphreys & Zeanah, 2015; McLaughlin & Sheridan, 2016; Teicher & Samson, 2016; Teicher, Samson, Anderson, & Ohashi, 2016). Future research should examine the differential effects of types of stress exposure, including exposure to threat and neglect, on neural functioning. Second, we did not examine effects of the timing of ELS severity or its chronicity in this study. Researchers have developed coding systems to describe more fully experiences of maltreatment; one notable example of this is the Maltreatment Classification System (MCS; Barnett, Manly & Cicchetti, 1993). Given our use of child-reported stressors and objectively-rated severity of stressors (as opposed to obtaining both child and parent reports, or reports from Child Protective Services for more severe stressors), we did not have sufficient details in our stress assessment to explore specific and differential effects of onset, chronicity, or developmental timing. It will be important in future research to elucidate how exposure to ELS at different points in development, as well as chronicity of adverse experiences, differentially affect corticolimbic circuitry, given that this system is still maturing through adulthood. In the present study, we defined ELS as any adverse experience prior to participating in the study, when adolescents were in early pubertal development. There is evidence in both human and rodent models, however, to suggest that the effects of adverse experiences on different neural systems depends on the individual’s developmental stage at the time of exposure (Andersen et al., 2008; Andersen & Teicher, 2008; Lupien et al., 2009; Pechtel, Lyons-Ruth, Anderson, & Teicher, 2014). Finally, it is important to mention the limitations of cross-sectional investigations such as the present study, which precludes our ability to draw causal inferences. It will be important in future research to understand how corticolimbic development across puberty differs in males and females, particularly with respect to its temporal relation to ELS and risk for internalizing problems. Elucidating deviations from normative neural development in individuals who are exposed to ELS will lead to a better understanding of the etiology of internalizing problems and will highlight potential ways to intervene in the association between ELS and internalizing problems.
Despite these limitations, this study documents the effects of a wide range of ELS exposure on corticolimbic function in early puberty, and elucidates how sex moderates the effects of ELS on corticolimbic function. These results are important in refining models describing the impact of ELS on development, and highlight the need to study homogenous samples of well-characterized individuals at specific points in development. Understanding how ELS affects psychobiological development over time will facilitate the generation of a model describing the emergence of internalizing disorders, and will help to identify specific points or sensitive periods at which intervention may be most effective.
Acknowledgments
We thank Holly Pham, Isabella Lazzareschi, Cat Camacho, Monica Ellwood-Lowe, Sophie Schouboe, Maddie Pollak and Morgan Popolizio for their assistance in scheduling and running the participants. We also thank the adolescents and families who participated in our study. This work was supported by NIMH (R01MH101495 to IHG, K01MH106805 to SJO, F32MH107129 to KLHN), the Brain & Behavior Research Foundation (Young Investigator Awards to SJO [23582] and KLH [23819]), the Klingenstein Third Generation Foundation (Fellowship Awards to SJO and KLH), the National Science Foundation (Graduate Fellowship Awards to NLC and LSK) and Stanford University (Gerald J. Lieberman Graduate Fellowship to NLC).
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRP Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 1991. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. http://doi.org/10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary Evidence for Sensitive Periods in the Effect of Childhood Sexual Abuse on Regional Brain Development. Journal of Neuropsychiatry. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. http://doi.org/10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9483683. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. http://doi.org/10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: the interface between policy and research. In: Cicchetti D, Toth SL, editors. Child Abuse, Child Development, and Social Policy. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. http://doi.org/10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: Adaptations mediated by the glucocorticoid receptor. Hormones and Behavior. 2012;62(3):210–218. doi: 10.1016/j.yhbeh.2012.02.024. http://doi.org/10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler Ja, … Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1–8. doi: 10.1038/nn.3257. http://doi.org/10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. http://doi.org/10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare Ta. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–26. doi: 10.1196/annals.1440.010. http://doi.org/10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Thomas KM. Inhibitory Control During Emotional Distraction Across Adolescence and Early Adulthood. 2013;0(0):1–13. doi: 10.1111/cdev.12085. http://doi.org/10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L, Coleman J. The measurement of puberty: a review. Journal of Adolescence. 2002;25:535–550. doi: 10.1006/jado.2002.0494. http://doi.org/10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … Kugel H. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. http://doi.org/10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. http://doi.org/10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Herrera-Melendez AL, Pestke K, Feeser M, Aust S, Otte C, … Grimm S. Early life stress modulates amygdala-prefrontal functional connectivity: Implications for oxytocin effects. Human Brain Mapping. 2014;35(10):5328–5339. doi: 10.1002/hbm.22553. http://doi.org/10.1002/hbm.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology. 2011;36(4):429–52. doi: 10.1080/87565641.2010.550178. http://doi.org/10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R. Psychophysiological and Modulatory Interactions in Neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. http://doi.org/10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depression and Anxiety. 2012;29(5):449–459. doi: 10.1002/da.21892. http://doi.org/10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH0J. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. http://doi.org/10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of Stressful Life Events and Depressive Symptoms During Adolescence. 1994;30(4) [Google Scholar]
- Gee DG, Casey BJ. The impact of developmental timing for stress and recovery. Neurobiology of Stress. 2015;1:184–194. doi: 10.1016/j.ynstr.2015.02.001. http://doi.org/10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. http://doi.org/10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2013;33(10):4584–93. doi: 10.1523/JNEUROSCI.3446-12.2013. http://doi.org/10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Karlsgodt KH, van Erp TGM, Bearden CE, Lieberman MD, Belger A, … Cannon TD. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophrenia Research. 2012;134(1):1–9. doi: 10.1016/j.schres.2011.10.005. http://doi.org/10.1016/j.schres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. http://doi.org/10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Green JG, Mclaughlin Ka, Berglund Pa, Gruber MJ, Sampson Na, Zaslavsky AM, Kessler RC. Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication I. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. http://doi.org/10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: description and possible explanations. Annals of Medicine. 1999;31(6):372–379. doi: 10.3109/07853899908998794. http://doi.org/10.3109/07853899908998794. [DOI] [PubMed] [Google Scholar]
- Hariri A, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–8. doi: 10.1097/00001756-200001170-00009. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10683827. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- Hayward C, Gotlib IH, Schraedley PK, Litt IF. Ethnic differences in the association between pubertal status and symptoms of depression in adolescent girls. Journal of Adolescent Health. 1999;25:143–149. doi: 10.1016/s1054-139x(99)00048-8. Retrieved from http://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pubmed/10447041. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology. 2012;233(1):102–11. doi: 10.1016/j.expneurol.2011.10.032. http://doi.org/10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. http://doi.org/10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depression and Anxiety. 2002;15(3):117–25. doi: 10.1002/da.10015. http://doi.org/10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy Ca, Stodola DE, Davidson RJ, Essex MJ. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. http://doi.org/10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ. Enhanced Prefrontal-Amygdala Connectivity Following Childhood Adversity as a Protective Mechanism Against Internalizing in Adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1(4):326–334. doi: 10.1016/j.bpsc.2016.03.003. http://doi.org/10.1016/j.bpsc.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Zeanah CH. Deviations from the Expectable Environment in Early Childhood and Emerging Psychopathology. Neuropsychopharmacology. 2015;40(1):154–170. doi: 10.1038/npp.2014.165. http://doi.org/10.1038/npp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. http://doi.org/10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. http://doi.org/10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. http://doi.org/10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593. doi: 10.1001/archpsyc.62.6.593. http://doi.org/10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Estan N, Weinberger DR, Casey BJ. Adolescent mental health--Opportunity and obligation. Science. 2014;346(6209):547–549. doi: 10.1126/science.1260497. http://doi.org/10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18(5):421–8. doi: 10.1111/j.1467-9280.2007.01916.x. http://doi.org/10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. http://doi.org/10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M, Tottenham N, Casey BJ. Translational developmental studies of stress on brain and behavior: Implications for adolescent mental health and illness? Neuroscience. 2013;249:53–62. doi: 10.1016/j.neuroscience.2013.01.023. http://doi.org/10.1016/j.neuroscience.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W, Tanner J. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. http://doi.org/10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W, Tanner J. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2020414&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood Trauma Exposure Disrupts the Automatic Regulation of Emotional Processing. Neuropsychopharmacology. 2014;40(5):1250–1258. doi: 10.1038/npp.2014.311. http://doi.org/10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan A, Cicchetti D. Impact of child maltreatment and interadult violence on children’s emotion regulation abilities and socioemotional adjustment. Child Development. 2002;73(5):1525–1542. doi: 10.1111/1467-8624.00488. http://doi.org/http://dx.doi.org/10.1111/1467-8624.00488. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, … Viding E. Amygdala activation in maltreated children during pre-attentive emotional processing. British Journal of Psychiatry. 2013;202(4):269–276. doi: 10.1192/bjp.bp.112.116624. http://doi.org/10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McEwen B, Morrison J. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. http://doi.org/10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood Adversities and First Onset of Psychiatric Disorders in a National Sample of US Adolescents. Archives of General Psychiatry. 2012;69(11):1151. doi: 10.1001/archgenpsychiatry.2011.2277. http://doi.org/10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child Maltreatment and Neural Systems Underlying Emotion Regulation. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010. http://doi.org/10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA. Beyond Cumulative Risk: A Dimensional Approach to Childhood Adversity. Current Directions in Psychological Science. 2016;25(4):239–245. doi: 10.1177/0963721416655883. http://doi.org/10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. http://doi.org/10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, … Ochsner KN. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. http://doi.org/10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, … Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. http://doi.org/10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negriff S, Susman EJ. Pubertal timing, depression, and externalizing problems: A framework, review, and examination of gender differences. Journal of Research on Adolescence. 2011;21(3):717–746. http://doi.org/10.1111/j.1532-7795.2010.00708.x. [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. Journal of Clinical Psychiatry. 2004;65(SUPPL 1):18–28. [PubMed] [Google Scholar]
- Novais A, Monteiro S, Roque S, Correia-Neves M, Sousa N. How age, sex and genotype shape the stress response. Neurobiology of Stress. 2016 doi: 10.1016/j.ynstr.2016.11.004. http://doi.org/10.1016/j.ynstr.2016.11.004. [DOI] [PMC free article] [PubMed]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7(5):604–609. doi: 10.1093/scan/nss055. http://doi.org/10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. http://doi.org/10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel AJ, Verhulst FC, Ormel J. Mental health problems during puberty: Tanner stage-related differences in specific symptoms. The TRAILS study. Journal of Adolescence. 2011;34(1):73–85. doi: 10.1016/j.adolescence.2010.01.010. http://doi.org/10.1016/j.adolescence.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37(8):1135–1157. doi: 10.1016/j.psyneuen.2012.01.002. http://doi.org/10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: The role of maltreatment in preadolescence. NeuroImage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. http://doi.org/10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. http://doi.org/10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done? Neuropsychologia. 2010;48(12):3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. http://doi.org/10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research. 2002;15(1):31–45. doi: 10.1016/s0926-6410(02)00214-8. http://doi.org/10.1016/S0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Peters S, Jolles DJ, Van Duijvenvoorde ACK, Crone EA, Peper JS. The link between testosterone and amygdala–orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. http://doi.org/10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, present, and future. Social Cognitive and Affective Neuroscience. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. http://doi.org/10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. http://doi.org/10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Raffaelli B, Strache N, Parchetka C, Artiges E, Banaschewski T, Bokde A, … Gallinat J. Sex-related differences in frequency and perception of stressful life events during adolescence. Journal of Public Health. 2016;24(5):365–374. http://doi.org/10.1007/s10389-016-0731-x. [Google Scholar]
- Ribbe D. Psychometric review of Traumatic Events Screening Inventory for Children (TESI-C) In: Stamm B, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidran; 1996. pp. 386–387. [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Development and Psychopathology. 2007;19(2):497–521. doi: 10.1017/S0954579407070241. http://doi.org/10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and Gender as Determinants of Stress Exposure, Generation, and Reactions in Youngsters: A Transactional Perspective. Child Development. 1999;70(3):660–677. doi: 10.1111/1467-8624.00048. http://doi.org/10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: The developmental context of stress generation. Development and Psychopathology. 2000;12(2):S0954579400002066. doi: 10.1017/s0954579400002066. http://doi.org/10.1017/S0954579400002066. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of Corticosteroid Receptors in the Rhesus Brain: Relative Absence of Glucocorticoid Receptors in the Hippocampal Formation. Journal of Neuroscience. 2000;20(12):4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. http://doi.org/20/12/4657 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff Ea, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Development. 2009;80(2):327–37. doi: 10.1111/j.1467-8624.2009.01263.x. http://doi.org/10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, … Ochsner KN. vlPFC–vmPFC–Amygdala Interactions Underlie Age-Related Differences in Cognitive Regulation of Emotion. Cerebral Cortex. 2016:1–13. doi: 10.1093/cercor/bhw073. http://doi.org/10.1093/cercor/bhw073. [DOI] [PMC free article] [PubMed]
- Slora EJ, Bocian AB, Herman-Giddens ME, Harris DL, Pedlow SE, Dowshen Sa, Wasserman RC. Assessing inter-rater reliability (IRR) of Tanner staging and orchidometer use with boys: a study from PROS. Journal of Pediatric Endocrinology & Metabolism : JPEM. 2009;22(4):291–9. doi: 10.1515/jpem.2009.22.4.291. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19554802. [DOI] [PubMed] [Google Scholar]
- Somerville L, Hare T, Casey B. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. http://doi.org/10.1162/jocn.2010.21572.Frontostriatal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Forbes EE, Ladouceur CD, Worthman CM, Olino TM, Ryan ND, Dahl RE. Pubertal Testosterone Influences Threat-Related Amygdala-Orbitofrontal Coupling. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu062. http://doi.org/10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed]
- Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: Does pubertal development alter threat processing? Developmental Cognitive Neuroscience. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. http://doi.org/10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. http://doi.org/10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, Barch DM. Early Life Stress and Trauma and Enhanced Limbic Activation to Emotionally Valenced Faces in Depressed and Healthy Children. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(7):800–813. e10. doi: 10.1016/j.jaac.2014.04.013. http://doi.org/10.1016/j.jaac.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural Responses to Emotional Stimuli Are Associated with Childhood Family Stress. Biological Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. http://doi.org/10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 2003;27(1):33–44. doi: 10.1016/s0149-7634(03)00007-1. http://doi.org/10.1016/S0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry. 2016;57(3):241–266. doi: 10.1111/jcpp.12507. http://doi.org/10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. http://doi.org/10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Galván A. Stress and the adolescent brain. Neuroscience & Biobehavioral Reviews. 2016;70:217–227. doi: 10.1016/j.neubiorev.2016.07.030. http://doi.org/10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. Behavioral Assessment of Emotion Discrimination, Emotion Regulation, and Cognitive Control in Childhood, Adolescence, and Adulthood. Frontiers in Psychology. 2011;2(39):1–9. doi: 10.3389/fpsyg.2011.00039. http://doi.org/10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. http://doi.org/10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, … Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. http://doi.org/10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. http://doi.org/10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, Dalgleish T, van der Wee NJA, Veltman DJ, Aleman A, … Elzinga BM. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience. 2014;9(12):2026–2033. doi: 10.1093/scan/nsu008. http://doi.org/10.1093/scan/nsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A-L, van Tol M-J, Demenescu LR, van der Wee NJa, Veltman DJ, Aleman A, … Elzinga BM. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience. 2013;8:362–369. doi: 10.1093/scan/nss007. http://doi.org/10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Longhurt J, Mazure C. Childhood Sexual Abuse as a Risk Factor for Depression in Women: Psychosocial and Neurobiological Correlates. American Journal of Psychiatry. 1999;156(6):816–828. doi: 10.1176/ajp.156.6.816. Retrieved from http://journals.psychiatryonline.org/article.aspx?articleid=173486. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ. Prefrontal–Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41(10):822–831. doi: 10.1038/npp.2015.209. http://doi.org/10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. http://doi.org/10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. http://doi.org/10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. http://doi.org/10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an introduction to methods. New York, NY: Oxford University Press; 2001. pp. 251–270. [Google Scholar]
- Yurgelun-Todd DA, Killgore WDS. Fear-related activity in the prefrontal cortex increases with age during adolescence: A preliminary fMRI study. Neuroscience Letters. 2006;406(3):194–199. doi: 10.1016/j.neulet.2006.07.046. http://doi.org/10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]