Abstract
Acute Kidney Injury (AKI) after cardiac surgery is associated with increased morbidity and mortality. Methods for measuring urine output in real-time may better ensure renal perfusion perioperatively in contrast to the current standard of care where urine output is visually estimated after empiric epochs of time. In this study we describe an accurate method for monitoring urine output continuously during cardiopulmonary bypass (CPB). This may provide a means for setting patient-specific targets for blood pressure and CPB flow as a potential strategy to reduce the risk for AKI.
Introduction
Acute kidney injury (AKI) is a devastating complication of cardiac surgery associated with prolonged hospitalization and short- and long-term mortality.1,2 There is growing emphasis on the early diagnosis of AKI and defining conditions which precede injury.3 In prior studies we identified that both excursions of mean arterial pressure (MAP) below the threshold of cerebral autoregulation and low urine flow rate during cardiopulmonary bypass (CPB) were identified risk factors for postoperative AKI.4,5 Our results suggest that close monitoring of urine flow rate might allow for individualizing MAP targets during CPB to ensure optimal renal perfusion. This practice would differ from current standards of care where urine output is imprecisely measured at arbitrary time epochs (e.g., hourly, etc). Visually estimating urine output from urine drainage bags, in fact, is reported to have a 26% measurement error.6 Our team developed a novel device to monitor minute-to-minute urine flow rate utilizing a high-precision digital scale. The purpose of this study was to test the accuracy of this device and the feasibility of its clinical use.
Methods
The urine measurement device is comprised of a 3D printed platform (Dimension 1200, Eden Prairie, MN) interfaced to an aluminum stand and a high precision, 0.1 g resolution digital scale (Ohaus ScoutPro 4001, Parsippany, NJ) that provides real time measurements of weight (Figure 1A). Urine bag weight from the scale is streamed via RS232 protocol to a laptop running ICM+ software (Cambridge, UK) used in our cerebral autoregulation studies.4,5 A threshold filter with alpha of 20 was applied to the highest and lowest 10% of measurements from the previous 10 seconds to exclude outliers resulting from mechanical interference by clinical personnel. The mean value of the remaining measurements, along with six previous 10-second mean values are used to calculate the average urine flow rate. This value was divided by the patient’s weight to report urine flow rates in ml·kg−1·min−1 or ml·kg−1·hr−1 displayed graphically through ICM+ (Figure 1B).
Figure 1.
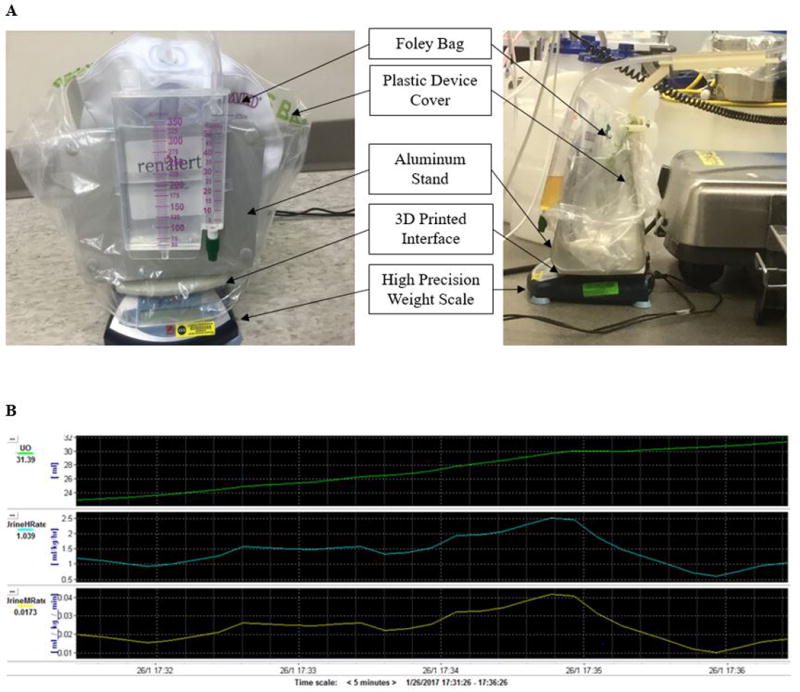
A. Real time urine output gravitational device
The urinary drainage bag is held on a 3D printed stand that sits on a high precision scale (Ohaus ScoutPro 4001, Parsippany, NJ) that is subsequently interfaced with a laptop computer. A representative view of the device underneath the operating table during clinical use is provided (right panel).
B. Laptop computer display of urine flow rate using ICM+ software program (University of Cambridge, UK). The top graph displays a clinical example of urine output (green), the second and third graphs show urine flow rate in ml·kg−1·hr−1 (blue) and ml·kg−1·min−1 (yellow) respectively.
In-Vitro Validation
The gravimetric system was tested in a series of blinded experiments using 0.9% normal saline infused via standard intravenous tubing and an infusion pump (Alaris 8100 series, Becton-Dickinson, Franklin Lakes, NJ) connected to a urine drainage system (Foley Tray ADVANCE BARDEX, C.R. Bard, Murray Hill, NJ). The specific gravity of saline is 1.006; the specific gravity of urine is 1.005–1.035.6 Computer generated randomized flow rates were programmed to mimic clinical urine flow between 0 ml·kg−1·hr−1 to 2.52 ml·kg−1·hr−1 for a hypothetical 70 kg patient. The latter urine flow rate was deemed to be a “high flow rate” in our previous study.5 For the first simulation, 25 randomized flows equivalent to 0.72 to 2.52 ml·kg−1·hr−1 for a 70 kg patient were evaluated over 75 minutes. The system outputs a value every 10 seconds, resulting in 451 measurements used to compare percent difference between infused and measured volumes. Subsequently, a period of 12 low flow rates was simulated utilizing rates from 0 to 1.3 ml·kg−1·hr−1 for a 70 kg patient over 134 minutes, yielding 805 comparisons.
Pilot Clinical Use
The device complied with FDA 21 CFR 812.3 guidelines for a non-significant-risk device and was approved by Johns Hopkins Medical Institution institutional review board (email address jhmeirb@jhmi.edu; phone: 410-955-3008). Using an IRB approved protocol that required written informed consent, 30 patients were recruited as a sub-study to a randomized trial patients undergoing surgery using CPB (www.clinicaltrials.gov NCT00981474) (Table 1). The device was placed underneath the operating table. Urine output measured with the device during CPB were compared to visual measurements recorded in the medical record. Visual measurements were made by clinicians from the calibrated collection chamber of a urine drainage system using usual clinical care. Clinicians were blinded to urine output measured by the gravimetric device. They were aware, however, that their urine measurements would be compared with measurements from the device. The IRB waived the need for informed consent of clinicians for this comparison due to the routine clinical practice of these measurements.
Table 1.
Demographic and intraoperative variables of the study cohort.
| Entire Cohort (N=30) |
|
|---|---|
| Age (yr)★ | 70±7.4 |
| Male gender, n(%) | 23 (76.7) |
| Chronic obstructive pulmonary disease, n (%) | 1 (3.6) |
| Diabetes, n(%) | 15 (51.7) |
| Coronary artery disease, n (%) | 21 (70.0) |
| Congestive heart failure, n(%) | 6 (22.2) |
| Hypertension, n(%) | 28 (100.0) |
| Baseline estimated GFR (ml/min/1.73 m2) ★ | 69.7±19.03 |
| Pulse pressure (mmHg) ★ | 61±17.0 |
| Preoperative systolic blood pressure (mmHg) ★ | 135±22.0 |
| Prior cardiac surgery, n(%) | 5 (17.2) |
| Prior carotid endarterectomy, n(%) | 2 (6.9) |
| Prior cerebral vascular accident, n(%) | 3 (10.7) |
| Peripheral vascular disease, n(%) | 4 (14.8) |
| Smoking history, n(%) | 13 (44.8) |
| Preoperative Medication | |
| ACE inhibitors-I, n(%) | 10 (41.7) |
| Anti-lipidemics, n(%) | 1 (4.2) |
| Statin, n(%) | 21 (84.0) |
| Aspirin, n(%) | 20 (76.9) |
| Beta-adrenergic receptor blocker, n(%) | 21 (84.0) |
| Calcium channel blocker, n(%) | 6 (25.0) |
| Diuretics, n(%) | 5 (20.0) |
| Heparin, n(%) | 8 (36.4) |
| Surgical procedure | |
| CABG, n(%) | 19 (63.3) |
| CABG+AVR/MVR, n(%) | 1 (3.3) |
| AVR/MVR, n(%) | 7 (23.4) |
| Aortic root, n(%) | 2 (6.7) |
| Aortic, n(%) | 1 (3.3) |
| Cardiopulmonary bypass duration (min)‡ | 107 (82–151) |
| Cross clamp (min) ‡ | 67 (54–100) |
| Lowest hemoglobin (g/dl) ‡ | 8.9 (8.2–9.5) |
| Urine Output on CPB Measured by Device (ml/kg/min) ‡ | 0.022 (0.016–0.035) |
Mean±SD.
Median, interquartile range.
ACE, angiotensin-converting enzyme; Aortic = aortic surgery; AVR = aortic valve replacement; CABG = coronary artery bypass graft; GFR, glomerular filtration rate; MVR = mitral valve replacement or repair.
Data Analysis
The in-vitro testing was compared using percent difference and confidence interval analysis between the programmed infusion pump rate and the measured urine flow rate to verify device accuracy. The widths of the confidence intervals were compared to the 95% confidence interval of the infusion pump accuracy to verify adequate sample size. Percent difference was used to compare visual urine volume measurements during CPB with that measured with the gravimetric device. Results were analyzed using Microsoft Excel (Seattle, WA) and STATA (College Station, TX).
Results
In Vitro Results
Data for low and high urine flow rate simulations are shown in Figure 2. The gravimetric system accurately tracked the manipulations to the infusion rate and volume. The median percent error across the high flow rate trial’s 451 comparisons (−2.71% difference; 95% CI, −2.81% to −2.51% difference) and the low flow rate trial’s 805 sample pairs (2.46% difference; 95% CI, 2.03% to 2.88% difference), was within the infusion pump accuracy’s 95% confidence interval (± 5% infusion error). This analysis demonstrates that the urine measurement system could accurately track flow in a dynamic simulation.7 For reference, a previously marketed device (Urinfo 2000, Baxter, Inc, Deerfield, IL) showed a measurement error of 8% per hour.8
Figure 2. In-Vitro Validation of Urine Output Device Measurement Accuracy.
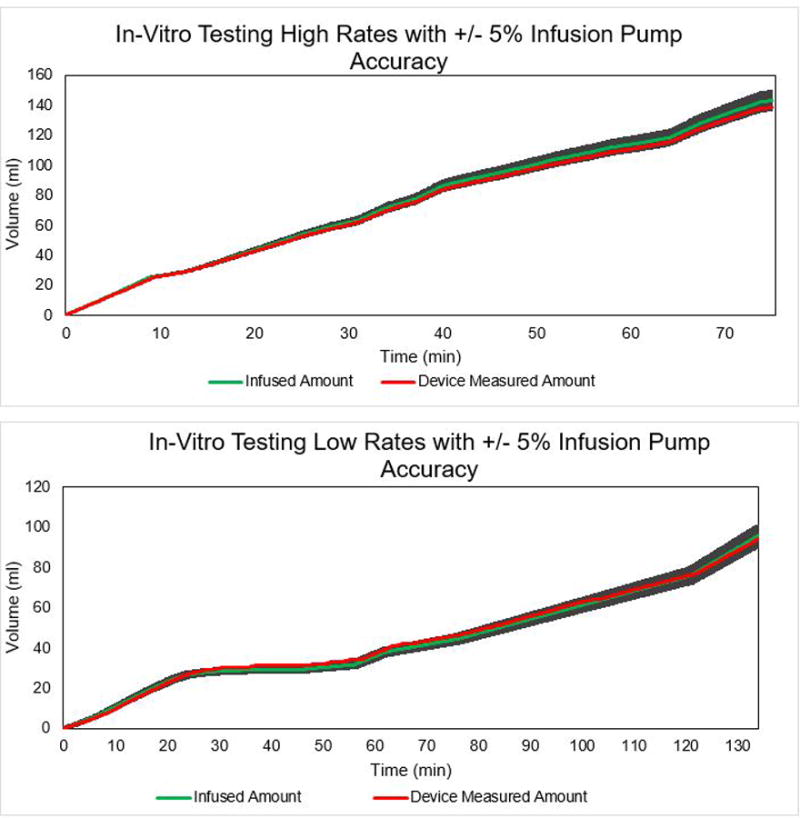
Time versus volume of 0.9% saline measured with the gravitational collection system at various flow rates programmed with a standard intravenous infusion device (Alaris 8100 series, Becton-Dickinson, Franklin Lakes, NJ). The y-axis is the cumulative fluid volume in ml simulating a range of clinically important low- and high-range urine flow rates (0 ml·kg−1·hr−1 to 2.52 ml·kg−1·hr−1 for a 70 kg patient). In the high rate trial (top panel), the total infused volume and respective rate was 143.2 ml (1.91 ml·hr−1) and the measured amount was 138.6 ml (1.85 ml·hr−1) for 3.21% difference in volume and 3.14% difference in overall rate. In the low rate trial (bottom panel), the total infused volume and respective rate was 95.7 ml (43.3 ml·hr−1) and the total measured amount was 94.2 ml (42.2 ml·hr−1) for 1.59 % difference in volume and 2.5% difference in overall rate. The shaded area of the graph represents the +/- 5% error rate which corresponds to the 95% confidence interval of the IV pump accuracy, and it can be seen that device tracks the infused data and the measured amount is virtually always within the accuracy range.
In Vivo Results
The urine measurement device was used successfully in all 30 patients. In two instances, urine output value was undecipherable from the handwritten perfusion record, underscoring the importance of automated, electronic record keeping. The median percent error in urine output measured between the device and 28 visual urine measurements was 19.3% (IQR 7.2% to 54.6%).
Discussion
Based on in vitro testing we found low measurement discrepancies between the volume of 0.9% saline infused into a urine drainage bag and that measured by the gravimetric device. Clinically, the device integrated into the operating room (OR) workflow. Given its height of 1.2 feet and footprint of <1 ft2, the device could be placed below the head of the operating table without interfering with its positioning and without being susceptible to inadvertent movement by the OR team. There was a nearly 20% median difference (IQR 7.21% to 54.6%) between the clinically measured urine volume and that measured digitally with the gravimetric device similar to that previously reported.8
In a previous study, we found that patients developing AKI after cardiac surgery had lower urine flow rates during CPB than those not developing AKI.5 When urine flow was < 1.5 ml·kg−1·hr−1, every 0.5 ml/kg/h increase in urine flow rate reduced the risk for AKI by 30%. We found that urine flow rate < 0.5 ml·kg−1·hr−1 occurred in only 5.8% of the nearly 30% of patients with AKI suggesting that oliguria based definitions for AKI may be imprecise for the intraoperative setting.5,9 Other devices for measurement of urine output are available (Accuryn, Potrero Medical, San Francisco, CA; Criticore, Bard, Murray Hill, NJ; Renalguard, Renalguard Solutions Inc., Milford, MA; Output Medical, Chicago, IL). However, unlike our device, they do not integrate with one-piece closed urinary catheter systems most commonly used in surgical patients. Given our observed error between clinical and digital urine volume measurements, the use of a more precise, real-time monitor of urine flow may provide clinicians with a means to measure urine output more precisely and more timely.
Limitations to this study include the failure to consider the effect of varying specific gravity on urine volume inferred from a weight measurement. We are not aware of data on the rapidity of urine specific gravity changes during CPB or a means to measure it continuously. This likely would add minimal error to the volume and rate of urine during CPB over the range associated with AKI.5 An approach to remedy this limitation would be scalar multiplication of urine weight by the preoperative urine specific gravity or by 1.02 which would assume a ± 1.5% error given a urine specific gravity range of 1.005 to 1.035.6 We observed negative percent error calculations during the low-flow in vitro experiments. This likely results from back-pressure generated by the infusion pumps during changes in infusion rate that were detected by the high-precision scales.7 Regardless, the device tested in this study was able to provide more granular and accurate real time measurement of urine output, than usual clinical measurements. Incorporation of these data into an electronic medical record may allow for more precision in patient management.
Acknowledgments
Disclosure of Funding:
Wallace H. Coulter Foundation Seed Grant
Japan Heart Association/Bayer Yakuhin Grant for Abroad (Daijiro Hori, Yohei Nomura)
Dr. Hogue is the principal investigator on a grant from the National Institutes of Health (RO1 092259)
Footnotes
Conflicts of Interest:
Prototype under development for commercial consideration by Aaron Chang
Clinical Trial Number and Registry URL: Substudy to NCT00981474, https://clinicaltrials.gov/ct2/show/NCT00981474?term=charles+hogue&rank=2
References
- 1.Shaw A. Update on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2012;143:676–81. doi: 10.1016/j.jtcvs.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Dasta JF, Kane-Gill SL, Durtschi AJ, et al. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–4. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 3.Katz N, Ronco C. Acute kidney stress – a useful term based on evolution in the understanding of acute kidney injury. Crit Care. 2016:20–23. doi: 10.1186/s13054-016-1184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono M, Arnaoutakis GJ, Fine DM, et al. Blood Pressure Excursions Below the Cerebral Autoregulation Threshold During Cardiac Surgery Are Associated with Acute Kidney Injury. Crit Care. 2013;41(2):464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hori D, Katz NM, Fine DM, et al. Urine Flow Rate and Cerebral Autoregulation Monitoring as a Guide of Renal Perfusion and Predictor for Cardiac Surgery-Associated Acute Kidney Injury. Br J Anaesth. 2016;117(6):733–740. doi: 10.1093/bja/aew340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otero A, Palacios F, Akinfiev T, Fernandez R. A Device for Automatically Measuring and Supervising the Critical Care Patient’s Urine Output. Sensors. 2010;10(1):934–951. doi: 10.3390/s100100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Users Manual, Alaris System with Guardrails Suite MX. Carefusion Web Site. http://www.carefusion.com/Documents/guides/user-guides/IF_Alaris-System-8015-v9-19_UG_EN.pdf. Published December 2015. Accessed October 2, 2016.
- 8.Hersch M, Einav S, Gabriel I. Accuracy and ease of use of a novel electronic urine output monitoring device compared with standard manual urinometer in the intensive care unit. J Crit Care. 2009;24(4):14–17. doi: 10.1016/j.jcrc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204.a. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]


