Abstract
Objectives
This study aimed to assess the independent associations of serum levels of vitamin B12 and plasma concentrations of homocysteine with gait speed decline.
Design, Setting, Participants
This study utilized longitudinal analysis of participants 50 years or older from The Baltimore Longitudinal Study of Aging, N=774.
Measurements
Gait speed (m/s) was assessed using the 6-meter usual pace test. Vitamin B12 and homocysteine concentrations were collected using standard clinical protocols. Linear mixed effects regression was stratified by baseline age category (50–69, 70–79, and ≥80 years old).
Results
Mean follow-up time for the total study sample was 5.4 ± 2.0 years. No association between vitamin B12 and gait speed decline over the follow-up time for any age group was found. Elevated homocysteine concentrations were associated with decline in gait speed after adjustment for covariates (50–69: β= −0.005, p=.057; 70–79: β= −0.013, p<.001, ≥80: β= −0.007, p=.054).
Conclusion
Homocysteine and vitamin B12 are inversely related, yet only homocysteine was associated with gait speed decline in this population of healthy older adults. Given these results, future research should be directed towards investigating the relationship in populations with greater variation in vitamin B12 concentrations and other mechanisms influencing homocysteine concentrations.
Keywords: Vitamin B12, homocysteine, gait speed
Introduction
In older adults, gait speed assessed at a single time point is a powerful predictor of many adverse health outcomes, including: disability, falls, hospitalization, cognitive impairment (1), mobility limitations (2), and mortality (3). Gait speed in old age likely reflects some level of decline or loss in gait speed that occurs insidiously as part of the normal aging process and in response to compounding pathologies. Gait speed decline may emerge as early as age 60 (4), thus identifying factors possibly slowing the rate of gait speed decline in older adults could potentially delay onset of many geriatric conditions.
The commonality of diet and nutritional factors suggest a potentially useful area of investigation. Micronutrient deficiencies are highly prevalent in older adults and may increase the risk of certain diseases and conditions like frailty, through inflammation, altered muscle and bone metabolism, and oxidative stress. Vitamin B12 deficiency in adults aged 65 years or older ranges from 12% to 40% (5, 6). Elevated concentrations of plasma homocysteine, which is metabolized into methionine and cysteine via vitamin B12, have been consistently associated with slow gait speed and reduced physical function, Figure 1 (7–10). As illustrated in Figure 1, elevated levels of homocysteine can result from vitamin B12 deficiency (11), which in turn is a risk factor for peripheral neuropathy (12–14), through its association with nerve demyelination in the central and peripheral nervous systems (15). Peripheral neuropathy itself has been shown to be associated with gait speed decline in older adults, independent of other disease conditions including Type 2 Diabetes (16).
Figure 1.
Potential mechanisms of decreased physical function through elevated homocysteine, reduced folate and vitamin B12
GPCR: G-protein Coupled Receptor; ROS: Reactive Oxygen Species; WMH: White Matter Hyperintensities
The specific relationship between vitamin B12 and gait speed has yet to be investigated. Cross-sectional studies assessing vitamin B12 serum levels and measures of physical function have conflicting results. In an analysis of adults aged 55 years and older in Singapore, vitamin B12 concentration examined as a continuous variable was not associated with changed in the Performance Oriented Mobility Assessment score of gait performance (9); whereas, vitamin B12 deficiency (defined as serum B12 <200 pmol/L and serum homocysteine >20 μmol/L) was associated with peripheral neuropathy and disability in a representative population of U.S. adults aged 60 years and older (12). Likewise, van Schoor et al., found women in the lowest quartile of serum vitamin B12 concentration were more likely to score lower on a walking test than women in the highest quartile, but this association was only in the cross-sectional analysis, not longitudinal analysis (17). Previous research has mainly been cross-sectional studies, further research is needed to determine if low and deficient vitamin B12 is a risk factor for age related gait speed decline in a longitudinal context.
This study aims to assess the independent associations of serum levels of vitamin B12 and plasma concentrations of homocysteine with gait speed decline in older adults participating in The Baltimore Longitudinal Study of Aging. Vitamin B12 and homocysteine could both be important independent modifiable risk factors for physical function decline opening opportunities for novel treatment and prevention approaches for mobility impairment and disability in the elderly.
Methods
The National Institute on Aging Intramural Research Program conducts the Baltimore Longitudinal Study of Aging (BLSA), which was initiated in 1958 (18). The BLSA is an open-enrollment cohort of community-dwelling adults, at least 20 years old. Participants are free of disease, cognitive and functional impairments, non-morbidly obese (body mass index; BMI <40 kg/m2), and have no reported difficulties in self-care or instrumental activities of daily living at the time of enrollment. BLSA follow-up schedules vary by participant age; those 20–59 years old are seen every 4 years, 60–79 years old are seen every 2 years, and 80 years or older are seen annually. More than 3,000 men and women have been enrolled since onset of the study.
This analysis includes BLSA participants aged 50 years and older, who have at least two observations with complete measures of gait speed, plasma homocysteine, and serum vitamin B12 between December 2004 and March 2015. Any participant who reached the age of 50 on or after December 1, 2004 with at least two complete observations was included (N=774). The final sample was reached after exclusion of a single complete observation without follow-up observations (n=162) and participants with multiple observations but only one complete observation (n=87). One participant with severe hyperhomocysteinemia (>100 μmol/L) was excluded. Participants with a single observation were unable to complete a subsequent follow-up observation due to death during the study period (n=6), their next follow-up visit was scheduled for after the end of the study observation period (n=14), or they were overdue for their next visit (n=142). Among excluded participants with multiple observations 40% were missing follow-up data on gait speed, 27% were missing data on homocysteine and vitamin B12, 25% were missing data on gait speed and vitamin B12, and 8% were missing data on all three measures.
This study was approved by the University of Texas Houston Health Science Center Committee for Protection of Human Subjects and the National Institute of Environmental Health Sciences Institutional Review Boards (IRB). All participants signed BLSA IRB approved informed consent forms at enrollment and follow-up visits.
Measurements
Gait speed was measured in meters per second (m/s). Participants completed a 6-meter normal pace walk test. To begin, participants stood with their toes touching the starting line and were timed from the first footfall to the first footfall across or touching the finish line. The use of a walking aid was allowed. Two trials were completed and the fastest trial was used in analyses. Gait slowness was defined using the Foundation for the National Institutes of Health Sarcopenia Project (FNIH) criteria of ≤0.8m/s.
Fasting serum and plasma samples were collected according to standard protocols at each BLSA visit. Vitamin B12 levels were measured using competitive protein-binding assays (Modular Analytics E170, Roche Diagnostics) (19). Total plasma homocysteine was determined using a fully automated fluorescence polarization immunoassay (Abbott Diagnostics, Abbott Park, IL, USA) (20). High homocysteine was defined as >13 μmol/L (21). Low vitamin B12 was defined as <200 pg/ml and vitamin B12 deficiency was defined as serum vitamin B12 <270 pg/ml & total homocysteine >20 μmol/l (22).
Data on demographics, health behaviors, and medical conditions were collected through an interviewer-administered questionnaire at each data collection time point. Variables included age, sex, marital status, education, household income, race, alcohol intake, smoking status (current vs former/never), meeting physical activity guidelines (≥1000 kcal/wk) in the past 12 months (23, 24), and self-reported physician diagnosed chronic diseases (cardiovascular disease, stroke, hypertension, osteoarthritis, Parkinson’s disease, any cancer, diabetes, and peripheral neuropathy). Height and weight measured by study staff were used to calculate BMI (kg/m2) and standard cut points for overweight and obesity were used to categorize individuals (25). A quantitative, self-administered food frequency questionnaire (FFQ), developed by Tufts University, was used to collect data on usual diet and supplement intake in the past 12 months (26, 27). The FFQ recorded frequency and quantity of intake for 313 food and beverage items. Daily nutrient intake of individual foods and the total diet was quantified using the Minnesota Nutrient Data System at the Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging in Boston (28). Serum folate was measured using competitive protein-binding assays (19).
Statistical Analyses
Baseline characteristics of the study sample are presented as means and percentages by baseline age category (50–69, 70–79, ≥80 years old) and differences were tested using Student’s t and chi-squared tests, as appropriate. Linear mixed effects models were used to estimate the associations of vitamin B12 and homocysteine with gait speed while accounting for the lack of independence between repeated measures. Additionally, linear mixed effects models easily accommodate the BLSA unbalanced and unequally spaced observation intervals. Models were stratified by baseline age category given gait speed declines at different rates by age groups. Random effects for the intercept and slope (follow-up time in years) were utilized to account for the excess variation implicit in the study design.
Model building followed a stepwise approach. Vitamin B12, homocysteine, and all other covariates, excluding education and race, were time-dependent. In univariate analyses, each covariate was modelled with the follow-up time variable and included in the full model if p<0.20. The full models included fixed effects, interaction terms (p<0.05), and random effects. Random effects were first assessed for removal from the full model using restricted maximum likelihood and tested using a likelihood ratio test (p<0.05). Likelihood ratio tests and maximum likelihood estimator were used to test fixed effects and interaction terms, further reducing the model. The final models include statistically and clinically significant covariates. Linear regression assumptions were checked and adjustments to the functional form of covariates were done as necessary. All statistical analyses were performed using Stata v.12 (StataCorp, College Station, TX) and alpha was set at 0.05.
Results
A comparison of excluded and included participants by baseline age category showed excluded participants in the 50–69 year-old age group had a lower mean age (p<0.001). Among participants aged 70–79 years old, excluded participants had a higher BMI (p=0.007), slower gait speed (p=0.046), and had a higher prevalence of clinically significant slowness (p=0.027) than included participants. Among those ≥80 years old, excluded participants were older (p=0.06), had a slower gait speed (p<0.001), a higher prevalence of clinically significant slowness (p<0.001), and a higher mean homocysteine (p=0.017). There was no difference in mean vitamin B12 concentrations between included and excluded participants, regardless of baseline age category.
Baseline characteristics of the study sample by age group are presented in Table 1. Mean follow-up time for the total study sample was 5.4 ± 2.0 years (range: 0.9 to 9.1 years). Participants, 80 years and older, had shorter mean follow-up time (4.6 ± 2.3 years) than participants younger than 80 years, (5.6 ± 1.9 years). Mean gait speed was above the threshold for clinically significant slowness (≤0.8 m/s) for all age groups, however, as age increased mean gait speed was slower and prevalence of clinically significant slowness higher. Mean vitamin B12 levels for all age groups, were above cut points for low vitamin B12 (29). Homocysteine concentrations ranged from 5.2 to 39.4 μmol/L. The majority of all participants at baseline were white, financially secure, non-smokers, moderate drinkers (less than 8 drinks per week), and had a BMI <30.0 kg/m2. Over 60% of participants <80 years old engaged in sufficient physical activity to meet physical activity guidelines (23, 24).
Table 1.
Baseline Characteristics of BLSA Participants by Age Category, N=774, 2004–2015
| 50–69 years old n=394 | 70–79 years old n=246 | ≥80 years old n=134 | |
|---|---|---|---|
| mean ± sd | |||
| Follow-up time, years | 5.7 ± 1.7 | 5.4 ± 2.0 | 4.6 ± 2.3 |
| Age, years | 61.2 ± 5.0 | 74.1 ± 2.9 | 84.0 ±3.5 |
| Gait Speed, m/s | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| Vitamin B12, pg/ml | 602.2 ± 280.5 | 647.3 ± 314.4 | 638.5 ± 410.5 |
| Homocysteine, μmol/L | 9.8 ± 2.8 | 10.8 ± 2.7 | 11.4 ± 3.1 |
| Folate, pg/ml | 23.2 ± 13.7 | 28.0 ± 15.0 | 26.9 ± 13.9 |
| BMI, kg/m2 | 27.8 ± 4.9 | 26.5 ± 4.3 | 25.8 ± 3.7 |
|
| |||
| n (%) | |||
|
| |||
| Women | 233 (59.1) | 102 (41.5) | 57 (42.5) |
| Slowness* | 5 (1.3) | 10 (4.0) | 18 (13.4) |
| Low B12** | 4 (1.0) | 2 (1.2) | 1 (0.8) |
| High Homocysteine*** | 34 (8.6) | 39 (15.8) | 30 (22.4) |
| White | 223 (56.6) | 171 (69.5) | 122 (91.0) |
| Houshold Income ≥$50,000 | 323 (82.8) | 185 (76.5) | 83 (63.9) |
| Graduate Degree | 247 (62.7) | 159 (64.6) | 86 (64.2) |
| Married | 288 (73.1) | 181 (73.6) | 64 (47.8) |
| BMI Categories, kg/m2 | |||
| ≤24.9 | 121 (30.7) | 95 (38.6) | 65 (48.5) |
| 25.0–29.9 | 157 (39.9) | 107 (43.5) | 50 (37.3) |
| ≥30.0 | 116 (29.4) | 44 (17.9) | 19 (14.2) |
| Adequate Physical Activity**** | 236 (60.1) | 153 (62.2) | 64 (47.8) |
| Alcoholic Drinks per week | |||
| None | 59 (15.0) | 39 (15.9) | 22 (16.4) |
| 3 or less | 201 (51.0) | 100 (40.6) | 66 (49.3) |
| 4–7 | 71 (18.0) | 59 (24.0) | 28 (20.9) |
| 8 or more | 63 (16.0) | 48 (19.5) | 18 (13.4) |
| Current Smoker | 13 (3.3) | 2 (0.8) | 1 (0.7) |
| Any Reported Chronic Disease | 314 (79.7) | 219 (89.0) | 125 (93.3) |
Missing: Household Income (n=12), Current Smoking (n=4);
Slowness: Gait Speed ≤0.8 m/s;
Low Vitamin B12; Vitamin B12 <200 pg/ml;
High Homocysteine: Homocysteine > 13 μmol/L;
Adequate Physical Activity defined as meeting recommended physical activity guideline, ≥1000 kcal/wk
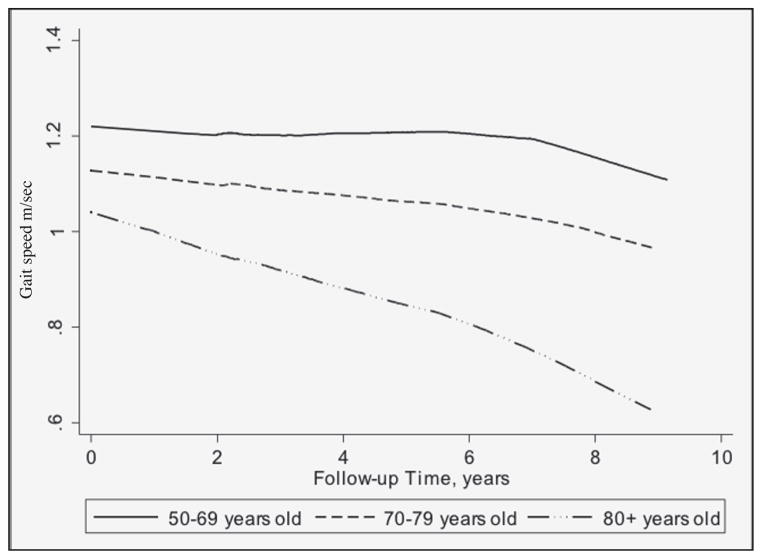
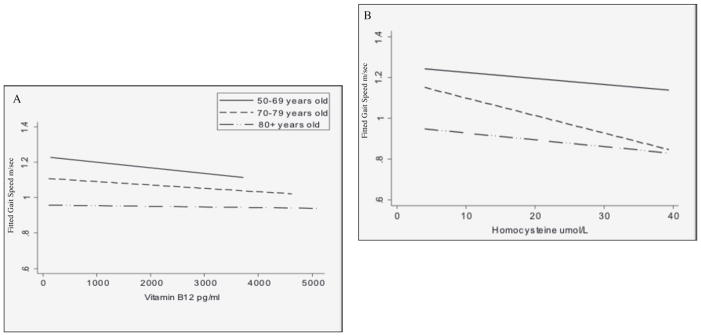
Gait speed decline by age group is illustrated in Figure 2. Participants aged 50–69 years exhibited little decline and those aged 70–79 showed gradual, yet small, decline. The oldest age group had the most and steepest decline in gait speed over the follow-up time compared to the other age groups. In Figure 3, predicted gait speed for each age category by concentrations of vitamin B12 and homocysteine are presented. Some decline in gait speed was observed with increasing vitamin B12 for all age categories with declines in gait speed among participants aged 50–69 and 70–79 most notable. As for homocysteine, an inverse relationship with gait speed was observed overall and among participants aged 70–79, after adjustment of covariates. Less steep declines in gait speed with increasing homocysteine were seen for participants 50–59 and ≥80 years old.
Figure 2.
Gait speed over the follow-up time by baseline age category, 2004–2015
Figure 3.
Fitted gait speed by (a) vitamin B12 and (b) homocysteine concentrations by baseline age category, adjusted for follow-up time, sex, race, BMI categories, physical activity, reported chronic disease, and alcohol intake
Results from linear mixed effects regression, Table 2, showed no association between vitamin B12 concentrations and gait speed decline over the follow-up time for any age group. However, elevated homocysteine concentrations were associated with decline in gait speed, Table 3, after adjustment for clinically and statistically significant covariates. Among participants aged 70–79 years old, homocysteine and gait speed over time had an inverse relationship with a one-unit increase in homocysteine associated with a decrease of 0.013 m/s in gait speed. This relationship was borderline significant among the youngest (50–69 years old) and the oldest (≥80 years old) participants.
Table 2.
Linear Mixed Effects Regression Results for Association Between Vitamin B12 and Gait Speed by Baseline Age Category
| 50–69 years old | 70–79 years old | ≥80 years old | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | se | p-value | β | se | p | β | se | p-value | |
| Follow-up Time, years | −0.0015 | 0.002 | .427 | −0.007 | 0.002 | .005 | −0.029 | 0.004 | <.001 |
| Vitamin B12. pg/ml | −0.0000006 | 0.00002 | .970 | 0.00002 | 0.00001 | .090 | −0.000005 | 0.00002 | .772 |
| Women | −0.043 | 0.016 | .009 | −0.074 | 0.024 | .002 | −0.053 | 0.035 | .129 |
| White | 0.088 | 0.017 | <.001 | 0.094 | 0.025 | <.001 | 0.166 | 0.060 | .005 |
| BMI Categories, kg/m2 | |||||||||
| ≤24.9 | Reference | Reference | Reference | ||||||
| 25.0–29.9 | −0.026 | 0.015 | .082 | −0.034 | 0.018 | .058 | −0.048 | 0.023 | .038 |
| ≥30.0 | −0.056 | 0.018 | .002 | −0.075 | 0.026 | .004 | −0.100 | 0.035 | .005 |
| Adequate Physical Activity | 0.024 | 0.011 | .026 | 0.023 | 0.014 | .101 | 0.011 | 0.016 | .487 |
| Alcoholic Drinks per week | |||||||||
| None | Reference | Reference | Reference | ||||||
| 3 or less | 0.053 | 0.018 | .003 | 0.009 | 0.022 | .671 | 0.0008 | 0.024 | .732 |
| 4–7 | 0.051 | 0.022 | .019 | 0.014 | 0.026 | .558 | 0.029 | 0.031 | .341 |
| 8 or more | 0.034 | 0.024 | .151 | 0.027 | 0.028 | .345 | 0.052 | 0.035 | .143 |
| Any Reported Chronic Disease | −0.028 | 0.018 | .117 | −0.047 | 0.032 | .136 | −0.067 | 0.049 | .170 |
Table 3.
Linear Mixed Effects Regression Results for Association Between Homocysteine and Gait Speed by Baseline Age Category
| 50–69 years old | 70–79 years old | ≥80 years old | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | se | p-value | β | se | p | β | se | p-value | |
| Follow-up Time, years | −0.001 | 0.003 | .624 | −0.010 | 0.004 | .009 | −0.033 | 0.006 | <.001 |
| Homocysteine, μmol/L | −0.005 | 0.003 | .057 | −0.013 | 0.003 | <.001 | −0.007 | 0.004 | .054 |
| Women | −0.050 | 0.017 | .005 | −0.096 | 0.025 | <.001 | −0.073 | 0.035 | .040 |
| White | 0.090 | 0.018 | <.001 | 0.087 | 0.025 | .001 | 0.146 | 0.060 | .015 |
| BMI Categories, kg/m2 | |||||||||
| ≤24.9 | Reference | Reference | Reference | ||||||
| 25.0–29.9 | −0.023 | 0.017 | .199 | −0.031 | 0.021 | .140 | −0.047 | 0.027 | .086 |
| ≥30.0 | −0.047 | 0.020 | .018 | −0.075 | 0.028 | .007 | −0.103 | 0.041 | .011 |
| Adequate Physical Activity | 0.028 | 0.013 | .028 | 0.015 | 0.018 | .393 | −0.001 | 0.020 | .941 |
| Alcoholic Drinks per Week | |||||||||
| None | Reference | Reference | Reference | ||||||
| 3 or less | 0.058 | 0.020 | .004 | 0.024 | 0.026 | .372 | 0.006 | 0.032 | .849 |
| 4–7 | 0.050 | 0.025 | .041 | 0.029 | 0.030 | .327 | 0.018 | 0.039 | .646 |
| 8 or more | 0.037 | 0.027 | .161 | 0.052 | 0.033 | .118 | 0.054 | 0.045 | .222 |
| Any Reported Chronic Disease | −0.030 | 0.019 | .125 | −0.071 | 0.035 | .042 | −0.073 | 0.057 | .204 |
Discussion
In this population of healthy adults aged 50 years or older, elevated homocysteine but not vitamin B12, was associated with gait speed decline over an average follow-up of 5.4 years. The results of this study support previous research identifying elevated homocysteine levels as a risk factor for gait speed and physical function decline (7–9, 20, 30). The lack of a relationship between vitamin B12 and gait speed has not been previously explored. Yet, these results are consisted with other null results investigating the associations between vitamin B12 and physical function (9, 17, 31), balance (9, 12), and gait performance (9). Vitamin B12 deficiency has been shown to be a risk factor for frailty in older women (32). Gait speed is often used in the measurement of frailty in addition to muscle strength, endurance, physical activity, and weight (33). In light of the present results, the relationship between vitamin B12 and frailty is probably not mediated through gait speed.
The multiple mechanisms depicted in Figure 1 by which homocysteine could influences gait speed are most likely mediated by impaired muscle, vascular, and nerve function. In a previous analysis of this study population, elevated homocysteine was associated with decline in muscle strength in both older adult men and women over time (34). Altered G-protein coupled receptor and increased TGF-b signaling from elevated homocysteine could result in reduced muscle regeneration. Elevated homocysteine also reduces blood flow to muscle cells through decreased bioavailability of nitric oxide (11). Additionally, elevated homocysteine is a known risk factor for stroke, vascular diseases (35), and white matter hyperintensities (WMH) (36). WMH are correlated with poor lower extremity function (37) through disruption of frontal lobe circuits that control gait and balance and interference with motor fibers important for lower extremity motor control (20). Recent analyses from the InCHIANTI study showed elevated homocysteine to be associated with worse sensory and motor peripheral nerve function (38), which has also been associated with poor physical function in older adults (16, 39).
Vitamin B12 and folate are necessary for the metabolism and removal of homocysteine, which implies a direct inverse relationship. Yet in the present study, elevated vitamin B12 was not protective against gait speed decline in the magnitude that elevated homocysteine was observed to be detrimental. This may be explained by differences in the relative levels of vitamin B12 and folate levels, which were largely within normal limits in contrast to homocysteine concentrations in which 13.3% were in the abnormally high range, >13 μmol/L. Previous randomized control trials of homocysteine lowering therapies, i.e. vitamin B12 and folate supplementation, have not consistently shown reductions in cognitive decline (40, 41). physical function decline (42), nor reduced risk of cardiovascular disease (43, 44). Alternatively, homocysteine might be a marker of disease severity rather than a causal agent of disease. Concerns of reverse causality or residual confounding in observational studies might be contributing to the results seen here and in other research. These findings call into question the comprehensiveness of the model presented in Figure 1 and suggest a need to identify a broader set of other factors that may influence homocysteine concentrations.
Additional factors influencing the variation in homocysteine include genetic (MTHFR C677T), behavioral (smoking, coffee and alcohol consumption) (46), and nutritional factors. Both vitamin B6 and fat intake could contribute to variation in homocysteine concentrations. Similar to vitamin B12, vitamin B6 has an inverse relationship with homocysteine as it serves as a cofactor of an enzyme that metabolizes of homocysteine into cysteine (47). In a subgroup of participants aged 60 years or older in this analysis with a FFQ (n=549), B vitamin intake from food and supplements was negatively correlated with homocysteine concentrations (vitamin B6: r= −0.13, p=0.002; vitamin B12: r= −0.03, p=0.443; folate: r= −0.12, p=0.004). Observational studies of total fat intake and homocysteine concentration have noted positive associations between saturated fats and monounsaturated fat,(48–50) and an inverse association with very-long-chain n-3 fatty acids (VLC n-3 PUFA) (48). When stratified by total intake of all B vitamins, the association between VLC n-3 PUFA and homocysteine remained only in the highest quartile of vitamin B intake (48). This has important implications for the present study since the majority of participants had high vitamin B12 and folate levels. In the same subgroup of participants with FFQ data, VLC n-3 PUFAs were negatively and total fat, saturated fat, and monounsaturated fat positively correlated with homocysteine concentrations (data not shown). Thus, in populations with high levels of folate and vitamin B12, fat intake could explain variation in homocysteine concentration. The biological mechanisms of this relationship are not well understood and further research into the associations between fat intake, homocysteine, and physical function is needed to address this hypothesis.
The lack of association between vitamin B12 and age-related gait speed decline in the present analysis may stem from the lack of variation in vitamin B12 concentrations with very few participants having low vitamin B12 (<200 pg/ml; 1%) and none exhibiting vitamin B12 deficiency (serum vitamin B12 <270 pg/ml & total homocysteine >20 μmol/L). As a further complication, vitamin B12 deficiency due to malabsorption occurs over a long time. The time it takes for the body to deplete stores of vitamin B12 and deficiency symptoms to appear can be between 2 to 5 years (51). Thus, malabsorption must occur for many years before enough depletion has occurred to cause a detectable deficiency.
Many older adults are at risk for vitamin B12 deficiency as increasing age is associated with changes in absorption and metabolism of vitamin B12. Hydrochloric acid and gastric protease in the stomach releases vitamin B12 bound to protein in food. Supplements of vitamin B12 and fortified foods are in free form; thus separation is not necessary. Free vitamin B12 attaches to intrinsic factor and is then absorbed. Malabsorption can occur when intrinsic factor is unavailable to attach to vitamin B12 and can result in vitamin B12 deficiency. Adults aged 65 years or older are at particular risk of malabsorption due to a reduction in hydrochloric acid secretion as age increases (52). Thus, the Institute of Medicine recommends older adults obtain vitamin B12 from supplements or fortified foods (53).
Clinical measurement of vitamin B12 may inaccurately describe vitamin B12 levels if supplements containing vitamin B12 were recently taken. Thus, supplement use prior to testing for deficiency can mask the presence of a deficiency. In the subgroup of participants with data from a FFQ (n=549), 81% reported supplement use in the past 12 months. While, there was a significant difference in mean vitamin B12 concentration between participants who did (645.3 pg/ml) and did not (526.3 pg/ml) take supplements (p<0.001), both groups had mean serum concentrations of vitamin B12 above dietary reference intakes (53). Thus the lack of participants with vitamin B12 deficiency is not likely a false negative due to recent supplement intake.
There are some limitations to take into account when interpreting these results. Excluded participants ≥80 years old had slower gait speed and higher homocysteine concentrations at baseline and excluded participants aged 70–79 had slower gait speeds than included participants. The excluded participants may have been less healthy than included participants and thus unable to fully complete a second observation. The observed significant relationship between elevated homocysteine and gait speed decline in participants aged 70–79 years old may be explained by exclusion of participants with slower gait speeds at baseline, which could strengthen the observed relationship. As well, the BLSA study population is a more robust and healthy group of older adults with low prevalence of obesity and smoking and thus may not be comparable to the general population (54, 55).
Study strengths include the long follow-up time for measures of gait speed, homocysteine, and vitamin B12. Vitamin B12 was measured directly using standard protocols, which reduces potential bias associated with self-reported food and supplement intakes. Gait speed was also measured using standard protocols and is a robust tool for defining poor physical function in older adults. The use of linear mixed effects models allowed for repeated measures of vitamin B12, homocysteine, and gait speed to be analyzed and relationships over time to be taken into account.
In conclusion, this study found no association between serum vitamin B12 concentrations and gait speed decline in a population of well-functioning older adults over time. Yet, there was an inverse relationship between homocysteine and gait speed, which is consistent with previous research. Given these results, future research should be directed towards investigating the relationship between vitamin B12 in other populations with more variation in vitamin B12 as well as among older adults who have vitamin B12 deficiency. Investigation of other mechanisms that may contribute to elevated homocysteine levels aside from low vitamin B12 are also warranted.
Footnotes
Conflict of Interest: MLV, KPG, STL, EMS, & RSD have no conflicts of interest to declare
Ethical standard: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board at the National Institutes of Health. Written informed consent was obtained from all participants.
Disclosure: Dr. Vidoni has nothing to disclose. Dr. Pettee Gabriel has nothing to disclose. Dr. Luo has nothing to disclose. Grants/Research: Funding to University of Texas Health Science Center, School of Public Health from NIH, National Parkinson Foundation, International Parkinson and Movement Disorder Society, and some of these funds support Dr. Luo’s salary as well as his research efforts. He participates in the translation program for the MDS-UPDRS and UDysRS and receives funds to conduct and present the research directed to University of Texas Health Science Center, School of Public Health from the International Parkinson and Movement Disorder Society (IPMDS) for this effort. Salary: University of Texas Health Science Center, School of Public Health. Dr. Simonsick has nothing to disclose. Dr. Day has nothing to disclose.
References
- 1.Van Kan GA, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 2.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people: results from The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 3.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shumway-Cook A, Guralnik JM, Phillips CL, Coppin AK, Ciol MA, Bandinelli S, et al. Age-associated declines in complex walking task performance: The Walking InCHIANTI Toolkit. J Am Geriatr Soc. 2007;55(1):58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baik H, Russell R. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19(1):357–77. doi: 10.1146/annurev.nutr.19.1.357. [DOI] [PubMed] [Google Scholar]
- 6.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994 Jul;60(1):2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Soumare A, Elbaz A, Ducros V, Tavernier B, Alperovitch A, Tzourio C. Cross-sectional association between homocysteine and motor function in the elderly. Neurology. 2006 Sep 26;67(6):985–90. doi: 10.1212/01.wnl.0000237325.16502.08. [DOI] [PubMed] [Google Scholar]
- 8.Rolita L, Holtzer R, Wang C, Lipton RB, Derby CA, Verghese J. Homocysteine and mobility in older adults. J Am Geriatr Soc. 2010;58(3):545–50. doi: 10.1111/j.1532-5415.2010.02718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng TP, Aung KC, Feng L, Scherer SC, Yap KB. Homocysteine, folate, vitamin B-12, and physical function in older adults: cross-sectional findings from The Singapore Longitudinal Ageing Study. Am J Clin Nutr. 2012 Dec;96(6):1362–8. doi: 10.3945/ajcn.112.035741. [DOI] [PubMed] [Google Scholar]
- 10.Gerdhem P, Ivaska KK, Isaksson A, Pettersson K, Väänänen HK, Obrant KJ, et al. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. Journal of Bone and Mineral Research. 2007;22(1):127–34. doi: 10.1359/jbmr.061003. [DOI] [PubMed] [Google Scholar]
- 11.Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. 2013;14(7):15074–91. doi: 10.3390/ijms140715074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberlin BS, Tangney CC, Gustashaw KA, Rasmussen HE. Vitamin B12 deficiency in relation to functional disabilities. Nutrients. 2013;5(11):4462–75. doi: 10.3390/nu5114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saperstein DS, Barohn RJ. Peripheral neuropathy due to cobalamin deficiency. Current Treatment Options in Neurology. 2002;4(3):197–201. doi: 10.1007/s11940-002-0036-y. [DOI] [PubMed] [Google Scholar]
- 14.Leishear K, Boudreau RM, Studenski SA, Ferrucci L, Rosano C, Rekeneire N, et al. Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. J Am Geriatr Soc. 2012;60(6):1057–63. doi: 10.1111/j.1532-5415.2012.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weir DG, Scott JM. Brain function in the elderly: role of vitamin B12 and folate. Br Med Bull. 1999;55(3):669–82. doi: 10.1258/0007142991902547. [DOI] [PubMed] [Google Scholar]
- 16.Strotmeyer ES, de Rekeneire N, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: The Health, Aging, and Body Composition (Health ABC) Study. Diabetes Care. 2008 Sep;31(9):1767–72. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Schoor N, Swart K, Pluijm S, Visser M, Simsek S, Smulders Y, et al. Cross-sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr. 2011;66(2):174–81. doi: 10.1038/ejcn.2011.151. [DOI] [PubMed] [Google Scholar]
- 18.Shock NW. Normal human aging: The Baltimore Longitudinal Study of Aging. JAMA. 1986 Feb 21;255(7):960. [Google Scholar]
- 19.Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from The Baltimore Longitudinal Study of Aging. PLoS One. 2010;5(4):e10099. doi: 10.1371/journal.pone.0010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo HK, Liao KC, Leveille SG, Bean JF, Yen CJ, Chen JH, et al. Relationship of homocysteine levels to quadriceps strength, gait speed, and late-life disability in older adults. J Gerontol A Biol Sci Med Sci. 2007 Apr;62(4):434–9. doi: 10.1093/gerona/62.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Second national report on biochemical indicators of diet and nutrition in the US population: 2012. Atlanta, GA: US Department of Health and Human Services; 2012. [Google Scholar]
- 22.Clarke R, Grimley Evans J, Schneede J, Nexo E, Bates C, Fletcher A, et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004 Jan;33(1):34–41. doi: 10.1093/ageing/afg109. [DOI] [PubMed] [Google Scholar]
- 23.Tucker JM, Welk GJ, Beyler NK. Physical activity in US adults: compliance with the physical activity guidelines for Americans. Am J Prev Med. 2011;40(4):454–61. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Havlik RJ, Simonsick EM, Sutton-Tyrrell K, Newman A, Danielson ME, Brock DB, et al. Association of physical activity and vascular stiffness in 70-to 79-year-olds: The Health ABC Study. J Aging Phys Act. 2003;11(2):156–66. [Google Scholar]
- 25.Panel, NHLBI Obesity Education. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. 1998 [PubMed] [Google Scholar]
- 26.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 27.Kristal AR, Shattuck AL, Henry HJ, Fowler AS. Rapid assessment of dietary intake of fat, fiber, and saturated fat: Validity of an instrument suitable for community intervention research and nutritional surveillance. Am J Health Promot. 1990;4(4):288–95. doi: 10.4278/0890-1171-4.4.288. [DOI] [PubMed] [Google Scholar]
- 28.Berndt SI, Carter HB, Landis PK, Tucker KL, Hsieh LJ, Metter EJ, et al. Calcium intake and prostate cancer risk in a long-term aging study: The Baltimore Longitudinal Study of Aging. Urology. 2002;60(6):1118–23. doi: 10.1016/s0090-4295(02)01991-x. [DOI] [PubMed] [Google Scholar]
- 29.Clarke R, Sherliker P, Hin H, Nexo E, Hvas AM, Schneede J, et al. Detection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalamin. Clin Chem. 2007 May;53(5):963–70. doi: 10.1373/clinchem.2006.080382. [DOI] [PubMed] [Google Scholar]
- 30.Kado DM, Bucur A, Selhub J, Rowe JW, Seeman T. Homocysteine levels and decline in physical function: MacArthur Studies of Successful Aging. Am J Med. 2002;113(7):537–42. doi: 10.1016/s0002-9343(02)01269-x. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary F, Allman-Farinelli M, Petocz P, Samman S. Nutritional status but not vitamin deficiencies are associated with low functional scores. J Aging Res Clin Pract. 2013;2:216–20. [Google Scholar]
- 32.Matteini A, Walston J, Fallin M, Bandeen-Roche K, Kao W, Semba R, et al. Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging. 2008;12(5): 303–8. doi: 10.1007/BF02982659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson ML, Gabriel KP, Luo ST, Simonsick EM, Day RS. Relationship between homocysteine and muscle strength decline: The Baltimore Longitudinal Study of Aging. 2016 doi: 10.1093/gerona/glx161. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002 Nov 23;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan A, Hunt BJ, O’Sullivan M, Bell R, D’Souza R, Jeffery S, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2004 Jan;127(Pt 1):212–9. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]
- 37.Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996 Aug;27(8):1274–82. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 38.Leishear K, Ferrucci L, Lauretani F, Boudreau RM, Studenski SA, Rosano C, et al. Vitamin B12 and homocysteine levels and 6-year change in peripheral nerve function and neurological signs. J Gerontol A Biol Sci Med Sci. 2012 May;67(5):537–43. doi: 10.1093/gerona/glr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: The Women’s Health and Aging Study. Diabetes Care. 2000 Nov;23(11):1642–7. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 40.Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJ, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr. 2014 Aug;100(2):657–66. doi: 10.3945/ajcn.113.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith AD, Refsum H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu Rev Nutr. 2016;36(1) doi: 10.1146/annurev-nutr-071715-050947. [DOI] [PubMed] [Google Scholar]
- 42.Swart KM, Ham AC, van Wijngaarden JP, Enneman AW, van Dijk SC, Sohl E, et al. A randomized controlled trial to examine the effect of 2-year vitamin B12 and folic acid supplementation on physical performance, strength, and falling: additional findings from the B-PROOF Study. Calcif Tissue Int. 2015:1–10. doi: 10.1007/s00223-015-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–6. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 44.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170(18):1622–31. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 45.Guttormsen A, Svarstad E, Ueland P, Refsum H. Elimination of homocysteine from plasma in subjects with endstage renal failure. Irish J Med Sci. 1995;164(Suppl 15):8–9. [Google Scholar]
- 46.Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006 Jun;136(6 Suppl):1731S–40S. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- 47.Kuo HK, Sorond FA, Chen JH, Hashmi A, Milberg WP, Lipsitz LA. The role of homocysteine in multisystem age-related problems: a systematic review. J Gerontol A Biol Sci Med Sci. 2005 Sep;60(9):1190–201. doi: 10.1093/gerona/60.9.1190. [DOI] [PubMed] [Google Scholar]
- 48.Berstad P, Konstantinova SV, Refsum H, Nurk E, Vollset SE, Tell GS, et al. Dietary fat and plasma total homocysteine concentrations in 2 adult age groups: The Hordaland Homocysteine Study. Am J Clin Nutr. 2007 Jun;85(6):1598–605. doi: 10.1093/ajcn/85.6.1598. [DOI] [PubMed] [Google Scholar]
- 49.Li D, Mann NJ, Sinclair AJ. A significant inverse relationship between concentrations of plasma homocysteine and phospholipid docosahexaenoic acid in healthy male subjects. Lipids. 2006;41(1):85–9. doi: 10.1007/s11745-006-5074-x. [DOI] [PubMed] [Google Scholar]
- 50.Villegas R, Salim A, Collins M, Flynn A, Perry I. Dietary patterns in middle-aged Irish men and women defined by cluster analysis. Public Health Nutr. 2004;7(08):1017–24. doi: 10.1079/PHN2004638. [DOI] [PubMed] [Google Scholar]
- 51.Allen LH. Causes of vitamin B12 and folate deficiency. Food and nutrition bulletin. 2008;29(2 suppl1):S20–34. doi: 10.1177/15648265080292S105. [DOI] [PubMed] [Google Scholar]
- 52.National Institutes of Health. Vitamin B12: Dietary Supplement Fact Sheet. http://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/. Updated June, 24 2011.
- 53.Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 54.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 55.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, et al. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012 Jan 3;125(1):45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]