Abstract
Patterns and features of substance use and abuse vary across the menstrual cycle in humans. Yet, little work has systematically examined the within-person relationships between ovarian hormone changes and alcohol use across the menstrual cycle. Our study was the first to examine the roles of within-person levels and changes in estradiol (E2) and progesterone (P4) in relation to daily alcohol use and binge drinking in young women. Participants were 22 naturally-cycling women, ages 18–22, recruited through a university subject pool who reported any alcohol use and who completed a screening visit assessing study eligibility, followed by 35 subsequent days of data collection. E2 and P4 were obtained via enzyme immunoassay of saliva samples collected by participants each morning, thirty minutes after waking. Presence and degree of daily substance use were obtained using an adaptation of the Timeline FollowBack Interview completed daily. Results indicated that elevated E2 in the context of decreased P4 levels were associated with higher risk of drinking and binge drinking. These effects were present only on weekend days. Results are suggestive of a dual risk model in which both ovulatory E2 increases and perimenstrual P4 decreases increase risk for drinking. Differential associations of steroids with drinking across the menstrual cycle may suggest the need for clinical assessment of substance use to take into account hormone dynamics and menstrual cycle phase.
Keywords: ovarian steroid hormones, estradiol, progesterone, alcohol use, binge drinking, menstrual cycle
Substance abuse affects at least 8% of individuals over their lifetime and is highly costly to society in terms of lost productivity, treatment costs, and health care and mortality costs (Compton et al., 2007; SAMHSA, 2001). Although males are approximately 2 to 3 times as likely to exhibit problems with substance abuse, female substance abuse is on the rise (Brady & Randall, 1999). Females acquire problems with substance abuse at a much more rapid rate than their male counterparts, find it more difficult to quit, and relapse more frequently following periods of abstinence in both humans and animals (reviewed by Anker & Carroll, 2010; Becker & Hu, 2008; Hudson & Stamp, 2011). Furthermore, females often exhibit more severe brain, heart, muscle, and liver consequences than their male counterparts and exhibit more functional impairment in relationships (Mann et al., 2005). These effects are particularly pronounced and well-established in relation to stimulants, although these effects have been noted in numerous other substance classes as well (Becker & Hu, 2008; Roth, Cosgrove, & Carroll, 2004).
Patterns and associated features of substance abuse appear to vary cyclically across the menstrual cycle in humans. Across different substances, craving and the subjective effects of substances are reported to be higher during the follicular phase, when estradiol [E2] is rising and progesterone [P4] is low, and during cycle phases in which P4 is declining, such as during the week leading up to menses (Evans, Haney, & Foltin, 2002; Schiller et al., 2012; Sofuoglu et al., 1999; Terner & de Wit, 2006; Weinberger et al., 2015). Further, quit attempts for smoking are more successful during periods in which P4 is elevated relative to E2 (Mazure et al., 2011; Saladin et al., 2015).
Experimental animal work extends human research on menstrual phase influences on substance abuse by suggesting that higher circulating levels of E2, relative to P4, enhance motivation to abuse substances in females (Hu & Becker, 2003; Lynch et al., 2001). This has also been demonstrated with regard to risky behaviors in women (e.g., borderline personality disorder symptoms; Eisenlohr-Moul et al., 2015; emotional eating; Klump et al., 2013). Yet, these interactive effects of E2 and P4 have not been evaluated in relation to substance abuse and specifically on drinking in humans. The only known study to examine hormonal effects on drinking found an association between higher E2 and increased drinking among 60 premenopausal women using an assessment of two time points one year apart, averaged together, with no consideration of P4 (Muti et al., 1998). In sum, the results of previous studies in animals and humans, most of which have utilized cycle phase, suggest that elevated E2 and lower P4 may be risk factors for substance abuse. Therefore, the current study examined the dynamic effects of E2 and P4 across the menstrual cycle on a particularly common forms of substance use and abuse in women: alcohol use and binge drinking.
Hormonal effects on human behavior are often strongly shaped by environmental factors. For example, the influence of cyclical hormonal status on female sexual behavior has been found to differ according to romantic relationship status (Haselton & Gandestad, 2006). In college student populations, drinking is most pronounced on the weekends, likely due to increased drinking opportunities (Del Boca, Darkes, Greenbaum, & Goldman, 2004). Therefore, in our study, weekend status was considered as important permissive environmental factor likely to facilitate an impact of hormonal states on drinking. With regard to alcohol use in young women, the availability of drinking opportunities may be a particularly important environmental factor, including whether one’s friends drink, attendance at social events, and availability of alcohol. In the present study, we also considered a variety of possible covariates that may influence drinking, hormones, or both, including legal drinking status, BMI, race, daily medication use, and daily positive and negative affect. These covariates were retained when they demonstrated significant associations with drinking or hormones.
Our study was the first to examine ovarian hormonal effects across the menstrual cycle on alcohol use and binge drinking within young adult women by repeatedly evaluating levels of E2 and P4 as well as drinking across the menstrual cycle. Although no experimental data are available to speak to the temporal delay of behavioral ovarian steroid effects in humans, animal data indicate a lag time of roughly 24 hours (Bless et al., 1997); therefore, a one-day lag was used to evaluate the effects of yesterday’s hormone levels on today’s alcohol use. Consistent with the existing evidence, our hypotheses were that the likelihood of drinking and binge drinking will be increased when E2 levels are high and P4 levels are low, an interactive effect that is consistent with a late follicular, or preovulatory, phase effect. We also hypothesized that weekend status will play a permissive role in the association between hormones and drinking, such that the links between yesterday’s hormones and today’s drinking will be stronger on weekends versus weekdays. An additional set of analyses was carried out to confirm the directionality of hormone-drinking associations by predicting tomorrow’s hormone levels from today’s alcohol use (reverse directionality).
Method
Participants
The original sample was comprised of 33 naturally-cycling women recruited through a university subject pool and flyers posted around the university and surrounding areas. The sample utilized in the present study consisted of the subsample of 22 women (67%) who reported at least one episode of drinking in daily reports. This subsample of 22 women were aged 18–22 (Mean = 19.99, SD = 1.48). They identified as Caucasian (70.6%), African American (18.2%) or other (11.8%), and 1 participant identified as Hispanic, approximately representative of the university population (i.e., 75% Caucasian, 15% African-American, 4% Asian, and 6% Hispanic or Latino). Average BMI was 24.10 (SD = 4.92).
Procedure
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 and was approved by the local university IRB. After signing informed consent consistent with local IRB, APA, and NIH guidelines, participants completed a screening visit during which study eligibility criteria were assessed. Exclusionary criteria included primary sensorimotor handicap, neurological disorder (e.g., seizure disorders, brain tumor, cerebral palsy, hydrocephalus, head injury with loss of consciousness), known pervasive developmental disorder (i.e., autism, Asperger’s, Rett’s, childhood disintegrative disorder), reported psychosis (i.e., schizophrenia, hallucinations, delusions), diagnosed intellectual disability, known hormonal abnormalities (e.g., Polycystic Ovarian Syndrome, thyroid conditions), including irregular cycles (i.e., cycles shorter than 21 days or longer than 35 days or fluctuating by more than 10 days across cycles, per self report), and current medical use of hormonal preparations (e.g., oral contraceptives), psychostimulants, or antipsychotic medications. Other medication use was measured daily in an open-ended manner. 3 women in the drinking subsample reported using prescribed SSRIs daily (between-person variable). Of note, SSRI use was not associated with mean drinking or hormone levels across the cycle. At the screening appointment, participants’ weight and height were also measured since Body Mass Index (BMI) can impact hormone levels (Lukanova et al., 2004).
Eligible and interested participants subsequently completed 35 days of daily data collection. Every morning, participants provided saliva samples (described below), as well as a short questionnaire about medication use, food intake, and onset of menses. All participants reported taking some form of medication over the course of the study. The most common included over-the-counter medications for pain, gastrointestinal distress, cold and flu, and allergies. No participants reported use of corticosteroids. None of the participants reported eating immediately upon waking, suggesting that saliva collection directions were followed. At this time, participants also completed a measure of drinking, described below. These measures were time- and date-stamped, and daily reminders about data collection were provided by email. Participants who completed any follow-up data collection (i.e., completed at least one additional questionnaire or provides at least one additional saliva sample) received $50, participants who completed over 50% of data collection received $75, and participants who completed all follow-up data collection received $100.
Measures
Cycle Phase Coding
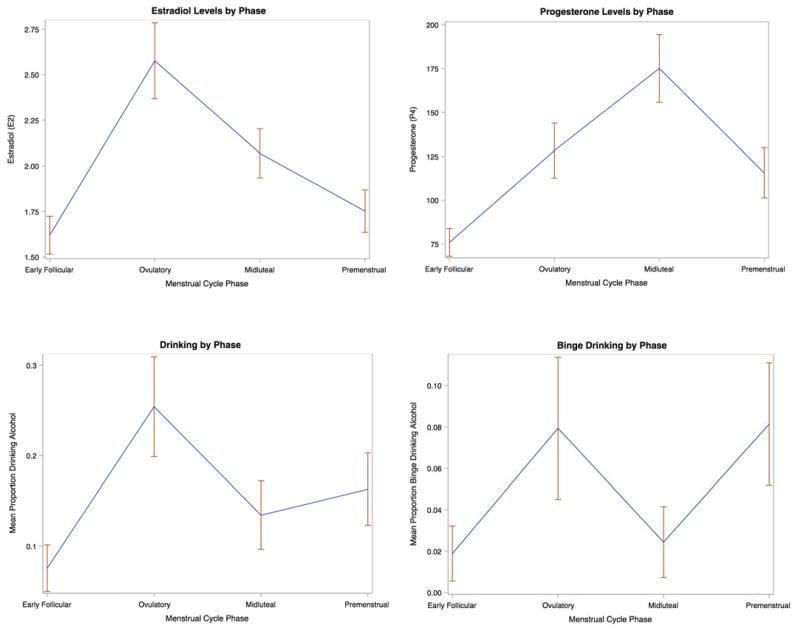
Cycle day was coded using methods described by Edler (2003). The first day of self-reported menses was coded as 1; from this day 1, cycle day was counted backward to −15, and forward to +10. There was no day 0. Cycle phases were coded as Early Follicular (days +3 to +7), Ovulatory (−15 to −12), Midluteal (−9 to −5) and Premenstrual (−3 to +1). Appropriate categorization was verified using visual inspection of hormone data to confirm that (1) the ovulatory phase contained the peak E2 level within a given person and that (2) the midluteal phase contained the peak level of P4 within a given person. Grand means and SDs for E2 and P4 by cycle phase are as follows (graphed in Figure 1): early follicular levels of E2 (M = 1.61, SD = .73) and P4 (M = 75.60, SD = 53.88) were low; ovulatory levels of E2 (M = 2.57, SD = 1.16) were high, and ovulatory levels of P4 were moderate (M = 128.54, SD = 87.79); midluteal levels of E2 (M = 2.06, SD = .89) were moderate, and midluteal levels of P4 were high (M = 175.43, SD = 120.11); finally, premenstrual levels of E2 (M = 1.75, SD = .72) and P4 (M = 115.59, SD = 89.97) were moderate (see Figure. Therefore, E2 and P4 showed expected trajectories across the menstrual cycle (Strauss & Barbieri, 2013).
Figure 1.
Means and SDs for Estradiol and Progesterone (pg/mL; TOP ROW) and Drinking Variables (BOTTOM ROW) across the menstrual cycle.
Means and Standard Deviations for Estradiol, Progesterone, Probability of Drinking, and Probability of Binge Drinking Across Menstrual Cycle Phases.
Daily Salivary E2 and P4
Saliva samples were collected via passive drool by participants each morning thirty minutes after waking and subsequently frozen. Samples from every other day were assayed due to cost. Participants were instructed not to eat, drink, brush teeth, or smoke before saliva collection. No participant reported violation of this morning protocol in daily diaries. Salivary E2 (pg/mL) and P4 (pg/mL) were determined using enzyme immunoassay kits available through Salimetrics and assayed through campus Clinical Center for Translation Science. For E2, the Salimetrics 17β-Estradiol immunoassay kit had a sensitivity of 0.1 pg/mL, and the sample precision (% coefficient of variation) ranged from 0.7 to 14.5. For P4, the Salimetrics immunoassay kits had a sensitivity of 5 pg/mL (from 0), and the sample precision (% coefficient of variation) ranged between 1.05 and 14.8. These saliva-based assays demonstrate high and significant correlations of 0.8 (p<.01) with serum levels of estradiol and progesterone, based on validity data provided by the kit manufacturer (Salimetrics). All participants showed peak P4 levels consistent with an ovulatory cycle (Howards et al., 2009).
Daily Alcohol Use and Binge Drinking
Each morning, participants reported the number of units of alcohol that they had consumed in the past 24 hours using an adaptation of the Timeline Followback Interview (Sobell & Sobell, 1994; Sobell et al., 1996). This interview has good reliability and validity in this population (Tonigan, Miller, & Brown, 1997) and for this purpose (Del Boca, Darkes, Greenbaum, & Goldman, 2004). Units were defined as one shot, one beer, one glass of wine, or one mixed drink. Two daily binary outcomes were defined from this response: (1) whether or not the woman drank alcohol at all on the previous day, and (2) whether the woman drank 4 or more drinks (binge drinking) on the previous day. Although binge drinking has been defined in a variety of ways, this corresponds to the definition provided by the CDC (http://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm). In analyses, the drinking variables were lagged so they were outcomes occurring 1–2 days subsequent to the measured hormone predictors (see supplemental schematic). We also examined whether participants were of legal age to drink (i.e., over the age of 21) in analyses since this could impact drinking frequency.
Daily Positive and Negative Affect
Daily affect was measured via daily online survey using the 20-item version of the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). This measure asks the individual to indicate the extent to which they have experienced each of 20 affective states on a scale from 1 (very slightly or not at all) to 5 (extremely). Ratings for the 10 positive affective states (e.g., proud, strong) and the 10 negative affective states (e.g., irritable, afraid) were averaged to arrive at a daily positive affect score and a daily negative affect score.
Analytic Plan
Operationalization of Ovarian Steroid Levels
Steroid levels were defined as levels of E2 and P4 one day prior to the drinking outcome day, standardized within person using a woman’s individual mean and standard deviation for E2 or P4 across all observations; this allows for the sensitive detection of effects related to deviations from a woman’s hormonal equilibria across the month (Klump et al., 2013)1. Therefore, negative values on a hormone variable indicate that the hormone level on the day before the drinking outcome was lower than the woman’s average level of the hormone across the entire cycle, whereas positive values indicate that the hormone level on the day before the drinking outcome was higher than the woman’s average level of the hormone across the entire cycle. Therefore, these variables have been standardized to remove all between-person variance in hormone levels, and represent pure within-person variance in hormone levels across the menstrual cycle.
Multilevel Models
Model Specification Details
Initially, normal multilevel models were examined using the daily number of drinks variable as an outcome; however, this resulted in non-normally distributed residuals, indicating a need to use zero-inflated models, for which statistical power was inadequate. Therefore, logistic models were utilized to predict daily drinking and daily binge drinking using log-link, binary outcome multilevel models in SAS PROC GLIMMIX, in which daily observations were nested within women. In all models, random intercepts were specified. Random slopes for the effects of steroid predictors were considered but eliminated in each case due to nonsignificance and failure to improve model fit; retention of these nonsignificant random slopes did not substantively alter results. Because hormonal predictors were person-standardized, coefficients and odds ratios for hormonal predictors in Table 1 can be interpreted as the effect of a one person-SD increase in the predictor relative to one’s person mean.
Table 1.
Models Predicting Daily Alcohol Use from Weekend Status and Recent Ovarian Steroid Levels
|
|
||||
|---|---|---|---|---|
| Outcome | ||||
|
| ||||
| Parameter | Daily Alcohol Use | Daily Binge Drinking (>=4 Drinks) | ||
| Estimate | SE | Estimate | SE | |
| Intercept | −2.33** | 0.40 | −4.72** | 0.85 |
| BMI | 0.11 | 0.22 | −.20 | .65 |
| Legal Drinking Status | 0.14* | 0.06 | 0.02 | .69 |
| Weekend Status | 0.93* | 0.37 | 2.20* | 0.71 |
| Recent E2 | 0.28* | 0.12 | 1.00** | 0.33 |
| Recent P4 | −0.08 | 0.18 | 0.37* | 0.18 |
| E2 × P4 | −0.08 | 0.12 | −0.05 | 0.22 |
| Wknd × E2 | 0.00 | 0.01 | −0.33 | 0.59 |
| Wknd × P4 | 0.00 | 0.28 | −0.99** | 0.38 |
| Wknd × E2 × P4 | 0.04** | 0.01 | −0.12* | 0.06 |
|
Simple Effects of Within-Person E2 Levels on the Weekend
| ||||
| At Low P4 | 0.23* | 0.11 | 0.81* | 0.36 |
| Odds Ratio (95% CI) | 1.25 (1.02 to 1.56) | 2.24 (1.11 to 4.55) | ||
| At High P4 | 0.17* | 0.083 | 0.24* | 0.11 |
| Odds Ratio (95% CI) | 1.18 (1.01 to 1.39) | 1.27 (1.02 to 1.57) | ||
Note.
p < .05
p < .01
p < .001.
All hormones are standardized within person (calculated as today’s value minus one’s person mean, divided by one’s person standard deviation). E2 = Estradiol, P4 = Progesterone. Low P4 = 1 standard deviation below the person mean; High P4 = 1 standard deviation above the person mean. Significant fixed effects are shown in bold.
Preliminary analyses were first carried out in SAS PROC GLIMMIX and SAS PROC MIXED to examine bivariate associations of potential covariates with hormones and drinking. Additional preliminary descriptive analyses in SAS PROC GLIMMIX examined the influence of day of the week and menstrual cycle phase on daily drinking outcomes.
Primary analyses testing hypotheses were carried out in SAS PROC GLIMMIX predicting daily alcohol variables from (1) standardized BMI and legal drinking status (0 = Underage 1 = Legal Age) as person-level covariates selected on the basis of significant bivariate associations with estradiol and drinking, respectively, (2) daily dichotomous weekend status (Thursday–Sunday = 1; Monday–Wednesday=02 ) as a time-varying covariate, (3) yesterday’s levels of E2, (4) yesterday’s levels of P4, (5) their interaction, and (6) the two- and three-way interactions of steroid levels with weekend status.
Secondary analyses in SAS PROC MIXED were examined to rule out an influence of yesterday’s dichotomous alcohol on today’s hormone levels (reverse directionality).
Results
Descriptive Information
The drinking subsample (N=22) included 707 total days, with a mean of 32 days provided per woman. Because hormone data was available only for every other day, the final sample of days included in analyses included 353 days, including an average of 16 days from each woman; the number of days contributed by each woman ranged from 11 to 19. This included 97 drinking reports (13.7% of days; range = 0–15 days of drinking) and 31 binge drinking reports (4.3% of days). Twelve women (54% of the drinking sample of 22) reported at least one episode of binge drinking. Mean drinks per day on drinking days in this drinking subsample was 3.14 (SD = 2.80); among women who ever reported binge drinking, the mean drinks per day on drinking ways was 6.19 (SD = 3.01). Sixteen participants (73%) in the drinking subsample reported being under the legal U.S. drinking age.
Preliminary Analyses: Bivariate Associations of Potential Covariates with Drinking and Hormones
Preliminary analyses were conducted predicting daily hormones (normal multilevel models with hormone levels as outcome) and daily drinking (logistic multilevel models with daily drinking as outcome) from potential covariates in order to determine their relevance to later analyses. Some previous work has documented low levels drinking in African American students (Siebert et al., 2003); however, in this sample, non-white race was not significantly associated with mean daily drinking, nor was it associated with mean hormone levels (p’s > .35). Daily medication use was also not significantly associated with daily drinking or hormone variables on the same or the following day (p’s > .52).
We also examined associations of hormones with daily affect and drinking, given that some women demonstrate effects of hormones on mood (Epperson et al., 2012). In normal multilevel models, we predicted today’s positive or negative affect from yesterday’s person-standardized hormonal predictors. Consistent with the notion that not all women experience psychiatric hormone sensitivity (Schmidt et al., 1998), there were no significant effects of yesterday’s E2 or P4 on today’s positive or negative affect in this sample of healthy women (all p’s > 0.26). Next, we predicted daily drinking from daily person-standardized positive and negative affect, but found no significant associations (all p’s > .47).
BMI was not a significant predictor of mean drinking (p < .22). However, consistent with previous work (Lukanova et al., 2004), we found that standardized BMI was associated with significantly higher mean levels of E2 (Estimate: .51, SE = .12, t(20) = 4.22, p < .0001) but was not significantly related to P4 (p < .39). Therefore, BMI was included as a covariate in further analyses to exclude variance in hormonal predictors that may be due to BMI rather than the influence of the menstrual cycle. Legal drinking status is of primary theoretical importance as a covariate given that legal drinking status increases access to alcohol. Indeed, legal drinking status predicted greater mean levels of drinking (Estimate: .26, SE = .05, X2 = 4.81, p = .02, Odds Ratio = 1.30) in this sample. Therefore, both BMI and legal drinking age were retained as a covariate in further analyses.
Preliminary Analysis: Does Day of the Week Predict Alcohol Use?
Consistent with reports by others (Del Boca et al., 2004), alcohol use in the present sample followed a weekly cycle in which alcohol use was substantially higher on Thursday through Saturday, with intermediate levels of drinking on Sunday, and much lower levels of drinking Monday through Wednesday. Dichotomous weekend status (where Thursday through Sunday = 1) strongly predicted the likelihood of drinking (γ = 1.36, SE = 0.30, t(685) = 4.53, p < .0001, Odds Ratio: 3.90, 95% CI for Odds Ratio: 2.16 to 7.04) and binge drinking today (γ = 2.33, SE = 0.48, t(685) = 4.83, p <.0001, Odds Ratio: 10.34, 95% CI for Odds Ratio: 4.00 to 26.72). Therefore, this variable was retained for moderation analyses.
Preliminary Analysis: Does Menstrual Cycle Phase Predict Alcohol Use?
Grand means and SDs for drinking risk and binge drinking risk across the four menstrual cycle phases were as follows, and are graphed in Figure 1: early follicular drinking risk (M = 0.047, SD = 0.21) and binge drinking risk (M = 0.026, SD = 0.16) were low; ovulatory drinking risk (M = 0.13, SD = 0.33) and binge drinking risk (M = 0.040, SD = 0.19) were high; midluteal drinking risk (M = 0.075, SD = 0.26) was moderate and midluteal binge drinking risk (M = 0.013, SD = 0.11) was low, and premenstrual drinking risk was moderately elevated (M = 0.099, SD = 0.30) while premenstrual binge drinking risk (M = 0.051, SD = 0.21) was high. These between-person descriptives were suggestive of increased drinking and binge risk in the ovulatory and premenstrual phases, but required confirmation using within-person models that account for the nested structure of the data.
Results of within-person models comparing the effects of the four coded menstrual cycle phases (early follicular, ovulatory, midluteal, and premenstrual; Edler et al., 2003) on daily drinking also revealed patterns consistent with our hypotheses. With regard to the likelihood of drinking at all on a given day, the ovulatory phase was associated with a greater likelihood of drinking compared with the early follicular phase (γ = 1.32, SE = 0.44, t(121.8) = 2.97, p = 0.0035, Odds Ratio: 3.75, 95% CI for Odds Ratio: 1.55 to 9.01), and the premenstrual phase was also associated with a greater likelihood of drinking compared with the early follicular phase (γ = 0.92, SE = 0.43, t(132) = 2.09, p = 0.038, Odds Ratio: 2.50, 95% CI for Odds Ratio: 1.05 to 5.98); other contrasts between phases were not statistically significant. With regard to the likelihood of binge drinking on a given day, the ovulatory phase was associated with a greater likelihood of binge drinking compared with the early follicular phase (γ = 1.42, SE = 0.70, t(134) = 2.01, p = 0.048, Odds Ratio: 4.19, 95% CI for Odds Ratio: 1.03 to 18.34), and the premenstrual phase was also associated with a greater likelihood of drinking compared with the early follicular phase (γ = 1.58, SE = .71, t(138.9) = 2.21, p = 0.028, Odds Ratio: 4.87, 95% CI for Odds Ratio: 1.18 to 20.06); other contrasts between phases were not statistically significant. In order to determine whether the rising E2 or falling E2 days of the ovulatory phase were responsible for the effect of ovulatory phase on drinking, we conducted additional follow-up analyses in which the ovulatory phase was split into two windows based on the hormonal data: pre-E2 peak and post-E2 peak. Results clearly indicated that all results of ovulation described above could be accounted for by greater drinking and binge drinking risk on pre-E2 peak days (e.g., just preovulatory).
Primary Analysis: Do Daily Ovarian Steroids Predict Alcohol Use or Binge Drinking?
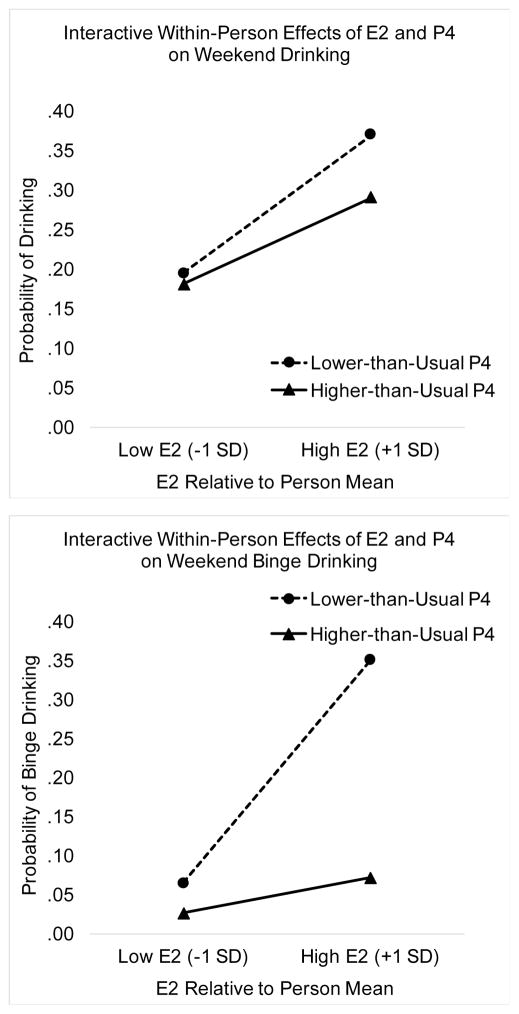
Results of models predicting both daily drinking and daily binge drinking from E2 and P4 levels and interactions of steroid levels with weekend status are presented in Table 1. Significant interactions are depicted in Figure 2, and are further characterized using simple slope analyses at the bottom of Table 1. As hypothesized, there were significant three-way interactions between E2, P4, and weekend status predicting both drinking and binge drinking. Steroid levels were predictive of drinking outcomes only on weekend days. Simple slope analyses, presented at the bottom of Table 1, indicated that high E2 on weekend days predicted a greater likelihood of drinking and binge drinking; these effects of E2 were strongest when P4 was low (i.e., presumably driven by days prior to ovulation, when P4 remains at low levels; see Figure 1) and were attenuated but still significant when P4 was high (i.e., presumably driven by the luteal phase, when the corpus luteum produces high levels of P4; see Figure 1). Odds ratios for these simple slope analyses indicated that a one person-SD elevation above the person mean of E2 was associated with a 26% increase in the probability of drinking and a 125% increase in the probability of binge drinking when P4 was 1 person-SD below the person mean. A similar elevation of E2 was associated with a 19% increase in the probability of drinking and a 27% increase in the probability of binge drinking when P4 was 1 person-SD above the person mean. Thus, high E2 exacerbated drinking, particularly in the context of low P4, but high P4 attenuated the effects of high E2, with all effects stronger on weekends.
Figure 2.
Interactive Within-Person Effects of Recent E2 Levels and Recent P4 Levels on Drinking (TOP) and Binge Drinking (BOTTOM) on Weekend Days.
On Weekend Days, E2 was positively associated with risk for drinking, and this was especially true when P4 was low (top panel); E2 was also positively associated with binge drinking on weekend days, although this was only true when P4 was low (lower panel).
Secondary Analysis: Does drinking or binge drinking today predict tomorrow’s hormone levels?
In order to rule out the possibility that drinking or binge drinking on a given day impacted hormonal levels on the following day, reversed models were conducted. These models specified steroid levels on the following day as a function of dichotomous daily drinking or binge drinking today and revealed no significant prospective effects of drinking or binge drinking on levels of E2 and P4 (both p’s > .58) or P4 (both p’s > .49). Therefore, while yesterday’s steroid levels prospectively predicted today’s drinking, yesterday’s drinking did not prospectively predict today’s hormone levels.
Post-Hoc Power Analyses
Post-hoc power analysis for multilevel models can be conducted as sensitivity analyses in which one determines the smallest detectible effect size given the observed intraclass correlation (ICC) observed for the outcome. Intraclass correlation coefficients for both daily drinking (ICC = 0.15) and daily binge drinking (ICC = 0.23) indicated that the majority of the variance in drinking was at the within-person level, leading to an adjusted N of 108 observations for the drinking outcome, and an adjusted N of 79 observations for the binge drinking outcome. Sensitivity analyses using G*Power indicated that detectible ranges of effect sizes (in this case, odds ratios) for drinking (ORs below 0.48 and ORs above 2.06) and binge drinking (ORs below 0.42 and ORs above 2.37) were consistent with an ability to detect conventionally small-to-medium effects (Chen, Cohen, & Chen, 2010) of a single predictor in a model in which other predictors account for 25% of the variance in drinking.
Discussion
Overall, study results suggested that within-person fluctuations in the ovarian steroids E2 and P4 influenced alcohol use across the menstrual cycle. Specifically, high E2 on the weekend was associated with increased risk of drinking and binge drinking. Though this effect of E2 was significant at all levels of P4, the effect was stronger when P4 was low and weaker when P4 was high, likely reflecting the antagonistic influence of P4 on E2. These steroid effects were present only on weekend days, presumably when there were more opportunities to drink. Such effects were consistent with our menstrual cycle phase findings, which demonstrated preovulatory and premenstrual increases in drinking. Importantly, odds ratios suggest that these could be relatively large effects, implying possible clinical and practical importance.
Overall, results indicating effects of elevated E2, especially in the context of low P4, on drinking are consistent with increases in female risk-taking around ovulation (Ellis et al., 2012; Geary, 2010). Animal studies suggest biological pathways through which these effects may occur. High E2 decreases GABA neurotransmission, which increases dopamine release in the striatum and nucleus accumbens (Becker & Hu, 2008; Lynch, Roth, & Carroll, 2002). In addition, high E2 downregulates dopamine receptor binding (Becker & Hu, 2008). Abnormalities in dopaminergic neurotransmission and elevated E2 can increase reward sensitivity, which is a key mechanism of substance craving, use, and other risk-taking behaviors (Dreher et al., 2007; Hyman, Malenka, & Nestler, 2006; Koob & Le Moal, 2001; Löfgren et al., 2009; Volkow et al., 2010). This might also provide a partial explanation for increases in alcohol use and other risk-taking behaviors around puberty (Spear, 2010), again potentiated by rapidly rising levels of sex steroids.
High P4 may antagonize these E2 effects by moderating E2 dopaminergic effects on substance abuse in the early and mid-luteal phase. P4 declines in the late luteal (premenstrual) phase may also directly motivate alcohol use. Alcohol and the neuroactive steroid metabolites of P4 (especially allopregnanolone, or ALLO) share GABAergic mechanisms of action (Ginsburg et al., 2008; Torres & Ortega, 2003). Therefore, P4/ALLO declines might increase alcohol consumption as a method of compensating for decreased GABAergic activity, which may cause both aversive arousal and increased risk taking (Keisner et al., 2012; Löfgren et al., 2006). Such effects of P4 declines on alcohol use are consistent with both animal work and research in women with premenstrual dysphoric disorder (PMDD). Of note, however, exploratory analyses did not reveal any main or interactive effects of hormones on distress (PANAS negative affect) in the present study; therefore, further work is needed to investigate the possibility of affective mediation, and to examine whether alternative mechanisms, such as changes in executive cognitive control over risk taking, may be relevant.
Although the majority of work on the menstrual cycle focuses on the perimenstrual period as a source of risk, the present results suggest that future work should also focus on the role of the preovulatory period in risk for impulsive behaviors and alcohol use. In the present sample, ovulatory E2 peaks generally ranged between two and three person-standard deviations above a woman’s E2 mean. More specifically, the mean of the person-standardized E2 values in the ovulatory phase was 2.53 (indicating that the average E2 level during the ovulatory phase is 2.53 person-standard deviations above one’s average E2 across the cycle). Extrapolating from the size of the probed coefficient for E2 on weekend days when P4 is low (i.e., OR = 2.24 in Table 1), we estimated that the increased odds of binge drinking on a weekend day in the ovulatory phase (a 2.53-SD increase in E2) are approximately 5.66. This is a large, potentially clinically significant effect with possible clinical implications. Of course, these results need to be replicated in a larger, clinical sample before making firm conclusions about clinical implications.
However, if results were replicated, these results might suggest the importance of considering hormonal profiles or menstrual cycle phase when assessing substance abuse. This is in contrast with current assessment practices, which assume that the behavioral manifestation of substance abuse is relatively static across the menstrual cycle. Such changes in magnitude of drinking risk with hormonal changes across the menstrual cycle also suggests that there may very well be changes in the magnitude or even direction of sex differences in drinking if men and women are compared at different points of women’s menstrual cycles (or menstrual cycle phase if hormone levels are not taken into account). In addition, results might also suggest the importance of both psychosocial and pharmacologic treatments targeted around ovulation and the perimenstrual period, and other important reproductive periods characterized by rapid changes in sex hormones, such as puberty with perhaps specific targeting of women who exhibit larger fluctuations in sex hormones, similar to postmenopausal hormone treatment for cognitive function (e.g., Resnick et al., 2006; Rocca, Grossardt, & Shuster, 2011). Furthermore, such results suggest a large impact of environmental influences, such as opportunity for drinking during social events and availability of alcohol on weekends on college campuses. This suggests the importance of work more carefully assessing specific environmental risk factors influencing hormonal effects on drinking and teaching women strategies for managing environmental factors particularly during particular points of their menstrual cycle. Finally, our results also suggest that further studies should explore a potentially therapeutic impact of hormone stabilization on substance abuse.
Crucially, it remains unclear whether drinking associated with menstrual cycle phase and ovarian steroid levels observed in the present study are due to ovarian steroid levels, absolute changes in ovarian steroids (i.e., destabilizing effects of change not dependent on direction; as in Schiller et al., 2014 and Gordon et al., 2015), or directional changes in ovarian steroids (i.e., effects of hormones that depend on their direction of change; as in McNamara et al., 2014 and Gordon et al., 2016). Determining whether levels or changes are responsible for potential ovarian steroid hormone effects on substance use is a critical question for future research, as it would have important implications for eventual translation of this work to treatment strategies for women with substance abuse. If levels are responsible, increasing or decreasing mean levels of hormones may be therapeutic. However, if absolute or directional change is responsible, then stabilization of hormone levels, regardless of levels, might be therapeutic.
The current study had a number of strengths. It was the first study to examine daily alcohol use in relation to repeated measures of ovarian steroids. Yet, the study was limited in its use of every-other-day hormone samples, an inexact measure of binge drinking, and a relatively small sample of drinkers who were mostly underage which did not provide adequate power to detect small higher-order interaction effects and might impact generalizability of study results.
Conclusions
In summary, elevated E2, particularly in the context of low P4, predicted increased drinking in young adult females, effects that were mirrored in increased drinking on pre-ovulatory and premenstrual days. Therefore, clinicians might consider assessing menstrual cycle phase and/or hormone levels during clinical evaluations of drinking in young women and consider taking such information into account in determination of substance use disorder diagnosis. Further work is needed to evaluate whether hormone stabilization interventions could be beneficial for women with substance use disorders.
Supplementary Material
Acknowledgments
The authors wish to thank Gregory T. Smith and Jessica R. Peters for their thoughtful comments on an earlier version of this manuscript.
Sources of Funding: This work was supported by the National Institutes of Health (M.M., UL1R000117), National Institute of Mental Health (T.E., T32MH093315; K99MH109667), and National Institute on Drug Abuse (M.M., DA005312; DA035150).
Footnotes
Use of person-centered rather than person-standardized coefficients did not substantively change results for models using within-person levels as predictors; therefore, person-standardized variables were retained in final models in order to maximize specificity to each woman’s endocrine environment (Klump et al., 2008).
An alternative definition of weekend status excluding Thursday from the weekend (i.e., where Friday–Sunday=1 and Monday–Thursday=0) did not substantively change results.
Contribution Statement: All authors contributed substantially to the design of the study, drafting of the paper, and final approval of paper.
Contributor Information
Michelle M. Martel, University of Kentucky.
Tory Eisenlohr-Moul, University of North Carolina at Chapel Hill
Bethan Roberts, University of Kentucky
References
- Anker JJ, Carroll ME. Biological Basis of Sex Differences in Psychopharmacology. Springer; Berlin Heidelberg: 2010. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones; pp. 73–96. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in substance abuse. Frontiers in Neuroendocrinology. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bless EP, McGinnis KA, Mitchell AL, Hartwell A, Mitchell JB. The effects of gonadal steroids on brain stimulation reward in female rats. Behavioural brain research. 1997;82(2):235–44. doi: 10.1016/s0166-4328(96)00129-5. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatric Clinics of North America. 1999;22(2):241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics—Simulation and Computation®. 2010;39(4):860–864. [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV substance abuse, and dependence in the United States: Results from the National Epidemiological Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J, Greenbaum PE, Goldman MS. Up close and personal: temporal variability in the drinking of individual college students during their first year. Journal of Consulting and Clinical Psychology. 2004;72(2):155. doi: 10.1037/0022-006X.72.2.155. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neuroscience Letters. 1997;230:140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, DeWall CN, Girdler SS, Segerstrom SC. Ovarian hormones and borderline personality disorder features: preliminary evidence for interactive effects of estradiol and progesterone. Biological Psychology. 2015;109:37–52. doi: 10.1016/j.biopsycho.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Dishion TJ, Figueredo AJ, Gray P, Griskevicius V, … Wilson DS. The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Developmental Psychology. 2012;48(3):598–623. doi: 10.1037/a0026220. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychological Methods. 2007;12(2):121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. American Journal of Psychiatry. 2012;169(5):465–475. doi: 10.1176/appi.ajp.2012.11081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Geary DC. Male, female: The evolution of human sex differences. American Psychological Association; Washington, D.C: 2010. [Google Scholar]
- Ginsburg BC, Gerak LR, McMahon LR, Roache JD. Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders. Springer; Netherlands: 2008. Neurosteroids in alcohol and substance use; pp. 509–538. [Google Scholar]
- Gonzales JE, Ferrer E. Efficacy of methods for ovulation estimation and their effect on the statistical detection of ovulation-linked behavioral fluctuations. Behavior Research Methods. 2015:1–20. doi: 10.3758/s13428-015-0638-4. [DOI] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, Wisner KL. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. American Journal of Psychiatry. 2015;172(3):227–236. doi: 10.1176/appi.ajp.2014.14070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, Girdler SS. Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clinical Psychological Science. 2016;4(5):919–935. doi: 10.1177/2167702616647924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, Wisner KL. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. American Journal of Psychiatry. 2015;172(3):227–36. doi: 10.1176/appi.ajp.2014.14070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton MG, Gangestad SW. Conditional expression of women’s desires and men’s mate guarding across the ovulatory cycle. Hormones and Behavior. 2006;49(4):509–518. doi: 10.1016/j.yhbeh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. American Journal of Epidemiology. 2009;169(1):105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. The Journal of Neuroscience. 2003;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Stamp JA. Ovarian hormones and propensity to drug relapse: a review. Neuroscience & Biobehavioral Reviews. 2011;35(3):427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Reviews of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kiesner J. Affective response to the menstrual cycle as a predictor of self-reported affective response to alcohol and alcohol use. Archives of Women’s Mental Health. 2012;15(6):423–432. doi: 10.1007/s00737-012-0303-1. [DOI] [PubMed] [Google Scholar]
- Klump KK, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. 2013;122(1):131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Substance addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Löfgren M, Johansson IM, Meyerson B, Lundgren P, Bäckström T. Progesterone withdrawal effects in the open field test can be predicted by elevated plus maze performance. Hormones and behavior. 2006;50(2):208–215. doi: 10.1016/j.yhbeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Löfgren Mm, Johansson IM, Meyerson B, Turkmen S, Bäckström T. Withdrawal effects from progesterone and estradiol relate to individual risk-taking and explorative behavior in female rats. Physiology & Behavior. 2009;96(1):91–97. doi: 10.1016/j.physbeh.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. European Journal of Endocrinology. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in substance abuse: Preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior. 2001;68(4):641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcoholism: Clinical and Experimental Research. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Progress in Hormone Research. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McNamara A, Moakes K, Aston P, Gavin C, Sterr A. The Importance of the Derivative in Sex-Hormone Cycles: A Reason Why Behavioural Measures in Sex-Hormone Studies Are So Mercurial. PloS one. 2014;9(11):e111891. doi: 10.1371/journal.pone.0111891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug and Alcohol Dependence. 2011;114(1):68–72. doi: 10.1016/j.drugalcdep.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muti P, Trevisan M, Micheli A, Krogh V, Bolelli G, Sciajno R, … Berrino F. Alcohol consumption and total estradiol in premenopausal women. Cancer Epidemiology Biomarkers & Prevention. 1998;7(3):189–193. [PubMed] [Google Scholar]
- Nyberg S, Wahlström G, Bäckström T, Poromaa IS. Altered sensitivity to alcohol in the late luteal phase among patients with premenstrual dysphoric disorder. Psychoneuroendocrinology. 2004;29(6):767–777. doi: 10.1016/S0306-4530(03)00121-5. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, … Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. The Journal of Clinical Endocrinology & Metabolism. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain research. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to substance abuse: A review of preclinical studies. Neuroscience and Biobehavioral Reviews. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, Gray KM. Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine & Tobacco Research. 2015;17(4):398–406. doi: 10.1093/ntr/ntu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Saladin ME, Gray KM, Hartwell KJ, Carpenter MJ. Association between ovarian hormones and smoking behavior in women. Experimental and Clinical Psychopharmacology. 2012;20(4):251–257. doi: 10.1037/a0027759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New England Journal of Medicine. 1998;338(4):209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Siebert DC, Wilke DJ, Delva J, Smith MP, Howell RL. Differences in African American and White college students’ drinking behaviors: Consequences, harm reduction strategies, and health information sources. Journal of American College Health. 2003;52(3):123–129. doi: 10.1080/07448480309595734. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel modeling: An introduction to basic and advanced multilevel modeling. London: Sage; 1999. [Google Scholar]
- Spear L. The behavioral neuroscience of adolescence. WW Norton & Company; 2010. [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and Alcohol Dependence. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback (TLFB) user’s manual. Toronto, Canada: Addiction Research Foundation; 1994. [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Experimental and Clinical Psychopharmacology. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Strauss JF, III, Barbieri RL. Yen & Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. Elsevier Health Sciences; 2013. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Summary of Findings from the 2000 National Household Survey on Substance Abuse. Office of Applied Studies; Rockville, Md: 2001. NHSDA Series H-13, DHHS Publication No. (SMA) 01-3549. [Google Scholar]
- Terner JM, De Wit H. Menstrual cycle phase and responses to substances of abuse in humans. Substance and Alcohol Dependence. 2006;84(1):1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: An instrument for assessing alcohol treatment outcome. Journal of Studies on Alcohol. 1997;58:358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28(6):1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32(9):748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, Mazure CM, Wetherington CL, McKee SA. Systematic and Meta-Analytic Review of Research Examining the Impact of Menstrual Cycle Phase and Ovarian Hormones on Smoking and Cessation. Nicotine & Tobacco Research. 2015;17(4):407–421. doi: 10.1093/ntr/ntu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.