This birth surveillance study compares the risk for selected birth outcomes by maternal antiretroviral therapy regimen among pregnant women with HIV infection in Botswana.
Key Points
Question
Among pregnant women infected with human immunodeficiency virus who start antiretroviral treatment before pregnancy, do adverse birth outcomes differ by antiretroviral treatment regimen?
Findings
In this birth surveillance study of 47 027 pregnant women in Botswana, preterm birth, very preterm birth, small and very small size for gestational age, stillbirth, and neonatal death were evaluated. The risk for any adverse or severe adverse birth outcome was lower among infants exposed to a combined regimen of tenofovir, emtricitabine, and efavirenz.
Meaning
Adverse birth outcomes may differ by antiretroviral treatment regimen used in pregnancy.
Abstract
Importance
Maternal antiretroviral treatment (ART) started before conception may increase the risk for adverse birth outcomes among women with human immunodeficiency virus (HIV) infection, but whether the risk differs by ART regimen is unknown.
Objective
To compare the risk for selected birth outcomes by maternal ART regimen.
Design, Setting, and Participants
This observational birth outcomes surveillance study compared all live births and stillbirths with a gestational age of at least 24 weeks in 8 geographically dispersed government hospitals throughout Botswana (approximately 45% of births nationwide). Data were collected from August 15, 2014, through August 15, 2016.
Exposures
Births among HIV-infected women who started 3-drug ART regimens before their last menstrual period and did not switch or stop ART in pregnancy were considered to be ART exposed from conception.
Main Outcomes and Measures
The primary outcomes were any adverse birth outcome, including stillbirth, preterm birth (<37 weeks), small size for gestational age (SGA; <10th percentile of weight for gestational age) or neonatal death (<28 days from delivery), and any severe adverse outcome, including very preterm birth (<32 weeks), very SGA (<3rd percentile of weight for gestational age), stillbirth, and neonatal death.
Results
Information was available for 47 027 of 47 124 births (99.8%) at surveillance maternity hospitals (mean [SD] age of mothers, 26.86 [6.45] years). Among 11 932 HIV-exposed infants, 5780 (48.4%) were ART exposed from conception. Adverse birth outcomes were more common among HIV-exposed infants than HIV-unexposed infants (39.6% vs 28.9%; adjusted relative risk [ARR], 1.40; 95% CI, 1.36-1.44). The risk for any adverse birth outcome was lower among infants exposed from conception to tenofovir disoproxil fumarate, emtricitabine, and efavirenz (TDF-FTC-EFV) (901 of 2472 [36.4%]) compared with TDF-FTC and nevirapine (NVP) (317 of 760 [41.7%]; ARR, 1.15; 95% CI, 1.04-1.27); TDF-FTC and lopinavir-ritonavir (TDF-FTC–LPV-R) (112 of 231 [48.5%]; ARR, 1.31; 95% CI, 1.13-1.52); zidovudine, lamivudine, and NPV (ZDV-3TC-NVP) (647 of 1365 [47.4%]; ARR, 1.30; 95% CI, 1.20-1.41); or ZDV-3TC–LPV-R (75 of 167 [44.9%]; ARR, 1.21; 95% CI, 1.01-1.45). The risk for any severe adverse outcome was also lower among infants exposed from conception to TDF-FTC-EFV (303 of 2472 [12.3%]) compared with TDF-FTC-NVP (136 of 760 [17.9%]; ARR, 1.44; 95% CI, 1.19-1.74), TDF-FTC–LPV-R (45 of 231 [19.5%]; ARR, 1.58; 95% CI, 1.19-2.11), ZDV-3TC-NVP (283 of 1365 [20.7%]; ARR, 1.68; 95% CI, 1.44-1.96), or ZDV-3TC–LPV-R (39 of 167 [23.4%]; ARR, 1.93; 95% CI, 1.43-2.60) from conception. Compared with TDF-FTC-EFV, all other regimens were associated with higher risk for SGA; ZDV-3TC-NVP was associated with higher risk of stillbirth, very preterm birth, and neonatal death; and ZDV-3TC-LPV-R was associated with higher risk for preterm birth, very preterm birth, and neonatal death.
Conclusions and Relevance
Among infants exposed to ART from conception, TDF-FTC-EFV was associated with a lower risk for adverse birth outcomes than other ART regimens.
Introduction
World Health Organization (WHO) treatment guidelines recommend 3-drug antiretroviral therapy (ART) for all persons infected with human immunodeficiency virus (HIV), including more than 1.5 million HIV-infected women who become pregnant every year. However, maternal ART taken during pregnancy may increase the risk for adverse birth outcomes, including preterm birth, stillbirth, and small size for gestational age (SGA) after in utero exposure, particularly among those exposed from conception.
Information on the comparative safety of specific ART regimens used during pregnancy is lacking. In utero exposure to protease inhibitors has been associated with an increased risk for preterm birth, and recent data from the PROMISE (Promoting Maternal and Infant Survival Everywhere) Study raise concerns about the combination of lopinavir and ritonavir (LPV-R) with tenofovir disoproxil fumarate and emtricitabine (TDF-FTC). However, only limited data have evaluated the safety of TDF-FTC in combination with efavirenz (TDF-FTC-EFV), which is the WHO-recommended regimen now used by most HIV-infected pregnant women throughout the world, and no studies compare the safety of different ART regimens taken from before conception.
Botswana offers a unique opportunity to study the comparative safety of in utero exposure to ART regimens from conception. Botswana has a high prevalence of HIV infection (approximately 22% of adults), high uptake of HIV testing and ART in pregnancy (99% undergoing testing and >90% receiving ART), a high proportion of hospital deliveries (95%), and a long-standing HIV treatment program (>50% of HIV-infected pregnant women receive ART before conception). Botswana’s national HIV treatment guidelines recommended TDF-FTC-EFV for all adults and pregnant women from 2012 through 2016 but did not recommend switching older regimens among stable HIV-infected individuals. Therefore, women receiving ART at conception in Botswana receive a variety of antiretrovial drugs, including EFV, nevirapine (NVP), and LPV-R combined with TDF-FTC or zidovudine and lamivudine (ZDV-3TC). Taking advantage of this unique landscape in Botswana, we performed birth outcomes surveillance to study differences in birth outcomes by specific ART regimens started before conception.
Methods
Study Population
We abstracted data from obstetric records of all women who delivered live-born or stillborn infants at 8 government maternity wards in Botswana, which represent approximately 45% of all births in the country. Princess Marina Hospital in Gaborone and Nyangabwe Hospital in Francistown were tertiary referral centers, whereas Letsholathebe II Memorial Hospital in Maun, Sekgoma Memorial Hospital in Serowe, Selebi-Phikwe Hospital in Selebi-Phikwe, Mahalapye Hospital in Mahalpye, Scottish Livingstone Hospital in Molepolole, and Ghanzi Primary Hospital in Ghanzi were district and primary-level hospitals. Births that occurred before arrival at the hospital and at a gestational age of less than 24 weeks were excluded. Ethical approval for this study was granted by Human Research and Development Council in Botswana and by the institutional review board of Harvard T. H. Chan School of Public Health, Boston, Massachusetts, which waived the need for informed consent for this observational study of deidentified data.
Botswana citizens receive free antenatal care services, HIV testing, and medications, including ART. Before 2012, ART was available for HIV-infected adults with low CD4 cell counts (<350/mm3 in 2011-2012 and <250/mm3 before 2011 [to convert to ×109 per liter, multiply by 0.001]) and generally included ZDV-3TC or TDF-FTC with NVP or LPV-R. From 2012 until May 2016, national HIV treatment guidelines recommended TDF-FTC-EFV for adults with CD4 cell counts of 350/mL or less and for all pregnant women regardless of CD4 cell count. In May 2016, guidelines were updated and recommended TDF-FTC with dolutegravir for all HIV-infected adults regardless of CD4 cell count or pregnancy status; births with this new regimen were not captured during the study period.
Data Collection
Study data were collected from August 15, 2014, through August 15, 2016. At each site, deidentified data were abstracted from obstetric cards (used throughout pregnancy) at the time of discharge from the postnatal ward. Information included maternal demographic data, maternal medical history, medications prescribed at the time of conception, and self-reported alcohol and tobacco use. The obstetric card also included laboratory values measured in pregnancy (levels of hemoglobin and rapid plasma reagin); serial blood pressure and weights; maternal diagnoses during pregnancy; medications prescribed during pregnancy with start dates; and the infant birth record with type of delivery, Apgar scores, gestational age, birth weight, congenital abnormalities, and vital status of the infant(s) at the time of discharge. For HIV-infected women, the obstetric card captured the date of diagnosis of HIV infection, most recent CD4 cell count, and history of ART use (including start date, regimen, and any switch or discontinuation during pregnancy).
The estimated gestational age was documented by nurses at the time of delivery using the estimated date of delivery calculated during antenatal care, based on last menstrual period documented at the first antenatal care visit and confirmed by ultrasonography when available. If the date of the last menstrual period was unknown or suspected to be incorrect and if no ultrasonographic record was available, fundal height measurements were occasionally used by midwives to estimate gestational age.
Exposure Definitions
Births were considered to be HIV exposed with documentation of a positive maternal HIV test result during or before pregnancy and HIV unexposed with documentation of a negative maternal HIV test result during pregnancy. If the start of maternal ART occurred before the calculated date of the last menstrual period according to neonatal gestational age at delivery, births were categorized as exposed to ART from conception. Births with a maternal ART start date after the calculated last menstrual period were categorized as ART exposed after conception. Births to HIV-infected mothers with no ART before delivery were considered to be ART unexposed. Births to mothers taking a nonstandard ART regimen (<3 ART medications) or who switched or stopped ART were excluded from ART-related analyses.
Outcome Definitions
The primary outcomes were the combined end point of any adverse outcome, which included stillbirth, preterm birth, SGA, or neonatal death, and the combined end point of any severe outcome, which included stillbirth, very preterm birth, very SGA, or neonatal death. Secondary analyses included individual evaluation of stillbirth, preterm birth, very preterm birth, SGA, very SGA, and neonatal death. Stillbirth was defined as fetal death (Apgar scores of 0, 0, and 0); preterm birth, a birth at a gestational age of less than 37 weeks; very preterm birth, a birth at a gestational age less than 32 weeks; SGA, birth weight below the 10th percentile for gestational age; and very SGA, birth weight below the 3rd percentile for gestational age using WHO criteria (defined from 24 to 42 weeks of gestation). Neonatal deaths included deaths before 28 days of age among infants who had never left the hospital. Congenital abnormalities will be reported at a planned 4-year analysis when we project to have sufficient sample size and power.
Statistical Analysis
Birth outcomes were calculated by HIV exposure and ART status for all births and separately among singleton births only. To compare the risk for each birth outcome, we fit log-binomial regression models to obtain the unadjusted and adjusted risk ratio (ARR) among singleton births. Covariates for adjusted analyses were preselected based on data from a similar large birth outcomes studies in Botswana and included maternal age (<18, 18-35, or >35 years), gravida (1, 1-5, or >5), and low educational attainment (none or primary vs secondary or higher). Models comparing outcomes by ART regimen from conception were limited to ART regimens with at least 160 exposures to have 80% power to detect a relative risk of 1.33 or higher in combined adverse outcomes. Given limited CD4 cell count availability, CD4 cell count was not included in primary models but was instead included in sensitivity analyses. Statistical analyses were performed using SAS software (version 9.3; SAS Institute Inc). All reported P values are based on a 2-sided test, with P < .05 indicating statistical significance.
Results
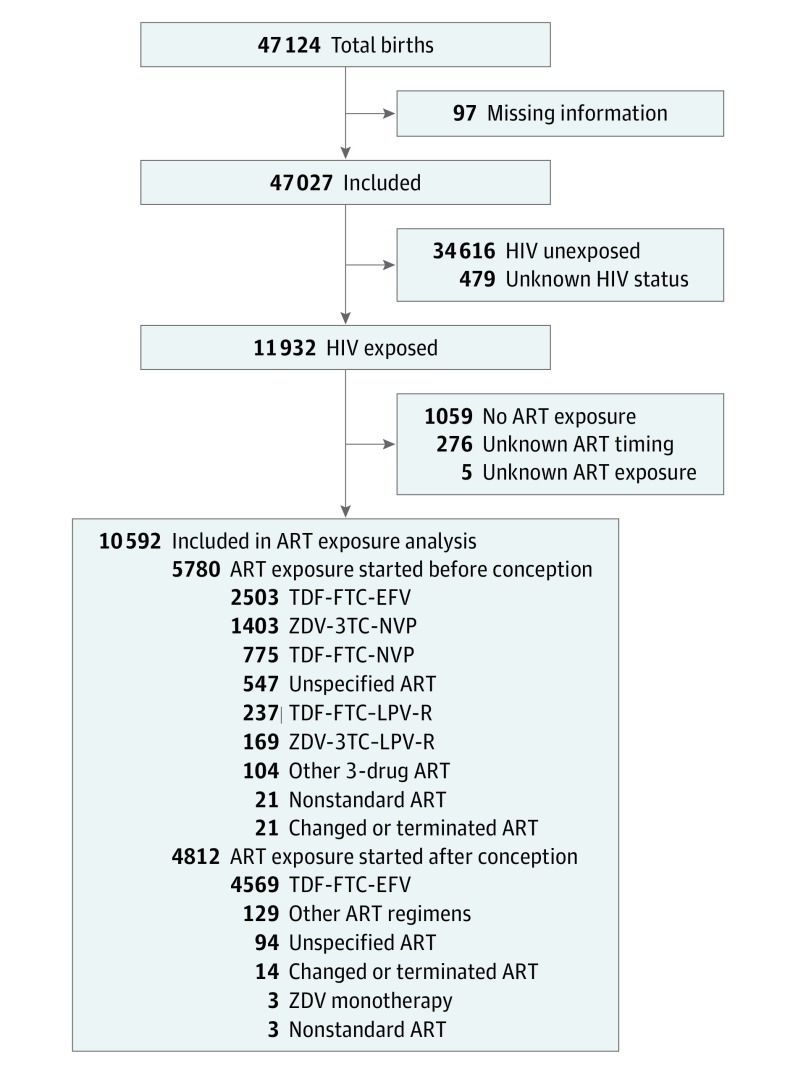
During the study period, 47 124 births occurred at surveillance maternity wards, and information was available for 47 027 (99.8%) (mean [SD] age of mothers, 26.86 [6.45] years) (Figure). Of these, 34 616 (73.6%) were HIV unexposed, 11 932 (25.4%) were HIV exposed, and 479 (1.0%) had unknown HIV exposure status. Among HIV-exposed infants, 5780 (48.4%) were ART exposed at the time of conception, 4812 (40.3%) were ART exposed after conception, 276 were ART exposed with unknown timing (2.3%), 1059 (8.9%) had no ART exposure, and 5 (0.04%) had unknown ART exposure. The 5 most common regimens, accounting for 5087 (88.0%) of all known standard ART started before conception, were TDF-FTC-EFV (n = 2503), TDF-FTC-NVP (n = 775), TDF-FTC–LPV-R (n = 237), ZDV-3TC-NVP (n = 1403), and ZDV-3TC–LPV-R (n = 169) (Figure).
Figure. Flowchart of the Study Population.
ART indicates antiretroviral therapy; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; LPV-R, lopinavir-ritonavir; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; 3TC, lamivudine; and ZDV, zidovudine.
Demographic characteristics of the entire study population are shown in Table 1 and for HIV-infected women who started ART before conception in Table 2. The HIV-uninfected pregnant women were more likely to be younger, have higher educational attainment, and be primiparous compared with HIV-infected women (Table 1). Among women starting ART before conception, women receiving TDF-FTC-EFV were younger and less likely to be married and had the shortest median duration of HIV infection and ART before conception (Table 2). Women receiving LPV-R–based regimens had higher median CD4 cell counts, and women receiving ZDV-3TC-NVP had the longest duration of ART before conception.
Table 1. Baseline Demographic Characteristics Among All Births by HIV Exposure Status and Timing of ART Exposurea.
| Characteristic | HIV Exposure Status | Maternal ART Regimen in HIV-Exposed Group | |||
|---|---|---|---|---|---|
| Unexposed (n = 34 616) |
Exposed (n = 11 932) |
No ART in Pregnancy (n = 1059) |
ART Started in Pregnancy (n = 4812) |
ART From Conception (n = 5780) |
|
| Maternal demographic | |||||
| Maternal age, median (IQR), y | 25 (21-30) | 31 (26-35) | 28 (23-33) | 28 (24-32) | 33 (29-37) |
| Married | 3883 (11.2) | 1153 (9.7) | 82 (7.7) | 346 (7.2) | 702 (12.1) |
| No or primary educational attainment | 2126 (6.1) | 1566 (13.1) | 170 (16.1) | 468 (9.7) | 881 (15.2) |
| Occupationb | |||||
| Housewife/none | 18 312 (55.5) | 6574 (57.9) | 635 (65.9) | 2643 (59.27) | 3132 (56.9) |
| Student | 3324 (10.1) | 329 (2.9) | 32 (3.3) | 200 (4.5) | 94 (1.7) |
| Salaried | 11 333 (34.4) | 4457 (39.2) | 296 (30.7) | 1791 (40.1) | 2274 (41.3) |
| Noncitizen | 1203 (3.5) | 334 (2.9) | 151 (14.3) | 88 (1.8) | 86 (1.5) |
| Obstetric and medical history | |||||
| Primiparous | 15 000 (43.3) | 1767 (14.8) | 187 (17.7) | 1140 (23.7) | 415 (7.2) |
| Grand multiparous (≥5 pregnancies) | 2361 (6.8) | 2411 (20.2) | 182 (17.2) | 581 (12.1) | 1567 (27.1) |
| Gestational age at antenatal care presentation, median (IQR), wk | 17 (13-22) | 17 (13-22) | 22 (15-28) | 17 (12-22) | 17 (12-21) |
| Received no prenatal care | 854 (2.5) | 393 (3.3) | 220 (20.8) | 26 (0.5) | 121 (2.1) |
| Alcohol consumption or smoking during pregnancy | 2557 (7.4) | 967 (8.1) | 75 (7.19) | 494 (10.3) | 374 (6.5) |
| Birth via cesarean delivery | 7948 (23.0) | 2830 (23.7) | 227 (21.4) | 1147 (23.8) | 1386 (23.9) |
| Scheduled cesarean delivery, No. (%) of all cesarean deliveries | 2241 (28.2) | 878 (31.0) | 62 (27.3) | 351 (30.6) | 442 (31.9) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range.
Includes data from all births in the cohort (singleton and multiple). Unless otherwise indicated, data are expressed as number (percentage) of patients.
Owing to missing data, numbers do not sum to totals in column heads.
Table 2. Baseline Demographic Characteristics Among All Births Exposed to Continuous ART From Conception by ART Regimena.
| Characteristic | ART Regimen | ||||
|---|---|---|---|---|---|
| TDF-FTC-EFV (n = 2503) |
TDF-FTC-NVP (n = 775) |
TDF-FTC–LPV-R (n = 237) |
ZDV-3TC-NVP (n = 1403) |
ZDV-3TC–LPV-R (n = 169) |
|
| Maternal demographic | |||||
| Age, median (IQR), y | 31 (27-36) | 34 (30-37) | 33 (30-37) | 35 (31-38) | 33 (30-37) |
| Married | 251 (10.0) | 108 (13.9) | 36 (15.2) | 209 (14.9) | 24 (14.2) |
| No or primary educational attainment | 342 (13.7) | 106 (13.7) | 35 (14.8) | 243 (17.3) | 22 (13.0) |
| Occupationb | |||||
| Housewife/none | 1417 (59.4) | 380 (51.1) | 129 (57.6) | 729 (54.3) | 94 (58.4) |
| Student | 50 (2.1) | 12 (1.6) | 6 (2.7) | 19 (1.4) | 0 |
| Salaried | 919 (38.5) | 352 (47.3) | 89 (39.7) | 593 (44.2) | 67 (41.6) |
| Noncitizen | 51 (2.0) | 5 (1.0) | 1 (0.4) | 15 (1.1) | 3 (1.8) |
| Obstetric history | |||||
| Primiparous | 198 (7.9) | 51 (6.6) | 21 (8.9) | 71 (5.1) | 14 (8.3) |
| Grand multiparous (≥5 pregnancies) | 595 (23.8) | 180 (23.2) | 64 (27.0) | 471 (33.6) | 49 (29.0) |
| Gestational age at antenatal care presentation, median (IQR), wk | 17 (13-22) | 15 (12-20) | 17 (12-21) | 16 (13-20) | 17 (12-22) |
| Received no prenatal care | 72 (2.9) | 4 (0.5) | 6 (2.5) | 21 (1.5) | 1 (0.6) |
| Alcohol consumption or smoking during pregnancy | 165 (6.6) | 47 (6.1) | 15 (6.3) | 86 (6.1) | 7 (4.1) |
| Birth via cesarean delivery | 568 (22.7) | 204 (26.3) | 56 (23.6) | 392 (27.9) | 33 (19.5) |
| Scheduled delivery section, No. (%) of all cesarean deliveries | 204 (35.9) | 65 (31.9) | 14 (25.0) | 114 (29.1) | 7 (21.2) |
| HIV history | |||||
| Time from HIV diagnosis to conception, median (IQR), wk | 174 (101-329) | 319 (233-470) | 392 (274-524) | 417 (310-521) | 346 (250-493) |
| Time from HIV diagnosis to conception, y | |||||
| <2 | 569 (22.7) | 12 (1.5) | 6 (2.5) | 16 (1.1) | 2 (1.2) |
| 2-5 | 877 (35.0) | 205 (26.5) | 39 (16.5) | 177 (12.6) | 38 (22.5) |
| >5 | 745 (29.8) | 475 (61.3) | 156 (65.8) | 1032 (73.6) | 106 (62.7) |
| Duration of ART before conception, median (IQR), wk | 112 (65-175) | 266 (179-364) | 312 (206-469) | 379 (277-479) | 287 (196-408) |
| Duration of ART before conception, y | |||||
| <2 | 1097 (43.8) | 45 (5.8) | 19 (8.0) | 35 (2.5) | 9 (5.3) |
| 2-5 | 1001 (40.0) | 319 (41.2) | 56 (23.6) | 248 (17.7) | 61 (36.1) |
| >5 | 281 (11.2) | 385 (49.7) | 147 (62.o) | 1074 (76.6) | 92 (54.4) |
| CD4 cell count obtained during pregnancy | 605 (24.2) | 212 (27.4) | 61 (25.7) | 353 (25.2) | 44 (26.0) |
| CD4 cell count in pregnancy, median (IQR), No./mm3 | 478 (363-600) | 508 (388-685) | 638 (492-735) | 504 (408-625) | 609 (414-798) |
| <200 | 34 (5.6) | 4 (1.9) | 1 (1.6) | 7 (1.9) | 2 (4.5) |
| 200-349 | 107 (17.7) | 27 (12.7) | 4 (6.6) | 41 (11.6) | 6 (13.6) |
| 350-499 | 200 (33.1) | 72 (34.0) | 12 (19.7) | 126 (35.7) | 6 (13.6) |
| ≥500 | 264 (43.6) | 109 (51.4) | 44 (72.1) | 179 (50.7) | 30 (68.2) |
Abbreviations: ART, antiretroviral therapy; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; IQR, interquartile range; LPV-R, lopinavir-ritonavir; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; 3TC, lamivudine; ZDV, zidovudine.
SI conversion factor: To convert CD4 cell count to ×109 per liter, multiply by 0.001.
Includes data from all births in the cohort (singleton and multiple). Unless otherwise indicated, data are expressed as number (percentage) of patients.
Owing to missing data, numbers do not sum to totals in column heads.
Adverse Outcomes Among All Births
Overall, 15 266 of all births (32.5%) resulted in any adverse birth outcome, and 5346 (11.4%) resulted in a serious adverse birth outcome. Among 710 sets of twins or triplets, a higher overall prevalence of any adverse birth outcome occurred compared with singleton births (541 of 710 [76.2%] vs 14 725 of 46 317 [31.8%]) and increased preterm (324 of 705 [46.0%] vs 8059 of 45 781 [17.6%]) and very preterm (71 of 705 [10.1%] vs 1943 of 45 781 [4.2%]) births. No differences were observed by maternal HIV status.
Adverse Birth Outcomes by HIV Exposure Status
Among 47 846 singleton births, the occurrence of any adverse birth outcome (39.6% vs 28.9%; ARR, 1.40; 95% CI, 1.36-1.44) or any severe adverse birth outcome (14.8% vs 9.9%; ARR, 1.50; 95% CI, 1.41-1.59) was higher among HIV-exposed than among HIV-unexposed infants (Table 3). Stillbirth (3.4% vs 2.1%; ARR, 1.48; 95% CI, 1.30-1.68), preterm birth (22.5% vs 15.6%; ARR, 1.39; 95% CI, 1.33-1.46), very preterm birth (5.4% vs 3.7%; ARR, 1.42; 95% CI, 1.29-1.57), SGA (20.5% vs 14.8%; ARR, 1.47; 95% CI, 1.40-1.54), very SGA (8.4% vs 5.4%; ARR, 1.63; 95% CI, 1.51-1.77), and neonatal death (1.8% vs 1.3%; ARR, 1.22; 95% CI, 1.03-1.45) were more common among HIV-exposed than HIV-unexposed infants.
Table 3. Birth Outcomes by HIV Status and ART Regimen at Conception.
| Adverse Birth Outcome | HIV Exposure Status | Maternal ART Regimen at Conception | |||||
|---|---|---|---|---|---|---|---|
| Unexposed (n = 34 138) |
Exposed (n = 11 708) |
TDF-FTC-EFV (n = 2472) |
TDF-FTC-NVP (n = 760) |
TDF-FTC–LPV-R (n = 231) |
ZDV-3TC-NVP (n = 1365) |
ZDV-3TC–LPV-R (n = 167) |
|
| Any, No. (%)a | 9851 (28.9) | 4641 (39.6) | 901 (36.4) | 317 (41.7) | 112 (48.5) | 647 (47.4) | 75 (44.9) |
| Severe, No. (%)b | 3394 (9.9) | 1731 (14.8) | 303 (12.3) | 136 (17.9) | 45 (19.5) | 283 (20.7) | 39 (23.4) |
| Anya | |||||||
| RR (95% CI) | 1 [Reference] | 1.37 (1.34-1.41) | 1 [Reference] | 1.14 (1.04-1.26) | 1.33 (1.15-1.53) | 1.30 (1.20-1.40) | 1.23 (1.03-1.50) |
| ARR (95% CI) | 1 [Reference] | 1.40 (1.36-1.44) | 1 [Reference] | 1.15 (1.04-1.27) | 1.31 (1.13-1.52) | 1.30 (1.20-1.41) | 1.21 (1.01-1.45) |
| Severeb | |||||||
| RR (95% CI) | 1 [Reference] | 1.49 (1.41-1.57) | 1 [Reference] | 1.46 (1.21-1.76) | 1.59 (1.20-2.11) | 1.69 (1.46-1.96) | 1.91 (1.42-2.56) |
| ARR (95% CI) | 1 [Reference] | 1.50 (1.41-1.59) | 1 [Reference] | 1.44 (1.19-1.74) | 1.58 (1.19-2.11) | 1.68 (1.44-1.96) | 1.93 (1.43-2.60) |
Abbreviations: ARR, adjusted relative risk; ART, antiretroviral therapy; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; LPV-R, lopinavir-ritonavir; NVP, nevirapine; RR, relative risk; TDF, tenofovir disoproxil fumarate; 3TC, lamivudine; ZDV, zidovudine.
Includes stillbirth, neonatal death, preterm birth, and small size for gestational age.
Includes stillbirth, neonatal death, very preterm birth, and very small size for gestational age.
Among the HIV-exposed infants, those exposed to ART from conception were more often SGA (1262 of 5588 [22.6%] vs 871 of 4644 [18.8%]; ARR, 1.25; 95% CI, 1.15-1.36) and very SGA (565 of 5588 [10.1%] vs 317 of 4644 [6.8%]; ARR, 1.53; 95% CI, 1.33-1.77) and had more neonatal deaths (92 of 5463 [1.7%] vs 61 of 4598 [1.3%]; ARR, 1.26; 95% CI, 0.89-1.77) compared with those whose ART exposure started after conception. Stillbirth and preterm birth could not be compared because of the greater opportunity for these outcomes among those exposed from conception (ie, among women initiating ART in pregnancy, stillbirth or preterm birth could occur before ART initiation). Infants who were HIV exposed but not ART exposed had the worst birth outcomes (524 of 1059 [49.5%] total and 220 of 1059 [20.8%] severe adverse outcomes) but were not directly comparable to ART-exposed infants owing to extreme differences in baseline characteristics, including citizenship and lack of antenatal care (Table 1).
Adverse Birth Outcomes by ART Regimen From Conception
Among singleton infants exposed to ART from conception, any adverse birth outcome occurred in 901 of 2472 (36.4%) exposed to TDF-FTC-EFV, 317 of 760 (41.7%) exposed to TDF-FTC-NVP, 112 of 231 (48.5%) exposed to TDF-FTC–LPV-R, 647 of 1365 (47.4%) exposed to ZDV-3TC-NVP, and 75 of 167 (44.9%) exposed to ZDV-3TC–LPV-R (Table 3). Severe birth outcomes exhibited a similar pattern, with the fewest events among infants exposed to TDF-FTC-EFV (303 of 2472 [12.3%]) compared with TDF-FTC-NVP (136 of 760 [17.9%]), TDF-FTC–LPV-R (45 of 231 [19.5%]), ZDV-3TC-NVP (283 of 1365 [20.7%]), and ZDV-3TC–LPV-R (39 of 167 [23.4%]) (Table 3).
After adjustment for maternal age, gravida, and educational attainment, singleton infants exposed to TDF-FTC-EFV from conception were less likely to have any adverse birth outcome or any severe adverse birth outcome compared with infants exposed to TDF-FTC-NVP (ARR, 1.15; 95% CI, 1.04-1.27), TDF-FTC–LPV-R (ARR, 1.31; 95% CI, 1.13-1.52), ZDV-3TC-NVP (ARR, 1.30; 95% CI, 1.20-1.41), or ZDV-3TC–LPV-R (ARR, 1.21; 95% CI, 1.01-1.45) (Table 3). Exposure to TDF-FTC-EFV from conception was less likely to be associated with very SGA compared with exposure to all other regimens from conception and with SGA compared with all other regimens except ZDV-3TC–LPV-R. Exposure to ZDV-3TC-NVP from conception was associated with greater risk for stillbirth (ARR, 2.31; 95% CI, 1.64-3.26), preterm birth (ARR, 1.14; 95% CI, 1.01-1.29), neonatal death (ARR, 1.94; 95% CI, 1.13-3.33), and very preterm birth (ARR, 1.44; 95% CI, 1.07-1.95) compared with exposure to TDF-FTC-EFV. Infants exposed to ZDV-3TC–LPV-R also had a greater risk of preterm birth (ARR, 1.36; 95% CI, 1.06-1.75), neonatal death (ARR, 4.01; 95% CI, 1.78-9.11), and very preterm birth (ARR, 2.21; 95% CI, 1.29-3.79) compared with infants exposed to TDF-FTC-EFV (Table 4).
Table 4. Adverse Birth Outcomes Among Singleton Births by ART Exposurea.
| Adverse Birth Outcome | TDF-FTC-EFV (n = 2472) |
TDF-FTC-NVP (n = 760) |
TDF-FTC–LPV-R (n = 231) |
ZDV-3TC-NVP (n = 1365) |
ZDV-3TC–LPV-R (n = 167)] |
|---|---|---|---|---|---|
| Preterm birthb | |||||
| No. (%) | 529 (21.4) | 145 (19.1) | 55 (23.8) | 338 (24.8) | 49 (29.3) |
| RR (95% CI) | 1 [Reference] | 0.88 (0.75-1.04) | 1.11 (0.87-1.41) | 1.15 (1.02-1.30) | 1.36 (1.07-1.74) |
| ARR (95% CI) | 1 [Reference] | 0.88 (0.75-1.05) | 1.12 (0.88-1-43) | 1.14 (1.01-1.29) | 1.36 (1.06-1.75) |
| Very preterm birthc | |||||
| No. (%) | 101 (4.1) | 39 (5.1) | 12 (5.2) | 80 (5.9) | 15 (9.0) |
| RR (95% CI) | 1 [Reference] | 1.25 (0.87-1.79) | 1.26 (0.71-2.27) | 1.43 (1.07-1.90) | 2.19 (1.30-3.67) |
| ARR (95% CI) | 1 [Reference] | 1.23 (0.84-1.80) | 1.36 (0.76-2.45) | 1.44 (1.07-1.95) | 2.21 (1.29-3.79) |
| SGAd | |||||
| No. (%) | 419 (16.9) | 189 (24.9) | 64 (27.7) | 385 (28.2) | 34 (20.4) |
| RR (95% CI) | 1 [Reference] | 1.44 (1.24-1.68) | 1.62 (1.29-2.03) | 1.65 (1.46-1.86) | 1.19 (0.87-1.63) |
| ARR (95% CI) | 1 [Reference] | 1.44 (1.24-1.68) | 1.56 (1.25-1.97) | 1.66 (1.46-1.87) | 1.13 (0.82-1.56) |
| Very SGAe | |||||
| No. (%) | 176 (7.1) | 85 (11.2) | 31 (13.4) | 176 (12.9) | 21 (12.6) |
| RR (95% CI) | 1 [Reference] | 1.55 (1.21-1.98) | 1.87 (1.31-2.67) | 1.80 (1.47-2.19) | 1.75 (1.15-2.67) |
| ARR (95% CI) | 1 [Reference] | 1.52 (1.18-1.94) | 1.81 (1.26-2.59) | 1.76 (1.44-2.16) | 1.70 (1.10-2.62) |
| Stillbirth | |||||
| No. (%) | 59 (2.4) | 22 (2.9) | 10 (4.3) | 83 (6.1) | 6 (3.6) |
| RR (95% CI) | 1 [Reference] | 1.21 (0.75-1.97) | 1.81 (0.94-3.50) | 2.55 (1.84-3.53) | 1.51 (0.66-3.44) |
| ARR (95% CI) | 1 [Reference] | 1.15 (0.70-1.89) | 1.81 (0.94-3.50) | 2.31 (1.64-3.26) | 1.53 (0.67-3.49) |
| Neonatal deathf | |||||
| No. (%) | 29 (1.2) | 13 (1.7) | 4 (1.7) | 28 (2.1) | 7 (4.2) |
| RR (95% CI) | 1 [Reference] | 1.46 (0.77-2.80) | 1.50 (0.53-4.24) | 1.82 (1.09-3.04) | 3.64 (1.62-8.17) |
| ARR (95% CI) | 1 [Reference] | 1.57 (0.81-3.06) | 1.60 (0.56-4.56) | 1.94 (1.13-3.33) | 4.01 (1.78-9.11) |
Abbreviations: ARR, adjusted relative risk; ART, antiretroviral therapy; EFV, efavirenz; FTC, emtricitabine; LPV-R, lopinavir-ritonavir; NVP, nevirapine; RR, relative risk; SGA, small size for gestational age; TDF, tenofovir disoproxil fumarate; 3TC, lamivudine; ZDV, zidovudine.
Includes singleton births only, and missing data are excluded. All models are adjusted for maternal age, gravidity, and low educational attainment.
Indicates gestational age less than 37 weeks.
Indicates gestational age less than 32 weeks.
Indicates less than 10th percentile of weight for gestational age.
Indicates less than third percentile of weight for gestational age.
Indicates death at less than 28 days.
Separate sensitivity analyses (data in eTable in the Supplement) of birth outcomes recategorizing unspecified ART as TDF-FTC-EFV (if it was started in 2012 or later), redefining conception exposure to include ART started within 4 weeks after conception, adjusting for duration of maternal ART and calendar year of delivery, and using generalized estimating equations and the robust variance estimator to account for clustering by maternity ward site did not change the direction or substantially change the magnitude of the results. Sensitivity analyses that included CD4 cell count in pregnancy (available for 1275 of 5087 [25.1%]) did not change the overall magnitude or direction of any adverse outcome or any severe outcome. We found no statistically significant association between years of maternal ART before conception (<2, 2-5, or >5 years) and adverse birth outcomes (eTable in the Supplement).
Discussion
We performed surveillance for adverse outcomes among almost 45% of the births in Botswana during a 2-year period. The changing ART landscape in Botswana allowed us to perform first-ever comparisons of adverse birth outcomes across multiple ART regimens, and we found that TDF-FTC-EFV exposure from conception was associated with a decreased risk for overall and severe adverse birth outcomes.
Our results extend prior findings on the safety of TDF-FTC-EFV regimens started during pregnancy and provide reassurance for the more than 90% of HIV-infected women who live in countries that follow WHO recommendations to use TDF-FTC-EFV. However, as reported in previous studies of birth outcomes throughout the world, infants exposed to any ART regimen from conception (including TDF-FTC-EFV) had worse birth outcomes than did HIV-unexposed infants. Further research is needed to understand the mechanism of adverse birth outcomes among these infants, and we believe that the distinct associations seen with different ART regimens in this study may provide guidance for future research.
Differences between TDF-FTC-EFV and other ART regimens were greater for SGA than for preterm birth. This finding may suggest a drug-specific mechanism at the placental level because the health of the placenta is directly related to fetal growth. No research has been published on the effects of specific antiretroviral drugs on the placenta, but placental insufficiency has been associated with ART among women with stillbirth. We hypothesize that some ART regimens may lead to placental insufficiency via impaired endothelial function by a direct effect on the endothelium or indirectly through an immunologic or hormonal mechanism. Nevirapine-based ART has been associated with an increased risk for essential hypertension among nonpregnant women. An ART effect at the level of the placenta may also explain why women receiving ART before conception have more adverse outcomes than those who start ART after conception because endothelial dysfunction during placentation would be expected to have a more detrimental effect on the pregnancy.
Our finding that ZDV-3TC-NVP was highly associated with an increased risk for stillbirth is concerning. The overall rate of stillbirth among births exposed to ZDV-3TC-NVP was 6.1% (83 of 1365), similar to the stillbirth rate of 6.3% among births exposed to ART from conception in a prior birth outcomes study in Botswana from 2009 to 2011, when 87% of ART used was ZDV-3TC-NVP. This regimen could explain much of the increased risk for stillbirth seen among births exposed to ART at conception in prior studies.
The ZDV-3TC–LPV-R regimen was most associated with preterm birth, consistent with the findings of prior studies of protease inhibitors. We also found that this regimen was associated with very preterm birth and increased risk for neonatal death. Our findings differ somewhat from the PROMISE trial, which found that women with CD4 cell counts greater than 350/mm3 randomized after 14 weeks of pregnancy to receive LPV-R paired with the TDF-FTC backbone had more severe adverse birth outcomes and neonatal deaths than did those receiving the ZDV-3TC backbone. However, this discrepancy could be attributable to preconception vs postconception ART exposure, restriction to women with a CD4 cell count greater than 350/mm3, or the higher LPV-R dose used in the PROMISE study.
Strengths and Limitations
Because a randomized clinical trial starting ART before conception and comparing birth outcomes is not feasible, we must rely on observational studies to assess the safety of ART from conception. Our study has several strengths, including a sample size large enough to evaluate severe birth outcomes; a short, 2-year time frame without the need for historic controls; a population with relatively homogenous sociodemographic characteristics and low levels of substance use; and the completeness of HIV and ART exposure data.
Limitations of this study relate to the observational study design, which decreased our ability to establish causation because of potential unmeasured confounding and indication bias. We attempted to address potential biases in several ways. Our analysis was restricted to infants with ART exposures from conception who had similar opportunities for adverse outcomes. We also adjusted for age and potential sociodemographic confounders, and we performed sensitivity analyses that included duration of HIV infection, duration of ART, calendar year of delivery, maternity ward site, and CD4 cell count in pregnancy (eTable in the Supplement). In each analysis, we found consistent associations with maternal ART regimen.
We were unable to account for the nadir of the CD4 cell count or immunologic aging, which may have affected birth outcomes. However, NVP-based regimens (started at the lowest CD4 cell count) were not uniformly worse than LPV-R–based regimens (started at a higher CD4 cell count), and no risk was identified among the subset of women receiving TDF-FTC-EFV with the lowest CD4 cell count, arguing against a nadir effect of the CD4 cell count.
Additional limitations included reliance on existing data from antenatal clinics and delivery sites and inability to document early fetal loss (<24 weeks), measure therapy adherence, or capture deaths outside the hospital. Our study findings may be difficult to integrate into settings with ART regimen choices beyond those available in Botswana. Whether the magnitude of the differences we found in Botswana will be similar in higher-resource settings is unclear. Finally, EFV has historically been avoided in the first trimester of pregnancy because of concerns about neural tube defects, and the present analysis does not directly address congenital abnormalities; however, neural tube defects will be evaluated in the final study analysis.
Conclusions
The most commonly prescribed ART in women of reproductive age, TDF-FTC-EFV, was associated with fewer adverse birth outcomes than other commonly used ART regimens in Botswana. This finding should provide reassurance to the global community. However, because specific ART regimens may be associated with worse adverse birth outcomes, increased efforts are needed to perform birth outcome surveillance as new antiretroviral medications are used in resource-rich and resource-limited settings. Our results also suggest that HIV infection and ART may play a more important role in adverse birth outcomes than was previously recognized and could help explain the lack of significant improvement in stillbirth and neonatal death rates throughout sub-Saharan Africa during the past 2 decades.
eTable. Additional Analyses
References
- 1.World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization Programme, Geneva, Switzerland. http://www.who.int/hiv/pub/arv/arv-2016/en/. June 2016. Accessed January 2, 2017.
- 2.UNICEF. For every child, end AIDS: seventh stocktaking report, 2016. http://st7.childrenandaids.org/sites/default/files/STR%202016%20Report%2012_9%20LR_.pdf. December 2016. Accessed January 2, 2016.
- 3.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206(11):1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zash R, Souda S, Chen JY, et al. Reassuring birth outcomes with tenofovir/emtricitabine/efavirenz used for prevention of mother-to-child transmission of HIV in Botswana. J Acquir Immune Defic Syndr. 2016;71(4):428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler MG, Qin M, Fiscus SA, et al. ; IMPAACT 1077BF/1077FF PROMISE Study Team . Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibiude J, Warszawski J, Tubiana R, et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clin Infect Dis. 2012;54(9):1348-1360. [DOI] [PubMed] [Google Scholar]
- 7.Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV. 2017;4(1):e21-e30. [DOI] [PubMed] [Google Scholar]
- 8.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. 2007;21(8):1019-1026. [DOI] [PubMed] [Google Scholar]
- 9.Cotter AM, Garcia AG, Duthely ML, Luke B, O’Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193(9):1195-1201. [DOI] [PubMed] [Google Scholar]
- 10.Powis KM, Kitch D, Ogwu A, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. 2011;204(4):506-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS. 2004;18(17):2337-2339. [DOI] [PubMed] [Google Scholar]
- 12.Ravizza M, Martinelli P, Bucceri A, et al. ; Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy . Treatment with protease inhibitors and coinfection with hepatitis C virus are independent predictors of preterm delivery in HIV-infected pregnant women. J Infect Dis. 2007;195(6):913-914. [DOI] [PubMed] [Google Scholar]
- 13.Fiore S, Ferrazzi E, Newell ML, Trabattoni D, Clerici M. Protease inhibitor–associated increased risk of preterm delivery is an immunological complication of therapy. J Infect Dis. 2007;195(6):914-916. [DOI] [PubMed] [Google Scholar]
- 14.Ouattara EN, Anglaret X, Wong AY, et al. Projecting the clinical benefits and risks of using efavirenz-containing antiretroviral therapy regimens in women of childbearing age. AIDS. 2012;26(5):625-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussmann H, Wester CW, Wester CN, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. J Acquir Immune Defic Syndr. 2007;45(3):269-273. [DOI] [PubMed] [Google Scholar]
- 16.Ekouevi DK, Coffie PA, Ouattara E, et al. ; International Epidemiological Database to Evaluate AIDS West Africa; ANRS 1269 and ANRS 12136 Study Groups in Abidjan . Pregnancy outcomes in women exposed to efavirenz and nevirapine: an appraisal of the IeDEA West Africa and ANRS databases, Abidjan, Côte d’Ivoire. J Acquir Immune Defic Syndr. 2011;56(2):183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford N, Calmy A, Mofenson L. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2011;25(18):2301-2304. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Sando MM, Spiegelman D, et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis. 2016;213(7):1057-1064. [DOI] [PubMed] [Google Scholar]
- 19.UNAIDS HIV and AIDS estimates (2015). http://www.unaids.org/en/regionscountries/countries/botswana. 2015. Accessed January 2, 2017.
- 20.World Health Organization. Botswana: WHO statistical profile. http://www.who.int/gho/countries/bwa.pdf?ua=1&ua=1. Updated January 2015. Accessed January 2, 2017.
- 21.National AIDS Coordinating Agency. Progress report of the national response to the 2011 declaration of commitments on HIV and AIDS. http://www.unaids.org/sites/default/files/country/documents/BWA_narrative_report_2015.pdf. June 2015. Accessed January 2, 2017.
- 22.Ministry of Health and Wellness. Botswana National HIV & AIDS Treatment Guidelines. 2012. Version. http://www.moh.gov.bw. April 1, 2012. Accessed December 7, 2016.
- 23.Republic of Botswana. Health Statistics Report 2009. http://www.statsbots.org.bw/sites/default/files/publications/Health%2520Statistics%2520Annual%2520Report_2009%5B2%5D.pdf. December 2012. Accessed January 2, 2017.
- 24.Republic of Botswana. Handbook of the Botswana 2016 Integrated HIV Clinical Care Guidelines. http://apps.who.int/medicinedocs/documents/s22413en/s22413en.pdf. Accessed January 2, 2017.
- 25.Villar J, Cheikh Ismail L, Victora CG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857-868. [DOI] [PubMed] [Google Scholar]
- 26.Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH; INTERGROWTH-21st Consortium . INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387(10021):844-845. [DOI] [PubMed] [Google Scholar]
- 27.Interagency Task Team. 2015 IATT Annual Report. http://www.emtct-iatt.org/wp-content/uploads/2016/08/IATT-2015-Annual-Report.pdf. February 26, 2015. Accessed June 26, 2017.
- 28.Rollins NC, Coovadia HM, Bland RM, et al. Pregnancy outcomes in HIV-infected and uninfected women in rural and urban South Africa. J Acquir Immune Defic Syndr. 2007;44(3):321-328. [DOI] [PubMed] [Google Scholar]
- 29.Machado ES, Hofer CB, Costa TT, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. 2009;85(2):82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro RL, Souda S, Parekh N, et al. High prevalence of hypertension and placental insufficiency, but no in utero HIV transmission, among women on HAART with stillbirths in Botswana. PLoS One. 2012;7(2):e31580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Halloran JA, Dunne E, Gurwith M, et al. The effect of initiation of antiretroviral therapy on monocyte, endothelial and platelet function in HIV-1 infection. HIV Med. 2015;16(10):608-619. [DOI] [PubMed] [Google Scholar]
- 32.Blodget E, Shen C, Aldrovandi G, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7(8):e42624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiore S, Newell ML, Trabattoni D, et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J Reprod Immunol. 2006;70(1-2):143-150. [DOI] [PubMed] [Google Scholar]
- 34.Papp E, Mohammadi H, Loutfy MR, et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis. 2015;211(1):10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaffer D, Hughes MD, Sawe F, et al. Cardiovascular disease risk factors in HIV-infected women after initiation of lopinavir/ritonavir- and nevirapine-based antiretroviral therapy in sub-Saharan Africa: A5208 (OCTANE). J Acquir Immune Defic Syndr. 2014;66(2):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germain AM, Romanik MC, Guerra I, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49(1):90-95. [DOI] [PubMed] [Google Scholar]
- 37.Lawn JE, Blencowe H, Waiswa P, et al. ; Lancet Ending Preventable Stillbirths Series Study Group; Lancet Stillbirth Epidemiology Investigator Group . Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387(10018):587-603. [DOI] [PubMed] [Google Scholar]
- 38.Lawn JE, Cousens S, Zupan J; Lancet Neonatal Survival Steering Team . 4 Million neonatal deaths: when? where? why? Lancet. 2005;365(9462):891-900. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro R, Dryden-Peterson S, Powis K, Zash R, Lockman S. Hidden in plain sight: HIV, antiretrovirals, and stillbirths. Lancet. 2016;387(10032):1994-1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Additional Analyses