Summary
Reduced habituation of the nociceptive blink reflex (NBR) is considered a trait marker for genetic predisposition to migraine. In this open-label randomized controlled study, we aimed to test the efficacy of a biofeedback training based on learning of habituation of the NBR (NBR biofeedback) compared with pharmacological (topiramate) treatment and NBR biofeedback plus topiramate treatment in a cohort of migraine without aura patients eligible for prophylaxis. Thirty-three migraine patients were randomly assigned to three months of treatment with: 1) NBR biofeedback, 2) NBR biofeedback plus topiramate 50 mg (b.i.d.), or 3) topiramate 50 mg (b.i.d.). Frequency of headache and disability changes were the main study outcomes. Anxiety, depression, sleep, fatigue, quality of life, allodynia and pericranial tenderness were also evaluated.
NBR biofeedback reduced the R2 area, without improving R2 habituation. However, it reduced the frequency of headache and disability, similarly to the combined treatment and topiramate alone.
Reduced habituation of the NBR is a stable neurophysiological pattern, scarcely modifiable by learning procedures. Training methods able to act on stress-related responses may modulate cortical mechanisms inducing migraine onset and trigeminal activation under stressful trigger factors.
Keywords: biofeedback, migraine, nociceptive blink reflex, topiramate
Introduction
Biofeedback-related approaches to headache therapy fall into two broad categories: general biofeedback techniques and methods linked more directly to the pathophysiology underlying headache. General biofeedback-assisted relaxation techniques for headache have been evaluated extensively by expert panels and in meta-analyses, and found to be potentially useful for enhancing patient outcomes, albeit with the limit of a lack of significant relief in a sizeable number of patients (Andrassik, 2010). The biofeedback methods most frequently used for migraine treatment have been peripheral skin temperature biofeedback, blood volume pulse (BVP) and electromyography feedback (Nestoriuc et al., 2008). Neurofeedback sessions combined with thermal biofeedback were effective in reducing the frequency of migraine (Stokes and Lappin, 2010). Biofeedback methods that more directly target headache pathophysiology have focused chiefly on migraine, and especially on BVP biofeedback (Friar and Beatty, 1976) and EEG feedback with training involving habituation of contingent negative variation (CNV) (Siniatchkin et al., 2000), or pain experience through EEG rhythms control (Jensen et al., 2008).
Habituation, “a response decrement as a result of repeated stimulation” (Harris, 1943), is a multifactorial event whose underlying plastic neural mechanisms are still not completely understood. According to the “dual-process” theory, two separate processes, depression (habituation) and facilitation (sensitization), compete to determine the final behavioral outcome after a sequence of repetitive stimuli (Groves and Thompson, 1970). Lack of habituation is a reproducible abnormality found in migraine between attacks, and attains to a model of behavior and a learning process that seem to predispose to attack occurrence. There is strong evidence that lack of habituation during stimulus repetition, despite an initial normal or low response amplitude, is a functional, probably genetically determined, hallmark of the migrainous brain between attacks. (Coppola et al., 2009). In addition, incapacity to progressively reduce pain-related responses under repetitive stimulation (Valeriani et al., 2003) may favor mechanisms of central sensitization (Burstein et al., 2000).
Methods for studying the nociceptive system are based on the employment of stimuli which activate preferentially the A-delta and C afferents. The nociceptive blink reflex (NBR) is elicited by a special stimulation electrode with high current density that rather selectively activates A-delta fibers, eliciting the R2 component (Kaube et al., 2000; Katsarava et al., 2002). Although this stimulation modality lacks the selectivity to evoke reliable pain-related cortical responses (de Tommaso et al., 2011), it may be efficaciously employed for the elicitation of a muscle response under trigeminal nociceptive activation (Kaube et al., 2000; Katsarava et al., 2002). A pivotal study on migraine pathophysiology described amplitude and habituation abnormalities of the NBR in asymptomatic subjects with first-degree inheritance for migraine; these were similar to the abnormalities found interictally in subjects with active disease, indicating NBR dis-habituation as a genetic predisposing trait (Di Clemente et al., 2007). The ability to reduce and control a reflex response to nociceptive trigeminal stimulation may prevent migraine occurrence. In fact, even though reduced habituation to multimodal stimuli seems to be a genetic trait of migraine, habituation may be restored by preventive pharmacological treatments (Ferraro et al., 2012). Consequently, a training setting able to restore this behavior may result in an improvement of migraine outcome.
This open-label randomized controlled study was conducted with the aim of testing the efficacy of a biofeedback training based on learning of habituation of the NBR (NBR biofeedback) compared with pharmacological treatment (topiramate) and the same biofeedback procedure associated with topiramate (NBR biofeedback + topiramate) in a cohort of migraine without aura patients eligible for prophylaxis.
Materials and methods
Cases
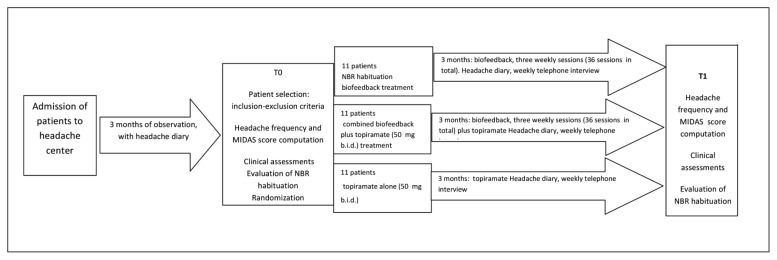
Thirty-three migraine without aura patients (9 males) attending the Neurophysiopathology of Pain Unit of the SMBNOS Department of Bari University were consecutively selected for the study after three months of observation during which they were required to scrupulously keep a headache diary and fill in an allodynia questionnaire (Jakubowski et al., 2005) (Fig. 1). The inclusion criteria were: a diagnosis of migraine without aura (Headache Classification Committee, 2004) and eligibility for migraine prophylaxis (≥ 4 disabling migraine attacks per month or, if < 4 per month, in the case of poor response to symptomatic treatment), according to Sarchielli et al. (2012).
Figure 1.
Summary of the experimental procedure.
The clinical assessments comprised: total tenderness score (TTS) (Langermark and Olesen, 1987), Short-Form 36 (SF-36) Health Survey (Ware et al., 2000), depression (self-rating depression scale (SDS)] and anxiety [self-rating anxiety scale (SAS)] scale (Zung, 1965, 1976), the sub-items sleep problems index (SLP9), and sleep quantity (SLPQ) of Medical Outcomes Study—Sleep Scale (MOS) (Hays and Stewart, 1992), and Multidimensional Assessment of Fatigue (MAF) (Belza et al., 1993). Headache frequency was considered the average number of days with headache/month, computed in the previous three months. The MIDAS score was calculated using the Italian version of the Migraine Disability Assessment Scale (MIDAS) (D’Amico et al., 2001).
The exclusion criteria were: general medical problems (including kidney stones) and psychiatric and other neurological diseases, psychoactive drug intake in the previous three months. After the three-month observation, serving to confirm the diagnosis and the criteria for migraine prophylaxis eligibility, at study phase T0 the patients were randomly assigned to the following treatments: 1) biofeedback 2) biofeedback + topiramate 50 mg (b.i.d.), or 3) topiramate 50 mg (b.i.d. ). We also selected 8 healthy non-migraine subjects (2 males), aged 18–40 years (mean age 39.9±4.56), who were not submitted to biofeedback treatment, but served for the evaluation of normal NBR parameters. The study was conducted in accordance with the Declaration of Helsinki (www.wma.net) and approved by the ethics committee of Bari Policlinico General Hospital. All subjects gave their informed consent before participation.
At the time of treatment assignation (T0), the patients underwent a clinical evaluation, according to de Tommaso et al. (2009, 2011). Briefly, this consisted of the following evaluations: total tenderness score (TTS) (Langermark and Olesen, 1987), Short-Form 36 (SF-36) Health Survey (Ware et al., 2000), self-rating depression scale (SDS) and self-rating anxiety scale (SAS) (Zung, 1965, 1976) — these are useful to evaluate anxiety and depression traits in non-psychiatric subjects —, the Medical Outcomes Study – Sleep Scale (MOS) sub-items sleep problems index (SLP9) and sleep quantity (SLPQ) (Hays and Stewart, 1992), and the Multidimensional Assessment of Fatigue (MAF) (Belza et al., 1993).
The Migraine Disability Assessment Scale (MIDAS), in the Italian version (D’Amico et al., 2001), was used to quantify headache-related disability. The same evaluations were performed at study phase T1, i.e. after three months of the randomly assigned treatments. The frequency of migraine and MIDAS scores computed at T1 were taken as the main outcome indexes. During the three months of treatment, the patients were contacted weekly by telephone, in order to check the reliability of their compilation of the headache diary, which they continued to keep throughout the treatment period (see Fig. 1 for the details of experimental procedure).
As a result of the random assignation, 11 patients were assigned to Group 1: NBR habituation biofeedback treatment (3 males, age 42.13 + 5.51); 11 to Group 2: NBR biofeedback + topiramate treatment 50 mg (b.i.d.) (2 males, age 40.5 + 9.9), and 11 to Group 3: topiramate alone 50 mg (b.i.d.) (4 males, age 39.38 + 11.50; ANOVA with age as a factor: F=0.73, n.s.). The frequency of headache reported in the previous three months — we considered the average number of days with headache in a month —, was also similar across the groups.
For Group 1, it was 11.9 + 5.15, for Group 2, 12.9 + 8.1, and for Group 3, 13.1 + 8.2 (ANOVA with frequency as a factor: F= 0.49, n.s.). Even though 11 of the 33 patients were not assigned to the NBR biofeedback, to ensure patient homogeneity from a neurophysiological perspective, we submitted all of them to NBR habituation exploration at T0 and T1.
Nociceptive blink reflex habituation procedure
The NBR evaluation was performed according to Di Clemente et al. (2007). A MICROMED SYSTEM PLUS apparatus (Micromed S.P.A., Mogliano Veneto, TV-Italy) was employed.
The nociceptive electrical stimulation was delivered by a custom-built planar concentric electrode assembly comprising a central metal cathode (D: 10.5 mm), an isolation insert (D: 5 mm), and an external anode ring (D: 6 mm) providing a stimulation area of 19.6 mm2). It provided a high current density at low intensities to stimulate the supra-orbital region.
Perception and pain thresholds were determined on both sides of the forehead with stimuli’s sequence of ascending and descending intensity (in 0.2mA steps). For the patients’ comfort and to avoid a too lengthy procedure the electrophysiological study and biofeedback training were restricted to a unilateral right-sided stimulation. The stimulus intensity was set at 1.5 times the individual pain threshold.
Monopolar square pulses of 0.3 ms duration were delivered with pseudo-randomized interstimulus intervals (ISIs) of 15–17 s. By means of surface electrodes, 10 blocks of six rectified EMG responses with an interblock interval (IBI) of 2 minutes were recorded over the orbicularis oculi muscle.
Data processing
For each sweep, 150 ms of the post-stimulus period were collected and filtered off-line (1 Hz–1 kHz). Five responses were rectified and averaged for each block (Fig. 1), as the first sweep was excluded from the signal analysis to avoid contamination with startle responses (Kaube et al., 2000; Katsarava et al., 2002), according to Di Clemente et al. (2007). In order to evaluate the global EMG activity generated during the R2 reflex, we measured the area under the curve (the response area: RA) in mV2.
For each averaged block, the R2 area between 27 and 87 ms (Ellrich and Treede, 1998) was measured off-line by an investigator blinded to the subjects’ identities and treatment. Habituation of the NBR R2 was defined as the percentage change of the R2 area between the first and the tenth block of recordings.
Biofeedback training
After the NBR evaluation and treatment assignment, biofeedback training consisted of repeated session three times a week for a period of three months. The patient was positioned in front of a monitor on which his/her NBR responses were displayed. In particular, he/she was instructed in the task, which consisted of progressive reduction of his/her own reflex response in order to obtain habituation. He/she received the noxious stimulus, which was set at the intensity employed in the T0 evaluation, with eyes closed, and was invited to relax and to reduce blinking, and then to open his/her eyes and check for his/her response. Ten blocks of six rectified EMG responses with an IBI of 2 min were recorded in each session. The monitor reproduced the average across the five responses making up the single block, with the RA between 27 and 87 ms represented by a green area, accompanied by its value reported in mA2. Patients were specifically instructed to progressively reduce the green area across the ten blocks. At the end of the session, an automatic program reported the value of the total area of the first and of the tenth block and the habituation index, expressed as the percentage change of the R2 area between the first and the tenth block of recordings.
We chose to submit patients to NBR biofeedback training interictally (at least 48 hours after one attack and before the next), therefore sessions were scheduled, in part, according to migraine episode occurrence.
Statistical analysis
All the variables introduced in the analysis were submitted to the Lilliefors (Kolmogorov-Smirnov) test to check their normal distribution prior to the ANOVA analysis. The NBR R2 habituation index and area were compared between patients and controls employing the one-way ANOVA with group as the main factor. In addition, t-test for single comparison was also used to assess whether R2 habituation failure occurred in randomized patients.
The percentage changes in headache frequency and MIDAS scores between T0 and T1 were taken as the main outcomes of the study, for which the sample size was calculated taking into consideration a 95% power for one-way ANOVA and a post-hoc Bonferroni test. Headache frequency and MIDAS, as well as allodynia, SF-36 and PCS score, SAS and SDS scores, and also the NBR area and habituation index were compared across Groups 1, 2 and 3 by means of two-way ANOVA with phase T0 vs T1 and treatment x phase as factors, in order to establish the trend of changes, despite the low power estimation for the factor treatment x phase. The Spearman correlation test showed that the percentage change of headache frequency between T0 and T1 was correlated with the percentage change of NBR habituation index and area in the single groups.
Results
All patients in Groups 1 and 2 completed their biofeedback training. One patient in Group 3 was lost to three-month follow-up, due to the occurrence of side effects, i.e. mild sedation, which led the patient to discontinue the treatment.
With regard to the NBR, we found no difference in detection or pain threshold between the patients and controls at T0.
The detection threshold was 1.2 + 0.22 mA in controls, 1.1 + 0.2 mA in Group 1, 0.98 + 0.12 in Group 2 and 1.12 + 0.11 in Group 3 (ANOVA F= 0.85 n.s.), while the pain threshold was 1.68 + 0.44 mA in controls, 1.56 + 0.21 mA in Group 1, 1.75 + 0.32 mA in Group 2, and 1.67 + 0.31 mA in Group 3 (ANOVA: F= 0.98 DF, 1 n.s.). The R2 area was not significantly different between migraine patients and controls (controls: 14.1 + 6.61 mV2; migraine: 15.12 + 6 mV2, ANOVA: F=1.1 DF, 1 n.s.). At T0, the abituation index was significantly reduced in all the patients vs controls. Habituation in normal subjects: range 31.20–51.10, mean 41.67 + 11.20; migraine patients: range 12–25.81, mean 18.91 + 13.60, ANOVA: F= 16.33 DF 1, p 0.001.
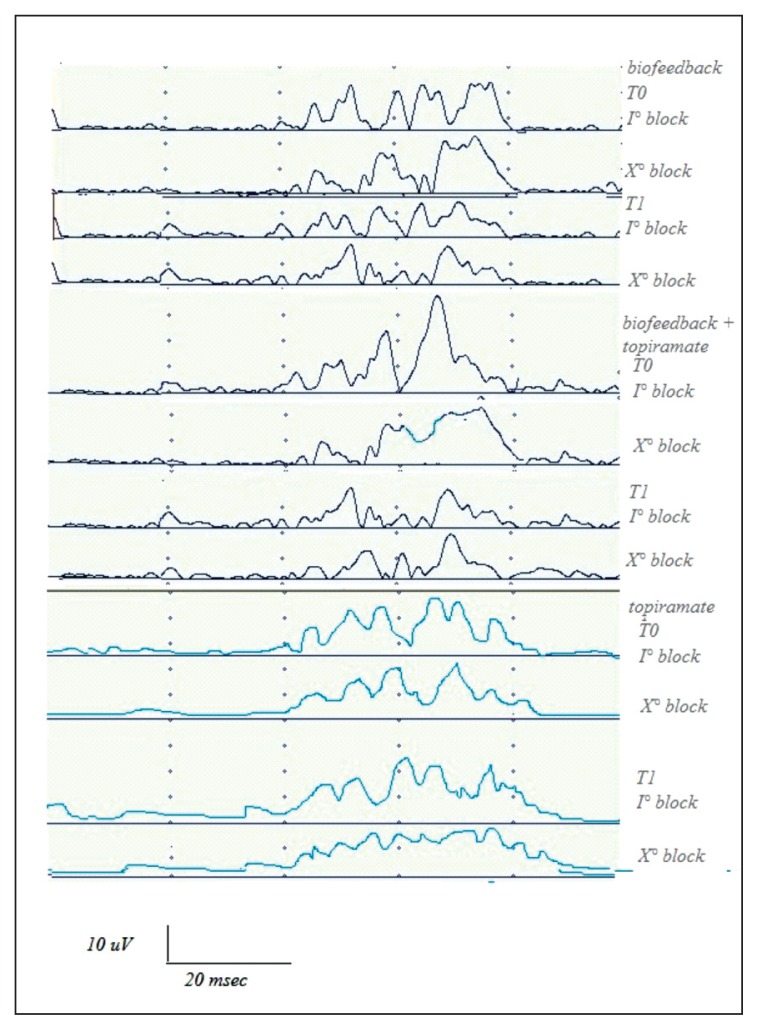
At three months (T1), we found that the R2 area was significantly reduced in migraine patients submitted to NBR biofeedback, compared with patients who took topiramate alone (Figs. 2, 3, Table I).
Figure 2.
Nociceptive blink reflex examples in three representative patients in basal condition (T0) and after 3 months (T1) of biofeedback, biofeedback + topiramate and topiramate treatment. The first and the tenth block of five rectified R2 responses is reported to show the effects of treatment on the R2 area and habituation.
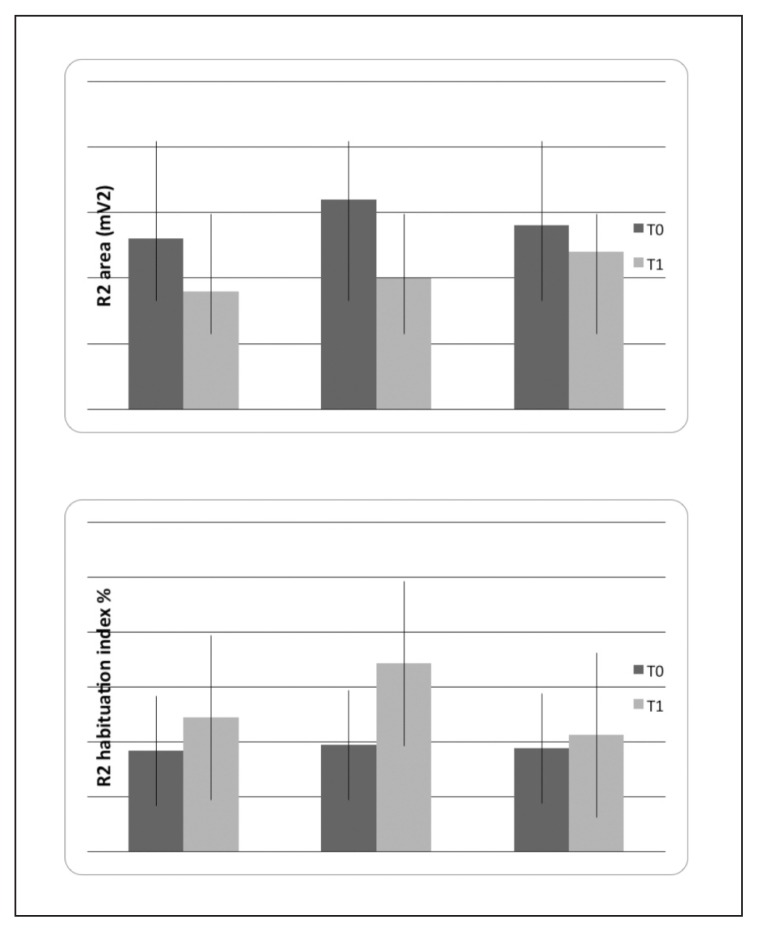
Figure 3.
Mean values and standard deviations of nociceptive blink reflex (NBR) features in basal condition (T0) and after 3 months (T1) of biofeedback (N° 11), biofeedback + topiramate (N° 11) and topiramate (N° 10) treatments. The results of two-way ANOVA with phase as factor are reported: *p<0.05. For the detailed statistical results, see Table I.
Table I.
Two-way ANOVA results with phases T0 vs T1 and treatments as factors (NBR biofeedback, 11 patients; NBR biofeedback+topiramate, 11 patients; topiramate alone, 10 patients)
| Variables Fixed factors |
Pain threshold | R2 area | R2 habituation index | Frequency of headache | MIDAS | Allodynia | TTS | SAS | SDS | SPL9 | Sleep quantity | SF-36 PCS | SF-36 MCS | MAF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Treatment | ||||||||||||||
|
| ||||||||||||||
| Phase | ||||||||||||||
| T0 vs Tl DF 1 | ||||||||||||||
| F | 0.85 | 4.73 | 3.21 | 4.86 | 10.15 | 1.23 | 0.45 | 0.67 | 1.45 | 0.89 | 1.45 | 14.61 | 3.15 | 5.24 |
| P | n.s. | 0.016 | n.s. | 0.0036 | 0.0004 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.0001 | n.s. | 0.035 |
|
| ||||||||||||||
| Phase x treatment | ||||||||||||||
| DF 2 | ||||||||||||||
| F | 0.79 | 4.23 | 0.45 | 0.48 | 0.65 | 0.99 | 0.23 | 1.23 | 1.22 | 0.44 | 1.11 | 1.67 | 0.47 | 0.34 |
| P | n.s. | 0.049 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
Abbreviations: MIDAS=Migraine Disability Assessment Scale; TTS=total tenderness score; SAS=self-rating anxiety scale; SDS=self-rating depression scale; SLP9=sleep problems index (Medical Outcomes Study – Sleep Scale sub-item); PCS=Physical Component Summary; MCS=Mental Component Summary; MAF= Multidimensional Assessment of Fatigue. Frequency of headache= average number of days with headache/month computed in the three months preceding T0 and T1 phases)
The habituation index was increased at T1, especially in migraine patients submitted to biofeedback, though this change did not reach statistical significance (Fig. 3, Table I). The pain threshold was not significantly modified (1.55 + 0.22 mA in Group 1, 1.74 + 0.22 mA in Group 2, and 1.69 + 0.32 mA in Group 3) (Table I).
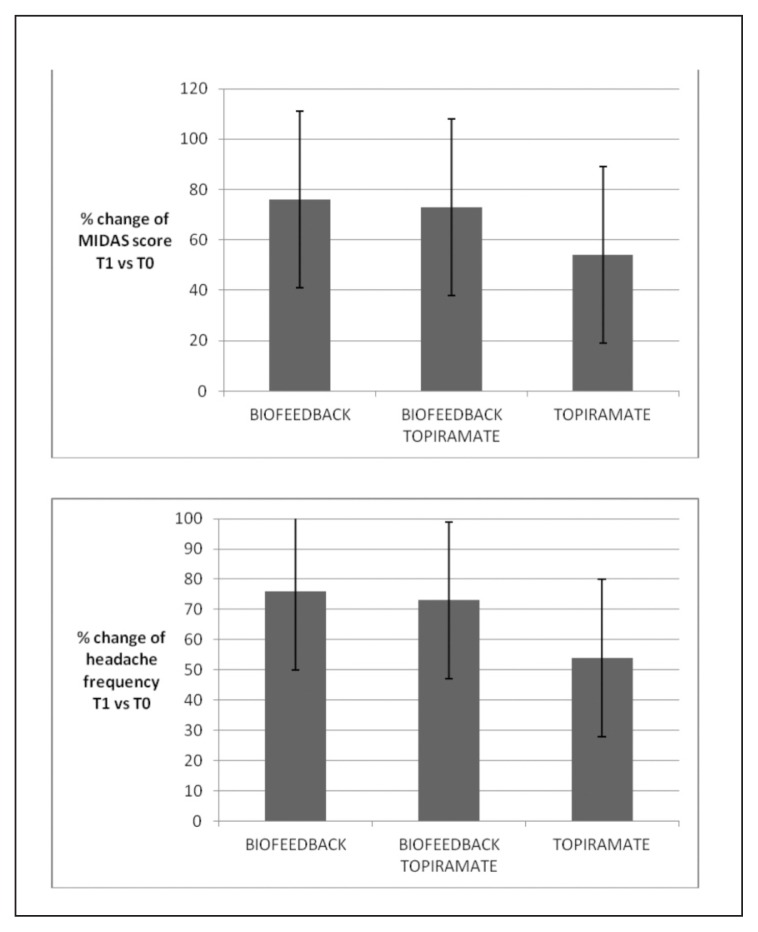
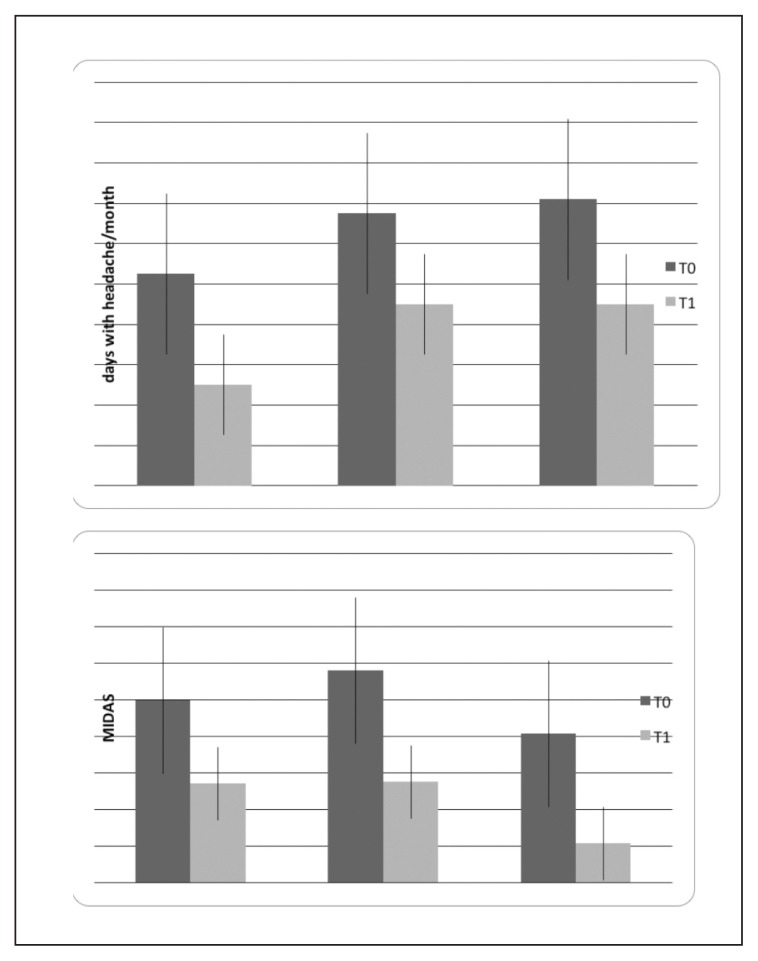
The percentage changes in headache frequency and MIDAS scores were not significantly different across treatments (one-way ANOVA with treatment as factor and percentage variation of headache frequency as variable: F=0.76 DF 2, n.s.; MIDAS score percentage change as factor: F= 0.87; DF 2, n.s. Post-hoc test: n.s.) (Fig. 4). The effect size was 0.67 for Group 1, 0.61 for Group 2, and 0.62 for Group 3. In Group 1, 10 of the 11 patients reported a 50% reduction in headache frequency, while the number of responders was 9 in Group 2, and 8 in Group 3 (Fig. 5, Table I).
Figure 4.
Mean values and standard deviation of percentage change in frequency of headache and MIDAS scores in the three migraine groups. One-way ANOVA with treatment as factor was not significant.
Figure 5.
Mean values and standard deviations of headache frequency and MIDAS scores, computed in the three months preceding the basal condition (T0) and at the T1 evaluation after three months of biofeedback (N° 11), biofeedback + topiramate (N° 11) and topiramate (N° 10) treatments. The results of two-way ANOVA with phase as factor are reported: ** p<0.001; p<0.001. For the detailed statistical results, see Table I.
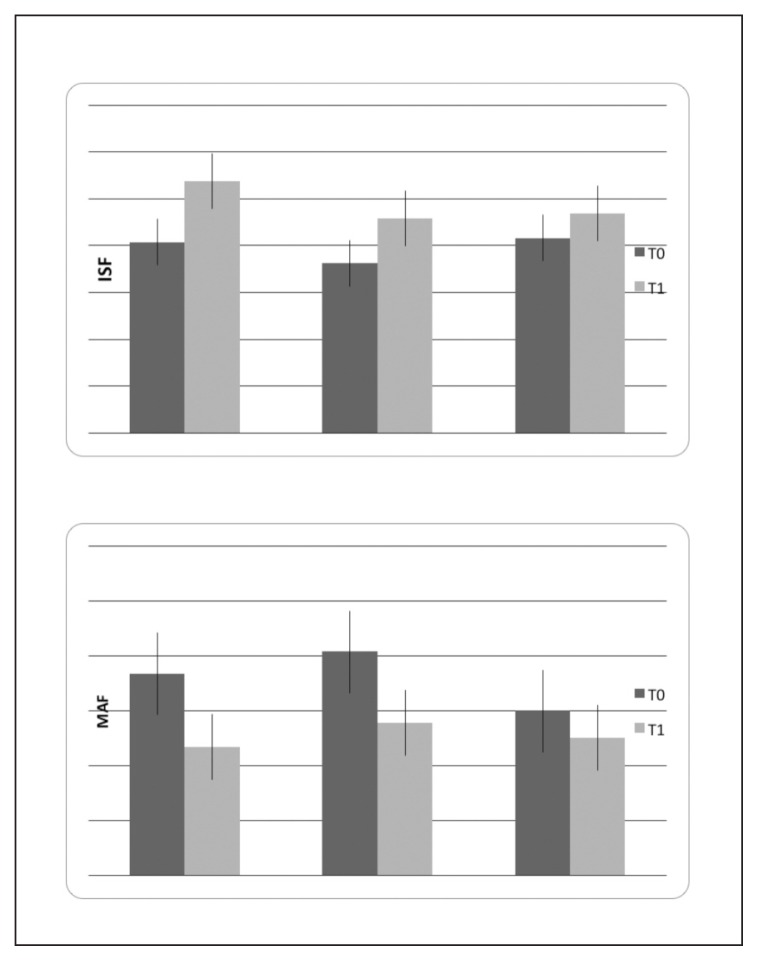
Allodynia, TTS, anxiety and depression scores, and sleep quality and duration did not change significantly after three months of treatment (Table I). Quality of life linked to physical conditions (Physical Component Summary – PCS of the SF-36 questionnaire) was significantly improved without differences across treatments, while health state linked to mental conditions (Mental Component Summary – MCS scores) was only slightly modified (Fig. 6, Table I). Fatigue as measured by MAF score was significantly reduced in all migraine patients after three months of treatment (Fig. 6, Table I). We did not observe significant correlations between the percentage change in headache frequency and percentage changes in R2 area and habituation index between the T0 and T1 phases in the two groups submitted to biofeedback (Spearman correlation test: percentage change in headache frequency vs percentage change in R2 area: 0.345, n.s.; habituation: 0.234, n.s.).
Figure 6.
Mean values and standard deviations of the Physical Component Summary (PCS) of SF-36 questionnaire and fatigue as measured by the MAF score in basal condition (T0) and after 3 months (T1) of biofeedback (N° 11), biofeedback + topiramate (N° 11) and topiramate (N° 10) treatments. The results of two-way ANOVA with phase as factor are reported: * p<0.001; *** p<0.0001. For the detailed statistical results, see Table I.
Discussion
This is the first study to apply a procedure of learning by biofeedback to reduced habituation of the NBR, which is considered a neurophysiological abnormality predisposing to migraine (Di Clemente et al., 2007). In our migraine patients, this pattern of reduced habituation was confirmed in all cases, but it was not found to be significantly modified by the NBR biofeedback training, either alone or in association with topiramate. Other studies exploring the effects of biofeedback training on habituation of CNV in children with migraine (Siniatchkin et al., 2000) reported a lack of effect when the feedback was removed, confirming that reduced habituation is a stable interictal pattern in migraine, and that it is scarcely modifiable by learning procedures. However, in that study the number of treatment sessions was very limited and probably not sufficient to provide stable effects on CNV habituation. In our study, a clear trend in habituation index change was seen in patients submitted to biofeedback, therefore it could be that a larger sample size would confirm the possibility of conditioning this genetic trait of migraine. In a previous study, we observed that topiramate was able to induce habituation of CNV, and that this effect was correlated with its clinical efficacy (de Tommaso, 2007, 2008), whereas the present results indicated a low effect of topiramate on R2 area and habituation index, despite the favorable clinical outcome. We can argue that antiepileptics may act on a late EEG response but are ineffective on reflex EMG responses and their modulation. The action of topiramate in migraine prevention is probably based on a specific inhibiting effect on the bioelectrical phenomena preceding the attack (Akerman and Goadbsy, 2005). Its efficacy could be linked to the reduction of cortical excitability and arousal that causes CNV abnormalities in migraine (Coppola and Schoenen, 2012). Although NBR habituation is dependent upon supra-segmental control, the blink reflex is a subcortical response (Ellrich and Treede, 1998; Elrich, 2002), which does not seem to be influenced by the central modulation exerted by topiramate. In the present study the main effect of the training procedure was exerted on the R2 area, which appeared to be significantly reduced in the groups treated by biofeedback alone or in association with topiramate, while topiramate alone confirmed its low efficacy in modifying the trigeminal reflex. The learning procedure probably reduced the reflex response to the nociceptive stimulus, counteracting the arousal and stress against pain, resulting in lower efficacy in restoring the pattern of reduced habituation. The lack of correlation between the size of the clinical effect and the changes in NBR habituation may indicate that in our procedure a generic mechanism of autogenic training and behavioral stress coping exerted on the trigeminal nociceptive system prevailed over a specific effect on NBR habituation. Even though our procedure did not act specifically on the NBR dis-habituation pattern, its effect on migraine frequency and disability was comparable to that exerted by topiramate. In fact, it reduced headache frequency by almost 50%, with an effect size (0.65) comparable to those of other training procedures (Nestoriuc et al., 2008). The association of NBR biofeedback with topiramate did not enhance the effect on migraine frequency. Holroyd et al. (1995) conducted a number of meta-analyses and randomized controlled trials that compared behavioral and pharmacological preventive treatments, as well as their used separately and in combination. These reviews and studies have consistently shown that outcomes for individual treatments are similar in magnitude and that combining behavioral and pharmacological treatments leads to even greater effects (Andrassik, 2010). At present, we cannot explain the lack of benefit of association therapy in our study, patients randomly assigned to the combination of biofeedback and topiramate, although not significant, might have contributed to this apparent lack of benefit from combining the treatments.
A limit of our study is the lack of placebo and of non-medicated patients in the control group. The biological basis of the placebo effect is currently accepted, and the effect of complex prophylactic procedures on migraine seems mostly linked to a placebo effect and non-specific psychological effects (Autret et al., 2012). In our opinion, the contribution of the placebo effect would not reduce the relevance of the clinical benefit induced by the NBR procedure. In a previous study, migraine patients did not seem easily conditioned by verbal suggestion of pain relief (de Tommaso et al., 2012), although the good effect of training procedures, including that performed in the present study, seems to suggest that conditioning of stress-related responses may act on cortical mechanisms subtending migraine onset and trigeminal system activation under stressful trigger factors Another flaw of our study was that it followed a non-blinded design, an element in favor of the above-mentioned placebo effect. The number of cases was also low, which could reduce the clinical relevance of the results. Indeed, the small sample size does not allow definite conclusions to be drawn from the comparison of the effects exerted by the three therapeutic approaches on the other clinical variables, although the trend of the changes may be interesting from the perspective of future studies. The effects of our NBR biofeedback procedure were not associated with reduction of anxiety and depression, differently from other biofeedback procedures (Nestoriuc et al., 2008). The main reason may be that our patients were not affected by psychiatric comorbidities. However, an improvement in anxiety and depression traits, was expected. In the same way, topiramate alone reduced headache frequency, without effects on anxiety and depression. Generally, these aspects are not considered in studies on migraine prophylaxis, which are exclusively based on headache frequency outcome, and also the effect of topiramate on anxiety and depression has not been fully clarified (Ettinger and Argoff, 2007; de Tommaso, 2012). The present study, with the limitations imposed by the small sample, seems to suggest that the effect of both topiramate and the biofeedback procedure on migraine frequency was not accompanied by an action on symptoms of anxiety and depression in non-psychiatric patients. Accordingly, the reduction of migraine frequency did not modify mental performances as tested by SF-36, rather it was associated with an improvement of quality of life related to physical conditions. The improvement of physical conditions linked to the reduction of migraine frequency was also confirmed by the reduction of fatigue, an effect slightly more prevalent in the biofeedback-treated patients. Allodynia was not modified by either biofeedback or topiramate. Even though allodynia is a sign of central sensitization, which can facilitate chronic headache occurrence, few studies have reported the effects of preventive treatments on this important feature of migraine (Mathew, 2011). In the present study, topiramate, both alone and in association with biofeedback, reduced migraine frequency but was ineffective on cutaneous allodynia, even though others have reported efficacy of topiramate in reducing symptoms of peripheral and central sensitization in neuropathic pain (Codd et al., 2008; Paranos et al., 2011). Our patients were requested to make a note of any allodynia symptom accompanying each single episode in a specific questionnaire, so the lack of effect of topiramate, NBR biofeedback, and their association on symptoms of central sensitization seems consistent and worthy of confirmation in a larger series. In addition, no effect emerged for pericranial tenderness, which is another objective sign of central sensitization at the level of the spinal dorsal horn/trigeminal nucleus (Bendtsen, 2000). In this regard, we can suppose that the learning procedure, aimed at improving modulation of trigeminal pain-related reflex responses, was ineffective in reducing the development of cutaneous allodynia and central sensitization during the migraine attack, but was able to prevent attack occurrence, probably by acting on the cortical mechanisms triggering trigeminal activation. Moreover, the NBR is not purely nociceptive, as the concentric electrode may activate mechanical as well as small myelinated afferents (de Tommaso et al., 2012). The training procedure probably acts on the generic phenomenon of arousal and supra-segmental control of the reflex response, without specifically involving the nociceptive component of the trigeminal system. Further evidence in patients with chronic migraine and more severe symptoms of central sensitization would help to confirm a low action of these pharmacological and nonpharmacological preventive strategies on these aspects of migraine.
Summarizing, we found that our biofeedback procedure, based on control of the reflex response and habituation to painful trigeminal stimulation, was as efficacious as topiramate in preventing migraine. Accordingly, NBR biofeedback improved migraine frequency and not allodynia or central sensitization, confirming an action on cortical factors that trigger migraine and trigeminal activation under stressful conditions, more than on mechanisms of pain control. This study confirms the usefulness of methods of autogenic training and behavioral stress coping able to condition cortical mechanisms driving trigeminal activation under the effect of stressful trigger factors. Further long-term trials may clarify the duration of clinical effects and the best treatment design.
Footnotes
Authors declare no conflict of interest.
References
- Akerman S, Goadsby PJ. Topiramate inhibits cortical spreading depression in rat and cat: impact in migraine aura. Neuroreport. 2005;16(12):1383–1387. doi: 10.1097/01.wnr.0000175250.33159.a9. [DOI] [PubMed] [Google Scholar]
- Andrassik J. Biofeedback in headache: An overview of approaches and evidence CLEV. CLIN J MED. 2010;77:S72–S76. doi: 10.3949/ccjm.77.s3.13. [DOI] [PubMed] [Google Scholar]
- Autret A, Valade D, Debiais S. Placebo and other psychological interactions in headache treatment. J Headache Pain. 2012;13(3):191–198. doi: 10.1007/s10194-012-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belza B, Henke C, Yelin E, Epstein WV, Gilliss CL. Correlates of fatigue in older adults with rheumatoid arthritis. Nursing Research. 1993;42:93–99. [PubMed] [Google Scholar]
- Bendtsen L. Central sensitization in tension-type headache--possible pathophysiological mechanisms. Cephalalgia. 2000;20(5):486–508. doi: 10.1046/j.1468-2982.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. Association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–616. [PubMed] [Google Scholar]
- Codd EE, Martinez RP, Molino L, Rogers KE, Stone DJ, Tallarida RJ. Tramadol and several anticonvulsants synergize in attenuating nerve injury-induced allodynia. Pain. 2008;134(3):254–262. doi: 10.1016/j.pain.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Coppola G, Pierelli F, Schoenen J. Habituation and migraine. Neurobiol Learn Mem. 2009;92:249–259. doi: 10.1016/j.nlm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Coppola G, Schoenen J. Cortical excitability in chronic migraine. Curr Pain Headache Rep. 2012;16(1):93–100. doi: 10.1007/s11916-011-0231-1. [DOI] [PubMed] [Google Scholar]
- D’Amico D, Mosconi P, Genco S, Usai S, Prudenzano AM, Grazzi L, et al. The Migraine Disability Assessment (MIDAS) questionnaire: translation and reliability of the Italian version. Cephalalgia. 2001;21:947–952. doi: 10.1046/j.0333-1024.2001.00277.x. [DOI] [PubMed] [Google Scholar]
- de Tommaso M. Effects of Antiepileptic Drugs on Neurophysiologic Abnormalities Subtending Migraine DRUG DEVELOP. RES. 2007;68:1–7. [Google Scholar]
- de Tommaso M, Federici A, Franco G, Ricci K, Lorenzo M, Delussi M, Vecchio E, Serpino C, Livrea P, Todarello O. Suggestion and pain in migraine: a study by laser evoked potentials. CNS Neurol Disord Drug Targets. 2012;11(2):110–126. doi: 10.2174/187152712800269759. [DOI] [PubMed] [Google Scholar]
- de Tommaso M, Federici A, Serpino C, Vecchio E, Franco G, Sardaro M, Delussi M, Livrea P. Clinical features of headache patients with fibromyalgia comorbidity. J Headache Pain. 2011;12(6):629–638. doi: 10.1007/s10194-011-0377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tommaso M, Guido M, Sardaro M, Serpino C, Vecchio E, De Stefano G, Di Claudio T, Specchio LM, Livrea P. Effects of topiramate and levetiracetam vs placebo on habituation of contingent negative variation in migraine patients. Neurosci Lett. 2008;12:81–85. doi: 10.1016/j.neulet.2008.06.076. [DOI] [PubMed] [Google Scholar]
- de Tommaso M, Santostasi R, Devitofrancesco V, Franco G, Vecchio E, Delussi M, et al. A comparative study of cortical responses evoked by transcutaneous electrical vs CO2 laser stimulation. Clin Neurophysiol. 2011;122:2482–2487. doi: 10.1016/j.clinph.2011.05.006. [DOI] [PubMed] [Google Scholar]
- de Tommaso M, Sardaro M, Serpino C, Costantini F, Vecchio E, Prudenzano M Pia, Lamberti P, Livrea P. Fibromyalgia comorbidity in primary headaches. Cephalalgia. 2009;29:453–464. doi: 10.1111/j.1468-2982.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- de Tommaso M. Prevalence, clinical features and potential therapies for fibromyalgia in primary headaches. Expert Rev Neurother. 2012;12(3):287–295. doi: 10.1586/ern.11.190. [DOI] [PubMed] [Google Scholar]
- Di Clemente L, Coppola G, Magis D, Fumal A, De Pasqua V, Di Piero V. Interictal habituation deficit of the nociceptive blink reflex: An endophenotypic marker for presymptomatic migraine? Brain. 2007;130:765–770. doi: 10.1093/brain/awl351. [DOI] [PubMed] [Google Scholar]
- Ellrich J. Trigeminal nociceptive reflexes. Mov Disord. 2002;17(2):S41–44. doi: 10.1002/mds.10057. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Treede RD. Characterization of blink reflex interneurons by activation of diffuse noxious inhibitory controls in man. Brain Res. 1998;803:161–168. doi: 10.1016/s0006-8993(98)00646-5. [DOI] [PubMed] [Google Scholar]
- Ettinger AB, Argoff CE. Use of antiepileptic drugs for nonepileptic conditions: psychiatric disorders and chronic pain. Neurotherapeutics. 2007;4(1):75–83. doi: 10.1016/j.nurt.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro D, Vollono C, Miliucci R, Virdis D, De Armas L, Pazzaglia C, Le Pera D, Tarantino S, Balestri M, Di Trapani G, Valeriani M. Habituation to pain in “medication overuse headache”: a CO2 laser-evoked potential study. Headache. 2012;52(5):792–807. doi: 10.1111/j.1526-4610.2012.02151.x. [DOI] [PubMed] [Google Scholar]
- Friar LR, Beatty J. Migraine: management by trained control of vasoconstriction. J Consult Clin Psychol. 1976;44:46–53. doi: 10.1037//0022-006x.44.1.46. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77(5):419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JE Jr, editors. Measuring functioning and well-being. Durham, NC: Duke University Press; 1992. pp. 235–239. [Google Scholar]
- Harris J. Habituatory response decrement in the intact organism. Psychol Bull. 1943;40:385–422. [Google Scholar]
- Headache Classification Committee. The International Classification of Headache Disorders II. Cephalalgia. 2004;24:24–136. [Google Scholar]
- Holroyd KA, France JL, Cordingley GE, et al. Enhancing the effectiveness of relaxation-thermal biofeedback training with propranolol hydrochloride. J Consult Clin Psychol. 1995;63:327–330. doi: 10.1037//0022-006x.63.2.327. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, Silberstein S, Ashkenazi A, Burstein R. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology. 2005;65:1419–1422. doi: 10.1212/01.wnl.0000183358.53939.38. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Hakimian S, Sherlin LH, Fregni F. New insights into neuromodulatory approaches for the treatment of pain. J Pain. 2008;9:193–199. doi: 10.1016/j.jpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Katsarava Z, Ellrich J, Diener HC, Kaube H. Optimized stimulation and recording parameters of human “nociception specific” blink reflex recordings. Clin Neurophysiol. 2002;113:1932–1936. doi: 10.1016/s1388-2457(02)00307-3. [DOI] [PubMed] [Google Scholar]
- Kaube H, Katsarava Z, Kaufer T, Diener HC, Ellrich J. A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol. 2000;111:413–416. doi: 10.1016/s1388-2457(99)00295-3. [DOI] [PubMed] [Google Scholar]
- Langermark M, Olesen J. Pericranial tenderness in tension headache. A blind, controlled study. Cephalalgia. 1987;7:249–255. doi: 10.1046/j.1468-2982.1987.0704249.x. [DOI] [PubMed] [Google Scholar]
- Mathew NT. Pathophysiology of chronic migraine and mode of action of preventive medications. Headache. 2011;51:84–92. doi: 10.1111/j.1526-4610.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- Nestoriuc Y, Martin A, Rief W, Andrasik F. Biofeedback treatment for headache disorders: a comprehensive efficacy review. Appl Psychophysiol Biofeedback. 2008;33:125–140. doi: 10.1007/s10484-008-9060-3. [DOI] [PubMed] [Google Scholar]
- Paranos SL, Tomić MA, Micov AM, Stepanović-Petrović RM. The mechanisms of antihyperalgesic effect of topiramate in a rat model of inflammatory hyperalgesia. Fundam Clin Pharmacol Dec. 2011;5 doi: 10.1111/j.1472-8206.2011.01018.x. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Granella F, Prudenzano MP, Pini LA, Guidetti V, Bono G, et al. Italian guidelines for primary headaches: revised version. J Headaceh Pain. 2012;13:S31–70. doi: 10.1007/s10194-012-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniatchkin M, Hierundar A, Kropp P, Kuhnert R, Gerber WD, Stephani U. Self-regulation of slow cortical potentials in children with migraine: an exploratory study. Appl Psychophysiol Biofeedback. 2000;25:13–32. doi: 10.1023/a:1009581321624. [DOI] [PubMed] [Google Scholar]
- Stokes DA, Lappin MS. Neurofeedback and biofeedback with 37 migraineurs: a clinical outcome study. Behav Brain Funct. 2010;6:9. doi: 10.1186/1744-9081-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriani M, de Tommaso M, Restuccia D, Le Pera D, Guido M, Iannetti GD, et al. Reduced habituation to experimental pain in migraine patients: A CO(2) laser evoked potential study. Pain. 2003;105:57–64. doi: 10.1016/s0304-3959(03)00137-4. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Dewey JE. How to score version 2 of the SF-36 (r) health survey. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- Zung WWK. SAS, self-rating anxiety scale. In: Guy W, editor. ECDEU assessment manual for psychopharmacology, revised edn. Rockville, MD: National Institute of Health, Psycho-pharmacology Research Branch; 1976. pp. 337–40. [Google Scholar]