Summary
An association of obstructive sleep apnea syndrome (OSAS) and other sleep-disordered breathing (SDB) with Parkinson’s disease (PD) has been reported in some small studies.
In the present study we investigated the occurrence of SDB in a large consecutive outpatient series. This is a case-control study in subjects attending a neurological clinic where all patients were screened for SDB by means of sleep-wake history, Epworth Sleepiness Scale, and full-night polysomnography, when indicated. 3194 patients were recruited. Of these, 194 were affected by PD and 77 by other parkinsonisms. Snoring, excessive daytime sleepiness and OSAS were more common in patients with PD or parkinsonisms (40.59, 5.9, and 4.06%) than in controls (35.58, 2.19, and 2.09%). Our study suggests an increased frequency of OSAS and other SDB in PD and parkinsonisms. Early detection and management of these disorders may have a substantial impact on quality of life and survival in these patients.
Keywords: non-motor symptoms, obstructive sleep apnea syndrome, parkinsonism, Parkinson’s disease, sleep-disordered breathing
Introduction
Non-motor symptoms of Parkinson’s disease (PD) and parkinsonisms have attracted growing attention because they are among the major factors impacting on quality of life of both affected individuals and their caregivers (Hoehn and Yahr, 1967; Global Parkinson’s Disease Survey Steering Committes 2002). Sleep disturbances are an important group of non-motor symptoms, being reported in 2/3 of PD patients (Tandberg et al., 1998) and in some cases anticipating the onset of the disease (Schenck et al., 1996). Sleep onset insomnia, sleep maintenance insomnia, excessive daytime sleepiness (EDS), REM sleep behavior disorder and sleep-disordered breathing (SDB) are sleep disorders commonly found in patients with neurodegenerative diseases (Schulte and Winkelmann, 2011). The term SDB refers to several chronic conditions in which snoring and partial or complete cessation of breathing during the night may result in daytime sleepiness and fatigue. Since snoring is reported by up to 70% of PD patients, it may reasonably be hypothesized that these patients have an increased risk of developing obstructive sleep apnea syndrome (OSAS), however available studies showed conflicting results in this regard (Braga-Neto et al., 2004; Zoccolella et al., 2011). Moreover, most of the studies were small and did not include a control group. Other studies were performed on case series or presented referral or selection biases, since they included non-consecutive patient series (Arnulf et al., 2002; Stevens et al., 2004; Diederich et al., 2005). Finally, no study in the literature has compared the prevalence of SDB between patients with PD and parkinsonisms. The aims of our study were to assess the association of OSAS and other forms of SDB with the presence of PD or parkinsonisms in a large consecutive series of patients and to perform comparisons between patients with PD and patients with parkinsonisms.
Materials and methods
All subjects admitted to the Neurology Department of Avezzano City Hospital between January 2010 and April 2013 were consecutively recruited and evaluated. Subjects affected by clinical disorders known to be associated with SDB (e.g. narcolepsy), or with conditions that might preclude an accurate and reliable medical history, were excluded. Patients with either PD or parkinsonisms were included in the case group. All the remaining subjects were included in the control group. The diagnosis of PD was made according to the UK Brain Bank criteria (Nice Guideline, 2006), which require the presence of bradykinesia plus at least one symptom from: rigidity, tremor at rest and postural instability, in association with three or more of the following signs: unilateral onset, tremor at rest, progression, persistent asymmetry, response (70–100%) to L-dopa, severe dyskinesia induced by L-dopa, response to L-dopa for five years or more, and clinical course of 10 years or more. Other causes of reversible PD (e.g. stroke, brain injury, tumors, neuroleptics treatments) had to be excluded. Cases with bradykinesia and at least one symptom among rigidity, tremor, rest and postural instability, but no associated signs, were considered not affected by PD (Movement Disorders Society Scientific Issues Committee report, 2003). Parkinsonism was diagnosed according to the criteria of the Movement Disorders Society Scientific Issues Committee (Movement Disorders Society Scientific Issues Committee report, 2003). The severity of PD symptoms was assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS) (Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease, 2003), while the staging was evaluated using the Hoehn and Yahr Scale (H&Y) (Goetz et al., 2004). A semi-structured interview was used to collect information on demographic characteristics, family history, risk factors, comorbidities, clinical signs and symptoms, final diagnosis and treatments. Sleep abnormalities were investigated through semi-structured interview, including bed partner and caregiver reports. Data were coded by two of the Authors on the basis of predefined criteria.
A subsample of 20 subjects was used to evaluate agreement between raters for training purposes.
Sleep impairments and SDB were assessed according to standard criteria (Maria et al., 2003). Participants complaining of daytime sleepiness, fatigue, snoring or sleep apnea were administered the Epworth Sleepiness Scale (ESS). Patients with an ESS score >10 underwent a full-night polysomnography (PSG). A diagnosis of OSAS was made in patients with an apnea/hypopnea index (AHI) > 5. Table I summarizes the diagnostic criteria of OSAS (The Report of an American Academy of Sleep Medicine Task Force, 1999). Since snoring is much more frequent than other forms of SDB, the chi-square test was used to compare separately the occurrence of snoring and other SDB in subjects with PD or other parkinsonisms; EDS and OSAS were subsequently analyzed separately because of their clinical relevance. Student’s t-test was used to compare continuous variables and the Mann-Whitney U-test to compare ordinal variables between each case group and controls. Multivariate analysis was performed to adjust the associations for demographic data and body mass index (BMI). Values are shown as mean and median. A p-value of less than 0.05 was considered significant.
Table I.
Diagnostic criteria and classification of OSAS.
|
Classification of severity of OSA on the basis of apnea-hypopnea index (AHI):
AHI 5–14 Mild
AHI 5–15 Moderate
AHI ≥ 30 Severe
Results
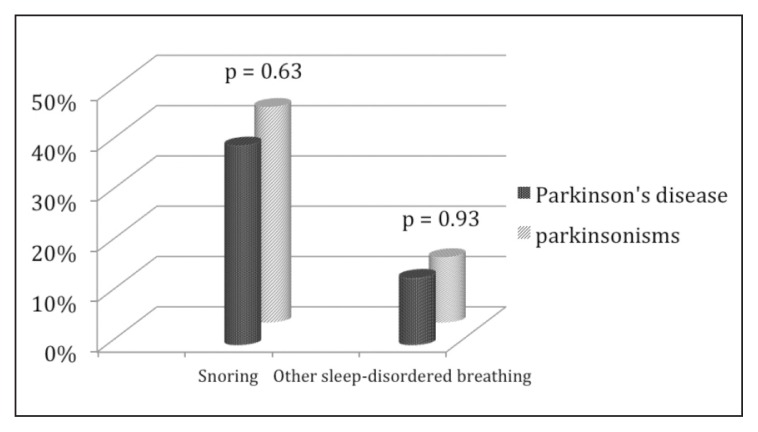
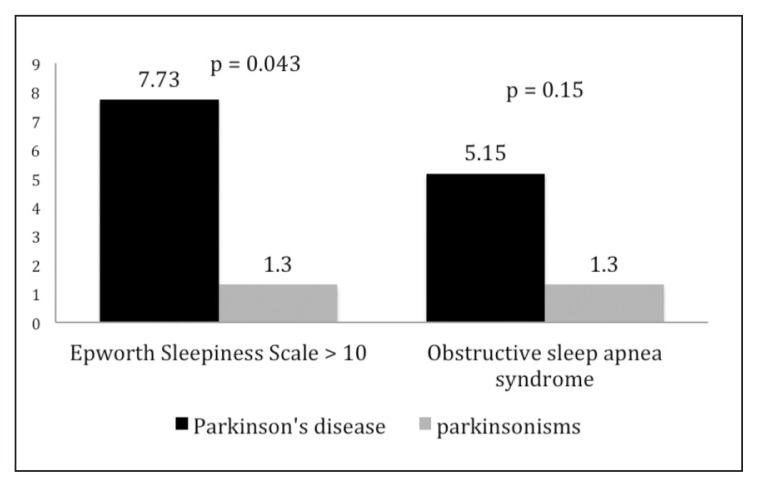
In total, 3405 subjects were recruited consecutively between January 2010 and April 2013; of these, 211 were excluded because they were affected by dementia or other conditions related to a previous stroke. Of the remaining 3194 enrolled patients, 271 (116 males, 155 females; mean age ± SD: 72.05 ± 11.74 years) were affected by PD or parkinsonisms. The patients with PD numbered 194 (89 males, 105 females; mean age ± SD: 73.29 ± 10.24 years), while 77 patients were affected by parkinsonisms (27 males, 50 females, mean age ± SD: 68.72 ± 14.60 years): 2 had progressive supranuclear palsy, 3 had multisystemic atrophy, and 72 had a vascular parkinsonism. The PD patients recorded a UPDRS total score mean value of 51.54 ± 27.84 and a H&Y mean value of 1.76 ± 0.94, while the corresponding values in the parkinsonism group were 10.51 ±18.99 and 0.36 ± 0.65 respectively. The control group included 2923 subjects, 1066 males and 1857 females, mean age ± SD: 53 ± 19.5 years (Table II). Snoring was the most common form of SDB in the whole study population, reported by 1040 subjects (32.56%); EDS was diagnosed, on the basis of an ESS score >10, in 80 subjects (2.5%) while OSAS was diagnosed, through PSG, in 72 subjects (2.25%). In the overall study population, OSAS (mean AHI 20.67) occurred more frequently than central sleep apneas (mean AHI 8.35). A non-significant increase in the occurrence of snoring was found in the patients with PD or parkinsonism versus the control subjects (40.59 vs 35.58), while other SDB (13.28% vs 8.48%), OSAS (4.06% vs 2.09%), and EDS (5.90% vs 2.19%) were significantly more common (Table III). The comparison between patients with PD and those with parkinsonisms showed that snoring and other SDB occurred at similar rates in these groups (42.86 vs 39.69%) (Fig. 1), while the prevalence rates of OSAS and EDS were rather higher in PD patients (5.15 and 7.73% respectively) than in patients with parkinsonisms (1.3 and 1.3%) (Fig. 2).
Table II.
Demographic and clinical characteristics of the subjects.
| Patients | Controls | PD | Parkinsonisms | PD and parkinsonisms | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean±SD | Mean±SD | p | Mean±SD | p | Mean±SD | p | |
|
| |||||||
| No. | 2923 | 194 | 77 | 271 | |||
| Age | 52.98 ± 19.59 | 73.29 ± 10.24 | <.0001 | 68.72 ± 14.60 | <.0001 | 72.05 ± 11.74 | <.0001 |
| Sex, female (%) | 63.5 | 54.1 | .008 | 64.9 | .8 | 57.2 | .03 |
| UPDRS | 51.54 ± 27.84 | 10.51 ± 18.99 | 47.40 ± 26.05 | <.001# | |||
| Hoehn &Yahr | 1.76 ± 0.94 | 0.36 ± 0.65 | 1.62 ± 0.88 | <.001# | |||
Abbreviations: PD=Parkinson’s disease; UPDRS=Unified Parkinson’s Disease Rating Scale.
based on Mann-Whitney U test.
Table III.
Sleep measures in controls, in Parkinson’s disease and in parkinsonisms.
| Patients | Controls | PD | Parkinsonisms | PD and parkinsonisms | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| p | p | p | |||||
|
| |||||||
| Snoring (%) | 35.58 | 39.69 | .24 | 42.86 | .18 | 40.59 | .1 |
| SDB (%) | 8.48 | 13.4 | .01 | 12.99 | .16 | 13.28 | .007 |
| OSAS (%) | 2.09 | 5.15 | .005 | 1.3 | .63 | 4.06 | .03 |
| ESS score >10 (%) | 2.19 | 7.73 | 1.97 | 1.3 | .59 | 5.90 | < 0.001 |
| Obstructive apnea, AHI (mean±SD) | 19.81 ± 10.02 | ||||||
| 36.67 ± 20.2 | .01 | 13 ± 9.89 | .35 | 27.20 ± 19.91 | .17# | ||
| Central apnea, AHI (mean±SD) | 7.92 ± 4.51 | ||||||
| 7.92 ± 4.51 | 15.33 ± 7.37 | .01 | 6 ± 2.82 | .55 | 11.60 ± 7.43 | .12# | |
Abbreviations: PD=Parkinson’s disease; SDB: sleep-disordered breathing other than snoring; OSAS=obstructive sleep apnea syndrome; ESS=Epworth Sleepiness Scale; AHI=apnea/hypopnea index.
based on Mann-Whitney U test.
Figure 1.
Prevalence of sleep-disordered breathing in Parkinson’s disease and parkinsonisms.
Figure 2.
Prevalence of excessive daytime sleepiness (Epworth Sleepiness Scale score ≥ 10) and obstructive sleep apnea syndromes in Parkinson’s disease and parkinsonisms.
The multivariate logistic regression analysis confirmed a significant association of PD and parkinsonisms with snoring and other SDB with similar odds ratios (ORs), while OSAS showed a very high OR for the association with parkinsonism (8.33), which was outside the confidence interval of the ORs for the associations with PD and PD + parkinsonisms (Table IV).
Table IV.
Results of multivariate analysis.
| OSAS | ||||
|---|---|---|---|---|
|
| ||||
| OR* | LL | UL | p | |
| PD + parkinsonisms | 2.81 | 1.33 | 5.95 | .006 |
| PD | 2.24 | 1.02 | 4.9 | .043 |
| Parkinsonisms | 8.33 | 1.09 | 62.5 | .041 |
|
| ||||
| SDB | ||||
|
| ||||
| PD + parkinsonisms | 2.57 | 1.63 | 4.04 | <.001 |
| PD | 2.39 | 1.43 | 3.98 | .001 |
| Parkinsonisms | 3.21 | 1.46 | 7.04 | .003 |
|
| ||||
| Snoring | ||||
|
| ||||
| PD + parkinsonisms | 12.7 | 7.9 | 20.4 | <.001 |
| PD | 14.7 | 9.1 | 24.4 | <.001 |
| Parkinsonisms | 10.2 | 5.5 | 18.9 | <.001 |
|
| ||||
| Excessive daytime sleepiness | ||||
|
| ||||
| PD + parkinsonisms | 1.71 | 0.88 | 3.3 | .107 |
| PD | 1.51 | 0.74 | 3.08 | .251 |
| Parkinsonisms | 2.73 | 0.78 | 9.52 | .113 |
Abbreviations: OSAS=obstructive sleep apnea syndrome; PD=Parkinson’s disease; SDB=sleep disordered breathing other than snoring; OR=odds ratio; UL=upper limit; LL=lower limit
Results adjusted for age and body mass index
Discussion
Although PD and parkinsonisms are defined by motor dysfunction, they are also characterized by a range of non-motor features including SDB, in particular OSAS (Chaudhuri and Schapira, 2009; Gaig and Iranzo, 2012). It is unclear whether OSAS is more prevalent in PD than in the general population, considering that obesity, a major risk factor for OSAS, is often absent in PD (Olson et al., 1995; Uc et al., 2006). However, several factors may predispose PD patients to develop OSAS. First, PD patients are usually elderly, and aging is a major risk factor for OSAS. The large number of subjects included in our research allowed us to perform a multivariate analysis, adjusted for age, which showed substantially different results. Second, PD patients have upper airway and pulmonary function abnormalities. Upper airway obstruction, assessed by spirometry, is present in 24 to 65% of PD patients, and this is thought to be related to hypokinesia and rigidity. Moreover, restrictive lung disease, probably due to chest wall rigidity and postural abnormalities of the trunk, can also occur in PD (Sabaté et al., 1996; Shill and Stacy, 1998). Finally, autonomic dysfunction in PD might also contribute to OSAS (Jost, 2003). Moreover, several studies have demonstrated, in PD, an extensive loss of neurons in extranigral sites, including brainstem nuclei involved in sleep physiology and respiratory control. Other studies have shown neurogenic myofiber atrophy possibly due to a PD-related loss of functioning motoneurons and/or degenerative alterations in the peripheral nerves innervating oropharyngeal muscles. Laryngeal and pharyngeal muscles receive their motor innervations from cranial nerve X and several studies have demonstrated alpha-synuclein in fibers in the cervical nerve X in PD (Liancai et al., 2012).
These mechanisms may determine not only dysphagia but also upper airway obstruction. Nevertheless, the clinical significance of OSAS in PD remains an issue of debate. Interpreting the clinical relevance of the AHI in PD can be particularly difficult. In PD, attributing EDS to an abnormal AHI detected by PSG can be challenging since EDS in this population may also be caused by many other factors, such as depression, use of dopaminergic drugs, restless legs syndrome, periodic limb movement syndrome, sleep onset and sleep maintenance problems, and sleep disruption because of hallucinations (Braga-Neto et al., 2004). Studies using overnight PSG to assess OSAS in PD have given conflicting results (Shpirer et al., 2006; Norlinah et al., 2009). Many of these studies were performed in small samples and did not include a control group. Other studies were retrospective or presented referral or selection biases since they included non-consecutive series of patients or used EDS as an exclusion criterion (Arnulf et al., 2002; Diederich et al., 2005). In these studies the mean AHI ranged from 3 to 16, and the frequency of OSAS (defined as an AHI ≥ 5) ranged from 26 to 60%. In our study we investigated the occurrence of all forms of SDB in a large consecutive outpatient series and we performed comparisons between patients with PD and patients with parkinsonisms, mainly of vascular etiology. We used a two-step screening approach and performed PSG only in patients with an ESS score >10. Even though patients with OSAS may not necessarily present with EDS, performing PSG in all the subjects was not feasible in our study. On the other hand, according to some studies, patients with OSAS and EDS are at higher risk of vascular complications (Empana et al., 2009; Garcia, 2009). We used a cut-off of 5 for AHI, thus including many mild cases. Had we used a more restrictive cut-off of 15, the OSAS distribution among groups would in any case have been very similar, although with a lower statistical power.
The results of our study revealed that PD and parkinsonisms were associated with OSAS, as well as with snoring and other forms of SDB, and that obstructive apnea was more frequent than central apnea, suggesting that obstructive phenomena may be the most important component in the pathophysiology of SDB. Probably the closer association of OSAS with parkinsonisms than with PD, found on the multivariate analysis adjusted for age and BMI, may be explained by the high prevalence of autonomic dysfunction in parkinsonian syndromes and to the well-known involvement of oropharyngeal muscles in multisystem atrophy (Jost, 2003). Moreover, the great majority of parkinsonisms in our study was due to vascular causes, and we suggest that the bulbar innervated muscles may be involved in patients with pseudobulbar palsy.
Evaluation of sleep in PD and in parkinsonism patients is important because sleep impairment is one of the major factors affecting quality of life throughout the course of the disease (Adler and Thorphy, 2010; Cocken et al., 2010). Moreover, OSAS is associated with many adverse health consequences, including an increased risk of all-cause mortality, stroke, hypertension, arrhythmias, myocardial infarction, insulin resistance, depression and worsening of cognitive function (Sabaté et al., 1996; Kielb et al., 2012). Several studies have demonstrated that risk factors for cardiovascular disease and cerebrovascular disease (CVD) are common in PD and in parkinsonism patients, possibly due to their older age (Perju-Dumbravă et al., 2014), and both orthostatic hypotension and supine hypertension coexist in these patients. A high nocturnal blood pressure is also associated with an increased prevalence of end-organ damage (Berganzo et al., 2013). Moreover, a large cross-sectional study with a cohort of PD patients provided evidence for an influence of specific comorbidities, particularly heart/circulatory comorbidities and diabetes, on general measures of cognition (Jones et al., 2012).
The onset of OSAS may probably help to explain the increased risk of cardiovascular disease and CVD in PD and parkinsonism patients. On the other hand, the sleep fragmentation secondary to OSAS provokes chronic fatigue and EDS, both of which interfere with motor symptoms and treatment. Fatigue and daytime sleepiness caused by sleep apnea usually progress over the years, reducing independence in activities of daily living and worsening cognitive impairment in PD and parkinsonism patients. However, these symptoms can be observed irrespective of the presence of sleep apnea and are frequently related to pharmacological treatment, in particular with dopaminergic agents. Therefore, an early diagnosis of OSAS could be very important to improve the quality of life of patients with PD and parkinsonisms, and to prevent the onset of the most serious complications. Our study demonstrates that sleep-wake history and ESS should be assessed whenever possible, and PSG should be performed whenever the presence of OSAS is suspected, in order to provide adequate therapeutic strategies.
In conclusion, the results of the present study show an increased frequency of OSAS and other forms of SDB in PD and in parkinsonisms. Early detection and efficient clinical management of these conditions should be strongly encouraged.
References
- Adler CH, Thorpy MJ. Sleep issues in Parkinson’s disease. Neurology. 2010;64(Suppl 3):S12–20. doi: 10.1212/wnl.64.12_suppl_3.s12. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology. 2002;58:1019–1024. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- Berganzo K, Díez-Arrola B, Tijero B, et al. Nocturnal hypertension and dysautonomia in patients with Parkinson’s disease: are they related? J Neurol. 2013;260:1752–1756. doi: 10.1007/s00415-013-6859-5. [DOI] [PubMed] [Google Scholar]
- Braga-Neto P, da Silva-Júnior FP, Sueli Monte F, et al. Snoring and excessive daytime sleepiness in Parkinson’s disease. J Neurol Sci. 2004;217:41–45. doi: 10.1016/j.jns.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Cochen D, Cock V, Abouda M, et al. Is obstructive sleep apnea a problem in Parkinson’s disease? Sleep Med. 2010;11:247–252. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Vaillant M, Leischen M, et al. Sleep apnea syndrome in Parkinson’s disease. A case-control study in 49 patients. Mov Disord. 2005;20:1413–1418. doi: 10.1002/mds.20624. [DOI] [PubMed] [Google Scholar]
- Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly. Stroke. 2009;40:1219–1224. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- Gaig C, Iranzo A. Sleep-disordered breathing in neurodegenerative diseases. Curr Neurol Neurosci Rep. 2012;12:205–217. doi: 10.1007/s11910-011-0248-1. [DOI] [PubMed] [Google Scholar]
- García CH. Sleep apnea-hypopnea syndrome without excessive daytime sleepiness. Arch Bronconeumol. 2009;45:240–244. doi: 10.1016/j.arbres.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Global Parkinson’s Disease Survey Steering Committee. Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord. 2002;17:60–67. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force on the Hoehn and Yahr Staging Scale: Status and Recommendations. Mov Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Jones JD, Malaty I, Price CC, et al. Health comorbidities and cognition in 1948 patients with idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:1073–1078. doi: 10.1016/j.parkreldis.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost WH. Autonomic dysfunctions in idiopathic Parkinson’s disease. J Neurol. 2003;250(Suppl 1):128–30. doi: 10.1007/s00415-003-1105-z. [DOI] [PubMed] [Google Scholar]
- Kielb SA, Ancoli-Israel S, Rebok GW, et al. Cognition in obstructive sleep apnea-hypopnea syndrome (OSAS): current clinical knowledge and the impact of treatment. Neuromolecular Med. 2012;14(3):180–193. doi: 10.1007/s12017-012-8182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liancai M, Stanislaw S, Jingming C, et al. Altered pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2012;71:520–530. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria B, Sophia S, Michalis M, et al. Sleep breathing disorders in patients with idiopathic Parkinson’s disease. Respir Med. 2003;97:1151–1157. doi: 10.1016/s0954-6111(03)00188-4. [DOI] [PubMed] [Google Scholar]
- Movement Disorders Society Scientific Issues Committee report. SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- Nice guideline. National Collaborating Centre for Chronic Conditions. London: Royal College of Physicians (UK); 2006. [Google Scholar]
- Norlinah MI, Afidah KN, Noradina AT, et al. Sleep disturbances in Malaysian patients with Parkinson’s disease using polysomnography and PDSS. Parkinsonism Relat Disord. 2009;15:670–674. doi: 10.1016/j.parkreldis.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Olson LG, King MT, Hensley MJ, et al. A community study of snoring and sleep-disordered breathing. Prevalence Am J Respir Crit Care Med. 1995;152:711–716. doi: 10.1164/ajrccm.152.2.7633731. [DOI] [PubMed] [Google Scholar]
- Perju-Dumbravă L, Muntean ML, Mureşanu DF. Cerebrovascular profile assessment in Parkinson’s disease patients. CNS Neurol Disord Drug Targets. 2014;13:712–717. doi: 10.2174/1871527313666140618110409. [DOI] [PubMed] [Google Scholar]
- Sabaté M, González I, Ruperez F, et al. Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. J Neurol Sci. 1996;138:114–119. doi: 10.1016/0022-510x(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Schulte EC, Winkelmann J. When Parkinson’s disease patients go to sleep: specific sleep disturbances related to Parkinson’s disease. J Neurol. 2011;258(Suppl 2):S328–335. doi: 10.1007/s00415-011-5933-0. [DOI] [PubMed] [Google Scholar]
- Shill H, Stacy M. Respiratory function in Parkinson’s disease. Clin Neurosci. 1998;5:131–135. [PubMed] [Google Scholar]
- Shpirer I, Miniovitz A, Klein C, et al. Excessive daytime sleepiness in patients with Parkinson’s disease: a polysomnography study. Mov Disord. 2006;21:1432–1438. doi: 10.1002/mds.21002. [DOI] [PubMed] [Google Scholar]
- Stevens S, Cormella CL, Stepanski EJ. Daytime sleepiness and alertness in patients with Parkinson disease. Sleep. 2004;27:967–972. doi: 10.1093/sleep/27.5.967. [DOI] [PubMed] [Google Scholar]
- Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson’s disease. Mov Disord. 1998;13:895–899. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- Uc EY, Struck LK, Rodnitzky RL, et al. Predictors of weight loss in Parkinson’s disease. Mov Disord. 2006;21:930–936. doi: 10.1002/mds.20837. [DOI] [PubMed] [Google Scholar]
- Zoccolella S, Savarese M, Lamberti P, et al. Sleep disorders and the natural history of Parkinson’s disease: the contribution of epidemiological studies. Sleep Med Rev. 2011;15:41–50. doi: 10.1016/j.smrv.2010.02.004. [DOI] [PubMed] [Google Scholar]