Summary
Patients with Parkinson’s disease (PD) and Pisa syndrome (PS) may present tonic dystonic or compensatory (i.e. acting against gravity) hyperactivity in the paraspinal and non-paraspinal muscles. Electromyographic (EMG) activity was measured in nine patients with PD and PS, three with PD without PS, and five healthy controls. Fine-wire intramuscular electrodes were inserted bilaterally into the iliocostalis lumborum (ICL), iliocostalis thoracis (ICT), gluteus medius (GM), and external oblique (EO) muscles. The root mean square (RMS) of the EMG signal was calculated and normalized for each muscle. In stance condition, side-to-side muscle activity comparisons showed a higher RMS only for the contralateral ICL in PD patients with PS (p=0.028). Moreover, with increasing degrees of lateral flexion, the activity of the EO and the ICL muscles progressively increased and decreased, respectively. The present data suggest that contralateral paraspinal muscle activity plays a crucial compensatory role and can be dysfunctional in PD patients with PS.
Keywords: electromyography, musculoskeletal equilibrium, paraspinal muscles, postural balance
Introduction
Pisa syndrome (PS) in Parkinson’s disease (PD) is clinically defined as a lateral trunk flexion of 10 degrees or more that can be completely alleviated by passive mobilization or supine position (Doherty et al., 2011). In our recent, multicenter observational study involving 1631 PD patients, we estimated a PS prevalence of 8.8% (Tinazzi et al., 2015).
Two different pathophysiological mechanisms for PS have been postulated: (1) a central mechanism arising from a defect in basal ganglia network function that results in dystonic activity and impaired sensorimotor integration (this hypothesis is supported by both experimental animal studies and clinical findings); and (2) a peripheral mechanism due to spinal osteoarticular changes and paraspinal myopathy (Tinazzi et al., 2016). The pathophysiology of PS is largely unknown, and its clinical variability makes the underlying mechanisms difficult to discern and characterize. The few studies that have investigated muscle activation patterns in PS have yielded conflicting results. This may be due to the variety of methodological approaches used, and the lack of detail provided about the electromyography (EMG) protocol and/or EMG data quantification (Bonanni et al., 2007, Di Matteo et al., 2011, Tassorelli et al., 2012, Tinazzi et al., 2013, Frazzitta et al., 2015). Previous studies identified two main patterns of muscle activation. Pattern I is characterized by hyperactivity of the lumbar paraspinal muscles ipsilateral to the trunk leaning side, and includes two subtypes, i.e, subpattern I-I with concomitant ipsilateral paraspinal thoracic muscle activity, and subpattern I–II with concomitant contralateral paraspinal thoracic muscle activity. Pattern II is displayed when there is hyperactivity of the paraspinal muscles contralateral to the trunk leaning side (Tinazzi et al., 2013). On the basis of these observations, it has been proposed that dystonic activity of the non-paraspinal muscles (e.g., external oblique, internal oblique and quadratus lumborum) might contribute to ipsilateral bending, while activation of the contralateral paraspinal muscles counteracts the effect of gravity (Tinazzi et al., 2013).
Although useful data about muscle activation can be obtained by conventional polygraphic EMG recording, this technique is often technically complex in PD patients with PS, making the EMG parameters difficult to interpret. Previous studies reported data on EMG recordings in static conditions, such as lying prone, and during dynamic tasks, like voluntary right and left lateral flexion of the trunk (Di Matteo et al., 2011; Tassorelli et al., 2012; Tinazzi et al., 2013; Frazzitta et al., 2015), but they were unable to discriminate dystonic muscles from those activated by compensatory mechanisms potentially serving to limit the lateral trunk flexion, and did not report quantitative data during stance (Tinazzi et al., 2013).
For this reason, in this pilot study, we measured and compared bilateral trunk muscle activity using quantitative data derived from kinesiologic EMG recordings in PD patients with PS. Since the leverage and moment arm of muscles change degree by degree, we hypothesized that in physiological conditions EMG quantification of muscle activity may vary with the level of lateral trunk flexion (Oddsson et al., 1990).
Furthermore, given that quantitative data in upright standing and during different lateral trunk flexions are lacking (Oddsson et al., 1990; Neumann, 2010), we also recorded EMG muscle activity in PD patients without PS and in healthy individuals. Generally, normalization of EMG activity is performed to allow comparisons of EMG activity between/within subjects (Soderberg et al., 2000) and it might help to clarify the side-to-side differences in trunk muscle activity in PS and how they differ from what is observed in healthy individuals. We hypothesized that in PD with PS, contralateral paraspinal muscle activity is essential to maintain upright posture, whereas non-paraspinal muscles could present abnormal hyperactivity.
Materials and methods
Patients
For this pilot study, 9 patients with PD and PS, 3 patients with PD without PS, and 5 healthy volunteers, who served as controls, were enrolled.
The patients and controls gave their written informed consent to participate in the study, which was performed according to the tenets of the Declaration of Helsinki and approved by the local ethics committee (project number: CE2399). All the patients were attending the outpatient clinic of the Movement Disorder Division, Neurorehabilitation Unit, Department of Neurosciences, Biomedicine and Movement Sciences, Verona University Hospital.
Neurological assessment was performed by neurologists experienced in evaluating movement disorders. The inclusion criteria were: a) confirmed diagnosis of PD according to the Movement Disorder Society clinical diagnostic criteria for PD (Postuma et al., 2015), b) PS defined as at least 10 degrees (PS≥10) of lateral bending of the trunk which could be reduced by passive mobilization or supine positioning (Doherty et al., 2011), c) modified Hoehn & Yahr stage <4 in the on medication phase. The exclusion criteria were: a) any change in drug intake in the 3 months preceding enrollment, b) severe dyskinesia or severe on-off fluctuations, c) need of assistive devices to rise from a chair/bed, d) vestibular disorders, e) any sensory impairment involving the lower limbs, f) other neurological, orthopedic (such as scoliosis confirmed by radiographs) or cardiovascular comorbidities as reported in the patient’s medical records.
Clinical evaluation
The following clinical and demographic variables were collected: age, gender, PD duration, dominant PD phenotype according to a data-driven approach (younger age at onset, tremor dominant, non-tremor dominant, and rapid disease progression) (Lewis et al., 2005), Unified Parkinson’s Disease Rating Scale part III (UPDRS III) score and modified Hoehn & Yahr stage in the on medication phase, the side of the body more affected by the PD, pharmacological therapy, degree of anterior trunk flexion, low back pain rated on a 1–10 visual analog scale. The PD patients with PS were evaluated for direction and degree of leaning at the thoracolumbar level, as measured with a pocket compass needle goniometer (IncliMed®, University of Padua) (Gravina et al., 2012), PS duration and any awareness of trunk misalignment.
EMG recording
Eight-channel simultaneous EMG polygraphic recordings (Keypoint Machine, Dantec Measurement Technology, Skovlunde, Denmark) were obtained using insulated fine wire electrodes with bare hook-shaped tips (SEI-EMG s.r.l., Padua, Italy). Electrodes were inserted bilaterally with a bipolar montage into the iliocostalis lumborum (ICL) (L2), iliocostalis thoracis (ICT) (T8), gluteus medius (GM), and external oblique (EO) muscles (Kumar et al., 2010). The muscles were chosen based on their biomechanical leverage and moment arm, able to induce a lateral trunk flexion (Neumann, 2010).
After insertion, the electrodes were checked for optimal positioning. The patients with PD and PS were evaluated in two different, consecutive conditions: first, during maximal isometric voluntary contraction (MIVC) of each muscle while lying on a physiotherapy bench, and then in their usual stance.
The patients performed MIVCs under optimal biomechanical conditions to elicit maximum muscle activation. To evaluate ICL and ICT muscle activation, trunk extensions were performed in prone position with the pelvis strapped to a frame and arms parallel to the body axis without any contact with the pad, which was positioned under the abdomen. Hip abduction and intra-rotation maneuvers were performed to assess maximum GM activation. To assess the EO muscles, isometric contralateral abdominal curls were performed supine in crook-lying position with arms folded across the chest.
MIVC of each muscle was performed three times, with an inter-trial interval of at least 3 minutes; in each trial the motor task was performed for 10 seconds against manual resistance. We felt that three trials would be sufficient for the purposes of this study since it has been demonstrated that the reliability of the procedure does not improve beyond three trials and that with this number of trials onset of fatigue is minimized (Yang et al., 1987, Deluca, 1997). The examiner gave verbal encouragement during manual resistance against MIVCs. The patients were then recorded during stance with arms at their side, as previously described (Di Matteo et al., 2011, Tinazzi et al., 2013). Evaluations were always performed by the same examiners between 10:00 and 17:00 to reduce possible bias related to circadian variability of lumbar spine properties (Adams et al., 1987) and in ON condition to minimize the possible effect of motor fluctuations. The subjects were familiarized with the procedures several days before the evaluation, and then again briefly the day before the evaluation (Kumar et al., 2010). The root mean square (RMS) of the EMG signal was calculated in both conditions and for each muscle. The RMS is a quantitative reference value that reflects the level of physiological activity in motor units during contraction (Soderberg et al., 2000). For each subject and muscle, the RMS values recorded during stance were ‘normalized’ to those obtained during MIVCs. The ratio was obtained by dividing the RMS obtained during stance with that recorded during MIVC (first condition) and expressed as percentage of EMG activity (Soderberg et al., 2000). EMG normalization was calculated for the purpose of quantifying and comparing bilateral muscle activity within the patient group (right muscle versus left muscle). Data were normalized by selecting the largest and most symmetrical RMS values from consecutive MIVCs (Shin et al., 2010). This choice was based on our preliminary EMG recordings and previous findings (Frazzitta et al., 2015), which demonstrated that patients with PD and PS may produce asymmetrical MIVCs between contralateral and ipsilateral paraspinal and non-paraspinal muscles. In a separate session, three PD patients without PS and five healthy volunteers (i.e., a 65-year-old woman and four young controls, mean age 28.75 years, SD 1.50) with no known neurological disorders were evaluated. This was done for explorative purposes, to gather physiological data in upright standing in PD without PS and in an age-matched control. In addition, because muscle activity differs with different degrees of lateral trunk flexion, we evaluated the kinesiologic EMG findings in the young subjects while they each mimicked the nine different lateral bending positions of the PS patients (simulation condition, SC). Finally, in keeping with the results of our previous study, we performed a descriptive analysis of muscle activity (+: sustained; −: mild) (Tinazzi et al., 2013). The complexity of the methodological procedures and ethical considerations precluded evaluation of a greater number of individuals and further statistical comparisons between PD patients with/without PS and healthy controls.
Statistical analysis
Descriptive statistics included frequency tables and calculation of means and standard deviation of MIVC normalized EMG data (%). Descriptive comparisons were made between the patients with PD without PS and the other groups (Tinazzi et al., 2013). Normal distribution of data was checked using the Shapiro-Wilk test. Nonparametric tests were applied because of the non-normal distribution of the data and small sample size in this study. Inter-side differences in muscle activity within patients and the single EMG recordings of normal subjects were compared using the Wilcoxon test. Spearman’s rank correlation coefficients were used to assess the correlation between the degree of lateral trunk flexion and MIVC normalized EMG data (%). The correlation coefficients were also calculated for two subgroups: PD patients with PS were stratified according to the side of trunk bending (right and left side), while the control group in the SC was stratified by degree of bending (≤ 20° and > 20°). Two experienced raters visually checked the muscle activity of each subject for any obvious differences across the conditions and decided these stratifications. The significance level was set at p<0.05. Statistical analyses were carried out using IBMR SPSSR Statistics version 16.0 for Mac.
Results
Table I presents the clinical findings and demographic characteristics of the PD patients with PS (5 men and 4 women, mean age 64.67±12.02 years, mean disease duration 7.22±4.06 years).
Table I.
Demographic and clinical features of patients with Parkinson’s disease and Pisa syndrome.
| Case | Age (yrs) | Gender | PD Features | PS Features | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD duration (yrs) | Type | UPDRS III | Mod. H&Y stage | More affected side | Direction (left/right) | Degrees (°) | PS duration | Therapy at onset (daily mg) | Anterior trunk flexion (°) | Back pain (0–10 VAS) | Awareness (Yes/No) | |||
| 1 | 72 | M | 16 yrs | NT | 16 | 3 | L | R | 10 | 6 yrs | Levodopa (600), Pramipexole (1.31) | 0 | 0 | Yes |
| 2 | 80 | M | 11 yrs | NT | 22 | 2.5 | R | R | 20 | 8 yrs | Levodopa (700), Pramipexole (1.31) | 20 | 0 | Yes |
| 3 | 72 | F | 3 yrs | NT | 35 | 2.5 | L | R | 30 | 1 yr | Levodopa (1900), Entacapone (200), Pramipexole (0.52) | 20 | 0 | Yes |
| 4 | 55 | M | 4 yrs | NT | 57 | 2.5 | L | R | 42 | 4 yrs | Pramipexole (3.15), Rasagiline (1) | 46 | 6 | Yes |
| 5 | 42 | M | 6 yrs | NT | 16 | 2.5 | L | R | 50 | 3 yrs | Levodopa (400), Pramipexole (3.15), Rasagiline (1) | 10 | 4 | Yes |
| 6 | 55 | F | 5 yrs | NT | 9 | 1 | L | L | 10 | 7 mths | Rasagiline (1), Pramipexole (1.57) | 22 | 3 | No |
| 7 | 75 | M | 8 yrs | TD | 54 | 3 | L | L | 16 | 2 yrs | Levodopa (400), Pramipexole (1.05) | 0 | 7 | Yes |
| 8 | 67 | F | 7 yrs | TD | 19 | 2.5 | R | L | 18 | 1 yr | Levodopa (800), Pramipexole (2,1), Rasagiline (1) | 8 | 3 | Yes |
| 9 | 64 | F | 5 yrs | NT | 30 | 2.5 | L | L | 28 | 4 mths | Levodopa (600), Pramipexole (2.1) | 12 | 9 | Yes |
Abbreviations: PD= Parkinson’s disease; PS=Pisa syndrome; UPDRS III=Unified Parkinson’s Disease Rating Scale, part III (motor subscale); R=right; L=left; TD=tremor dominant phenotype; NT=non-tremor dominant phenotype; Mod. H&Y stage=Modified Hoehn and Yahr stage; VAS=visual analog scale.
All the patients had participated in a previous study (Geroin et al., 2015). They were under chronic therapy with dopaminergic drugs and showed acceptable motor compensation. None had psychiatric disturbances, took neuroleptics or had undergone major spine surgery due to structural changes.
In the PD patients with PS, the PS duration was 2.88±2.67 years (range 0.33–8). Four patients presented lateral flexion of the trunk towards the left side (18±7.48°) and 5 towards the right side (30.4±16.15°). Anterior trunk flexion, differing in severity, was present in 7 of the 9 PS patients (19.71±12.83°, range 8–46) but without differences related to the side of bending. Camptocormia was present in 1 patient (case 4) (Doherty et al., 2011). The leaning side was contralateral to the more affected side in 5 of the 9 patients with PS (Table I).
In the PD patients with PS, there was a significant inter-side difference in ICL activity during upright stance (p=0.028), with greater activity contralateral to the side of bending, while the other muscles displayed similar activity (Table II).
Table II.
Mean normalized EMG data (%) of muscles in PS patients during upright standing.
| ICL | ICT | GM | EO | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | |
| Mean | *80.67 | 31.12 | 134.81 | 94.19 | 41.25 | 25.40 | 26.34 | 30.73 |
| SD | 61.19 | 52.85 | 261.90 | 168.28 | 34.35 | 27.64 | 21.21 | 20.72 |
| Pattern | + | − | + | − | + | − | − | + |
Abbreviations: PS=Pisa syndrome; ICL=iliocostalis lumborum; ICT=iliocostalis thoracis; GM=gluteus medius; EO=external oblique muscle; PS=Pisa syndrome; Ipsi=ipsilateral to the side of bending; Contra=contralateral to the side of bending; Pattern=categorization of muscle (EMG) activity as + sustained, or − mild.
Wilcoxon p<0.05 for left-right comparison
In the same stance position, PD patients without PS (subjects A, B, C) showed a higher intensity of muscle activity in almost all muscles under study than the 65-year-old healthy control (subject 1) (Table III). In the upright stance position, the young healthy controls (subjects 2–5) showed a lower intensity of muscle activity in several muscles under study than the 65-year-old healthy control (subject 1) and the PD patients without PS (subjects A, B, C) (Table III).
Table III.
Mean normalized EMG data (%) of muscles in upright standing in PD patients without PS and healthy controls.
| Subject | ICL | ICT | GM | EO | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| A | 43.21 | 13.21 | 18.29 | 21.67 | 13.44 | 4.74 | 3.59 | 5.42 |
| B | 34.41 | 38.48 | 56.43 | 45.96 | 33.72 | 31.31 | 1.22 | 1.08 |
| C | 58.36 | 19.36 | 7.33 | 7.52 | 58.57 | 59.44 | 2.76 | 2.89 |
|
| ||||||||
| 1 | 12.12 | 11.71 | 18.8 | 18.76 | 1.42 | 1.29 | 6.35 | 7.65 |
| 2 | 0.94 | 0.11 | 2.25 | 3.48 | 0.27 | 0.46 | 1.58 | 0.65 |
| 3 | 1.33 | 0.46 | 3.33 | 3.78 | 0.23 | 0.37 | 1.26 | 1.12 |
| 4 | 0.57 | 1.01 | 2.51 | 2.17 | 0.36 | 0.42 | 0.92 | 0.56 |
| 5 | 1.62 | 1.63 | 2.08 | 3.35 | 0.24 | 0.41 | 0.68 | 2.18 |
Abbreviations: PD=Parkinson’s disease; PS=Pisa syndrome; ICL=iliocostalis lumborum; ICT=iliocostalis thoracis; GM=gluteus medius; EO=external oblique muscle; A, B C=PD patients without PS; Subject 1= age-matched normal subject (i.e., elderly healthy control); Subjects 2–5= young healthy controls.
Comparison of muscle activity between sides in young healthy controls simulating PS and degree of anterior trunk flexion (SC) revealed significant side-to-side differences in the ICL (p<0.01), ICT (p<0.01), GM (p<0.01) and EO (p<0.01), with the higher intensity of muscle activity mainly contralateral to the side of bending, i.e. except in the GM (Table IV).
Table IV.
Mean normalized EMG data (%) of muscles in young healthy controls in different simulation conditions.
| ICL | ICT | GM | EO | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | |
| Mean | *32.54 | 2.92 | *19.24 | 4.11 | *0.61 | 2.41 | *8.23 | 2.11 |
| SD | 21.16 | 3.29 | 21.45 | 6.80 | 0.38 | 2.79 | 11.10 | 1.70 |
| Pattern | + | − | + | − | − | + | + | − |
Abbreviations: ICL=iliocostalis lumborum; ICT=iliocostalis thoracis; GM=gluteus medius; EO=external oblique muscle; Ipsi=ipsilateral to the side of bending; Contra=contralateral to the side of bending; Pattern=categorization of muscle (EMG) activity as + sustained, or − mild.
Wilcoxon p<0.01.
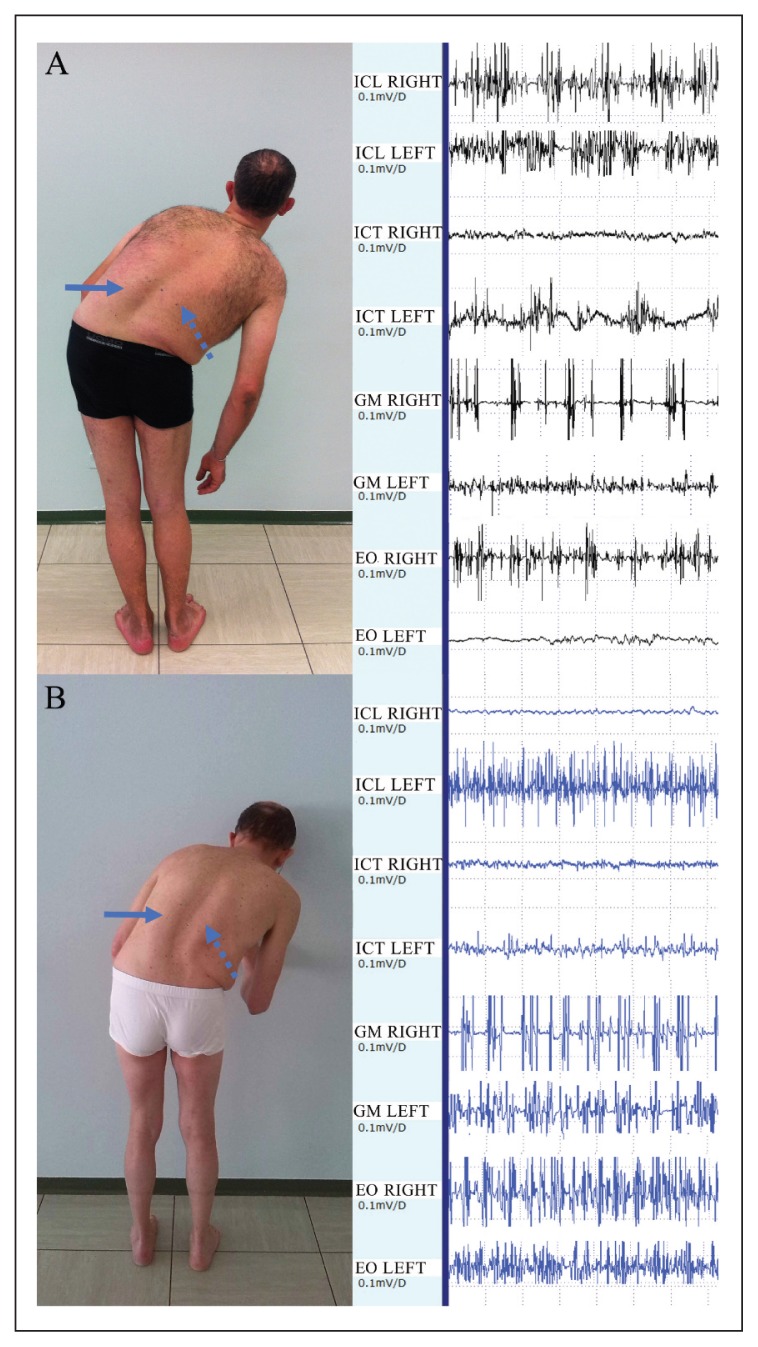
In keeping with the results of our previous study (Tinazzi et al., 2013), the patients with PS and PD displayed two main patterns of muscle activation (Fig. 1A and B). One patient had pattern I subtype II (Fig. 1A), and 8 had pattern II. Three of the patients with pattern II also showed ipsilateral hyperactivity of the ICT. The EO showed higher muscle activity contralateral to the trunk leaning side in 4 patients and higher ipsilateral muscle activity in 5 patients.
Figure 1.
A: case 5 with 50° of PS, pattern I (subtype II). He shows left (arrow) and right (dotted arrow) ICL hypertrophy. The intensity of contralateral ICL activation is reduced when compared with the right side, which indicates a possible impairment of the paraspinal compensatory mechanism. B: case 4 with 42° of PS, pattern II. He shows left (arrow) and right (dotted arrow) ICL hypertrophy. The intensity of contralateral ICL activation is increased when compared with the right side, which indicates possible integrity of the paraspinal compensatory mechanism. In both these PS patients there is hyperactivity of EO, right side.
In the young healthy controls mimicking the positions of the PS patients (Table V), the paraspinal muscles consistently demonstrated EMG activity similar to pattern II, while the non-paraspinal muscles showed bilateral activity in the GM and predominantly contralateral activity in the EO (Table V).
Table V.
Simulation conditions in four young healthy controls
| Degree of LF | Case | ICL | ICT | GM | EO | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | ||
| 10 | 2 | + | − | + | − | * | * | + | − |
| 3 | + | − | + | − | − | + | + | − | |
| 4 | + | − | + | − | − | + | + | − | |
| 5 | + | − | + | − | − | + | + | − | |
| 10 | 2 | + | − | + | − | − | + | * | * |
| 3 | + | − | + | − | − | + | + | − | |
| 4 | + | − | + | − | − | + | + | − | |
| 5 | + | − | + | − | * | * | + | − | |
| 16 | 2 | + | − | + | − | + | − | + | − |
| 3 | + | − | + | − | + | − | + | − | |
| 4 | + | − | + | − | + | − | + | − | |
| 5 | + | − | + | − | + | − | + | − | |
| 18 | 2 | + | − | + | − | − | + | + | − |
| 3 | + | − | + | − | − | + | * | * | |
| 4 | + | − | + | − | − | + | + | − | |
| 5 | + | − | + | − | − | + | + | − | |
| 20 | 2 | + | − | + | − | * | * | + | − |
| 3 | + | − | + | − | − | + | + | − | |
| 4 | + | − | + | − | − | + | + | − | |
| 5 | + | − | + | − | + | − | + | − | |
| 28 | 2 | + | − | + | − | − | + | + | − |
| 3 | + | − | + | − | − | + | + | − | |
| 4 | + | − | + | − | − | + | + | − | |
| 5 | + | − | + | − | − | + | + | − | |
| 30 | 2 | + | − | + | − | − | + | * | * |
| 3 | + | − | + | − | + | − | + | − | |
| 4 | + | − | + | − | + | − | + | − | |
| 5 | + | − | + | − | + | − | + | − | |
| 42 | 2 | + | − | + | − | − | + | * | * |
| 3 | + | − | + | − | − | + | * | * | |
| 4 | + | − | + | − | − | + | + | − | |
| 5 | + | − | + | − | − | + | + | − | |
| 50 | 2 | + | − | + | − | − | + | + | − |
| 3 | + | − | + | − | − | + | + | − | |
| 4 | + | − | + | − | − | + | + | − | |
| 5 | + | − | + | − | − | + | + | − | |
Abbreviations: LF=degree of lateral flexion (simulated); ICL=iliocostalis lumborum; ICT=iliocostalis thoracis; GM=gluteus medius; EO=external oblique muscle; Ipsi=ipsilateral to the side of bending; Contra=contralateral to the side of bending. Muscle (EMG) activity is categorized as: + sustained; − mild.
similar muscle activation on both sides.
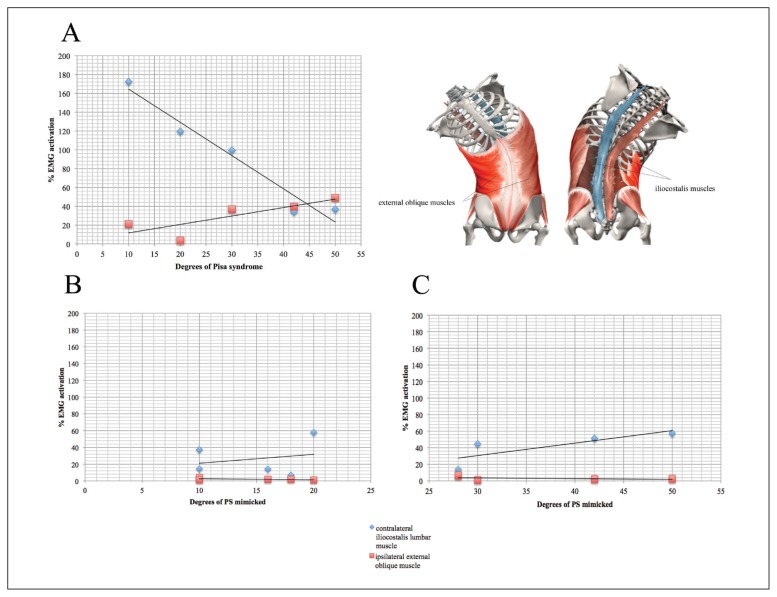
In the PD with PS, no significant correlation was found between the degree of PS and intensity of muscle activation. However, when the patients were stratified by side of bending, right trunk leaning was found to be significantly correlated with reduced contralateral ICL activity (p=0.037) and increased activity in the ipsilateral EO (p=0.037) (Fig. 2A). No significant correlation was found in patients with left trunk leaning.
Figure 2.
A= Correlation between the degree of trunk flexion and RMS of the contralateral ICL and the ipsilateral EO muscles in the right PS patients. B and C= Correlation between degree of trunk flexion and RMS of the contralateral ICL and the ipsilateral EO muscle in four young healthy controls (mean value) simulating PS from 0 to 25 degrees (B) and from 25 to 55 degrees (C).
By contrast, in the SC in the healthy young controls, a significant correlation was found between the degree of lateral trunk flexion and increased activity of the ipsilateral GM (p<0.01). Furthermore, when stratified by degree of bending, a lateral flexion ≤ 20° was associated with sustained muscle activity of the contralateral ICL and ipsilateral EO muscles (Fig. 2B). Interestingly, we observed a significant correlation between lateral trunk flexion > 20° and increased activity in the contralateral ICL (p< 0.01) (Fig. 2C).
Discussion
The main finding of this pilot study was that the PD patients with PS showed a significant inter-side difference in ICL during stance (p=0.028), in that EMG activity was significantly more intense contralateral to the side of bending, while the ICT, GM and EO displayed similar activity. These data confirm previous studies (Di Matteo et al., 2011, Tinazzi et al., 2013) suggesting that the presence of pattern II that is characterized by a significant contralateral muscle activity in the ICL, and co-contraction activity of the other muscles.
Another finding was that the muscle activity in upright stance was higher in the PD patients without PS than in the age-matched and young healthy controls, suggesting that PD and aging may both be associated with enhanced EMG activity.
As expected, the data from the young healthy controls mimicking the different degrees of PS showed that muscle activity varied with different degrees of lateral trunk flexion (Fig. 2B–C). These recordings indicated that side-to-side differences in paraspinal and non-paraspinal muscles exist and have a well-defined role in trunk muscle activity.
Indeed, in the SC, we found significant differences between the activation of the ipsilateral and contralateral paraspinal and non-paraspinal muscles. In particular, higher activity was observed in the contralateral ICL, ICT and EO, but not the GM, indicating a possible compensatory role of the contralateral muscles. By contrast, PD patients with PS showed no side-to-side difference, other than for the ICL, suggesting a possible impairment of trunk muscle compensatory mechanisms.
The GM is one the main stabilizers of the pelvic region, serving to maintain vertical femoropelvic alignment in the coronal plane during the middle phase of the gait cycle. Although PS patients did not show a significant side-to-side difference in muscle activity in the GM, its hyperactivity might be explained by its role in vertical femoropelvic alignment, and also by the fact that it counteracts the weight of worsening trunk bending due to gravity and contributes to keeping the center of pressure (CoP) within the base of support to avoid the risk of falling. We have previously demonstrated that biomechanical dysfunction of the GM results in increased CoP displacement in the anterior-posterior and mediolateral directions due to an altered use of the hip strategy (Geroin et al., 2015).
The EO induces ipsilateral trunk flexion followed by a forward flexion and contralateral trunk rotation (Neumann, 2010). The PD patients with PS showed bilateral activity of the EO with predominant, but not significant, ipsilateral hyperactivity (Table II), consistent with previous findings (Tassorelli et al., 2012; Tinazzi et al., 2013). In the context of PD-related postural abnormalities, the EO is one of the muscles primarily associated with the pathogenesis of upper camptocormia (Furusawa et al., 2012). In the SC in the young healthy controls, bilateral activation of the EO was recorded, with dominant contralateral activity due to the compensatory role of this muscle (Table IV and V).
Dystonia is considered one of the prevailing pathophysiological aspects of PS in PD. It is a movement disorder characterized by sustained or intermittent involuntary muscle contractions leading to abnormal posture and twisting movements (Albanese et al., 2013). PS can be classified as a generalized and persistent dystonia involving not only the trunk but also the lower limb muscles (Tinazzi et al., 2013; Geroin et al., 2015; Tinazzi et al., 2016).
Previous studies documented the presence of dystonia leading to abnormal EMG muscle activity in PS patients (Di Matteo et al., 2011; Tassorelli et al., 2012; Tinazzi et al., 2013). However, it is worth noting that other secondary muscle compensatory mechanisms (linked to the cocontraction phenomenon, the biomechanical function of muscles, the age effect and the clinical presentation of PS) might contribute to the abnormal EMG pattern.
Although our findings do not offer direct evidence, dys-regulation of control of spinal and supra-spinal reflexes may be involved in the pathogenesis of PS (Nardone et al., 2012). A recent study (Tinazzi et al., 2013) reported co-activation of the paraspinal and non-paraspinal muscles during trunk flexion contralateral to the leaning side in PD patients with PS. This might be the expression of pathological reflexes or the result of synergistic reflex connections that activate both sides of the body (Beith et al., 2004). Such patients may utilize a secondary ‘cocontraction’ strategy to counteract progression of lateral bending.
Muscle activation asymmetry may be due to an increase in lateral flexion, because the moment and leverage of muscle changes degree by degree (Oddsson et al., 1990). In PS patients with right trunk leaning, increasing degrees of flexion were correlated with greater activity of the ipsilateral EO and less activation of the contralateral ICL (Fig. 1A, Fig. 2A). By contrast, in the healthy controls in the SC, the increase in flexion from 0 to 20 degrees was associated with balanced and stable activity of both the ICL and the EO muscles (Fig. 2B). When the trunk was flexed more than 28 degrees, the activity of the contralateral ICL increased significantly while that of the EO progressively decreased, albeit not significantly (Fig. 2C). In this case, activation of the contralateral ICL may have a stabilizing role in counteracting the weight of the trunk against gravity.
The activity of the contralateral ICL might have been reduced in PS patients because of myoelectric cessation involving the low back extensor muscles at the maximal degrees of lateral bending, also known as the flexion-relaxation phenomenon (Fig. 2A) (Shin et al., 2010). This phenomenon was not observed in patients with left trunk bending, perhaps because of the small sample size or because the clinical phenotypes differed among the PS patients.
Another aspect that merits discussion is the age effect. We found larger EMG activity in the elderly control in comparison to the younger ones. With advancing age, there is a progressive reduction in joint space, muscle strength (Hasue et al., 1980), contractile speed (D’Antona et al., 2007), action potential velocity (Rivner et al., 2001; Quirk et al., 2014), and joint position sense, accompanied by changes in central nervous system recruitment (Van Impe et al., 2011). Axial rigidity can reduce passive stability (e.g., ligaments) and/or active stability (muscles, tendons and nerves), producing functional impairment of posture and locomotion (Wright et al., 2007), and resulting in pain (Geroin et al., 2016).
Finally, it is worth noting that the muscle activity in PS may change over time, given that PS has different clinical presentations. It can develop in a chronic fashion with subclinical onset and progressive worsening, or appear in a short space of time, showing a rapid progression within days or weeks (Tinazzi et al., 2016). In the chronic condition, other musculoskeletal changes (i.e., muscle-tendon retraction, articular ankyloses) might be involved and produce different patterns of muscle activation. In accordance with this line of reasoning, previous reports showed muscle activation patterns that differ from those we found in the present sample.
Our study has several limitations: 1) the small sample size, due primarily to the technically complicated, invasive, and time-consuming experimental protocol; 2) the difficulty obtaining MIVCs in PS patients; 3) a possible bias effect in the healthy controls mimicking PS postures (i.e. in the SC); 4) no evaluation of other postural muscles, such as the quadratus lumborum, that play an important role in torque production, especially in PS (Dupeyron et al., 2015).
These factors notwithstanding, this pilot study showed significant inter-side difference in ICL during stance in PD patients with PS, mainly consisting of increased contralateral muscle activity (pattern II), with co-contraction activity of the other muscles (ICT, GM and EO) as a result of dysfunctional compensatory mechanisms in the muscles.
Neurodegenerative disease and aging may also have contributed to the increased muscle activation. Moreover, in PD patients with PS, with increasing degrees of lateral flexion, the activity of the EO and the ICL muscles progressively increased and decreased, respectively, documenting hyperactivity of the non-paraspinal and hypofunction/weakness of the paraspinal muscles. Larger-scale studies in PD patients with and without PS and healthy controls, and also investigating other muscles, are needed to confirm our data.
To conclude, diagnostic procedures based on detailed EMG evaluation of paraspinal and non-paraspinal muscle function, as well as postural and gait assessment (Geroin et al., 2015), may aid in selecting a therapeutic approach appropriate for the individual patient, i.e., physiotherapy to strengthen weak compensatory muscles and/or botulinum toxin injection to reduce muscle dystonia.
References
- Adams MA, Dolan P, Hutton WC. Diurnal variations in the stresses on the lumbar spine. Spine (Phila Pa) 1987;1976;12:130–137. doi: 10.1097/00007632-198703000-00008. [DOI] [PubMed] [Google Scholar]
- Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beith ID, Harrison PJ. Stretch reflexes in human abdominal muscles. Exp Brain Res. 2004;159:206–213. doi: 10.1007/s00221-004-1948-4. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Thomas A, Varanese S, et al. Botulinum toxin treatment of lateral axial dystonia in Parkinsonism. Mov Disord. 2007;22:2097–2103. doi: 10.1002/mds.21694. [DOI] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino MA, Carlizzi CN, et al. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol. 2007;100:603–611. doi: 10.1007/s00421-007-0402-2. [DOI] [PubMed] [Google Scholar]
- DeLuca C. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- Di Matteo A, Fasano A, Squintani G, et al. Lateral trunk flexion in Parkinson’s disease: EMG features disclose two different underlying pathophysiological mechanisms. J Neurol. 2011;258:740–745. doi: 10.1007/s00415-010-5822-y. [DOI] [PubMed] [Google Scholar]
- Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011;10:538–549. doi: 10.1016/S1474-4422(11)70067-9. [DOI] [PubMed] [Google Scholar]
- Dupeyron A, Viollet E, Coroian F, et al. Botulinum Toxin-A for treatment of Pisa syndrome: A new target muscle. Parkinsonism Relat Disord. 2015;21:669–670. doi: 10.1016/j.parkreldis.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Frazzitta G, Balbi P, Gotti F, et al. Pisa Syndrome in Parkinson’s Disease: Electromyographic Aspects and Implications for Rehabilitation. Parkinsons Dis. 20152015:4371–4390. doi: 10.1155/2015/437190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Mukai Y, Kobayashi Y, et al. Role of the external oblique muscle in upper camptocormia for patients with Parkinson’s disease. Mov Disord. 2012;27:802–803. doi: 10.1002/mds.24930. [DOI] [PubMed] [Google Scholar]
- Geroin C, Gandolfi M, Bruno V, et al. Integrated Approach for Pain Management in Parkinson Disease. Curr Neurol Neurosci Rep. 2016;16:28. doi: 10.1007/s11910-016-0628-7. [DOI] [PubMed] [Google Scholar]
- Geroin C, Smania N, Schena F, et al. Does the Pisa syndrome affect postural control, balance, and gait in patients with Parkinson’s disease? An observational cross-sectional study. Parkinsonism Relat Disord. 2015;21:736–741. doi: 10.1016/j.parkreldis.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Gravina AR, Ferraro C, Frizziero A, et al. Goniometer evaluation of thoracic kyphosis and lumbar lordosis in subjects during growth age: a validity study. Stud Health Technol Inform. 2012;176:247–251. [PubMed] [Google Scholar]
- Hasue M, Fujiwara M, Kikuchi S. A new method of quantitative measurement of abdominal and back muscle strength. Spine (Phila Pa 1976) 1980;5:143–148. doi: 10.1097/00007632-198003000-00008. [DOI] [PubMed] [Google Scholar]
- Kumar S. EMG in rotation-flexion of the torso. J Electromyogr Kinesiol. 2010;20:1146–1154. doi: 10.1016/j.jelekin.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Nardone A, Pasetti C, Schieppati M. Spinal and supraspinal stretch responses of postural muscles in early Parkinsonian patients. Exp Neurol. 2012;237:407–417. doi: 10.1016/j.expneurol.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Neumann DA. Kinesiology of the Musculoskeletal System, Foundations for Physical Rehabilitation. Mosby Elsevier; 2010. [Google Scholar]
- Oddsson LI. Control of voluntary trunk movements in man. Mechanisms for postural equilibrium during standing. Acta Physiol Scand Suppl. 1990;595:1–60. [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–15601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- Quirk DA, Hubley-Kozey CL. Age-related changes in trunk neuromuscular activation patterns during a controlled functional transfer task include amplitude and temporal synergies. Hum Mov Sci. 2014;38:262–280. doi: 10.1016/j.humov.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24:1134–1141. doi: 10.1002/mus.1124. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Foltynie T, Blackwell AD, et al. Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatr. 2005;76:343e348. doi: 10.1136/jnnp.2003.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin G, D’Souza C. EMG activity of low back extensor muscles during cyclic flexion/extension. J Electromyogr Kinesiol. 2010;20:742–749. doi: 10.1016/j.jelekin.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Soderberg GL, Knutson LM. A guide for use and interpretation of kinesiologic electromyographic data. Phys Ther. 2000;80:485–498. [PubMed] [Google Scholar]
- Tassorelli C, Furnari A, Buscone S, et al. Pisa syndrome in Parkinson’s disease: clinical, electromyographic, and radiological characterization. Mov Disord. 2012;27:227–235. doi: 10.1002/mds.23930. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Fasano A, Geroin C, et al. Italian Pisa Syndrome Study Group. Pisa syndrome in Parkinson disease: An observational multicenter Italian study. Neurology. 2015;85:1769–1779. doi: 10.1212/WNL.0000000000002122. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Geroin C, Gandolfi M, et al. Pisa syndrome in Parkinson’s disease: An integrated approach from pathophysiology to management. Mov Disord. 2016;31:1785–1795. doi: 10.1002/mds.26829. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Juergenson I, Squintani G, et al. Pisa syndrome in Parkinson’s disease: an electrophysiological and imaging study. J Neurol. 2013;260:2138–2148. doi: 10.1007/s00415-013-6945-8. [DOI] [PubMed] [Google Scholar]
- Van Impe A, Coxon JP, Goble DJ, et al. Age-related changes in brain activation underlying single-and dual-task performance: visuomanual drawing and mental arithmetic. Neuropsychologia. 2011;49:2400–2409. doi: 10.1016/j.neuropsychologia.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel VS, Nutt J, et al. Axial hyper-tonicity in Parkinson’s disease: direct measurements of trunk and hip torque. Exp Neurol. 2007;208:38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Winter DA. Electromyography reliability in maximal and submaximal isometric contractions. Arch Phys Med Rehabil. 1987;1983;64:417–420. [PubMed] [Google Scholar]