Abstract
Background:
Maternal exposure to ambient air pollution has been associated with an increased risk of congenital heart defects in offspring; however, the results are inconsistent.
Objectives:
We investigated whether there is an association between prenatal exposure to particulate matter with diameter () during early pregnancy and fetal cardiovascular malformations.
Methods:
The gravidae from a hospital-based case–control study in Fuzhou, China, during 2007–2013 were assigned 10-d or 1-mo averages of daily using an air monitor–based inverse distance weighting method during early pregnancy. A total of 662 live-birth or selectively terminated cases and 3,972 live-birth controls were enrolled. The exposure was considered as a categorical variable. A multivariable logistic regression model was constructed to quantify the adjusted odds ratios (aORs) of the exposure to and the risks of fetal cardiovascular malformations.
Results:
levels were positively associated with the risks of atrial septal defect (aORs ranging from 1.29 to 2.17), patent ductus arteriosus [, 1.63; 95% confidence intervals (CIs): 1.17, 2.23; 1.06, 3.24], overall fetal cardiovascular malformations (; 95% CI: 1.03, 1.61), ventricular septal defect (; 95% CI: 1.00, 1.43), and tetralogy of Fallot (; 95% CI: 1.01, 2.19) in the various observed periods scaled by 10 d or 1 mo in the first and second gestation months. The strongest associations were observed for exposure to in the second quartile, whereas the associations were attenuated when higher concentrations of in the third and fourth quartiles of the exposure were evaluated. No correlations of levels with these cardiovascular malformations in the other time periods of gestation were observed.
Conclusions:
Our findings suggest some positive associations between maternal exposure to ambient during the first two months of pregnancy and fetal cardiovascular malformations. https://doi.org/10.1289/EHP289
Introduction
A growing body of epidemiological literature has suggested an association between ambient particulate matter (PM) exposure and fetal anomalies, particularly cardiovascular malformations. Evidence has indicated associations between in utero exposure to PM with an aerodynamic diameter () and the prevalence of fetal cardiovascular malformations, that is to say, associations between and ventricular septal defects, pulmonary valve stenosis (Padula et al. 2013), patent ductus arteriosus (Strickland et al. 2009), and multiple congenital heart defects (Agay-Shay et al. 2013). A meta-analysis of air pollutant–anomaly combinations found that exposure was related to an increased risk of atrial septal defects (Vrijheid et al. 2011). Evidence from a line of studies, however, failed to demonstrate an association between and fetal cardiovascular malformations (Ritz et al. 2002; Schembari et al. 2014; Stingone et al. 2014), and no definite link was indicated between ambient total suspended particles and congenital heart diseases in a study performed in Brindisi, Italy (Gianicolo et al. 2014). An inverse association was reported between and ventricular septal defects (Hansen et al. 2009) and between fine particulate matter (; with an aerodynamic diameter ) and atrial septal defects (Stingone et al. 2014), ventricular septal defects (Padula et al. 2013), and isolated patent ductus arteriosus (Agay-Shay et al. 2013). These inconsistent results may be attributed to heterogeneity in the sources, components, and exposure levels of PM, to the methods used, and to differences in demography, topography, meteorology, socioeconomic status, and individual lifestyle of the population surveyed (Gorini et al. 2014).
China has been experiencing exceptionally high levels of air pollution in recent years. A few metropolitan areas are among the most polluted in the world, with daily levels of averaged at during 2004–2008 in Beijing (Guo et al. 2013) and in autumn in Shanghai (Zhou et al. 2012). Annual levels of have exceeded in approximately one-third of the cities covered by the China National Ambient Air Quality Surveillance Network (Yang et al. 2011). is the only inhalable particulate matter that had been continuously monitored before 2012 within major cities in China. In Fuzhou, the capital city of Fujian Province, daily ambient levels vary between . These values may represent average levels across the majority of cities in mainland China (Yang et al. 2011).
Rates of birth defects in China have been reported at 5.12%, which is higher than in developed countries (Christianson et al. 2006). Birth defect rates have been higher in the coastal/eastern regions and in urban areas where environmental pollution, particularly ambient air pollution, is more severe than in western regions and in rural areas (Ministry of Health, People’s Republic of China 2011). Cardiovascular malformations, the most frequently occurring anomalies in fetuses and neonates, are the main causes of infant mortality (Dadvand et al. 2011b). In China, a national survey reported a prevalence of 7 to 8 cases of congenital heart diseases per 1,000 live births (China National Center for Cardiovascular Disease 2006); however, there have been few studies regarding associations between particulate air pollution and cardiovascular malformations in China. The air quality in China has been deteriorating over the last several decades because of the booming economy and has had a significant impact globally, particularly in neighboring countries. In the present study, we used a hospital-based retrospective case–control design to investigate whether there was an association between exposure to and risks of fetal cardiac malformations.
Methods
Study Area
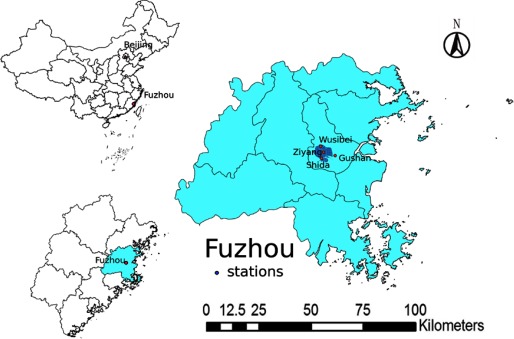
Fuzhou is the capital city of Fujian Province and is located in the coastal area of southeastern China. The city is surrounded on the northeast, northwest, and southwest by hills and mountains and faces southeast to the Taiwan Strait, with an administrative area of 11,968 and a population of 6.24 million. The built-up region of Fuzhou, which is in the central area of the city and has an area of ∼ 200 consists of a population of 1.95 million according to the Sixth National Population Census of the People’s Republic of China for Fuzhou in 2010 (Fuzhou Statistics Bureau 2011) (Figure 1). A built-up region is generally the central area of an urban field with the densest population. The built-up region of Fuzhou is relatively small compared with the entire city area (Figure 1). Fuzhou has a wet, monsoon-influenced, humid marine climate, characterized by a long summer and winter and a short spring and autumn. The temperature averages 19.6°C, with precipitation of 1,348.8 mm between 2007 and 2013 (Fuzhou Water Conservancy Bureau 2015). Ambient air pollution, including airborne PM, is among the average levels in China (Ministry of Environmental Protection, People?s Republic of China 2014).
Figure 1.
Location of monitoring stations in Fuzhou, China. Three monitoring stations are distributed within the built-up region of the city, which is in the central area of Fuzhou and has a high population density. One additional monitoring station (Gushan) is located at 450 m above sea level on a mountain outside the built-up region of the city, which serves as a reference for monitoring the background air pollution. Data obtained from the reference station do not enter the estimating system for evaluation of air quality.
Study Population
We collected the data from pregnant women who resided in Fuzhou, China during their pregnancy and were gestationally monitored and delivered at Fujian Maternity and Child Health Hospital or Fuzhou General Hospital. Both hospitals are large, Grade A tertiary hospitals in Fuzhou, Fujian Province, with a total of ∼15,000 births annually for both hospitals, accounting for nearly 2/3 of the total annual births in the built-up region of Fuzhou. All of the data were obtained from prenatal care and parturition records of the pregnant women of who delivered or selected termination at gestation weeks, with an estimated date of conception between 1 January 2007 and 31 December 2013. A total of 110,720 births were enrolled for the selection of cases and controls, in which 99,094 births (89.5%) lived in the built-up region of Fuzhou. The date of conception was estimated based on the hospital records (date of birth and gestational age) by assuming that conception occurred 14 d after the last menstrual period date. The Scientific Research Committees and Ethics Committees of Fuzhou General Hospital and Fujian Maternity and Child Health Hospital approved the study involving the use of the hospital records.
Cases consisted of a cohort clinically diagnosed with birth defects. We recorded the details regarding premature delivery, stillbirth, and term birth, late abortion and with antenatal diagnosis of congenital malformations. We also documented the mother’s general condition, including age, gravidity, parity, last menstrual period, residential address, history of abnormal pregnancy and labor, trimester in which prenatal care began, medication, concomitant disease(s) and if any, living habits, psychological status, nutritional status, and diet. The data of infants with any indications of fetal anomalies were also obtained from the hospital records. Fetal malformations were confirmed by echocardiogram, cardiac catheter surgical operation, or autopsy, or by a combination of these methods, with reference to ultrasonic examination, cytogenetic examination, medication administration records, and follow-up materials, whenever available.
Cases were excluded when they met one of the following criteria (Bassili et al. 2000; Jenkins et al. 2007; Strickland et al. 2009): a) missing important records of medical history, including equivocation of conceptus age (nine cases that met the criteria); b) maternal ill conditions, including gestation weeks or , gestational diabetes or diabetes with pregnancy, congenital cardiopathy with pregnancy, conditions of illness with fever (), exposure to pesticides within 3–8 gestation weeks, or history of consanguineous mating or genetic defects (18 cases); c) twin pregnancy (6 cases); and d) isolated patent ductus arteriosus in premature birth (5 cases).
Controls were randomly selected from the total live-born and nonmalformed infants by the date of birth sampling, which were independent of the cases at a ratio of 1:6 (cases:controls) to meet an accepted statistical power. Data for the control samples were derived from the medical records of the gravidae and the birth records from the two hospitals enrolled. All of the newborns in the control group were routinely examined to rule out cardiovascular malformations and other major anomalies.
Data for the numbers of fetal defects, the ages of the pregnant women, their parity, the longitude and latitude of the gravida’s habitation, the season of conception, and diagnoses for fetal malformations were collected and entered manually into an EpiData 3.0 database created using EpiData software (EpiData Association, Odense, Denmark). These data were obtained from the hospital records and the birth records.
Classification of Fetal Cardiovascular Malformations
Cardiovascular malformations were diagnostically categorized in the following nine subtypes: ventricular septal defect, atrial septal defect, patent ductus arteriosus, coarctation of aorta, pulmonary valve stenosis, tetralogy of Fallot, hypoplastic left heart syndrome, transposition of conducting arteries, and other uncommon subtypes such as ectopia cordis, ventricular double outlets, tricuspid atresia, inborn aortic arch anomalies, and others. (Strickland et al. 2009). The concomitance of cardiovascular abnormalities was recognized and counted only if these abnormalities were embryologically independent from each other (Riehle-Colarusso et al. 2007; Strickland et al. 2009). Temporarily isolated cardiovascular conditions in neonates, such as patent oval foramen, were considered to be normal, and neonates with identified trisomies, heterotaxy syndrome, or abnormal cardiac looping were not counted (Riehle-Colarusso et al. 2007; Strickland et al. 2009). Cardiovascular abnormalities combined with simultaneous fetal anomalies other than those in the cardiovascular system were excluded from further analyses because they might have more complicated causes (Agay-Shay et al. 2013).
Ambient Levels and Estimation of Maternal Exposure to PM10
Ambient levels were quantified by the Central Station of Environmental Monitoring of Fujian Province. levels were monitored and recorded hourly from three monitoring stations within the built-up region of Fuzhou, namely Wusibei, Ziyang, and Shida, all of which are part of the China National Ambient Air Quality Surveillance Network (Figure 1). Daily, monthly, and seasonal average levels of between 1 January 2007 and 31 December 2013 were calculated based on these measurements.
The residential address of each gravida during pregnancy was converted into longitude and latitude using Google Maps software (version 7.6.1) according to a detailed address, including house number, street, and block (Dadvand et al. 2011a). To estimate the individual exposure level, a station-based inverse distance weighting interpolation method was used to interpolate the daily concentrations from the three monitoring stations to the predicted residential site across Fuzhou (Padula et al. 2013). For each monitoring site, was used as the weighting factor, where d refers to the distance between the monitoring site and the predicted residential site (Wong et al. 2004). Data from the three monitoring stations were included in each interpolation. When transferring the data from the cases and controls and the corresponding levels into the database, logistic rectification was performed automatically to identify and correct potential errors in the data entry, which were manually rectified as soon as an error was found.
Statistical Analysis
Twenty-four-hour measurements of , hospital-based birth certificates, and birth defect records were used for our analyses. Maternal exposure and risk factors were classified at the individual level. The risk of fetal cardiovascular malformations versus exposure was analyzed by incorporating the variables using multivariable logistic regression analyses. Maternal education was coded as one of the three categories: , junior or senior high school, and . Seasons of conception were categorized as warm (May–October) or cool (November–April) based on the local climate conditions. The trimester in which prenatal care began was categorized as the first, second, or third trimester, or no care. Maternal variables, such as psychological status, nutritional status, prenatal folic acid and vitamin use, marital status, and history of abnormal pregnancy and labor, were treated as the dichotomous variables (Rankin et al. 2009). Potential covariates from the hospital records, which included maternal age, parity, educational attainment, prenatal care, prenatal folic acid and vitamin use, season of conception, and marital status, were entered for the final adjustments based on previous studies (Agay-Shay et al. 2013; Myers et al. 2011; Padula et al. 2013) and on the data available in Fuzhou, China.
Potential deviations in the estimation of the conception date may produce greater bias by using a shorter time scale for the analyses. Time lag may exist between the exposure and the body’s response. Considering these factors, we analyzed associations using a 10-d scale, rather than a seven-day (1-wk) scale, over postconception days 11–60 to increase the stability of the results. The association was analyzed by categorizing the exposure duration as gestation days 11–20, 21–30, 31–40, 41–50, and 51–60, and the first and second months of gestation. exposure estimates were examined as quartiles using the distribution among the controls. Because exposure estimates were verified to be normally distributed, they were entered into the regression model as categorical variables on the basis of the quartile distribution. The first quartile was used as a reference, and the other (higher) quartiles were compared with the reference to calculate the adjusted odds ratios (aORs) and 95% confidence intervals (CIs).
Comparisons were conducted in the ventricular septal defect, atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, and overall cardiovascular malformations subgroups because they consisted of relatively large sample sizes. The data were analyzed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) and the STATA statistical package (version 10.1; StataCorp LLC, College Station, TX, USA).
Results
Descriptive Statistics
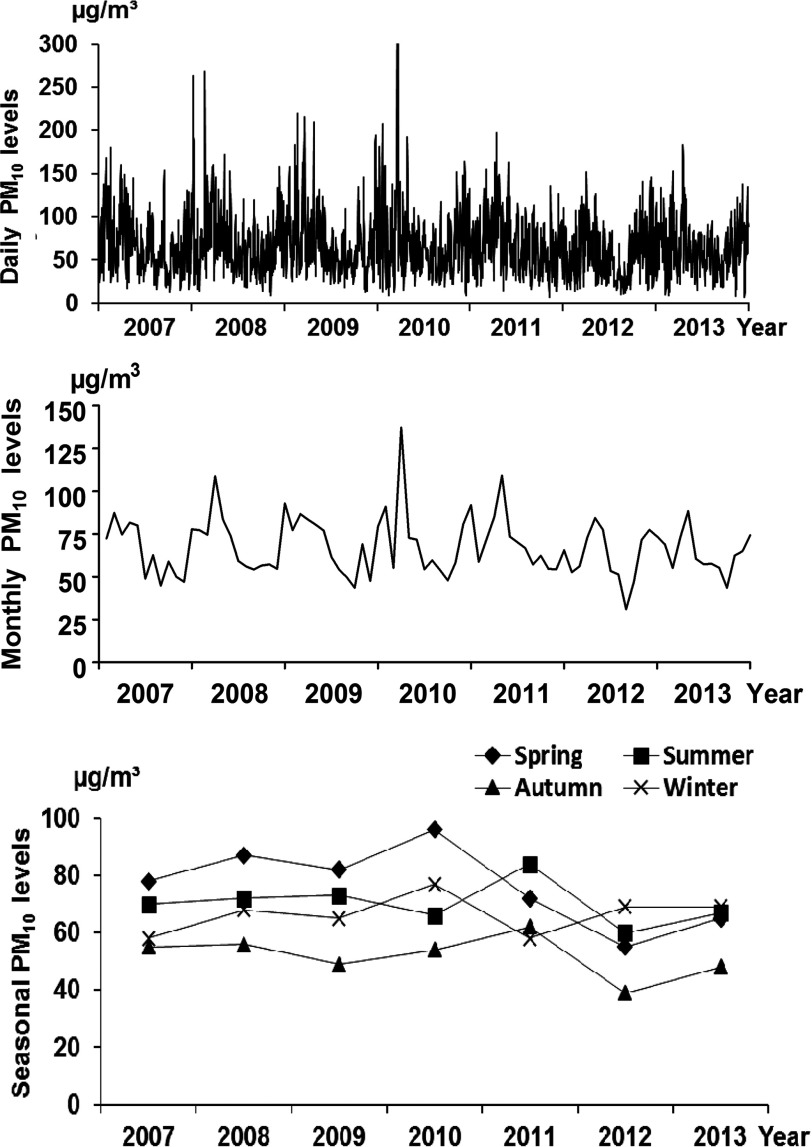
Daily levels in Fuzhou fluctuated with the average level at , between 1 January 2007 and 31 December 2013. The monthly levels were typically higher from February to April within a year, with the highest at in February 2010. The seasonal levels of ranged between 40 and 99.8 , with the highest levels occurring in the spring or summer and the lowest in the autumn; these are delineated in Figure 2.
Figure 2.
levels in Fuzhou, China during 2007–2013. levels in Fuzhou varied dramatically, with daily levels averaged at . Daily levels exceeded 250 µg/m3 on 9 January () and 23 February (), 2008; on 21 March () and 23 (), 2010, peaking on 22 March (), 2010; and were as low as on 10 November 2011 and 16 December 2013. Levels that exceeded are not fully shown in the figure. The average monthly levels ranged between 56.1 and , and the seasonal levels were generally higher in the spring and lower in the autumn.
All of the enrolled participants were Han, the ethnic majority in China. Before the exclusions, there was a liveborn and stillborn cohort of 110,720 births in Fujian Maternity and Child Health Hospital and Fuzhou General Hospital with the estimated dates of conception between 1 January 2007 and 31 December 2013. Among the 110,720 births, fetal malformations were diagnosed in 1,584 births without the evidence of trisomy. The overall prevalence of fetal malformations was 1.43% with an increasing tendency from 2007 to 2013 (see Table S1).
A total of 700 cases were diagnosed with fetal cardiovascular malformations among the 1,584 cases with fetal malformations (see Table S2). The subtypes of fetal cardiovascular malformations were ranked in a frequency order from high to low as ventricular septal defect, atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, and others (Table 1).
Table 1.
Subtype and prevalence of fetal cardiovascular malformation groupings from a total birth population of 110,720 in Fuzhou, China, between 2007 and 2013.
| Cardiovascular malformations | Number | Prevalence (Subtype/total birth population, %) | Ratio (Subtype/total, %) |
|---|---|---|---|
| Ventricular septal defect | 270 | 0.244 | 38.6 |
| Atrial septal defect | 145 | 0.131 | 20.7 |
| Patent ductus arteriosus | 107 | 0.097 | 15.3 |
| Tetralogy of Fallot | 81 | 0.073 | 11.6 |
| Pulmonary valve stenosis | 34 | 0.031 | 4.9 |
| Transposition of conducting arteries | 26 | 0.023 | 3.7 |
| Coarctation of aorta | 19 | 0.017 | 2.7 |
| Hypoplastic left heart syndrome | 14 | 0.013 | 2.0 |
| Others | 4 | 0.004 | 0.6 |
| Total | 700 | 0.632 | 100.0 |
Correlations of Exposure Levels and Risks for Fetal Cardiovascular Malformations
After screening for the exclusion criteria, 38 cases that met the exclusion criteria were excluded from the final analyses. Of the 700 cases with fetal cardiovascular malformations, 662 entered the final analyses; of these, 638 cases (96.4%) lived in the built-up region of Fuzhou. The remaining 24 cases lived outside the built-up region, including one living 41 km from the nearest monitoring station and one 33 living km from the nearest monitoring station; the others lived within 15–25 km of the nearest monitoring station. The locations of the pregnant women were geographically stochastically distributed in the city of Fuzhou other than proximal to the two hospitals. The local main emissions sources are the traffic and living sources, which are distributed roughly evenly throughout the observed region, and there are no major emissions sources from heavy industrial sectors in the region and nearby. Therefore, there was no tendency of mothers with different socioeconomic statuses to selectively distribute in places with different levels of . The general conditions of the cases and controls, including age, parity, and distribution of residential sites, are presented in Table S3.
As shown in Table 2, exposure levels were associated with the risks of atrial septal defect (: 1.19, 3.22) and patent ductus arteriosus (: 1.03, 3.00) in the second gestation month. Divided by shorter time scales (10 d), we observed elevated risks for atrial septal defect, with aORs ranging between 1.29 and 2.17, and for patent ductus arteriosus, with aORs at 1.54 (95% CI: 1.17, 2.23) and 1.63 (95% CI: 1.06, 3.24) in the various observed periods; for overall fetal cardiovascular malformations (: 1.03, 1.61) in gestation days 41–50; for ventricular septal defect (: 1.00, 1.43) in gestation days 41–50; and for tetralogy of Fallot (: 1.01, 2.19) in gestation days 31–40. We also observed an increased risk for atrial septal defect (: 1.05, 1.74) during gestation days 21–30 despite the absence of an increased risk in the first gestation month. Interestingly, the effect estimates were generally observed to be the highest in the second quartile, with exposure levels ranging from 41.9 to and attenuated in the third and fourth quartiles at higher exposure levels.
Table 2.
Adjusted odds ratios (95% confidence intervals) for congenital heart malformations by quartiles for concentrations () during the first 2 mo of gestation during 2007–2013 in Fuzhou, China (total birth population, 110,720).
| Time scale/Quartile of exposure level | Ventricular septal defect ()a | Atrial septal defect () | Patent ductus arteriosus ()b | Tetralogy of Fallot () | Overall fetal cardiovascular malformations () |
|---|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Days 11–20 | |||||
| Reference | Reference | Reference | Reference | Reference | |
| 1.14 (0.90, 1.29) | 1.20 (0.78, 1.92) | 1.18 (0.72, 1.51) | 1.26 (0.65, 2.24) | 1.35 (0.78, 2.34) | |
| 1.09 (0.79, 1.63) | 1.09 (0.80, 1.37) | 1.10 (0.89, 1.39) | 1.07 (0.64, 2.09) | 1.11 (0.70, 1.71) | |
| 0.89 (0.66, 1.07) | 0.98 (0.57, 1.61) | 1.14 (0.80, 1.46) | 1.04 (0.65, 1.59) | 1.15 (0.68, 1.78) | |
| Days 21–30 | |||||
| Reference | Reference | Reference | Reference | Reference | |
| 1.19 (0.78, 1.58) | 1.29 (1.05, 1.74) | 1.27 (0.80, 1.60) | 1.29 (0.76, 2.18) | 1.19 (0.67, 1.88) | |
| 1.14 (0.81, 1.53) | 1.24 (0.87, 1.55) | 1.08 (0.74, 1.37) | 1.13 (0.66, 2.17) | 1.11 (0.71, 1.49) | |
| 0.88 (0.67, 1.71) | 1.12 (0.90, 1.52) | 0.89 (0.67, 1.15) | 1.04 (0.77, 1.44) | 0.99 (0.70, 1.47) | |
| Days 31–40 | |||||
| Reference | Reference | Reference | Reference | Reference | |
| 1.21 (1.01, 1.53) | 2.17 (1.29, 3.64) | 1.54 (1.17, 2.23) | 1.44 (1.01, 2.19) | 1.55 (0.93, 2.47) | |
| 1.27 (0.88, 1.62) | 1.33 (1.07, 1.85) | 1.21 (0.78, 1.97) | 1.20 (0.76, 1.70) | 1.29 (0.75, 2.08) | |
| 1.23 (0.90, 1.52) | 1.04 (0.79, 1.46) | 1.07 (0.65, 1.82) | 1.07 (0.66, 1.87) | 1.11 (0.70, 1.96) | |
| Days 41–50 | |||||
| Reference | Reference | Reference | Reference | Reference | |
| 1.19 (1.00, 1.43) | 1.88 (1.22, 2.75) | 1.63 (1.06, 3.24) | 1.22 (0.94, 1.59) | 1.28 (1.03, 1.61) | |
| 1.19 (0.87, 1.58) | 1.18 (0.97, 1.63) | 1.26 (0.85, 2.39) | 1.25 (0.83, 1.91) | 1.12 (0.86, 1.41) | |
| 1.01 (0.83, 1.44) | 1.07 (0.88, 1.48) | 1.11 (0.64, 2.06) | 0.96 (0.68, 1.55) | 1.03 (0.84, 1.36) | |
| Days 51–60 | |||||
| Reference | Reference | Reference | Reference | Reference | |
| 1.11 (0.89, 1.38) | 1.33 (0.94, 1.68) | 1.30 (0.79, 1.75) | 1.39 (0.92, 1.86) | 1.20 (0.77, 1.91) | |
| 0.97 (0.79, 1.42) | 0.92 (0.80, 1.35) | 1.21 (0.67, 2.27) | 1.17 (0.67, 1.60) | 1.23 (0.84, 1.76) | |
| 1.04 (0.90, 1.31) | 1.22 (0.95, 1.48) | 1.05 (0.70, 1.71) | 0.88 (0.65, 1.37) | 1.09 (0.73, 1.70) | |
| First month | |||||
| Reference | Reference | Reference | Reference | Reference | |
| 1.16 (0.82, 1.50) | 1.31 (0.87, 1.72) | 1.26 (0.95, 1.74) | 1.33 (0.70, 2.06) | 1.19 (0.78, 1.89) | |
| 1.10 (0.79, 1.57) | 1.08 (0.85, 1.31) | 1.12 (0.71, 1.78) | 1.21 (0.59, 2.42) | 1.16 (0.72, 1.78) | |
| 0.93 (0.67, 1.41) | 1.00 (0.61, 1.54) | 0.90 (0.67, 1.31) | 0.82 (0.62, 1.27) | 1.07 (0.68, 1.55) | |
| Second month | |||||
| Reference | Reference | Reference | Reference | Reference | |
| 1.17 (0.95, 1.47) | 2.07 (1.19, 3.22) | 1.59 (1.03, 3.00) | 1.32 (0.96, 1.78) | 1.33 (0.98, 2.17) | |
| 1.21 (0.84, 1.60) | 1.44 (0.91, 2.05) | 1.16 (0.77, 2.11) | 1.20 (0.79, 1.71) | 1.31 (0.89, 2.20) | |
| 1.03 (0.86, 1.41) | 1.07 (0.87, 1.44) | 1.10 (0.72, 1.89) | 0.94 (0.71, 1.52) | 1.07 (0.78, 1.81) |
Notes: aOR, adjusted odds ratio; CI, confidence interval.
refers to the number of cases. The first quartile was used as a reference, and the higher quartiles were compared with the first quartile. Models were adjusted for maternal age, parity, educational attainment, prenatal care, prenatal folic acid and vitamin use, season of conception, and marital status.
Term infants with patent ductus arteriosus persisting for after birth.
Discussion
In the present study, we estimated associations between maternal exposure to ambient during the first 2 mo of pregnancy from 2007 to 2013 and the risks of fetal cardiovascular malformations. Our results indicate that exposure to ambient during early gestation may be associated with increased risk of fetal cardiovascular malformations during the observed time period in Fuzhou, China. The associations between maternal gestational exposure to and atrial septal defect, fetal patent ductus arteriosus, and overall congenital heart malformations provide further evidence that prenatal exposure to air pollution is associated with risks for fetal heart malformations.
The aORs in the second quartiles were higher than those in the third and fourth quartiles for the trends in most of the results, as shown in Table 2. This finding may suggest a nonmonotonic exposure response relating to other competing birth outcomes, such as spontaneous abortions and stillbirths, which were not considered in the present study (Gianicolo et al. 2014; Padula et al. 2013; Ritz et al. 2002; Vrijheid et al. 2011). Because gestation weeks 3–8 comprise the critical window for the formation of fetal cardiac chambers and inflow and outflow tracks, we intended to collect all the cases that were exposed to during this time period. However, early exposure during gestation may increase the risk of fetal abortion before gestation week 20 (Strickland et al. 2009). We therefore might have missed important data from these cases in our analyses. This missing data may be one of the causes of the higher aORs in the second quartiles compared with those in the third and fourth quartiles in our results. Paradoxical reversal of the risks for fetal cardiovascular malformations with excessively high levels of (Zhang et al. 2016) might also be interpreted as a result of nonmonotonic exposure response, which seems to be coincident with the nonlinearity of our results.
A number of studies have investigated associations between maternal exposure to ambient , combined with other air pollutants, and fetal congenital heart defects. Some original studies have reported increased risks of atrial septal defects (Gilboa et al. 2005; Hwang et al. 2015), patent ductus arteriosus (Strickland et al. 2009), and tetralogy of Fallot (Dolk et al. 2010) associated with maternal exposure. Our results were consistent with the results of these reports. In addition, our results also suggested a possible inverse association between exposure and ventricular septal defect, which also coincides with the findings of a previous report (Padula et al. 2013). The correlations that we observed between exposure and the risk of fetal heart malformations seemed to be comparable to or stronger than, and the exposure levels of were higher than, those reported by Gilboa et al. (: 1.43, 3.60 for isolated atrial septal defects; first quartile and fourth quartile ), Strickland et al. (: 1.11, 2.31 for patent ductus arteriosus; daily levels between ), Padula et al. (: 1.1, 3.9 for perimembranous ventricular septal defects; daily exposure levels ), and Hwang et al. (: 1.44, 4.42 for atrial septal defects; averaged level ). One report indicated an increased risk for tetralogy of Fallot in relation to exposure (:0.57,3.84) (Dolk et al. 2010). Our results showed a similar aOR value of 1.44 for exposure versus tetralogy of Fallot. A meta-analysis supported the correlation between maternal exposure and the increased risk of atrial septal defects (: 1.01, 1.28 for each increase) (Vrijheid et al. 2011). However, this finding was not confirmed by another meta-analysis that was conducted more recently (Chen et al. 2014).
Several reports of exposure and congenital heart defects did not observe a positive association (Dadvand et al. 2011a; Hansen et al. 2009; Ritz et al. 2002; Schembari et al. 2014; Stingone et al. 2014; Vinceti et al. 2016). Most of these reports had low exposure levels [i.e., levels averaging (Hansen et al. 2009); for , and for (Stingone et al. 2014); and with a median of (Schembari et al. 2014) and a minimum of and a maximum of (Dadvand et al. 2011a)] compared with those in the current study, except for one recent report by Zhang et al. (2016) in which the mean level was . Our results suggested that adverse associations between exposure and fetal cardiovascular malformations could also be observed during gestation days 21–30 (atrial septal defect), the earlier stage in which formation of the fetal heart occurs. Agay-Shay et al. (2013) reported an association between maternal exposure and multiple congenital heart defects, but not isolated atrial and atrial septal defects, ventricular septal defect, or patent ductus arteriosus, at an average exposure level of . One study did not report the exposure level (Ritz et al. 2002). Although there were various factors affecting the results, we considered a level of exposure that was 2.4–4.1 times higher than the exposure levels reported by most other studies during early pregnancy, which would therefore exert greater toxicities associated with fetal heart malformations. However, exorbitantly high-level exposure to might have reverse correlations with fetal cardiovascular malformations (Zhang et al. 2016), which is likely to be attributable to a nonlinear correlation between exposure and its cardiovascular effects.
Ventricular septal defect is the most common subtype of cardiovascular malformations, with a prevalence similar to that reported by Strickland et al. (2009). The proportions for ventricular septal defect and atrial septal defect were comparable to, whereas the proportions for patent ductus arteriosus and tetralogy of Fallot were higher than, those in other reports (Agay-Shay et al. 2013; Gilboa et al. 2005; Schembari et al. 2014; Strickland et al. 2009). The differences between our results and those of similar studies may arise from differences in sample collection and in the disease definition used. We studied term infants with patent ductus arteriosus persisting for after birth compared with in the study by Strickland et al. (2009). Furthermore, our hospital-based sample was derived from a relatively small region with limited representatives of the population of congenital heart defects, which might be another cause of the discrepancy in proportions.
We included approximately two-thirds of the regional births in our study. The remaining one-third of births and their mothers who entered other hospitals were not significantly different from those in our hospitals with regard to the mothers’ socioeconomic statuses, and the different residences of the mothers enrolled did not correlate with their socioeconomic statuses. A few pregnant women might transfer to a grade-A hospital from a lower-grade hospital when a complicated pregnancy condition is found. Considering the small percentage of gravidae in lower-grade hospitals and the small percentage of gravidae who incurred abnormal pregnancy including fetal cardiovascular malformations, the discrepancies among the gravidae choosing different grade hospitals, if present, would not significantly alter the outcomes in our analyses.
There are several limitations in the present study. Although we used a geographic technique coupled with an inverse-distance weighting method to evaluate the exposure levels, this model and analyses based on exposure estimates from ambient monitoring networks have been reported to produce somewhat wider 95% CIs than effect estimates based on land-use regression models even with similar estimates of the effect among them (Brauer et al. 2008). The present case–control study was based on cases with pregnancy durations . Some gravidae were excluded from the analyses, which might have led to bias in our results. No maternal residential history during the pregnancy was available. We took only the residential information from the women’s medical records at the early stages of pregnancy regardless of possible residential changes during the pregnancy. Thus, deviation of the exposure assessment with bias in an unknown direction might have occurred, although reports have shown no obvious impact on these assessments resulting from a change in living address during pregnancy (Lupo et al. 2010). Additional unmeasured covariates, such as the socioeconomic status, living habits, and dietary factors of the gravidae, could have affected our results, although we did not evaluate the co-effects from these factors in our analyses because of the limited amount of relevant data. The temporal factors in our study design along with the data obtained from a limited number of monitoring stations might also have compromised our exposure evaluation. We did not investigate other subtypes of cardiovascular malformations beyond the four major subtypes owing to the inadequate sample sizes; thus, we may have missed their possible associations with exposure. was found to be associated with pulmonary valve stenosis (Padula et al. 2013). Agay-Shay et al. (2013) reported an association of maternal exposure to increased concentrations of with multiple congenital heart defects. Associations were found between in utero exposure to , but not to coarse particulate matter with an aerodynamic diameter of 10–2.5 μm, and hypoplastic left heart syndrome (Stingone et al. 2014) and transposition of the great arteries (Padula et al. 2013). We did not observe these associations owing to the relatively small sample sizes.
A gravida generally lives inside the house during gestation, and indoor levels might be very different from outdoor levels, particularly in developing countries (Ezzati and Kammen 2002); this potentially overestimates, or more likely, underestimates the exposure that could bias our effect estimates. We assigned exposures using only the geographic technique regardless of the impact from local meteorological factors, traffic factors, social geography, and the spatiotemporal activity patterns of individual gravida. More importantly, we did not investigate the co-effects from other air pollutants such as carbon monoxide, nitrogen dioxide, and ozone, which are associated with fetal heart development (Gilboa et al. 2005). Therefore, our data did not represent all of the major air pollutants emitted and transported, which is another limitation of our study. levels were routinely monitored beginning in 2012 in Fuzhou, China. Because we lacked the entire serial monitoring data for during the study period, we could not evaluate the health impact of exposure in the present work.
Conclusions
In conclusion, exposure to ambient levels of during early pregnancy was shown to be associated with fetal cardiovascular malformations. The most significant association was found in the second quartile of the exposure levels, between 41.9 and , and was attenuated in the third and fourth quartiles at higher exposure levels. Our findings have potential impact on public health and policy making for emerging countries including China, whose standard criteria were revised in 2012 as daily levels at 50, and annual levels at 40, (Grade I, II), respectively (Ministry of Environmental Protection, People’s Republic of China 2012), and stricter criteria may be needed in the near future.
Supplemental Material
Acknowledgments
We thank J.-L. Zeng from Fujian Maternity and Child Health Hospital for his assistance with clinical data collection, H. Zheng from Fujian Kingshow Media Group Co. Ltd. for his assistance with plotting the schematic layout, B.-B. Lin and B.-Q. Liao for their assistance with patient interviews and data processing, and E. Strawser for her English editing. We thank L.E. Wold for his careful review and editing. This work was supported by research grants from the Chinese National Natural Science Fund (81172677), Fujian Science and Technology Bureau Unode Project (2007Y0017), Fujian Provincial Natural Science Fund (2015J01493), and Medical Science Technology Project of Nanjing Military Command (07M093), China. X.-Q. Chen was supported by the Fund of Public Welfare from the Ministry of Environmental Protection of China (201009004).
References
- Agay-Shay K, Friger M, Linn S, Peled A, Amitai Y, Peretz C. 2013. Air pollution and congenital heart defects. Environ Res 124:28–34, PMID: 23623715, 10.1016/j.envres.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Bassili A, Mokhtar SA, Dabous NI, Zaher SR, Mokhtar MM, Zaki A. 2000. Risk factors for congenital heart diseases in Alexandria, Egypt. Eur J Epidemiol 16:805–814, PMID: 11297222, 10.1023/A:1007601919164 [DOI] [PubMed] [Google Scholar]
- Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. 2008. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect 116:680–686, PMID: 18470315, 10.1289/ehp.10952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EK, Zmirou-Navier D, Padilla C, Deguen S. 2014. Effects of air pollution on the risk of congenital anomalies: A systematic review and meta-analysis. Int J Environ Res Public Health 11:7642–7668, PMID: 25089772, 10.3390/ijerph110807642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- China National Center for Cardiovascular Disease. 2006. Chinese Cardiovascular Disease Report 2005. Beijing: Encyclopedia of China Publishing House; 103–105. [Google Scholar]
- Christianson A, Howson CP, Modell B. 2006. March of Dimes Global Report on Birth Defects: The Hidden Toll of Dying and Disabled Children. Available: http://www.marchofdimes.com/downloads/Birth_Defects_Report-PF.pdf [accessed 15 February 2012].
- Dadvand P, Rankin J, Rushton S, Pless-Mulloli T. 2011a. Ambient air pollution and congenital heart disease: A register-based study. Environ Res 111:435–441, 10.1016/j.envres.2011.01.022 [DOI] [PubMed] [Google Scholar]
- Dadvand P, Rankin J, Rushton S, Pless-Mulloli T. 2011b. Association between maternal exposure to ambient air pollution and congenital heart disease: A register-based spatiotemporal analysis. Am J Epidemiol 173:171–182, 10.1093/aje/kwq342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolk H, Armstrong B, Lachowycz K, Vrijheid M, Rankin J, Abramsky L, et al. 2010. Ambient air pollution and risk of congenital anomalies in England, 1991–1999. Occup Environ Med 67:223–227, PMID: 19819865, 10.1136/oem.2009.045997 [DOI] [PubMed] [Google Scholar]
- Ezzati M, Kammen DM. 2002. The health impacts of exposure to indoor air pollution from solid fuels in developing countries: knowledge, gaps, and data needs. Environ health perspect 110:1057–1068, PMID: 12417475, 10.1289/ehp.021101057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzhou Statistics Bureau. 2011. The Sixth National Population Census for Fuzhou. Available: http://blog.sina.com.cn/s/blog_4b0a5e8a0100sqyr.html [accessed 7 July 2011].
- Fuzhou Water Conservancy Bureau. 2015. Fuzhou water resources bulletin. 2015. Available: http://www.doc88.com/p-8919202193452.html [accessed 10 March 2015]. [Google Scholar]
- Gianicolo EA, Mangia C, Cervino M, Bruni A, Andreassi MG, Latini G. 2014. Congenital anomalies among live births in a high environmental risk area–a case-control study in Brindisi (southern Italy). Environ Res 128:9–14, 10.1016/j.envres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Gilboa SM, Mendola P, Olshan AF, Langlois PH, Savitz DA, Loomis D, et al. 2005. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am J Epidemiol 162:238–252, 10.1093/aje/kwi189 [DOI] [PubMed] [Google Scholar]
- Gorini F, Chiappa E, Gargani L, Picano E. 2014. Potential effects of environmental chemical contamination in congenital heart disease. Pediatr Cardiol 35:559–568, PMID: 24452958, 10.1007/s00246-014-0870-1 [DOI] [PubMed] [Google Scholar]
- Guo Y, Li S, Tian Z, Pan X, Zhang J, Williams G. 2013. The burden of air pollution on years of life lost in Beijing, China, 2004–2008: Retrospective regression analysis of daily deaths. BMJ 347:f7139, PMID: 24322399, 10.1136/bmj.f7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CA, Barnett AG, Jalaludin BB, Morgan GG. 2009. Ambient air pollution and birth defects in Brisbane, Australia. PloS One 4:e5408, PMID: 19404385, 10.1371/journal.pone.0005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BF, Lee YL, Jaakkola JJ. 2015. Air pollution and the risk of cardiac defects: A population-based case-control study. Medicine (Baltimore) 94:e1883, PMID: 26554783, 10.1097/MD.0000000000001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, et al. 2007. Noninherited risk factors and congenital cardiovascular defects: current knowledge: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: Endorsed by the American Academy of Pediatrics. Circulation 115:2995–3014, 10.1161/CIRCULATIONAHA.106.183216 [DOI] [PubMed] [Google Scholar]
- Lupo PJ, Symanski E, Chan W, Mitchell LE, Waller DK, Canfield MA, et al. 2010. Differences in exposure assignment between conception and delivery: The impact of maternal mobility. Paediatr Perinat Epidemiol 4:200–208, 10.1111/j.1365-3016.2010.01096.x [DOI] [PubMed] [Google Scholar]
- Ministry of Environmental Protection, People’s Republic of China. 2012. GB3095-2012 Ambient Air Quality Standard. Available: http://wenku.baidu.com/link?url=Rbc0AnsLogD8nxHDnlrzWOeLgjXmc3bqUOuqM3Cd0fIgOJshbD2Ox4AvQ7FlKrFhJVx0ZojtX1nzbczSZCKPyhygw4CCBZrXd9pG6pFn4W7 [accessed 29 February 2012].
- Ministry of Environmental Protection, People's Republic of China. 2014. China Environment Status Bulletin for 2013. Available: http://www.zhb.gov.cn/hjzl/zghjzkgb/lssj/2013nzghjzkgb/ [accessed 5 June 2014]. [Google Scholar]
- Ministry of Health, People’s Republic of China. 2011. Report on women and children’s health development in China. Available: http://www.docin.com/p-454460607.html [accessed August 2011].
- Myers JA, Rassen JA, Gagne JJ, Huybrechts KF, Schneeweiss S, Rothman KJ, et al. 2011. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol 174:1213–1222, PMID: 22025356, 10.1093/aje/kwr364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, Tager IB, Carmichael SL, Hammond SK, Yang W, Lurmann F, et al. 2013. Ambient air pollution and traffic exposures and congenital heart defects in the San Joaquin Valley of California. Paediatr Perinat Epidemiol 27:329–339, 10.1111/ppe.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Chadwick T, Natarajan M, Howel D, Pearce MS, Pless-Mulloli T. 2009. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environ Res 109:181–187, PMID: 19135190, 10.1016/j.envres.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Riehle-Colarusso T, Strickland MJ, Reller MD, Mahle WT, Botto LD, Siffel C, et al. 2007. Improving the quality of surveillance data on congenital heart defects in the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res Part A Clin Mol Teratol 79:743–753, PMID: 17990334, 10.1002/bdra.20412 [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. 2002. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol 155:17–25, PMID: 11772780, 10.1093/aje/155.1.17 [DOI] [PubMed] [Google Scholar]
- Schembari A, Nieuwenhuijsen MJ, Salvador J, de Nazelle A, Cirach M, Dadvand P, et al. 2014. Traffic-related air pollution and congenital anomalies in Barcelona. Environ Health Perspect 122:317–323, PMID: 24380957, 10.1289/ehp.1306802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingone JA, Luben TJ, Daniels JL, Fuentes M, Richardson DB, Aylsworth AS, et al. 2014. Maternal exposure to criteria air pollutants and congenitalpdel heart defects in offspring: Results from the National Birth Defects Prevention Study. Environ Health Perspect 122:863–872, PMID: 24727555, 10.1289/ehp.1307289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland MJ, Klein M, Correa A, Reller MD, Mahle WT, Riehle-Colarusso TJ, et al. 2009. Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. Am J Epidemiol 169:1004–1014, 10.1093/aje/kwp011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Malagoli C, Malavolti M, Cherubini A, Maffeis G, Rodolfi R, et al. 2016. Does maternal exposure to benzene and PM10 during pregnancy increase the risk of congenital anomalies? A population-based case-control study. Sci Total Environ 541:444–450, PMID: 26410719, 10.1016/j.scitotenv.2015.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J, et al. 2011. Ambient air pollution and risk of congenital anomalies: A systematic review and meta-analysis. Environ Health Perspect 119:598–606, PMID: 21131253, 10.1289/ehp.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DW, Yuan L, Perlin SA. 2004. Comparison of spatial interpolation methods for the estimation of air quality data. J Expo Anal Environ Epidemiol 14:404–415, PMID: 15361900, 10.1038/sj.jea.7500338 [DOI] [PubMed] [Google Scholar]
- Yang F, Tan J, Zhao Q, Du Z, He K, Ma Y, et al. 2011. Characteristics of PM2.5 speciation in representative megacities and across China. Atmos Chem Phys 11:5207–5219, 10.5194/acp-11-5207-2011 [DOI] [Google Scholar]
- Zhang B, Liang S, Zhao J, Qian Z, Bassig BA, Yang R, et al. 2016. Maternal exposure to air pollutant PM2.5 and PM10 during pregnancy and risk of congenital heart defects. J Expo Sci Environ Epidemiol 10.1038/jes.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Chen C, Wang H, Huang C, Su L, Chen Y, et al. 2012. Chemical characteristics of particulate matters during air pollution episodes in autumn of Shanghai, China. Acta Scientiae Circumstantiae 32:81–92. (Chinese with the English abstract) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.