Abstract
We examined the modulation of persistent inward currents (PICs) by serotonin (5-HT) in spinal motoneurons of normal and chronic spinal rats. PICs are composed of both a TTX-sensitive persistent sodium current (Na PIC) and a nimodipine-sensitive persistent calcium current (Ca PIC), and we focused on quantifying the Na PIC (and its action on the total PIC), which is known to be critical in enabling repetitive firing. Intracellular recordings were made from motoneurons of the whole sacrocaudal spinal cord of normal adult rats after the cord was acutely transected at the S2 spinal level (acute spinal rat condition), removed from the animal, and then maintained in vitro. In vitro motoneuron recordings were likewise made from rats that had a sacral spinal transection 2 mo previously (chronic spinal rats). In motoneurons from acute spinal rats, moderately high doses of 5-HT (≥ 10 µM), or the 5-HT2 receptor agonist DOI (≥30 µM), significantly increased the total PIC, hyperpolarized the PIC onset voltage, and hyperpolarized the spike threshold, whereas lower doses had no effect. Both 5-HT and DOI specifically increased the Na PIC portion of the total PIC (tested with nimodipine blocking the Ca PIC). Additionally, 5-HT, but not DOI, depolarized the resting membrane potential (Vm) and increased the input resistance (Rm) in a dose-dependent manner. Therefore 5-HT2 receptor activation facilitated the Na PIC, whereas other 5-HT receptors modulated Vm and Rm. Motoneurons of chronic spinal rats responded to 5-HT and DOI in the same way, but with larger responses and at much lower doses (0.3–1 µM), thus exhibiting a 30-fold supersensitivity to 5-HT. Specifically the Na PIC was super-sensitive to 5-HT2 receptor activation with DOI. Also, Rm and Vm were supersensitive to 5-HT. Consistent with the known critical role of the Na PIC in repetitive firing, enhancement of the Na PIC by DOI or 5-HT facilitated the repetitive firing evoked by steady current injection and enabled repetitive firing in a subpopulation of motoneurons of acute spinal rats that were initially unable to produce sustained repetitive firing. We suggest that after spinal transection, residual endogenous spinal sources of 5-HT help facilitate the Na PIC and repetitive firing. With chronic injury, the developed 5-HT supersensitivity more than compensates for lost brain stem 5-HT, so that the Na PIC is large and motoneurons are very excitable, thus contributing to spasticity.
INTRODUCTION
Spinal motoneurons exhibit large persistent inward currents (PICs) that greatly augment synaptic input, enable sustained depolarizations (plateaus), and produce firing that outlasts a stimulation (self-sustained firing: Bennett et al. 1998; Hounsgaard et al. 1988) in unanesthetized animals (Lee and Heckman 1998; Li and Bennett 2003; Schwindt and Crill 1984), and even in awake humans (Gorassini et al. 2002). PICs are composed of two currents: a tetrodotoxin (TTX)-sensitive persistent sodium current (Na PIC) and a nimodipine-sensitive persistent calcium current (Ca PIC) (Carlin et al. 2000; Hsiao et al. 1998; Li and Bennett 2003). PICs are considered to be a latent property of normal motoneurons (see Heckman et al. 2004) that are regulated by the synaptic input of endogenous neuromodulators such as norepinephrine (NE), serotonin (5-HT), acetylcholine, and glutamate (Alaburda and Hounsgaard 2003; Delgado-Lezama et al. 1997; Hounsgaard and Kiehn 1989; Hultborn and Kiehn 1992; Lee and Heckman 1999). The brain stem–derived monoamines (5-HT and NE) play a major role in facilitating PICs because acute spinal cord transection largely eliminates plateaus and self-sustained firing associated with PICs (Harvey et al. 2006b; Hounsgaard et al. 1988) and subsequent application of monoamine agonists recovers plateau properties (Conway et al. 1988; Hounsgaard et al. 1988).
We know that 5-HT can induce Na and Ca PICs in spinal motoneurons of acute spinal animals or acutely sliced spinal cords (rat: Li et al. 2004c; turtle: Perrier and Hounsgaard 2003), as well as in brain stem motoneurons in brain stem slices (Hsiao et al. 1998). Some of the details of how Ca PICs are facilitated by neuromodulators have been described in turtle motoneurons; they involve 5-HT2 receptors and intracellular calcium and calmodulin (Perrier and Hounsgaard 2003; Perrier et al. 2000). The regulation of the Na PIC in motoneurons by 5-HT is less well understood. The first goal of the present paper was to quantify how 5-HT—and in particular 5-HT2 receptor agonists—modulate the Na PIC (and total PIC) in acute spinal rats, and how this affects firing. We studied motoneurons of the whole sacrocaudal spinal cord of adult rats that had been acutely transected at the upper sacral level, removed from the animal, and maintained in vitro (Bennett et al. 2001b; Li and Bennett 2003). We found that the natural ligand 5-HT had complex effects on the motoneurons, including increasing the Na PIC, increasing the input resistance, and depolarizing the resting membrane potential. In contrast, the 5-HT2 receptor agonist (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) selectively increased the Na PIC, without changing other membrane properties.
Curiously, motoneurons below a complete spinal transection spontaneously redevelop plateaus and large PICs in the months after injury (Li and Bennett 2003); this leads to the spastic syndrome seen in vivo, characterized by intense prolonged and uncontrolled muscle spasms (Bennett et al. 2001a; Li et al. 2004a). These large PICs are present despite the complete degeneration of brain stem axons in the chronic spinal state (Haggendal and Dahlstrom 1973), resulting in a loss of the major source of monoamines. With this dramatic reduction in 5-HT availability, motoneuron responses below the transection become much more sensitive to 5-HT in terms of increasing reflex excitability and spontaneous motoneuron firing (Barbeau and Bedard 1981; Li et al. 2004b). It may be that the motoneurons and the PICs themselves become supersensitive to 5-HT; thus the second goal of this paper was to examine that possibility. We found that the Na PIC (and total PIC) was a remarkable 30-fold more sensitive to 5-HT compared with acute spinal rats, such that the Na PIC was increased with very small doses of 5-HT (by the 5-HT2 receptors). Because PICs do indeed become supersensitive to 5-HT, residual 5-HT caudal to the injury (Hadjiconstantinuo et al. 1984; Newton and Hamill 1988) should in principle play a major role in producing the large PICs seen in the chronic spinal state; this idea is verified in a companion paper (Harvey et al. 2006a).
METHODS
Intracellular recordings were made from motoneurons in the in vitro sacrocaudal spinal cord of adult female Sprague–Dawley rats (200–600 g). Both normal adult rats (about 3 mo old) and rats with spasticity arising from chronic spinal cord injury (about 3 mo old) were included in the present study. For normal rats, the cord was transected at the S2 level at the time of removal of the sacrocaudal cord (acute spinal rats). For spastic chronic spinal rats, a complete transection at the S2 level was made at 40 to 55 days of age. Usually, within 1 mo, dramatic spasticity developed in the tail muscles, which are innervated by sacrocaudal motoneurons below the level of the injury (for details of the animal model and spasticity assessment, see Bennett et al. 1999, 2001a). Only rats >1.5 mo postinjury with clear spasticity were used for in vitro recording of the motoneurons. All experimental procedures were conducted in accordance with guidelines for the ethical treatment of animals issued by the Canadian Council on Animal Care and approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee.
In vitro preparation
Details of the in vitro procedures were described in a companion paper (Harvey et al. 2006b). Briefly, under urethane anesthesia (0.18 g/100 g), the sacrocaudal spinal cords of both normal and chronic spinal rats were removed to a dissection chamber containing modified artificial cerebrospinal fluid (mACSF), for preparation, and then transferred to a recording chamber containing normal artificial cerebrospinal fluid (nACSF). Residual anesthetic, and kynurenic acid from the mACSF, were allowed to wash out for 1 h in the recording chamber before the bathing solution was switched to nACSF containing a cocktail of fast synaptic transmission blockers (AP5, CNQX, strychnine, and picrotoxin; see following text) and recording then began. Often 15 µM nimodipine was also added at the beginning of the experiment to isolate the Na PIC by blocking the L-type calcium channels mediating the Ca PIC (Harvey et al. 2006b; Li and Bennett 2003).
Drugs and solutions
Two kinds of ACSF were used in these experiments: mACSF, designed to minimize potential excitotoxicity, was used in the dissection chamber before recording; nACSF was used in the recording chamber. Composition of the mACSF was (in mM) 118 NaCl, 24 NaHCO3, 1.5 CaCl2, 3 KCl, 5 MgCl2, 1.4 NaH2PO4, 1.3 MgSO4, 25 d-glucose, and 1 kynurenic acid. The nACSF was composed of (in mM) 122 NaCl, 24 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2, and 12 d-glucose. Both types of ACSF were saturated with 95% O2-5% CO2 and maintained at pH 7.4. The synaptic transmission blocking cocktail added to the nACSF contained 50 µM d-(−)-2-amino-5-phosphopentanoic acid (AP5, Tocris Cookson, Ellisville, MO), 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Tocris), 1 µM strychnine (Sigma, St. Louis, MO), and 100 µM picrotoxin (Tocris Cookson) to block N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate, glycine, and γ-aminobutyric acid type A (GABAA) receptors, respectively. Additional drugs were added as required, including 0.3–50 µM 5-hydroxytryptamine (5-HT; Sigma), 1–50 µM of the 5-HT2 receptor agonist (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI; Sigma), 10 µM of the 5-HT2A receptor antagonist ketanserin (Tocris Cookson), 2 µM of the 5-HT2C receptor antagonist RS 102221 (Tocris Cookson), 2 µM tetrodotoxin (TTX; Alamone Labs, Jerusalem, Israel) to block transient and persistent voltage-dependent Na channels, and 15 µM nimodipine (Tocris Cookson) to block voltage-dependent L-type Ca channels mediating the persistent calcium current.
Persistent inward current in voltage- and current-clamp recordings
Intracellular recording electrodes and methods were as described in a companion paper (Harvey et al. 2006b). Briefly, motoneurons were identified by antidromic stimulation of the ventral roots. Slow triangular current ramps (0.4 nA/s) in discontinuous current clamp (DCC; with 5- to 8-kHz switching rate, 3-kHz output bandwidth, and 10-kHz data acquisition rate) were used to measure firing, frequency–current (F–I) responses, and self-sustained firing (ΔI; difference between current to initiate firing and current to terminate firing) (Harvey et al. 2006b; Li and Bennett 2003). Slow voltage ramps (3.5 mV/s) in discontinuous single-electrode voltage clamp (SEVC; 5- to 8-kHz switching rate and 0.8- to 2.5-nA/mV gain) were used to measure the PICs, as previously described in detail (Harvey et al. 2006b; Li and Bennett 2003). In summary, to estimate the PIC, the leak current was first determined from a linear regression to the current response near rest and subthreshold to the PIC. Then this leak current was extrapolated to the potentials where the PIC was activated. Finally, the PIC was quantified as the initial maximum difference between the recorded current and the extrapolated leak current, at its initial peak (initial peak of PIC). In some cases before 5-HT (or DOI) application the voltage of the initial peak of the PIC was difficult to determine, and instead the PIC was quantified at a common potential before and after drug application (near −50 mV and < −40 mV, where the peak usually occurred). The onset voltage of the PIC (VSTART) was computed as described previously (Harvey et al. 2006b).
Basic cell properties, such as input resistance (Rm), resting potential (Vm), and spike threshold (Vth), were determined from the current-clamp ramps. Measurements and calculations were performed as described in detail in Harvey et al. (2006b), with the following additional measurements. The influence of agonists on the PIC was measured by taking the difference in maximum leak-subtracted current before and after drug application, and this was called the drug-induced PIC. When the PIC or firing was activated at rest (0-nA current, especially in drugs such as 5-HT), then the Rm was measured just subthreshold to this, and the drug-induced change in subthreshold potential was not measured at rest (0 nA), but with a small bias current (same before and after drug) to keep the cell subthreshold (still termed change in Vm). The spike afterhyperpolarization (AHP) was recorded after antidromic activation across a range of potentials. Ultimately the depth of the medium-latency AHP was quantified at −70 mV before and after 5-HT application to control for 5-HT–induced changes in resting membrane potential.
Data analysis
Data were analyzed in Clampfit 8.0 (Axon Instruments, Foster City, CA) and figures were made in Sigmaplot (Jandel Scientific, San Rafael, CA). Data are shown as means ± SD. The number of motoneurons tested is indicated as n; one cell was tested per rat per drug condition, so n also equals the number of rats tested in a given condition. Unless otherwise specified, a paired Student’s t-test was used to test for statistical differences before and after drug applications, with a significance level of P < 0.05. Unpaired t-tests were used to compare data in acute and chronic spinal rats. As required for the t-test, the data were verified to be normal, using a Kolmogorov–Smirnov test for normality, with a P < 0.05 level set for significance. In one case, the data set was not normal and thus, instead of a paired t-test, the nonparametric Wilcoxon rank-order test was used, which does not depend on the normality of the data (denoted Wilcoxon test in results; tested with the usual P < 0.05 significance level).
RESULTS
Intracellular recordings were made from motoneurons of the whole sacrocaudal spinal cord of adult rats after acute spinal transection and after chronic spinal transection. The basic membrane properties and persistent inward currents (PICs) in these motoneurons before the addition of 5-HT receptor agonists are detailed in a companion paper (Harvey et al. 2006b). Briefly, motoneurons from normal rats recorded in the acute spinal state were found to rest further from the spike threshold, have a lower input resistance, and require more current to recruit than in chronic spinal rats (Harvey et al. 2006b). Furthermore, after chronic spinal injury motoneurons had much larger PICs (arrow in control plot of Fig. 1B) than immediately after injury (acute spinal; Fig. 1A), with an amplitude large enough to produce a pronounced negative-slope region in the current trace (PIC about 1–3 nA; Fig. 1B, gray trace). In acute spinal rats, the mean PIC was only about half the size of those in chronic spinal rats, and not usually large enough to produce a negative-slope region (Fig. 1A). In both acute and chronic spinal rats, the total PIC was composed of about 60% Ca PIC and 40% Na PIC (Harvey et al. 2006b).
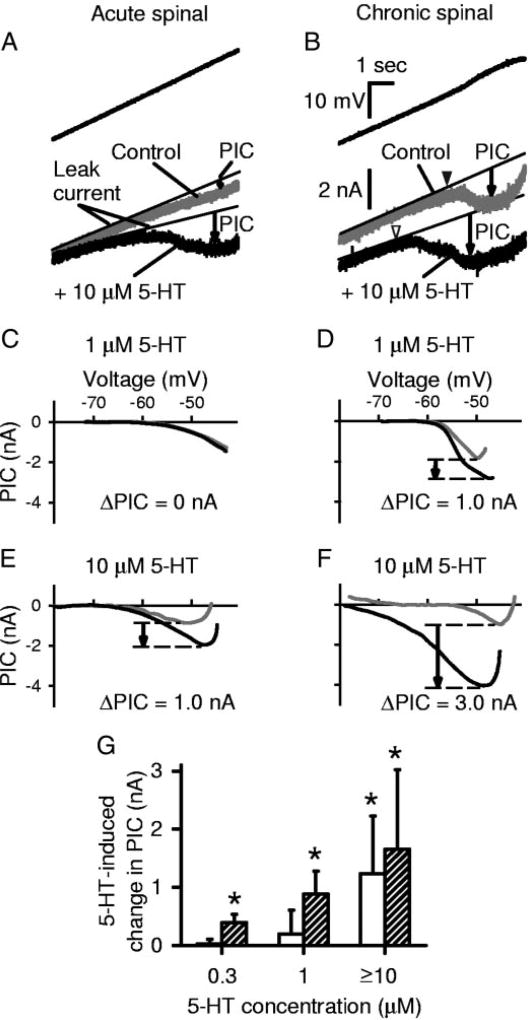
FIG. 1.
Persistent inward currents (PICs) in motoneurons are facilitated by serotonin (5-HT) and are supersensitive to 5-HT after chronic spinal transection. A: in motoneuron from acute spinal rat, a slow voltage ramp (3.5 mV/s) from −80 to −40 mV (top) was used to measure the PIC (bottom) before and after 5-HT application. PIC was quantified as the difference between the leak current (thin line) and the recorded current (downward arrow). Note that PIC was increased by 10 µM 5-HT (black trace) compared with control (gray trace). B: motoneuron from chronic spinal rat. Same format and scale as in A. 5-HT again increased the PIC amplitude and lowered onset voltage of the PIC (VSTART; black arrowhead: control; white arrowhead: 5-HT). Also, note in A and B the decrease in input conductance and depolarization of resting potential (at a given current) with 5-HT. C–F: filtered data from voltage ramps, with leak current subtracted, plotted against membrane potential. Typical motoneurons before (gray traces) and after (black traces) 5-HT application of varying doses. Drug-induced PIC (ΔPIC) was calculated as the difference in maximum leak-subtracted currents (downward arrow). Low-dose (1 µM) 5-HT did not change PIC (ΔPIC = 0 nA) in acute spinal rat (C), but did increase it in chronic spinal rat (D). High-dose 5-HT (≥ 10 µM) increased PIC in both acute (E) and chronic (F) spinal rats, but the effect was greater in chronic spinal rats. G: group data showing average change in PIC amplitude with various doses of 5-HT. Open bars: acute spinal; shaded bars: chronic spinal. Asterisk (*) indicates effect significantly greater than zero (P< 0.05). PIC amplitude significantly increased at low doses (≤1 µM) of 5-HT in motoneurons from chronic spinal rats, whereas high doses (≥ 10 µM) were required to facilitate PICs in acute spinal rats.
5-HT enhances total PIC in motoneurons of acute spinal rats
When 5-HT was applied to the spinal cord of acute spinal rats at moderate to high doses (10–50 µM, n = 9), the net PIC recorded in motoneurons was significantly increased by 1.23 ± 0.99 nA (mean ± SD; Fig. 1, A, E, and G, open bars; increase measured as the change in maximum leak-subtracted current induced by 5-HT; see methods). These recordings were made in the presence of a fast synaptic transmission blockade (see methods); thus the 5-HT–induced PIC likely resulted from the direct action of 5-HT on motoneurons, rather than on interneurons. The mean onset voltage of the PIC (VSTART) was also lowered by, on average, 4.3 ± 5.7 mV (at ≥10 µM), although this reduction was not significant (P = 0.1). The current threshold for activation of the PIC was significantly lowered by 2.3 ± 1.8 to 2.1 ±1.9 nA (n = 10). Lower doses of 5-HT (0.3 and 1.0 µM) had no significant effects on the PICs (n = 5 at each dose; Fig. 1, C and G).
5-HT also changes membrane properties, making the PIC more easily activated
5-HT had more complicated effects on motoneurons of acute spinal rats than merely facilitating the PIC, and these aid in the activation of PICs. Doses of 10–50 µM 5-HT produced a significant depolarization of the resting membrane potential by 3.2 ± 3.2 mV (Fig. 2A) and a significant increase in input resistance of 1.1 ± 1.2 MΩ (21.3% increase; Fig. 2B, n = 10). Ultimately, this helped to contribute to lower the current threshold for PIC activation, described above. Application of 5-HT (10–50 µM) in these cells also produced a significant reduction of spike voltage threshold (Vth) by 2.4 ±2.1 mV (Fig. 2C; similar to Fedirchuk and Dai 2004) and a significant increase of 2.5 ± 2.3 mV in the spike height, as measured by the spike overshoot [potential >0 mV, measured from first spikes evoked on current ramp as in Harvey et al. (2006b)]. Together, these effects made motoneurons considerably easier to depolarize and recruit after 5-HT application. Lower doses of 5-HT (0.3 and 1.0 µM) had no significant effects on these membrane (Vm, Rm) or spike properties (Vth or overshoot; n = 5 at each dose; Fig. 2) in acute spinal rats. Application of 5-HT at any dose did not significantly change the amplitude of the postspike AHP (measured at −70 mV).
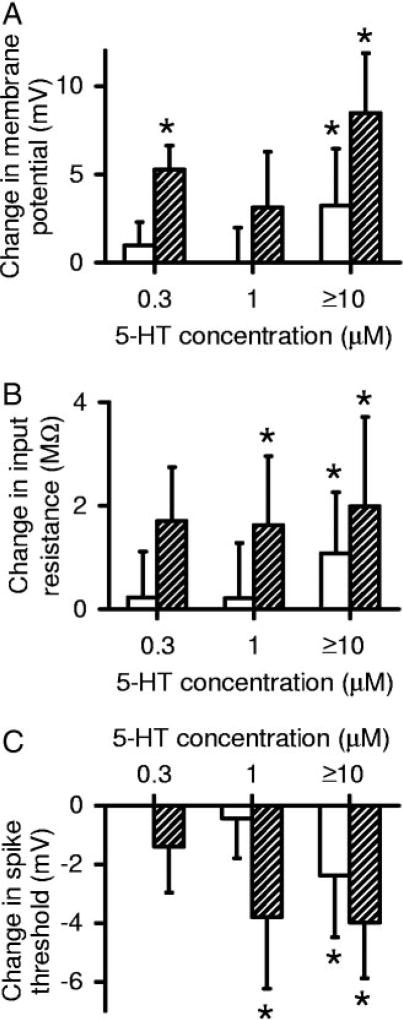
FIG. 2.
Motoneurons from chronic spinal rats are supersensitive to multiple effects of 5-HT. Group data are shown for average change in cell property with various doses of 5-HT. Open bars: acute spinal; shaded bars: chronic spinal. Asterisk (*) indicates effect significantly greater than zero (P< 0.05). Low doses (0.3–1 µM) of 5-HT significantly depolarized the resting potential (Vm; A), increased the input resistance (Rm; B), and lowered the spike voltage threshold (Vth;C) in chronic spinal rats, whereas high doses of 5-HT (>10 µM) were required to affect these parameters in acute spinal rats.
5-HT2 receptors facilitate the total PIC in acute spinal rats
Application of the 5-HT2 receptor antagonists ketanserin (10 µM; 5-HT2A) and RS 102221 (2 µM; 5-HT2C) reduced the PIC induced by 5-HT, and even reduced the spontaneously occurring PIC without 5-HT (see details in Harvey et al. 2006a), suggesting that 5-HT2 receptors are involved in regulating the PIC. To directly test this, we applied the 5-HT2 agonist DOI to motoneurons of acute spinal rats. At a 30-µM dose, DOI significantly increased the total PIC (measured in the absence of nimodipine) by 0.97 ± 0.85 nA (n = 7; see Fig. 6 described later), and significantly lowered the onset voltage of the PIC (VSTART; from −62.4 ± 3.1 to −69.0 ± 5.0 mV), suggesting that indeed 5-HT2 receptors modulate the total PIC (including Ca and/or Na PIC). Lower doses of DOI (1–10 µM; n = 5) had no effect on the PIC. To this point we have evaluated the action of 5-HT and DOI on the total PIC after acute spinal transaction because, ultimately, it is this total current that is important for motoneuron firing (Li et al. 2004a). In the next section we evaluate the specific action of 5-HT receptors on the Na PIC portion of the total PIC.
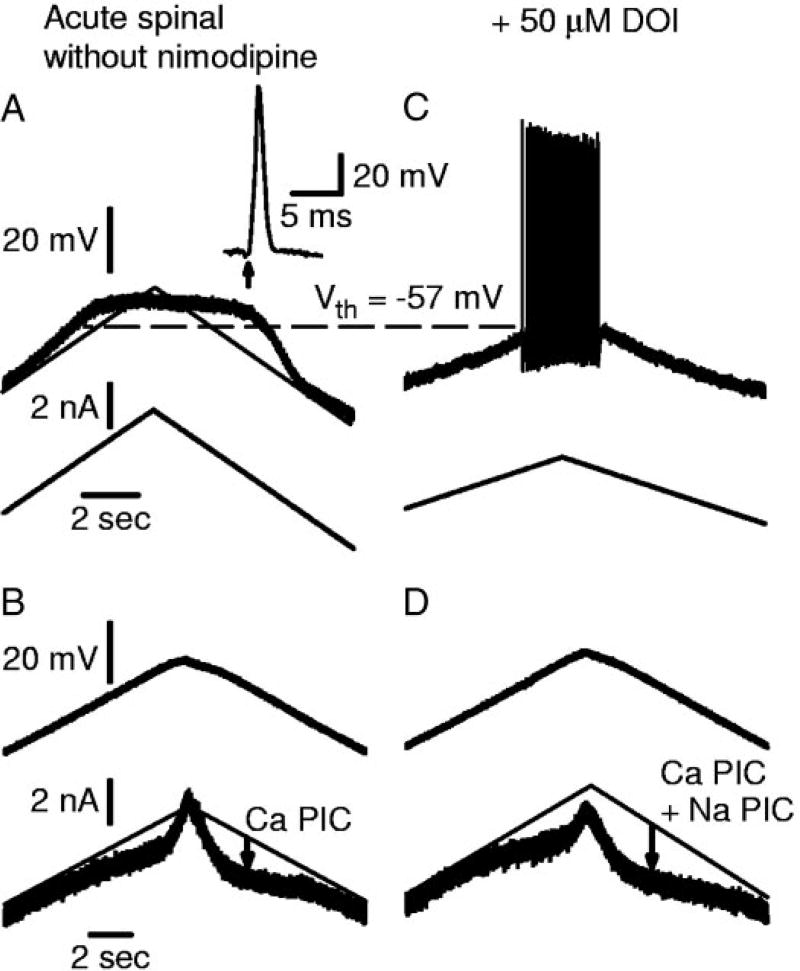
FIG. 6.
DOI restores firing ability in motoneuron with Ca PIC but no Na PIC. Motoneuron from acute spinal rat, recorded without nimodipine present, in same format as Fig. 5. A: motoneuron was unable to initiate action potentials or repetitive firing with slow current ramp. Note large Ca plateau. Normal size action potential was generated in response to antidromic stimulation (inset in A). B: without nimodipine, this motoneuron had a Ca PIC. C: DOI enabled repetitive firing with slow current ramps. D: amplitude of the total PIC was larger in DOI.
5-HT2 receptors facilitate the Na PIC in acute spinal rats
To specifically study the effect of 5-HT receptors on the Na PIC in isolation, we eliminated the Ca PIC by application of nimodipine (15 µM) (Harvey et al. 2006b). In motoneurons of acute spinal rats, the Na PIC was small, or occasionally even absent (Harvey et al. 2006b). When present, the Na PIC usually did not produce a negative-slope region, and instead produced only an inflection (flattening) in the I–V relation (gray line in Fig. 3A), as described in Harvey et al. (2006b). In all motoneurons tested in acute spinal rats DOI (30 µM) significantly increased the Na PIC by on average 0.75 ± 0.56 nA (n = 5; Fig. 3, A and E), and significantly lowered the Na PIC onset voltage VSTART from −62.3 ± 5.0 to −66.1 ± 4.9 mV. This DOI-induced PIC measured in nimodipine was blocked by subsequent application of TTX (Fig. 3C; n = 5), and thus was mediated by a TTX-sensitive Na PIC. A higher dose of DOI (50 µM; n = 5) had effects similar to those of a 30-µM dose of DOI (significant increase in Na PIC amplitude, with no significant difference between 30 and 50 µM doses), whereas lower doses of 1–10 µM DOI (n > 4 each dose) had no effect on the Na PIC (see Fig. 3E).
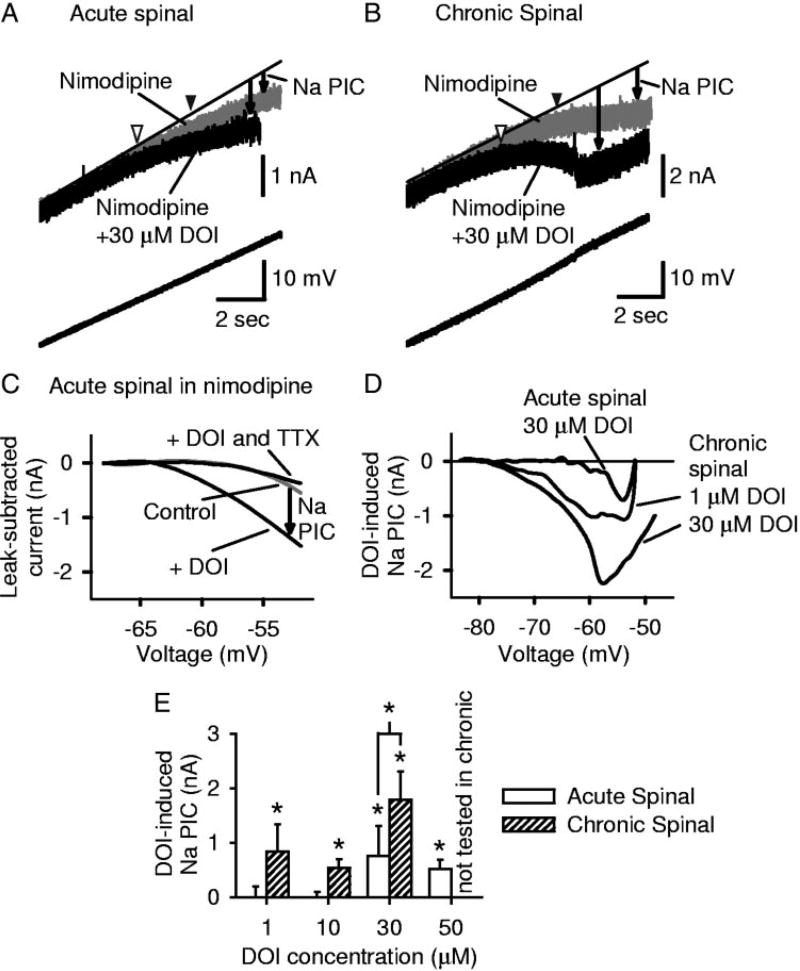
FIG. 3.
Persistent sodium current (Na PIC) in motoneurons is facilitated by the 5-HT2 receptor agonist (±)-1-(2,5-dime-thoxy-4-iodophenyl)-2-aminopropane (DOI), and motoneurons of chronic spinal rats are supersensitive to DOI. All recordings (A–D) done in 15 µM nimodipine to block persistent calcium current (Ca PIC; see methods). A: in acute spinal rat motoneuron, high-dose DOI (30 µM) facilitated the Na PIC and hyperpolarized Na PIC onset (VSTART; arrowheads: black: control; white: DOI). Same format as in Fig. 1A. B: in motoneuron after chronic injury, Na PIC amplitude was increased and PIC onset hyperpolarized by DOI. C: leak-subtracted current-voltage (I–V) curves of acute spinal rat motoneurons in nimodipine (control), after DOI application and after TTX application. Na PIC induced by high-dose DOI was subsequently blocked by tetrodotoxin (TTX). D: current induced by DOI (I–V curve in nimodipine subtracted from I–V curve in nimodipine + DOI). For chronic spinal rat motoneurons, higher doses of DOI induce larger increases in Na PIC amplitude (dose-dependent). Effect of high dose (≥30 µM) DOI in acute spinal rats was similar to low dose (≤10 µM) in chronic spinal rats. E: group data for DOI effect on Na PIC, measured in nimodipine. Significant effect of DOI for chronic spinal rat motoneurons at all doses tested. Only high doses (≥30 µM) were effective in acute spinal rat motoneurons. Asterisk (*) indicates significant effect of DOI.
5-HT2 receptor action is relatively specific to sodium currents, not affecting basic membrane properties
The 5-HT2 receptor agonist DOI had a relatively selective effect on the Na PIC because it did not influence many other motoneuron properties that were affected by 5-HT itself in acute spinal rats. Unlike 5-HT, DOI did not significantly affect the resting membrane potential (Vm), input resistance (Rm), and spike overshoot, whether tested with nimodipine (ΔVm = −2.1 ± 6.4 mV, ΔRm = 0.01 ± 1.03 MΩ, Δovershoot = −2.1 ± 4.3, n = 11; no effects significant; 30- and 50-µM doses combined), or without nimodipine (ΔVm = 4.4 ± 4.3 mV, ΔRm = 0.3 ± 0.7 MΩ, Δovershoot = 1.4 ± 5.8, n = 7; no effects significant).
However, DOI (30–50 µM) significantly hyperpolarized the voltage threshold for fast sodium spikes (Vth) by −2.6 ±3.7 mV (n = 17; responses with and without nimodipine were similar and combined) in motoneurons of acute spinal rats, consistent with the results of Fedirchuk et al. (2004). Further DOI significantly lowered the current threshold for spike activation during slow current ramps, from 3.9 ± 2.2 to 2.4 ± 0.9 nA (n = 17), making these cells easier to activate despite the lack of effect of DOI on the input resistance or resting potential. Also, DOI (30–50 µM) facilitated the fast sodium spike, in that the maximum rate of rise (dV/dtmax) of the first spike during our standard slow current ramp was significantly increased by 29.2 ± 9.9 V/s (dV/dtmax was 159.0 ± 31.7 V/s before DOI). This dV/dtmax (on the first spike) was previously argued to be linked to sodium channel inactivation during a slow ramp, before the first spike (Schlue et al. 1974), so the DOI-induced increase in dV/dtmax can be interpreted as a decreased tendency for sodium channel inactivation. In some cells, DOI was so effective in increasing the rate of rise of the spike that we encountered problems with proper voltage-clamping of the spikes after adding DOI, with more breakaway spikes during our slow voltage ramps, compared with before DOI (not shown).
The Na PIC is supersensitive to 5-HT2 receptor activation in chronic spinal rats
Motoneurons of chronic spinal rats responded to the 5-HT2 receptor agonist DOI in the same way as normal motoneurons in all respects, but with larger responses and much lower doses evoking responses, indicating that a classic denervation super-sensitivity had developed from the chronic loss of brain stem–derived 5-HT innervation. That is, high doses of DOI (30 µM) produced a significantly greater increase in the Na PIC amplitude in chronic spinal (1.78 ± 0.52 nA; n = 5) compared with acute spinal (0.75 ± 0.56 nA; n = 5) rat motoneurons (Fig. 3D; also compare Fig. 3B against 3A; Na PIC measured in nimodipine). Indeed, the DOI-induced Na PIC was significantly larger at all doses of DOI, when compared with equivalent doses in motoneurons of acute spinal rats (Fig. 3E; more than four cells at each dose). Furthermore, motoneurons of chronic spinal rats exhibited a nearly 30-fold supersensitivity to DOI, in that as little as 1 µM DOI induced a significant increase in the Na PIC in chronic spinal rats (n = 5), whereas the minimal effective dose in acute spinal rats was 30 µM (Fig. 3E). Further, this low 1 µM DOI dose had a similar effect in motoneurons of chronic spinal rats (0.84 ± 0.50 nA, n = 5) as the 30 µM dose did in acute spinal rats (n = 5; Fig. 3, D and E). Finally, as with acute spinal rat motoneurons, DOI significantly lowered VSTART of the Na PIC in chronic spinal rats (from, on average, −64.4 ± 5.4 to −69.9 ± 5.4 mV; Fig. 3B), but again it did so at a much lower dose (as low as 1 µM DOI combined, n = 15), compared with the high dose required in acute spinal rat motoneurons (≥30 µM DOI, n = 10).
Effects of DOI in motoneurons of chronic spinal rats are specific to Na channel facilitation
There were no significant effects of DOI (at any dose) on resting membrane potential Vm or input resistance Rm in motoneurons of chronic spinal rats (DOI-induced changes: ΔVm = −0.4 ± 3.5 mV, ΔRm = 0.3 ± 2.5 MΩ; not significant, n = 16 from 12 cells with nimodipine and four cells without), as with acute spinal rats. However, with DOI, the spike voltage threshold was significantly hyperpolarized by 1.9 ± 3.1 mV, and the spike height as measured by the overshoot was significantly increased by 2.3 ± 2.9 mV (n = 16 combined data with and without nimodipine; spikes evoked during ramp) as in acute spinal rats, although with effects at much lower doses than in acute spinal rats (1–10 µM; supersensitivity). Furthermore, the maximum rate of rise of the action potential (dV/ dtmax) measured on the first spike during firing (as above) was significantly increased by 22.3 ± 17.0 V/s with 1–30 µM DOI (n = 12 with nimodipine, dV/dtmax was 158.6 ± 37.2 V/s, n = 16 before DOI). Thus 5-HT2 receptor activation in chronic spinal rat motoneurons is specific to facilitating persistent and transient Na currents, as also found in motoneurons of acute spinal rats, although much lower doses are sufficient for facilitation.
PICs are also supersensitive to 5-HT itself in chronic spinal rats
The 30-fold supersensitivity to the 5-HT2 receptor agonist DOI was also seen when 5-HT itself was applied in chronic spinal rats (Fig. 1). That is, the PIC was significantly increased by low doses of 5-HT (0.3 and 1 µM, n > 4 each; Fig. 1, D and G), whereas ≥10 µM 5-HT was required to significantly increase the PIC in acute spinal rats. Further, the increase in PIC amplitude with 1 µM 5-HT (0.88 ± 0.40 nA, n = 7) was significantly greater in chronic spinal rats than the small insignificant effect in acute spinal rat motoneurons (0.20 ± 0.41 nA, n = 5; Fig. 1G). At doses ≥10 µM, 5-HT induced a PIC that was not significantly different between motoneurons of chronic (1.65 ± 1.38 nA, n = 6, Fig. 1, F and G) and acute (1.23 ± 0.99 nA, n = 9) spinal rats, suggesting a saturation in the response at these high doses. The onset voltage of the PIC, VSTART, was also significantly lowered by low doses of 5-HT that had no effect in acute spinal rats (VSTART lowered from −60.0 ± 5.0 to −67.1 ± 6.4 mV, n = 19, with doses as low as 0.3 µM 5-HT; Fig. 1B, arrowheads). In the presence of nimodipine to block the Ca PIC, the Na PIC alone was also significantly increased by 5-HT in chronic spinal rats (by 0.55 ± 0.38 nA), although the dose dependency of this effect was not evaluated (average for 1–10 µM combined, n = 6).
General supersensitivity to 5-HT after chronic injury
Not only was the PIC supersensitive to 5-HT in chronic spinal rat motoneurons, but the 5-HT–induced changes in resting membrane potential, input resistance, and spike voltage threshold were also supersensitive to 5-HT (Fig. 2). Very low doses of 5-HT significantly depolarized the resting membrane potential and increased the input resistance (Fig. 2, A and B; at 0.3 and 1 µM, respectively, n > 4 per dose), whereas motoneurons of acute spinal rats required ≥10 µM 5-HT to be affected (Fig. 2, A and B, n = 10). Further, at these higher doses (10–50 µM, n = 6), 5-HT depolarized motoneurons and increased their input resistance significantly more than in acute spinal rats (Fig. 2, A and B, n = 10).
Facilitation of the fast sodium spike by 5-HT also became supersensitive with chronic spinal transection. The spike voltage threshold (Vth; Fig. 2C) in chronic spinal rat motoneurons was significantly hyperpolarized by a low dose of 5-HT (1 µM, n = 7; shifted by −4.0 ± 2.3 mV from control), whereas over 10 times more 5-HT was required to significantly hyperpolarize Vth in acute spinal rat motoneurons (≥10 µM; see above; n = 10). The hyperpolarization of Vth was maximal at low doses (1 µM) in motoneurons from chronic spinal rats because higher doses of 5-HT (≥10 µM; n = 6) did not produce a significantly larger hyperpolarization (−4.0 ± 1.9 mV). At these higher doses, the effect of 5-HT on Vth was not significantly different from that in acute spinal rats (likely saturated; Vth lowered by −2.4 ± 2.1 mV with ≥10 µM 5-HT in acute spinal rats, n = 10; Fig. 2C). The threshold current required to initiate firing was also significantly reduced by 2.1 ± 1.0 nA (n = 7; Wilcoxon test) with low doses of 5-HT (1 µM) in chronic spinal rats. In fact, the average spike current threshold for chronic spinal rat motoneurons in 5-HT was −0.2 ±1.2 nA at 1 µM (n = 7) and −0.7 ± 1.4 nA at ≥10 µM (n = 6), which meant that many motoneurons in 5-HT were able to fire spontaneously at rest (although when not injecting current we held these cells below threshold to prevent Na channel inactivation).
The spike height (overshoot) was not supersensitive to 5-HT, not increasing significantly with a low dose (1 µM, n = 7), and only increasing significantly with high doses (≥10 µM) of 5-HT (by 3.4 ± 3.7 mV, n = 6, not shown), and this was not significantly different from the overshoot increase seen at this high dose in acute spinal rats (n = 10; see above). As in acute spinal rats, application of 5-HT at any dose did not significantly change the amplitude of the postspike AHP in chronic spinal rats (measured at −70 mV).
5-HT2 receptor agonists act slowly on the Na PIC in acute and chronic spinal rats
The facilitation of the Na PIC by 5-HT or DOI was slow relative to other effects of 5-HT (on Vm and Rm), in both acute and chronic spinal rats. Typically, the Na PIC was increased about 5–10 min after application of DOI or 5-HT (as measured in nimodipine), and maximum effects took up to 20 min (n = 15 chronic and 10 acute examined with DOI at all effective doses, and n = 6 chronic examined with 5-HT). Also, there was no desensitization, in that the Na PIC induced by 5-HT or DOI remained unattenuated for long periods (>1 h, n = 8, acute and chronic combined). After washout of 5-HT receptor agonists from the bath, long periods (≤45 min) were required for the Na PIC to return to control levels (n = 13, acute and chronic combined). The voltage threshold for spike activation Vth was modulated slowly with the Na PIC. Thus the facilitation of the persistent and transient Na channels by the Gq-protein–coupled 5-HT2 receptor appears to have substantially delayed onsets and offsets.
In contrast, the effects of 5-HT that do not depend on the 5-HT2 receptors (DOI-insensitive) occurred with shorter latencies. Changes in resting membrane potential and input resistance occurred within about 2 min of application of 5-HT (1–30 µM). This delay is considered very fast because it is similar to the time that it took TTX (2 µM) to block fast sodium spikes (see Li and Bennett 2003), and much of this delay likely represents the drug diffusion time into the whole sacrocaudal spinal cord preparation. In summary, 5-HT receptor activation much more rapidly affects the resting membrane potential and input resistance compared with the Na PIC. This further supports the notion that 5-HT2 receptors are involved in modulating the Na PIC, whereas other faster-acting 5-HT receptors modulate the input resistance and resting membrane potential.
5-HT2 receptor activation produces very slow firing mediated by the Na PIC
5-HT2 receptor activation (with DOI) significantly increased the self-sustained firing in acute spinal rats (ΔI increased significantly by 0.21 ± 0.21 nA with ≥30 µM DOI, n = 9 tested; compare Fig. 4, A and B) because the increased Na PIC with DOI (see above) enabled sustained slow firing before derecruitment (Fig. 4, B and C), not seen before DOI (Fig. 4A), when, as usual, measured in the presence of nimodipine to block the Ca PIC. These increases in self-sustained firing (ΔI) with DOI were significantly correlated to increases in Na PIC amplitude (slope = 0.41 nA/nA, r = 0.86, n = 9, P = 0.006). Interestingly, DOI had no significant effect on the F–I slope (in nimodipine), despite the large increases in the Na PIC. That is, the F–I slope was not significantly changed in acute spinal rats with DOI (≥30 µM; slope change −0.9 ± 2.4 Hz/nA, n = 6 tested), or in chronic spinal rats with DOI (≥1 µM; slope change −0.5 ± 0.8 Hz/nA, n = 11 tested). Thus this input–output gain (F–I slope) does not depend on the Na PIC.
FIG. 4.
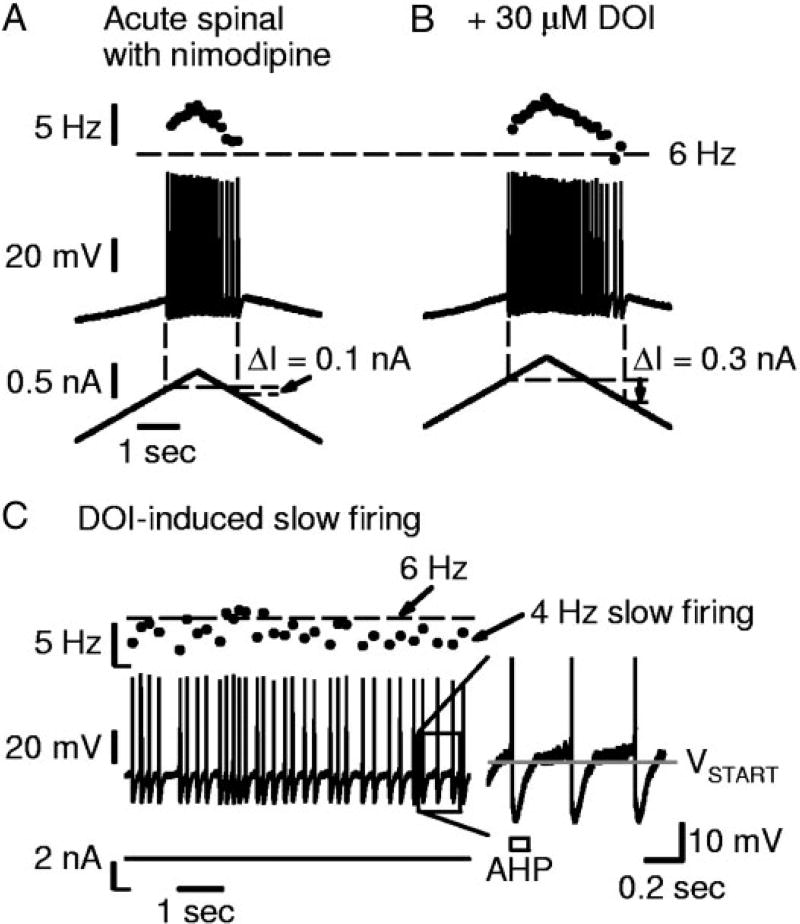
DOI increases self-sustained firing and enables very slow firing, in association with increases in the Na PIC. All recordings were done in motoneurons from acute spinal rats, with nimodipine blocking Ca PIC. A: slow triangular current ramp (bottom) elicited repetitive firing and a very small amount of self-sustained firing (middle; ΔI= 0.1 nA). Firing frequency (top) not <6 Hz (horizontal dashed line). B: from same motoneuron as in A; DOI increased Na PIC amplitude in this cell (not shown). ΔI was increased and firing occurred at <6 Hz just before derecruitment. C: different cell in DOI showing continuous firing <6 Hz with current held at threshold. Close up shown at right. Note interspike interval longer than AHP duration (open rectangle).
The major effect of DOI on firing was to lower the minimum firing rate at derecruitment (Fig. 4B). That is, DOI at high doses (≥30 µM) significantly lowered the minimum firing rate in motoneurons of acute spinal rats by 1.2 ± 1.2 Hz (n = 9). Similarly, 5-HT at high doses (≥10 µM; without nimodipine) also significantly lowered the minimum firing rate by 2.7 ±3.4 Hz (n = 11) in motoneurons of acute spinal rats. The lower minimum rate that results from an increase in Na PIC amplitude with DOI may seem counterintuitive, but occurs because a large Na PIC is critical in supporting low-frequency firing. As described by Li et al. (2004a), when the Na PIC is sufficiently large to produce a negative-slope region in the I–V relation, a subthreshold sodium plateau is produced by this Na PIC. Near threshold, this sodium plateau oscillates on and off to produce very slow firing (Fig. 4C, acute spinal rat motoneuron in 30 µM DOI). That is, each sodium plateau onset triggers a spike, which is followed by an AHP that turns off the sodium plateau, although the plateau is again turned on once the AHP ends (Fig. 4C, close-up on spikes at right). This oscillation produces very slow firing, which we define as firing with interspike intervals substantially longer than the AHP duration (<6 Hz firing, and as slow as 1 Hz; Li et al. 2004a). Thus the above finding that DOI lowered the minimum firing rate in acute spinal rat motoneurons is consistent with the large increase in the Na PIC and an associated increase in incidence of negative-slope regions with DOI [40.0% (4/10) of motoneurons in acute spinal rats had negative-slope regions in high dose DOI, compared with 9.1% (3/33) in control conditions (see Harvey et al. 2006b)]. Furthermore, all cells that exhibited a Na PIC negative-slope region in DOI (and nimodipine) also exhibited very slow firing (<6 Hz), with a mean minimum rate of 4.9 ± 0.9 Hz (n = 4). In contrast, cells that did not exhibit a Na PIC negative-slope region had a significantly higher minimum firing rate (7.0 ±1.8 Hz, n = 5 tested during firing). Basically, cells of acute spinal rats with an induced Na PIC large enough to produce a negative-slope region exhibit very slow firing similar to that described in chronic spinal rats (Li et al. 2004a).
In chronic spinal rats, dosages of DOI that were effective at increasing the Na PIC (≥ 1 µM) did not significantly lower the minimum firing rate (changed by −0.6 ±1.0 Hz; not significant) and did not significantly increase the self-sustained firing (ΔI increased by only 0.05 ± 0.18 nA, n = 12 tested). Although the Na PIC was increased significantly by DOI (see above), these cells already had a large enough Na PIC to exhibit the very slow steady firing phenomenon before DOI (minimum firing rate in control plus nimodipine was 4.6 ±1.9 Hz; see Harvey et al. 2006b), so that DOI did not further slow the firing. Likely the slow firing phenomena is all or nothing, simply requiring a Na PIC with a negative-slope region and associated sodium plateau; it does not matter so much how big the negative-slope region is (Harvey et al. 2006b; Li et al. 2004a).
Motoneurons with poor repetitive firing are rescued by increasing the Na PIC with 5-HT2 receptor activation
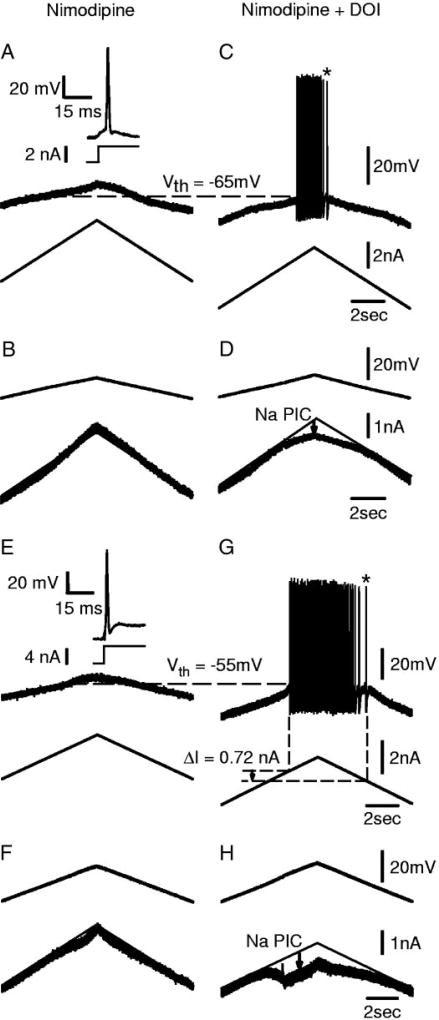
As described in Harvey et al. (2006b), a few motoneurons of acute spinal rats (8/34 in nimodipine) were unable to fire repetitively during our standard slow current ramps (Figs. 5A and 6A), although they could fire transiently with healthy spikes induced by fast current steps (inset of Fig. 5A) or antidromic activation (inset of Fig. 6A). Also, we encountered one unusual motoneuron of a chronic spinal rat that likewise lacked steady repetitive firing (Fig. 5E), even though all other chronic spinal rats had robust repetitive firing in motoneurons. These cells fired poorly because they also lacked a significant Na PIC (Fig. 5, B and F, linear I–V relation in nimodipine), which has been shown to be critical for spike initiation (Harvey et al. 2006b; Lee and Heckman 2001). Indeed, DOI was found to rescue such poor firing motoneurons by inducing a Na PIC (Fig. 5D), and thus ultimately enabling them to fire repetitively during current ramps (Fig. 5C; n = 4/4 tested with DOI). In two of these four cells, a large enough Na PIC was induced by DOI (especially Fig. 5H) to ultimately enable very slow self-sustained firing (at * in Fig. 5, C and G), as previously described for cells with a large Na PIC (Harvey et al. 2006b). Thus 5-HT2 receptor activation can turn any motoneuron with little or no repetitive firing ability into one that can readily fire repetitively, and in some cases (half) these cells exhibit self-sustained firing like that of a typical excitable motoneuron in a chronic spinal rat.
FIG. 5.
DOI rescues healthy motoneurons with no Na PIC and enables them to generate repetitive firing with slow current ramps. Sections A–D are recordings from the same cell. A: motoneuron from acute spinal rat recorded in nimodipine. Same format as in Fig. 4A. Slow current ramps well past normal threshold (Vth, horizontal dashed line measured in DOI) did not initiate action potentials or repetitive firing. Inset: full-height action potential triggered by current step. B: no Na PIC observed in voltage-clamp ramp before DOI. C: in this cell, DOI enabled repetitive firing with normal speed current ramps. D: onset of repetitive firing occurred at same time as facilitation of the Na PIC. E and F: same format as in A–D, but from an unusual chronic spinal rat motoneuron that did not exhibit any Na PIC when recorded in nimodipine. DOI facilitated self-sustained repetitive firing and slow firing (*) during current ramps (G), and induced a Na PIC large enough to produce a negative-slope region during voltage ramps (H).
These kinds of cells that initially had poor repetitive firing ability occurred both with (see Fig. 5 described above) and without the Ca PIC blocked with nimodipine (see Fig. 6A, 8/28 cells in acute spinal rats had no firing on slow ramps without nimodipine). The cells without nimodipine often had PICs (Fig. 6B), which we suppose were entirely Ca PICs, because of the lack of repetitive firing and because of their characteristic hysteretic current response on the downward voltage ramp (Li and Bennett 2003). In these cells with a Ca PIC present, DOI enabled repetitive firing (Fig. 6C) and increased the amplitude of the PIC (Fig. 6D), suggesting an enhancement of the Na PIC.
DISCUSSION
The present results demonstrate that 5-HT increases the PICs in motoneurons of both acute and chronic spinal rats and also modulates a number of other membrane properties that together make PICs more easily activated and the motoneurons more excitable. Specifically, 5-HT augments the Na PIC, lowers the spike threshold, and increases the spike height (overshoot), and these sodium channel-related effects are mediated by 5-HT2 receptors (mimicked by DOI and blocked by 5-HT2 antagonists). We also found that 5-HT depolarizes the motoneurons and increases their input resistance (consistent with other reports, reviewed in Rekling et al. 2000), but these effects are not mediated by 5-HT2 receptors (not mimicked by DOI). Interestingly, when 5-HT is applied to acute spinal rats, the combined effects of 5-HT make the motoneurons behave more like excitable motoneurons in chronic spinal rats, which rest closer to threshold, have a higher input resistance, and have much larger PICs than in acute spinal rats before 5-HT (Harvey et al. 2006b). Thus it is possible that motoneurons of chronic spinal rats are very excitable precisely because the 5-HT receptor-related intracellular pathways are strongly activated. Indeed, we also found that motoneurons of chronic spinal rats are supersensitive to very small amounts of 5-HT and thus the residual 5-HT below a transection (2–15% of normal; Schmidt and Jordan 2000) may be sufficient to endogenously activate the 5-HT receptors. This may ultimately lead to the highly excitable motoneurons in chronic spinal rats. We explore this possibility in a companion paper (Harvey et al. 2006a) and in the final section of the discussion.
5-HT2 receptor activation modulates Na channels
It has been shown that activation of 5-HT2 receptors on spinal motoneurons leads to facilitation of the Ca PIC (Perrier and Hounsgaard 2003) and that the intracellular signaling linking the receptors to the Ca channels involves intracellular Ca2+ and calmodulin (Perrier et al. 2000). Much less is known regarding modulation of the Na PIC in spinal motoneurons, although what is known is consistent with our findings that 5-HT2 receptors facilitate the Na PIC (DOI facilitates the Na PIC and 5-HT2 receptor antagonists inhibit the Na PIC; see results and Harvey et al. 2006a). That is, activation of 5-HT2A has been found in neonatal rat phrenic motoneurons to induce a tonic inward current (Lindsay and Feldman 1993) that is sodium-dependent (Lee et al. 1999). Also, 5-HT–treated tri-geminal motoneurons in guinea pigs exhibit a Na PIC, although the extent to which 5-HT caused these Na PICs is unclear (Hsiao et al. 1998). The Na PIC in cultured neonatal rat spinal motoneurons is also facilitated by 5-HT2 receptor activation, although this depends on intracellular calcium levels (Jiang et al. 2004; see following text). In other neurons, Pena and Ramirez (2002) report that 5-HT2A (although not 5-HT2C) receptors facilitate a Na PIC in interneurons involved in respiratory rhythms, and 5-HT enhances the Na PIC in leech locomotor neurons (Angstadt and Friesen 1993). On the other hand, 5-HT2A/2C receptor activation reduces the Na PIC in prefrontal cortex (Carr et al. 2002). In summary, at this stage, it is clear that the 5-HT2 receptor is involved in modulating persistent sodium currents and usually facilitates them in motoneurons.
The sodium channels mediating the Na PIC are likely to be similar, if not identical, to those involved in the fast sodium spike, albeit in a different activation state or mode that allows them to remain open without inactivating (e.g., modal current of transient Na channel; see Alzheimer et al. 1993; Crill 1996). Regardless of the specific mechanism, the idea that the same sodium channels mediate both the fast sodium spike and the Na PIC is consistent with the findings that 5-HT2 receptors have a general facilitatory effect on sodium currents in motoneurons; lowering the spike threshold (see results and Fedirchuk and Dai 2004), increasing the spike overshoot, decreasing the tendency for sodium channel inactivation during a slow ramp (rate of rise of first spike on ramp increased; see results), lowering the Na PIC threshold, and increasing the Na PIC amplitude (see results).
5-HT2 receptors are coupled to the Gαq-protein that triggers membrane phospholipid turnover (by phospholipase C activation), yielding the intracellular second messengers diacylglycerol and inositol-1,4,5-triphosphate (IP3), which respectively go on to activate intracellular protein kinase C (PKC) and regulate release of calcium from intracellular calcium stores (reviewed in Hille 2001). Thus it is not unexpected that 5-HT2 receptors modulate the persistent and transient sodium currents, given that sodium currents are also modulated by other Gq-coupled receptors, such as the muscarinic (Delmas et al. 1996) and dopaminergic (Gorelova and Yang 2000) receptors. Furthermore, PKC itself has been shown to phosphorylate the sodium channel and thus modulate its conductance (reviewed in Catterall 1999), altering activation and inactivation properties of the channels, and sometimes facilitating the persistent sodium current (Astman et al. 1998; Franceschetti et al. 2000; Numann et al. 1991; Patel et al. 2000). However, in many neuron systems, the net action of PKC is to inhibit the sodium channel (see Catterall 1999), in contrast to our results in motoneurons. Typically, when PKC is shown to inhibit the sodium channel in these neurons, it does so under nonphysiological conditions, where intracellular calcium is very low because all Ca currents are blocked (e.g., with Cd2+), or intracellular calcium is buffered. Considering that motoneurons buffer calcium very poorly (Lips and Keller 1998), they should reach substantial intracellular calcium levels with intracellular Ca2+ release, and thus should normally behave very differently than in preparations where Ca2+ signaling is impaired. Indeed, intracellular calcium chelation reduces the Na PIC in neocortical neurons (Li and Hatton 1996; Schwindt et al. 1992), and recent findings from cultured embryonic spinal motoneurons indicate that 5-HT2 receptor activation (with DOI) facilitates the Na PIC under normal conditions, but reverses to inhibit the Na PIC when intracellular calcium is low (i.e., buffered; Jiang et al. 2004).
The Na PIC is also facilitated by the Gi-coupled GABAB receptor in motoneurons (Li et al. 2004c). Considering that Gi receptors, by the Gβγ subunit, can positively modulate the PKC pathway activated by Gq-coupled receptors (Boyer et al. 1992), it seems likely that sodium currents in motoneurons are ultimately modulated in a PKC-dependent manner. In summary, 5-HT2 receptor activation—and perhaps more generally any receptor coupled to PKC—modulates sodium currents (transient and persistent) in motoneurons and other neurons.
5-HT2 receptors selectively modulate Na currents, whereas other 5-HT receptors modulate the input resistance and resting potential
We have found that 5-HT has several effects on rat motoneurons that are not reproduced by the 5-HT2 agonist DOI and that occur much faster than the DOI-induced changes in the Na currents. Thus the 5-HT2 receptor appears to relatively selectively modulate the Na PIC and the sodium spike. Other 5-HT receptors (non–5-HT2) must modulate the resting membrane potential and input resistant in motoneurons because 5-HT affects these parameters and DOI does not. 5-HT receptors that have so far been identified on spinal motoneurons include the 1A, 1B, 2A, and 2C subtypes (Rekling et al. 2000); however, the specific receptors involved in modulating these nonsodium channel-related parameters are not yet clear for motoneurons. We know in motoneurons that the 5-HT-induced depolarization of the resting potential is in part explained by facilitation of an Ih current (Larkman and Kelly 1997; McLarnon 1995) and this can occur by 5-HT1A receptor activation (Takahashi and Berger 1990). A reduction in resting potassium conductance, likely from closure of TASK-1 channels (Talley et al. 2000), is likely also partly responsible for the observed 5-HT–induced depolarization (Vandermaelen and Aghajanian 1982). A few reports have concluded that the potassium channel closure leading to depolarization and decreased conductance is mediated by 5-HT2 receptors on brain stem motoneurons (Garratt et al. 1993; Hsiao et al. 1997), in contrast to our observations. However, in turtle spinal motoneurons, depolarization and the increase in input resistance are mediated by 5-HT receptors other than 5-HT2 (Perrier and Hounsgaard 2003), as in our results, so the discrepancy may be related to differences in cell type and/or physiological role (brain stem motoneurons controlling facial and tongue movements vs. spinal motoneurons involved in limb movement).
In contrast to our findings in adult rat motoneurons, a reduction of the spike afterhyperpolarization (AHP) and associated calcium-activated potassium currents (KCa) is commonly observed with 5-HT application in many neurons (Ballerini et al. 1994) and is thought to be mediated by 5-HT4 (Andrade and Chaput 1991) or 5-HT7 receptors (Inoue et al. 2002). However, neither of these receptors has been identified on spinal motoneurons (although 5-HT7 is present on hypoglossal motoneurons; see Rekling et al. 2000), which is consistent with our finding that the AHP was not affected by 5-HT. Bayliss and colleagues (1997) reported that, whereas 5-HT reduced AHP amplitude in rat neonatal hypoglossal motoneurons, it had no effect on adults because the 5-HT1A receptor mediating this effect is dramatically downregulated in adulthood. In contrast 5-HT, particularly the 5-HT1A receptor, has been found to inhibit the AHP in adult cat (White and Fung 1989), lamprey (Wikstrom et al. 1995), and turtle (Grunnet et al. 2004; Hounsgaard and Mintz 1988) motoneurons. In our motoneurons, there remains a remote possibility that 5-HT induced a reduction in the conductance of the channel carrying the AHP current, but this effect was obscured by the simultaneous reduction in cell conductance, such that the AHP amplitude did not change significantly.
Supersensitivity of motoneurons to residual 5-HT contributes to recovery of PICs after chronic injury
Months after spinal transection, in chronic spinal animals, the descending brain stem–derived axons degenerate (Haggendal and Dahlstrom 1973) and the total level of monoamines available in the spinal cord is substantially reduced (Cline-schmidt et al. 1971; Schmidt and Jordan 2000). However, there remains about 2–15% of the normal monoamines, as assessed by immunohistochemistry (Cassam et al. 1997; Newton and Hamill 1988), fluorometric analysis (Clineschmidt et al. 1971), or high-pressure liquid chromatography–electrochemical detection (Hadjiconstantinuo et al. 1984). These residual monoamines, at least in part, occur because there are intrinsic spinal 5-HT neurons, possibly associated with the autonomic system (Hadjiconstantinuo et al. 1984; Newton and Hamill 1988). Further, there are also intrinsic NE neurons normally present in the adult spinal cord, and the number of these NE neurons appears to increase substantially with long-term transection (Cassam et al. 1997).
The small but significant level of residual monoamines (about 2–15%) becomes particularly relevant when we consider our present finding that, after spinal transaction, the Na PIC becomes supersensitive to 5-HT and, in general, motoneuron responses are found to be supersensitive to both 5-HT and NE agonists (Advokat 2002; Barbeau and Bedard 1981; Li et al. 2004b). In particular, we found a 30-fold supersensitivity, in that only about 3% (1/30) of the usual 5-HT dose is required to induce PICs in chronic spinal rats, compared with normal. Taken together with the 2–15% residual monoamines after chronic injury, this 30-fold supersensitivity suggests that residual monoamines may facilitate the Na PIC to levels near, or even well in excess of, those in the normal intact animal (30 × 2% to 30 × 15% = 60 to 450%; up to 4.5-fold increase over normal). Thus the residual intrinsic monoaminergic neurons, combined with the motoneuron supersensitivity to monoamines, may explain the exaggerated Na PIC in chronic spinal rats. In a companion paper (Harvey et al. 2006a), we verify this idea by demonstrating that monoamine receptor antagonists block the naturally occurring Na PIC in the absence of exogenously applied monoamine agonists.
Finally, the present results evaluated the action of only three doses of 5-HT agonists (low, medium, high) on the PIC. Thus the precise shape of the usual sigmoidal dose–response curve cannot be determined, nor the precise shift in sensitivity to the agonists (shift in sigmoid curve). What we do know, however, is that doses over an order of magnitude higher are required to attain similar results in acute compared with chronic spinal rats, supporting the notion of supersensitivity.
Effects of supersensitivity of Rm and Vm to 5-HT in motoneurons with long-term injury
We found that most of the effects produced by 5-HT on motoneurons were supersensitive to very low doses of 5-HT in chronic spinal rats. Thus like the PIC discussed above, the general supersensitivity to 5-HT and residual intrinsic spinal 5-HT in the spinal cord after chronic injury should in principle affect the input resistance and membrane polarization, for example. Indeed, we found that the input resistance was significantly higher in chronic than in acute spinal rats and the resting membrane potential was closer to the firing threshold, consistent with a greater intrinsic 5-HT activation (Harvey et al. 2006b). The net effect was to make motoneurons from chronic spinal rats easier to activate using intracellular current injection.
Possible mechanisms of supersensitivity
Supersensitivity of spinal reflexes to 5-HT as a result of chronic spinal transection or targeted destruction of serotonergic fibers with 5,7-dihydroxytryptamine is well documented (Barbeau and Bedard 1981; Hains et al. 2003; Li et al. 2004b; Shibuya and Anderson 1968). However, the mechanisms of this supersensitivity are unclear. Monoamine binding sites (receptors) may be initially increased after transection but return to normal levels by 2 mo postinjury (Frazer and Hensler 1990; Giroux et al. 1999), so upregulation of receptor number is not a likely explanation (at least for the 5-HT1A receptor tested). An alternative explanation is that single receptors can become sensitized by developing higher affinity for ligands through receptor modification. For example, the 5-HT2C receptor pre-mRNA is edited in response to serotonin depletion, resulting in a receptor isoform that has more efficient coupling to G-protein activation, leading to supersensitivity without any change in cytoplasmic mRNA levels (Gurevich et al. 2002).
Interestingly, the 5-HT supersensitization does not depend on the presence of 5-HT in the spinal cord because destruction of the axons with 5,7-dihydroxytryptamine leads to supersensitivity (Barbeau and Bedard 1981), although p-chlorophenylalanine-induced 5-HT depletion from axon terminals, without destruction, does not lead to supersensitivity (Tremblay and Bedard 1995). Similarly, chronic infusion of the 5-HT precursor 5-HTP after chronic spinal transection does not prevent supersensitivity from developing (Tremblay et al. 1985). Rather, it appears that the peptides co-localized with 5-HT in raphespinal terminals (substance P or TRH) have a role in regulating sensitization to 5-HT agonists (for reviews see Eide and Hole 1993; Tremblay and Bedard 1995). However, it is not clear precisely what signal induces 5-HT supersensitivity in spinal motoneurons.
Functional implications of 5-HT activity on normal motoneurons
In normal motoneurons of acute spinal rats, much of the facilitation from 5-HT and NE is lost because of the acute loss of brain stem innervation. Accordingly, these neurons have small PICs, rest far from threshold, and have a low input resistance (Harvey et al. 2006b). Exogenous application of 5-HT increases the Na PIC and input resistance, depolarizes the membrane potential, and lowers the spike threshold. In general, this makes motoneurons more excitable by making them easier to recruit and more prone to sustained firing, which is likely how the neurons behave in the intact state with brain stem monoamine innervation.
Motoneurons need at least some small Na PIC to initiate repetitive firing (Harvey et al. 2006b; Lee and Heckman 2001); accordingly, we found that 5-HT2 receptor activation rescues motoneurons that do not have any firing ability or Na PIC, enabling them to fire by inducing a Na PIC. Further, the Na PIC is critical in slow firing (Harvey et al. 2006b; Li et al. 2004a), and thus DOI enables cells to fire at lower rates (see Fig. 4). Interestingly, increasing the Na PIC with 5-HT2 receptor activation does not change the F–I slope (gain), so increasing the Na PIC seems to widen the range over which firing occurs (lower minimum firing frequency), but does not change the gain (in contrast to the conclusions of Lee and Heckman 2001).
Supersensitivity causes spasticity
Recently, large PICs in motoneurons have been demonstrated to be primarily responsible for the spasticity (including debilitating muscle spasms) that occurs after chronic spinal cord injury in rats (Li et al. 2004a) and humans (Gorassini et al. 2004). The present results and those of the companion papers (Harvey et al. 2006a,b) take our understanding of spasticity one step further. That is, the large PICs that cause spasms result from the development of supersensitivity in motoneurons to residual 5-HT in the spinal cord; thus ultimately, supersensitivity to 5-HT plays a major role in the development of spasticity after injury. The heightened sensitivity of motoneurons to endogenous 5-HT makes them exhibit large PICs either tonically or at entirely inappropriate times, depending on the activity and/or spontaneous leak of 5-HT from endogenous 5-HT neurons and other sources, perhaps related to the autonomic system inputs (Harvey et al. 2006a; Newton and Hamill 1988). This can lead to intense and prolonged muscle contractions in response to any synaptic input that activates PICs. In a companion paper (Harvey et al. 2006a), we demonstrate that the Na PIC can be inhibited by drugs that act at the three major Gq-protein–coupled monoamine receptors (5-HT2A, 5-HT2C, and α1-NE receptors), and thus these drugs, and other Gq-related drugs, offer a novel approach to treating debilitating muscle spasms.
Acknowledgments
Special thanks to L. Sanelli for expert technical assistance.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant RO1 NS-47567-01, the Natural Sciences and Engineering Research Council, Canadian Foundation for Innovation, the Canadian Institutes of Health Research, and the Alberta Heritage Foundation for Medical Research.
References
- Advokat C. Spinal transection increases the potency of clonidine on the tail-flick and hindlimb flexion reflexes. Eur J Pharmacol. 2002;437:63–67. doi: 10.1016/s0014-2999(02)01259-1. [DOI] [PubMed] [Google Scholar]
- Alaburda A, Hounsgaard J. Metabotropic modulation of motoneurons by scratch-like spinal network activity. J Neurosci. 2003;23:8625–8629. doi: 10.1523/JNEUROSCI.23-25-08625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Chaput Y. 5-Hydroxytryptamine4-like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther. 1991;257:930–937. [PubMed] [Google Scholar]
- Angstadt JD, Friesen WO. Modulation of swimming behavior in the medicinal leech. II. Ionic conductances underlying serotonergic modulation of swim-gating cell 204. J Comp Physiol A Sens Neural Behav Physiol. 1993;172:235–248. doi: 10.1007/BF00189399. [DOI] [PubMed] [Google Scholar]
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. doi: 10.1152/jn.1998.80.3.1547. [DOI] [PubMed] [Google Scholar]
- Ballerini L, Corradetti R, Nistri A, Pugliese AM, Stocca G. Electrophysiological interactions between 5-hydroxytryptamine and thyrotropin releasing hormone on rat hippocampal CA1 neurons. Eur J Neurosci. 1994;6:953–960. doi: 10.1111/j.1460-9568.1994.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Bedard P. Denervation supersensitivity to 5-hydroxytryptophan in rats following spinal transection and 5,7-dihydroxytryptamine injection. Neuropharmacology. 1981;20:611–616. doi: 10.1016/0028-3908(81)90216-1. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Talley EM, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir Physiol. 1997;110:139–150. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini MA, Siu M. In vitro preparation to study spasticity in chronic spinal rats. Soc Neurosci Abstr. 1999;25:1394. [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol. 2001a;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001b;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Waldo GL, Harden TK. Beta gamma-subunit activation of G-protein-regulated phospholipase C. J Biol Chem. 1992;267:25451–25456. [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassam AK, Llewellyn-Smith IJ, Weaver LC. Catecholamine enzymes and neuropeptides are expressed in fibres and somata in the intermediate gray matter in chronic spinal rats. Neuroscience. 1997;78:829–841. doi: 10.1016/s0306-4522(96)00599-4. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Molecular properties of brain sodium channels: an important target for anticonvulsant drugs. Adv Neurol. 1999;79:441–456. [PubMed] [Google Scholar]
- Clineschmidt BV, Pierce JE, Lovenberg L. Tryptophan hydroxylase and serotonin in spinal cord and brain stem before and after chronic transection. J Neurochem. 1971;18:1593–1596. doi: 10.1111/j.1471-4159.1971.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol. 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Niel JP, Gola M. Muscarinic activation of a novel voltage-sensitive inward current in rabbit prevertebral sympathetic neurons. Eur J Neurosci. 1996;8:598–610. doi: 10.1111/j.1460-9568.1996.tb01245.x. [DOI] [PubMed] [Google Scholar]
- Eide PK, Hole K. The role of 5-hydroxytryptamine (5-HT) receptor subtypes and plasticity in the 5-HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13:75–85. doi: 10.1046/j.1468-2982.1993.1302075.x. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–361. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S, Taverna S, Sancini G, Panzica F, Lombardi R, Avanzini G. Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones. J Physiol. 2000;528:291–304. doi: 10.1111/j.1469-7793.2000.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A, Hensler JG. 5-HT1A receptors and 5-HT1A-mediated responses: effect of treatments that modify serotonergic neurotransmission. Ann NY Acad Sci. 1990;600:460–474. doi: 10.1111/j.1749-6632.1990.tb16902.x. discussion 474-465. [DOI] [PubMed] [Google Scholar]
- Garratt JC, Alreja M, Aghajanian GK. LSD has high efficacy relative to serotonin in enhancing the cationic current Ih: intracellular studies in rat facial motoneurons. Synapse. 1993;13:123–134. doi: 10.1002/syn.890130205. [DOI] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA. Autoradiographic study of alpha1-and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol. 1999;406:402–414. [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Gorelova NA, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol. 2000;84:75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Perrier JF. 5-HT1A receptors modulate small-conductance Ca2+-activated K+channels. J Neurosci Res. 2004;78:845–854. doi: 10.1002/jnr.20318. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci. 2002;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiconstantinuo M, Panula P, Lackovic Z, Neff NF. Spinal cord serotonin: a biochemical and immunohistochemical study following transection. Brain Res. 1984;322:245–254. doi: 10.1016/0006-8993(84)90114-8. [DOI] [PubMed] [Google Scholar]
- Haggendal J, Dahlstrom A. The time course of noradrenaline decrease in rat spinal cord following transection. Neuropharmacology. 1973;12:349–354. doi: 10.1016/0028-3908(73)90094-4. [DOI] [PubMed] [Google Scholar]
- Hains BC, Willis WD, Hulsebosch CE. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp Brain Res. 2003;149:174–186. doi: 10.1007/s00221-002-1352-x. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006a;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol. 2006b;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2004;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Del Negro CA, Trueblood PR, Chandler SH. Ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Curr Opin Neurobiol. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Inoue T, Itoh S, Wakisaka S, Ogawa S, Saito M, Morimoto T. Involvement of 5-HT7 receptors in serotonergic effects on spike afterpotentials in presumed jaw-closing motoneurons of rats. Brain Res. 2002;954:202–211. doi: 10.1016/s0006-8993(02)03286-9. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Kuo JJ, Heckman CJ. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Soc Neurosci; 2004. Regulation of 5-HT2 receptors on persistent sodium current and its intracellular signaling in cultured mouse spinal cord motoneurons; p. 875.16. [Google Scholar]
- Larkman PM, Kelly JS. Modulation of IH by 5-HT in neonatal rat motoneurones in vitro: mediation through a phosphorylation independent action of cAMP. Neuropharmacology. 1997;36:721–733. doi: 10.1016/s0028-3908(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Lee K, Dixon AK, Pinnock RD. Serotonin depolarizes hippocampal interneurones in the rat stratum oriens by interaction with 5HT2 receptors. Neurosci Lett. 1999;270:56–58. doi: 10.1016/s0304-3940(99)00449-8. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004a;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol. 2004b;91:2236–2246. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol. 2004c;92:2694–2703. doi: 10.1152/jn.00164.2004. [DOI] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Oscillatory bursting of phasically firing rat supraoptic neurones in low-Ca2+ medium: Na+ influx, cytosolic Ca2+ and gap junctions. J Physiol. 1996;496:379–394. doi: 10.1113/jphysiol.1996.sp021692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips MB, Keller BU. Endogenous calcium buffering in motoneurones of the nucleus hypoglossus from mouse. J Physiol. 1998;511:105–117. doi: 10.1111/j.1469-7793.1998.105bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon JG. Potassium currents in motoneurones. Prog Neurobiol. 1995;47:513–531. doi: 10.1016/0301-0082(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Newton BW, Hamill RW. The morphology and distribution of rat serotoninergic intraspinal neurons: an immunohistochemical study. Brain Res Bull. 1988;20:349–360. doi: 10.1016/0361-9230(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Numann R, Catterall WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Patel MK, Mistry D, John JE3rd, Mounsey JP. Sodium channel isoform-specific effects of halothane: protein kinase C co-expression and slow inactivation gating. Br J Pharmacol. 2000;130:1785–1792. doi: 10.1038/sj.bjp.0703487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol. 2000;528:107–113. doi: 10.1111/j.1469-7793.2000.t01-1-00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlue WR, Richter DW, Mauritz KH, Nacimiento AC. Responses of cat spinal motoneuron somata and axons to linearly rising currents. J Neurophysiol. 1974;37:303–309. doi: 10.1152/jn.1974.37.2.303. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Membrane properties of cat spinal motoneurons. In: Davidoff RA, editor. Handbook of the Spinal Cord. 2 and 3. New York: Marcel Dekker; 1984. pp. 199–242. [Google Scholar]
- Schwindt PC, Spain WJ, Crill WE. Effects of intracellular calcium chelation on voltage-dependent and calcium-dependent currents in cat neo-cortical neurons. Neuroscience. 1992;47:571–578. doi: 10.1016/0306-4522(92)90166-y. [DOI] [PubMed] [Google Scholar]
- Shibuya T, Anderson EG. The influence of chronic cord transection on the effects of 5-hydroxytryptophan, L-tryptophan and pargyline on spinal neuronal activity. J Pharmacol Exp Ther. 1968;164:185–190. [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Tremblay LE, Bedard PJ. Action of 5-hydroxytryptamine, substance P, thyrotropin releasing hormone and clonidine on spinal neuron excitability. J Spinal Cord Med. 1995;18:42–46. doi: 10.1080/10790268.1995.11719379. [DOI] [PubMed] [Google Scholar]
- Tremblay LE, Bedard PJ, Maheux R, Di Paolo T. Denervation supersensitivity to 5-hydroxytryptamine in the rat spinal cord is not due to the absence of 5-hydroxytryptamine. Brain Res. 1985;330:174–177. doi: 10.1016/0006-8993(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Serotonin-induced depolarization of rat facial motoneurons in vivo: comparison with amino acid transmitters. Brain Res. 1982;239:139–152. doi: 10.1016/0006-8993(82)90838-1. [DOI] [PubMed] [Google Scholar]
- White SR, Fung SJ. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyperpolarizing potentials. Brain Res. 1989;502:205–213. doi: 10.1016/0006-8993(89)90615-x. [DOI] [PubMed] [Google Scholar]
- Wikstrom M, Hill R, Hellgren J, Grillner S. The action of 5-HT on calcium-dependent potassium channels and on the spinal locomotor network in lamprey is mediated by 5-HT1A–like receptors. Brain Res. 1995;678:191–199. doi: 10.1016/0006-8993(95)00183-q. [DOI] [PubMed] [Google Scholar]