Abstract
Color vision relies on the ability to discriminate different wavelengths and is often improved in insects that inhabit well-lit, spectrally rich environments. Although the Opsin proteins themselves are sensitive to specific wavelength ranges, other factors can alter and further restrict the sensitivity of photoreceptors to allow for finer color discrimination and thereby more informed decisions while interacting with the environment. The ability to discriminate colors differs between insects that exhibit different life styles, between female and male eyes of the same species, and between regions of the same eye, depending on the requirements of intraspecific communication and ecological demands.
Introduction
Color vision is one of the main senses animals use for inter- and intraspecific communication and to evaluate relevant resources within their habitat, for example, in finding food, mates, shelter, and places for oviposition. During evolution, the color vision system was modified in response to changing environments allowing species to adapt to new ecological niches.
Color vision relies on the ability to discriminate between different wavelengths regardless of their intensity. The structural unit for vision in insects is the unit eye, or ommatidium, in the compound eye. Ommatidia can vary greatly in number and size, yet each ommatidium is usually composed of a fixed number of photoreceptor neurons. Color vision requires the presence of different photopigments located in specialized photoreceptor cells. Across insects, photopigments consist of an Opsin portion, a special type of G-protein coupled transmembrane receptor, which is linked to the light-detecting chromophore retinal. The crucial step of phototransduction (the translation of the light stimulus into an electrical signal) is achieved when the chromophore absorbs photons leading to its isomerization, which causes a conformational change and thus activates the Opsin. The sensitivity of any visual pigment for specific wavelengths is defined by the amino acid sequence of the Opsin protein. Evolutionary changes in the Opsins can therefore cause shifts of spectral sensitivity towards new wavelengths, thus allowing for the recognition and better discrimination of potentially beneficial stimuli in the environment.
Studies in Opsin evolution revealed a high conservation of c(ciliary)-Opsin as the main vertebrate visual pigment while r(rhabdomeric)-Opsins mainly mediates light-detection in arthropod photoreceptors [1]. The r-Opsin family in insects contains three paralogous groups with different wavelength sensitivity with absorbance peaks for the ultraviolet (UV), short (SW, blue) and long wavelengths (LW, green) [2]. Such studies suggest that the insect ancestor was a trichromat and possessed one of each of these three distinct types of Opsins (UV, SW and LW) covering a wavelength range between 300 and 700nm (Fig. 1A) [3,4,5,6]. Molecular phylogenetic analyses reveal a dynamic evolution through multiplications, functional diversification and losses of Opsin genes, especially adaptive evolution of UV-sensitive Opsins in day-flying insects in general and LW Opsins in Lepidoptera and dragonflies specifically [2,7**,8]. Coleoptera as well as arthropods closest related to insects, the Chelicerates (Spiders and scorpions), lack the blue pigment [9*,10**,11,12,13] (Fig. 1B) although some species can detect blue light through mechanisms that will be discussed below. Most of what we know about color vision in insects is based on studies in bees (honeybees and bumblebees), Lepidoptera (butterflies and moths), and flies [4,14,15,16,17]. Recent studies show how insects have reduced, enhanced or adjusted their color vision systems. For instance, Lepidoptera and dragonflies – usually regarded as having superior color vision – manifest a large number of Opsins and a high spectral diversity of photoreceptors (Fig. 1C) [7**,18,19,20]. On the other hand, nocturnal insects have often lost Opsins or have reduced Opsin expression, which can affect an entire phylogenetic branch or just a single species [2,21*]. Some insects exhibit strategies to enhance color vision or regain previously lost sensitivities for certain wavelengths.
Figure 1.
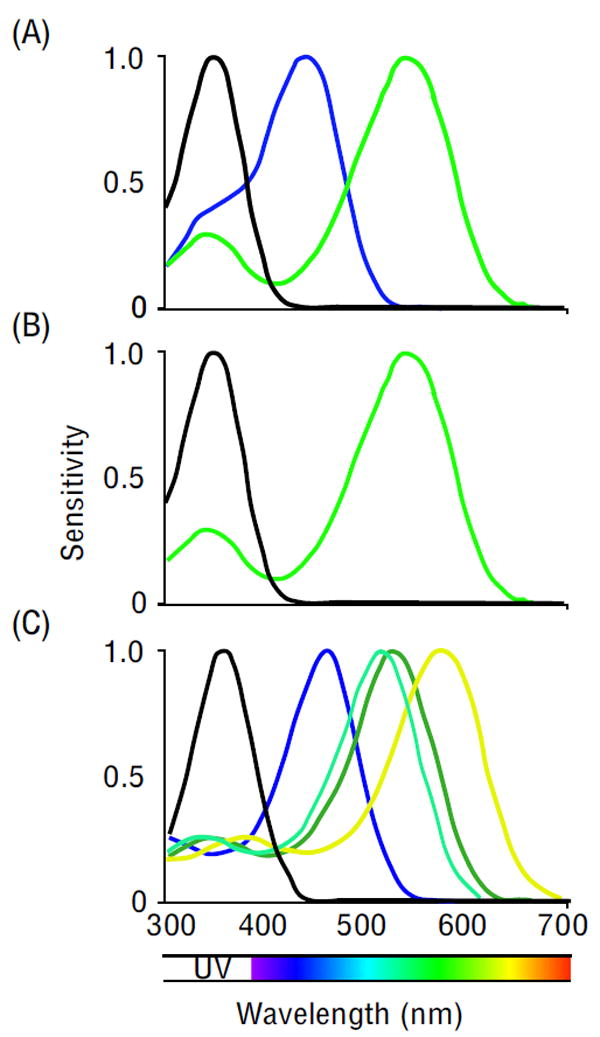
Spectral sensitivity of visual pigments in different insect species, normalized to have a peak value of 1. (A) The ancestral insect has three different Opsins that cover UV (black), SW (blue), and LW (green) and allow for the discrimination of wavelengths ranging from ~300-600 nm (figure adapted from [6]). With this combination of Opsins good color discrimination is already possible. (B) Most insects living under low-light conditions have reduced color vision due to, for example, the loss of blue Opsin. This is also the case for Coleopterans - although living under daylight conditions [9*,10**] (figure adapted from [6]). (C) Insects that primarily rely on color vision enhanced their ability to distinguish between different wavelengths by expressing additional Opsins. Some butterflies (Papilio Xuthus shown here) have improved color discrimination in the LW range (green and yellow lines) (figure taken from [18]).
Compared to retinal perception and behavioral responses to color stimuli, color processing in the brain is not well understood. The basic structure of the optic lobes is conserved among insects and even in arthropods, and comprises three neuropils: the lamina, the medulla, and the lobula. Each shows a columnar organization resembling the array of ommatidia in the retina [22]. In most insects six of the eight to nine photoreceptors are predominantly involved in motion vision and synapse first in the lamina, while the remaining color-sensitive PRs project directly to the medulla [16,23,24], the largest and most complex optic neuropil. In the medulla, different connections of color sensitive photoreceptors allow comparisons of wavelengths detected by two or more photoreceptors from the same and/or different ommatidia [25]. The color vision pathway has long been thought to be separate from the motion vision pathway. However crosstalk has been observed in flies [26,27]. Further connections target the lobula and color-opponent neurons from both neuropils send projections to different brain areas, e.g. the mushroom bodies and the anterior lateral and posterior protocerebrum [28,29].
To ensure true color vision, the responses of at least two photoreceptors sensitive to different colors must be compared. Two photoreceptors containing different Opsins with only slightly shifted spectral sensitivities allow for a finer discrimination and thus are adapted to detecting a more specific color range (Fig. 1) [15]. In contrast, comparisons between photoreceptors sensitive to distant wavelengths are less sensitive to fine differences but allow for a wider color range. Different types of photoreceptors are classified based on their absorbance spectrum, which is predominantly dictated by the Opsins they express. However, different modulating features exist in the eye (e.g. filtering pigments, antenna pigments, and chemical modifications of the chromophore). These features can further alter the spectral sensitivity of a specific photoreceptor type with quite dramatic effects, as we will discuss below. Taken together, the alteration of the spectral sensitivity based on biological demands is referred to as spectral tuning.
Spectral tuning to enhance color discrimination
In general, three different strategies have been described in insect color vision, which can modulate the spectral sensitivity of a photoreceptor (Fig. 2). The primary feature that specifies color sensitivity is the Opsin protein. Opsin evolution is thought to occur via gene duplication followed by subsequent subfunctionalization [8,30,31,32]. Especially changes in the amino acid sequence in close proximity to the chromophore-binding site can produce divergent spectral sensitivities [33,34]. For instance, Coleoptera that ancestrally lack SW Opsins have successfully reestablished their sensitivity for blue wavelengths by functionally diverged copies of former UV or LW Opsin genes (Fig. 2A) [9*,10**]. The same principle based on small changes in the amino acid sequence is responsible for the sensitivity shift of the human SW Opsin towards blue light despite its closer phylogenetic relation to the vertebrate UV Opsin [35].
Figure 2.

(A) Subfunctionalization of an Opsin due to specific amino acid substitution: (Ai) Two views (180° rotation) on a 3D model of the Coleopteran UVS2 (Acmaeodera diffusa) with relevant amino acid residues marked in green and the retinal chromophore in orange (taken from [9*], https://creativecommons.org/licenses/by/4.0/). (Aii) The dramatic blue shift in the Opsin sensitivity (shown here for Drosophila Rh3) is caused by a single amino acid mutation (taken from [89]). Spectral sensitivity curves are shown as solid lines and the absorption spectra of the unaltered or predicted visual pigment as dotted lines, curves are normalized to have a peak value of 1. (B) Co-expression of a red and a green Opsin yields a photoreceptor with broadband sensitivity (taken from [36]). (Ci) The sensitizing pigment, 3-hydroxyretinol attached to the outside of the Opsin, absorbs UV light and transfers its excitation energy to the main chromophore, adding a sensitivity peak in the UV (shown in Cii for Drosophila Rh1; modified after [37]). (D) Elements of spectral-tuning circuits in a model insect ommatidium. Schematic of a longitudinal view of the ommatidium and micrographs of relevant structures: (i) transmission electron micrograph of a longitudinal section of the facet lens, (ii) and (iii’) fluorescence micrographs, and (iii) light micrograph of transverse sections at different positions along the longitudinal axis of the ommatidia. Fluorescing (here UV and yellow), perirhabdomal, and filter pigments alter the light spectrum that reaches the Opsins. The three circles in (ii-iii’) indicate three different ommatidial types that exhibit unique combinations for spectral tuning. (Figure adapted from [74**] (https://creativecommons.org/licenses/by/4.0/legalcode); micrograph (i) taken from [41], and (ii-iii’) from [74**]). (E) Examples of how spectral-tuning affects the sensitivity of photoreceptors. Spectral sensitivity curves in solid lines and dotted lines as described above for (Ai, B, and Ci). (i) Colored lenses reduce transmittance of a small range of wavelengths by selective reflection (gray dashed line; figure modified from [41]). (ii) Fluorescent pigments and (iii) rhabdomeric pigments function as cut-off filters (grey dashed lines) isolating the Opsin from a broad part of the spectrum, thus causing a shift in spectral sensitivity (figures modified from [65]).
When discrimination is not required, co-expression of two or more Opsins within the same photoreceptor broadens the range of spectral sensitivity of a photoreceptor, such as in Papilio butterflies (Fig. 2B) [36]. An alternate way to broaden sensitivities has been described in flies: instead of expressing multiple overlapping Opsins in the same photoreceptor, they use an accessory pigment to modify the “Rh1/NinaE” Opsin. This “sensitizing pigment”, 3-hydroxy-retinol, is attached to and transfers energy obtained by the absorption of UV light to the visual pigment, thus adding a characteristic UV peak to the absorption spectrum and creating broadband-sensitivity (300-600nm) (Fig. 2C) [37,38,39]. In some vertebrates, the visual pigment’s chromophore can also tune the spectral sensitivity. Substitution of 11-cis retinal with 11-cis 3,4 didehydroretinal in photoreceptors of the eyes of zebrafish and bullfrogs cause a red-shifted sensitivity, most likely an adaption for vision under or into water [40]. Different types of chromophores, the retinal and two enantiomers of 3-hydroxyretinal have been described in insects, the latter causing a slight shift (4-12nm) towards shorter wavelengths relative to retinal [33]. Simultaneous use of two different chromophores could further enhance color vision, though the majority of insects use only one.
Certain anatomical structures can further sculpt the incoming light and thereby have drastic effects on the spectral sensitivity of the photoreceptors. The beautifully colored lenses of some flies, e.g. Tabanidae or Dolichopodidae, are composed of various structural layers with different refraction indices, which reflect and thus reduce the transmittance of a narrow-band of wavelengths (Fig. 2Di, Ei) [41,42,43,44]. Furthermore, pigmentation around the rhabdomeres, the Opsin-containing structures of photoreceptors, can alter the spectral sensitivity. Fluorescent pigments such as 3-hydroxy-retinol, present in the eyes of some Papilionidae and Pieridae butterflies absorb short wavelength light and emit light of longer wavelength. Located at the distal end of the photoreceptors these pigments filter out UV light (wavelengths below 350nm) and hence narrow the spectral sensitivity of the photoreceptor (Fig. 2Dii, Eii) [45]. Drosophila and Musca (houseflies) have photostable carotenoid pigments in some of their photoreceptors. These are blue light filters that produce a 20nm shift towards longer wavelengths [46,47]. Different compositions of carotenoids might define screening and improve color constancy and discrimination, as shown for the analogue oil droplets in the avian eye [48]. In Heliconius erato butterflies, screening pigments even produce two different photoreceptor subtypes although they all express the same LW Opsin. Due to long-pass filtering, pigments in one photoreceptor subtype cause a shift in spectral sensitivity, allowing for discrimination between green and red (Fig. 2Diii, Eiii) [49**]. Even small differences in the dispersion and position of perirhabdomal pigments along the vertical axis of the photoreceptors can affect the wavelength sensitivity of the ommatidia types in Colias erate butterflies [50]. Thus, screening and filtering can either shift or narrow the absorbance spectrum of an Opsin and define the spectral sensitivity of the photoreceptors.
Color vision in different insects
Vision is energetically costly in terms of metabolism in the retina as well as requiring large downstream computational neural networks [51,52]. As a consequence, subterranean animals that no longer require the ability to see often have lost their eyes completely and reducedsmaller the visual brain regions, e.g.for example, the Mexican cavefishes [53,54] and a suite of cave and subterranean insects, e.g.for example, cave beetles and cave crickets [55-57]. Similarly, animals are likely to reduce their ability to differentiate between wavelengths that are no longer ecologically relevant. Average color vision requires three different types of Opsins and higher quality sensitivity can be obtained via additional channels. Trichromacy is widely spread among insects and allows color discrimination based on three different channels, UV, blue and green as described for hymenoptera [4,6,18]. Interestingly most species include UV wavelengths in their range of spectral sensitivity, suggesting that UV is an important signal in insect ecology, e.g. for finding flowers in pollinating species, for intraspecific communication, or for detecting polarized light for orientation (reviewed in [58]). In general, insect life styles are quite diverse and color vision can take many forms, with the number of Opsins found in a species reduced or enhanced depending at least in part on behavioral ecology [7**,59].
How different color discrimination abilities are reflected in the architecture of the optic neuropils is difficult to evaluate since mainly insects with good color vision, e.g. bees, flies, and butterflies, have been investigated. Interestingly, color vision seems to be processed differently depending on the behavioral output. For instance UV attraction appears to be mediated by a direct pathway while color discrimination relies on more complex color vision pathways in the optic lobes (see also [60,61]). This means that rather simple pathways, i.e. the response to a specific wavelength without further processing could be the origin of color vision while enhanced color vision might have evolved by increased connectivity within and between the different neuropil structures and central brain areas. For instance, in insects like butterflies and dragonflies, color vision involves additional processing in the lamina, where large monopolar cells exhibit spectral sensitivity [62,63,64,65].
Species living under low-light conditions have often lost Opsins and/or use Opsins sensitive to broader wavelength ranges that are also more sensitive at lower light intensities. These species might have also reduced neuronal connectivity in the visual processing neuropils, i.e. in specific layers in the medulla and lobula. For instance, in Xenos peckii (Strepsiptera, sister group to Coleoptera), all PRs project to the lamina and no direct projections to the medulla exist [66]. Although X. peckii shows some preference for UV light, which is likely to be mediated by the UV-sensitive of one of the two Opsins found in the genome [67], it might only have basic color vision. Another reduction in connectivity can be observed in the fly medulla: While in the main eye, R7 and R8, the inner photoreceptors responsible for color detection, project to two different layers in the medulla (M6 and M3), the synapses collapse to one layer (M6) for monochromatic photoreceptors located in the dorsal rim area (DRA) [68] where the orientation of the vector of polarized light rather than colors is processed.
Changes in Opsin number can be observed even within closely related species that prefer different light conditions, as shown by the comparisons between surface and subterranean species of predatory diving beetles [21*]. However, scorpionflies provide a conflicting example: common scorpionflies (Panorpidae) have somewhat colored bodies and wings but have lost two Opsins (SW and UV) with only one (LW) left, making them monochromats [59,69]. In contrast, the closely related snow scorpionflies (Boreidae) are less colorful and thought to be less visually motivated, yet have the basic set of three Opsins [59]. How the neural connectivity of the optic lobes differ between these two species and how the ability to discriminate spectral cues influences their visual ecology remains to be elucidated. Opsin number is not always a reliable indicator of color discrimination ability or the importance of color vision to a species. The genome of dragonflies exhibits a very large number of up to 33 Opsins [7**]. However, despite highly diverse variations in the sequence of LW Opsins, they rarely differ in their spectral sensitivity [63,70]. The same is true for polymorphisms in SW Opsins across different species of the Limenitis butterfly genus, which correlate with discrete populations living in different environments along the US east coast [71**] as these mutations do not affect residues in the chromophore-binding pocket that would affect the absorbance spectra. These polymorphisms might instead provide other physiological adaptations, such as the rate or cost of regeneration of the active photopigment, its expression level or (thermo)stability over time. This might enhance fitness in order to adapt to distinct environmental challenges without a change in spectral discrimination and explain the evolution and maintenance of additional diversity in a single species or the small changes between Opsins of closely related species [19,71**].
Thus, although the number of Opsin variants might still provide a rough estimate of color vision abilities, it is not necessarily an indication of color-vision quality. Similarly, mantis shrimp, a crustacean example, are known to express a large number of Opsins yet have relatively poor color discrimination abilities [72]. This discrepancy might be caused to some extent by their peculiar scanning strategy, which has been proposed to allow very fast recognition of colors rather than fine discrimination [72,73]. In yet another counterexample, the butterfly species Graphium sarpedon has particularly impressive color vision using a limited number of Opsins [74**]. Instead of expressing an enormous number of different Opsins, this butterfly species produces 15 different types of photoreceptors based solely on 5 different Opsins in different combinations to develop specific spectral tuning. Thus, the estimation of variations in wavelength sensitivity from Opsin sequence data is not only problematic [75], but also has limited implications for visual ecology.
Ecological significance of color vision
Considering the high metabolic cost of vision and the wide range of ecological demands and behavioral requirements, it might be expected that photoreceptors are highly adapted to specific stimuli. Such adaptation can come at a cost, and specialization for one type of stimulus might require the loss of ability to detect another. In one example, Musca housefly males search and pursue females for mating. Males harbor a specialized region of the eye known as the “Love Spot” that is dedicated to detecting and chasing females. Such adaptations are sometimes accompanied by drastic morphological changes, e.g. the enlarged dorsal regions in Tabanid horse flies and the “turbinate” dorsal eyes of male mayflies [76]. Ommatidia within these dorsal eye regions are modified to improve motion vision, presumably at the expense of color vision [77,78]. Indeed these transformed color-sensitive photoreceptors project no longer to the medulla but to the lamina, together with motion and contrast processing photoreceptors [79].
Similar strategies have also evolved in favor of color vision. In butterflies of the species H. erato, photoreceptors in the female eye express an additional UV Opsin that is missing in the male eye [49**]. The butterfly ommatidia contain nine different photoreceptors (R1-9). The R1/R2 photoreceptor pair defines three different ommatidial subtypes by expressing blue/UV, UV/UV, or blue/blue [80**]. Surprisingly, the additional UV Opsin (UVRh1) in the female H. erato eye does not increase the number of different ommatidial subtypes, i.e. the combinations of Opsins, but simply replaces the UV Opsin in the blue/UV ommatidia. This new combination causes a second absorbance peak, and hence allows for finer discrimination in the UV wavelength range. This sexual dimorphism suggests there is a stimulus relevant only for females. This photoreceptor type represents half of the ommatidial types in the retina and does not show a restriction to a specific part of the eye. Its significance for the behavior of the animals remains to be tested. Males, manifest an increased number of blue receptors relative to females, suggesting a requirement for improved discrimination in the blue wavelength range [49**]. A conspicuous yellow bar on the hind wing of H. erato may be important for recognition of conspecifics. This bar reflects predominantly in the blue wavelength range but causes similar attraction of males and females when tested with artificial models [81].
Male and females of another butterfly, Colias erate, show indistinguishable expression patterns of Opsins [82,83], but produce different filtering pigments in different ommatidial types [50]. In females, these red or orange colored perirhabdomal pigments filter light in different photoreceptors to produce 3 distinct output peaks for the same LW Opsin, thus allowing finer discrimination of green and red. The fact that this sexual dimorphism is restricted to the ventral part of the eye suggests a specific role for sexual behavior (i.e., courtship or finding places for oviposition). In general the localization of specific photoreceptors within the eye corresponds to behaviors requiring the eye to face upwards towards the sky or downwards towards green foliage or other ground structures (reviewed in [23]).
Adaption of the color vision system is not only found across long-term evolutionary contexts, i.e. between species, but also for polyphenism at a shorter generation timescale within species or even during development of individuals. The African butterfly Bicyclus anynana exhibits two different phenotypes that are highly adapted to seasonal environmental factors, in the dry or wet season [84*,85]. Female butterflies raised under dry mating season conditions have their vision impaired, perhaps to save energy during long periods of reduced activity. Along with a general reduction in eye size and the number of ommatidia, expression of the blue Opsin is significantly reduced and leads to impaired color vision ability that is consistent with the idea that females may be less selective in mate choice during the dry season [84*]. The eight green and four blue photoreceptors in the fly larva constitute an even more extreme example: during metamorphosis the green photoreceptors die and the blue become green-sensitive to entrain the circadian clock [86]. Similarly, different Opsins are expressed during different developmental stages of dragonflies. Exploiting the high variety of Opsins found in the genome and in accordance with their visually-driven behavior, terrestrial adults in general express more Opsins while their aquatic larvae express fewer and preferably LW Opsins adapted to their life under water (LW light penetrate water better than SW or UV light) [63,70].
Conclusion and perspective
The insect eye is highly specialized both among and within species, allowing insects to adapt to specific environmental conditions and to produce ecologically relevant behaviors. A changing diversity of Opsins and spectral tuning strategies across groups allow for discrimination of sometimes specialized cues relevant to a particular species, from finding food or mates to avoiding predators. Despite the multiple strategies that allow the eye to adapt, the combination of Opsins or tuning strategies employed may be limited either by phylogenetic constraints or by the retinal region where such Opsins can be expressed. Drosophila are thought to make two main color comparisons: UV vs. Blue in one ommatidial subtype and a different UV vs. Green in a second subtype. A clever developmental modification allowed butterflies to add a third ommatidial type to their retinas enhancing their color vision by allowing for more different color comparisons to be made [80**,87].
Comparative studies of color processing pathways in the optic lobes of insects with basic or enhanced color vision might help address some of the open questions, especially the computation and processing of color vision stimuli: are color inputs compared only within a single ommatidial unit, between photoreceptors in adjacent ommatidia or can they be compared across regions or the entire retina? How does neuronal connectivity of the optic lobes reflect enhanced color vision abilities? Models designed to explain the neuronal processing of color vision include ideas that assume that noise is limited to the receptor level and downstream processing is noise-free (“RNL”, receptor noise limited model) and that discrimination between wavelengths occurs through color-opponent neurons (“COC”, color-opponent coding). The different parallel pathways to process colors might explain why the different models are only partially successful depending on the behavioral task [88].
From an ecological point of view, adaptation also means the ability to develop specificity for relevant wavelengths within specified areas of the eye, e.g. the ventral parts of the eye, which are dedicated to detect objects on the ground such as food or oviposition sites within a homogeneous background, or the dorsal parts to detect predators or prey above, against the sky. Convergently evolving strategies are used in different animals to enhance color vision to adapt to new ecological demands.
Highlights.
Color vision: more than just Opsins
Spectral tuning differs between sexes and species, and even within the same eye
The distribution of different photoreceptor types across the eye is adapted to specific ecological tasks
Acknowledgments
We thank Mike Perry and Mathias Wernet for valuable and helpful comments on the manuscript. This work was supported by the National Institutes of Health EY13010 to CD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, Robinson PR. Shedding new light on opsin evolution. Proceedings of the Royal Society Biological Sciences Series B. 2012;279:3–14. doi: 10.1098/rspb.2011.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feuda R, Marletaz F, Bentley MA, Holland PWH. Conservation, Duplication, and Divergence of Five Opsin Genes in Insect Evolution. Genome Biology and Evolution. 2016;8:579–587. doi: 10.1093/gbe/evw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briscoe AD. Reconstructing the ancestral butterfly eye: focus on the opsins. J Exp Biol. 2008;211:1805–1813. doi: 10.1242/jeb.013045. [DOI] [PubMed] [Google Scholar]
- 4.Briscoe AD, Chittka L. The evolution of color vision in insects. Annu Rev Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- 5.Yuan F, Bernard GD, Le J, Briscoe AD. Contrasting Modes of Evolution of the Visual Pigments in Heliconius Butterflies. Mol Biol Evol. 2010;27:2392–2405. doi: 10.1093/molbev/msq124. [DOI] [PubMed] [Google Scholar]
- 6.Peitsch D, Fietz A, Hertel H, Desouza J, Ventura DF, Menzel R. The Spectral Input Systems of Hymenopteran Insects and Their Receptor-Based Color-Vision. J Comp Physiol A. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- 7**.Futahashi R, Kawahara-Miki R, Kinoshita M, Yoshitake K, Yajima S, Arikawa K, Fukatsu T. Extraordinary diversity of visual opsin genes in dragonflies. P Natl Acad Sci USA. 2015;112:E1247–E1256. doi: 10.1073/pnas.1424670112. High variety of Opsins in dragonfly is expressed stage- and region-specific, in accordance with behavioral and ecological demands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaethe J, Briscoe AD. Early duplication and functional diversification of the opsin gene family in insects. Mol Biol Evol. 2004;21:1583–1594. doi: 10.1093/molbev/msh162. [DOI] [PubMed] [Google Scholar]
- 9*.Lord NP, Plimpton RL, Sharkey CR, Suvorov A, Lelito JP, Willardson BM, Bybee SM. A cure for the blues: opsin duplication and subfunctionalization for short-wavelength sensitivity in jewel beetles (Coleoptera: Buprestidae) BMC Evol Biol. 2016;16:107. doi: 10.1186/s12862-016-0674-4. RNA-seq data of Coleoptera (Buprestidae) reveal two copies in both UV and LW Opsin classes, due to these opsin copies with multiple amino acid substitutions blue light sensitivity might be recovered. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Sharkey CR, Fujimoto MS, Lord NP, Shin S, McKenna DD, Suvorov A, Martin GJ, Bybee SM. Overcoming the loss of blue sensitivity through opsin duplication in the largest animal group, beetles. Sci Rep. 2017;7 doi: 10.1038/s41598-017-00061-7. Highly divergent UV opsin duplicates emerged independently in different Coleoptera lineages and match recovered blue sensitivity in these species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyanagi M, Nagata T, Katoh K, Yamashita S, Tokunaga F. Molecular evolution of arthropod color vision deduced from multiple opsin genes of jumping spiders. J Mol Evol. 2008;66:130–137. doi: 10.1007/s00239-008-9065-9. [DOI] [PubMed] [Google Scholar]
- 12.Zopf LM, Schmid A, Fredman D, Eriksson BJ. Spectral sensitivity of the ctenid spider Cupiennius salei. J Exp Biol. 2013;216:4103–4108. doi: 10.1242/jeb.086256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zurek DB, Cronin TW, Taylor LA, Byrne K, Sullivan MLG, Morehouse NI. Spectral filtering enables trichromatic vision in colorful jumping spiders. Curr Biol. 2015;25:R403–R404. doi: 10.1016/j.cub.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Lunau K. Visual ecology of flies with particular reference to colour vision and colour preferences. Journal of Comparative Physiology A Sensory Neural and Behavioral Physiology. 2014;200:497–512. doi: 10.1007/s00359-014-0895-1. [DOI] [PubMed] [Google Scholar]
- 15.Kelber A, Vorobyev M, Osorio D. Animal colour vision--behavioural tests and physiological concepts. Biol Rev Camb Philos Soc. 2003;78:81–118. doi: 10.1017/s1464793102005985. [DOI] [PubMed] [Google Scholar]
- 16.Hempel de Ibarra N, Vorobyev M, Menzel R. Mechanisms, functions and ecology of colour vision in the honeybee. Journal of Comparative Physiology A. 2014;200:411–433. doi: 10.1007/s00359-014-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arikawa K. Spectral organization of the eye of a butterfly, Papilio. Journal of Comparative Physiology A Sensory Neural and Behavioral Physiology. 2003;189:791–800. doi: 10.1007/s00359-003-0454-7. [DOI] [PubMed] [Google Scholar]
- 18.Briscoe AD. Reconstructing the ancestral butterfly eye: focus on the opsins. J Exp Biol. 2008;211:1805–1813. doi: 10.1242/jeb.013045. [DOI] [PubMed] [Google Scholar]
- 19.Suvorov A, Jensen NO, Sharkey CR, Fujimoto MS, Bodily P, Wightman HM, Ogden TH, Clement MJ, Bybee SM. Opsins have evolved under the permanent heterozygote model: insights from phylotranscriptomics of Odonata. Mol Ecol. 2017;26:1306–1322. doi: 10.1111/mec.13884. [DOI] [PubMed] [Google Scholar]
- 20.Frentiu FD, Bernard GD, Sison-Mangus MP, Brower AVZ, Briscoe AD. Gene duplication is an evolutionary mechanism for expanding spectral diversity in the long-wavelength photopigments of butterflies. Mol Biol Evol. 2007;24:2016–2028. doi: 10.1093/molbev/msm132. [DOI] [PubMed] [Google Scholar]
- 21*.Tierney SM, Cooper SJ, Saint KM, Bertozzi T, Hyde J, Humphreys WF, Austin AD. Opsin transcripts of predatory diving beetles: a comparison of surface and subterranean photic niches. Roy Soc Open Sci. 2015;2:140386. doi: 10.1098/rsos.140386. The authors describe loss of opsins or of the complete eyes in diving beetle populations that colonized aquifiers as a new ecological niche. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinakevitch I, Douglass JK, Scholtz G, Loesel R, Strausfeld NJ. Conserved and convergent organization in the optic lobes of insects and isopods, with reference to other crustacean taxa. J Comp Neurol. 2003;467:150–172. doi: 10.1002/cne.10925. [DOI] [PubMed] [Google Scholar]
- 23.Wernet MF, Perry MW, Desplan C. The evolutionary diversity of insect retinal mosaics: common design principles and emerging molecular logic. Trends Genet. 2015;31:316–328. doi: 10.1016/j.tig.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinertzhagen IA. The organization of perpendicular fibre pathways in the insect optic lobe. Philos Trans R Soc Lond B Biol Sci. 1976;274:555–594. doi: 10.1098/rstb.1976.0064. [DOI] [PubMed] [Google Scholar]
- 25.Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Curr Biol. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnaitmann C, Garbers C, Wachtler T, Tanimoto H. Color Discrimination with Broadband Photoreceptors. Curr Biol. 2013;23:2375–2382. doi: 10.1016/j.cub.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Wardill TJ, List O, Li XF, Dongre S, McCulloch M, Ting CY, O’Kane CJ, Tang SM, Lee CH, Hardie RC, et al. Multiple Spectral Inputs Improve Motion Discrimination in the Drosophila Visual System. Science. 2012;336:925–931. doi: 10.1126/science.1215317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulk AC, Dacks AM, Phillips-Portillo J, Fellous JM, Gronenberg W. Visual processing in the central bee brain. J Neurosci. 2009;29:9987–9999. doi: 10.1523/JNEUROSCI.1325-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogt K, Aso Y, Hige T, Knapek S, Ichinose T, Friedrich AB, Turner GC, Rubin GM, Tanimoto H. Direct neural pathways convey distinct visual information to Drosophila mushroom bodies. Elife. 2016;5 doi: 10.7554/eLife.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154:459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briscoe AD. Functional diversification of lepidopteran opsins following gene duplication. Mol Biol Evol. 2001;18:2270–2279. doi: 10.1093/oxfordjournals.molbev.a003773. [DOI] [PubMed] [Google Scholar]
- 32.Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki T, Vogt K. Evolutionary aspects of the diversity of visual pigment chromophores in the class Insecta. Comp Biochem Phys B. 1998;119B:53–64. [Google Scholar]
- 34.Yokoyama S. Molecular evolution of vertebrate visual pigments. Prog Retin Eye Res. 2000;19:385–419. doi: 10.1016/s1350-9462(00)00002-1. [DOI] [PubMed] [Google Scholar]
- 35.Chang BSW, Crandall KA, Carulli JP, Hartl DL. Opsin phylogeny and evolution: a model for blue shifts in wavelength regulation. Mol Phylogen Evol. 1995;4:31–43. doi: 10.1006/mpev.1995.1004. [DOI] [PubMed] [Google Scholar]
- 36.Arikawa K, Mizuno S, Kinoshita M, Stavenga DG. Coexpression of two visual pigments in a photoreceptor causes an abnormally broad spectral sensitivity in the eye of the butterfly Papilio xuthus. J Neurosci. 2003;23:4527–4532. doi: 10.1523/JNEUROSCI.23-11-04527.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamdorf K, Hochstrate P, Hoglund G, Moser M, Sperber S, Schlecht P. Ultra-violet sensitizing pigment in blowfly photoreceptors R1-6; probable nature and binding sites. J Comp Physiol A. 1992;171:601–615. [Google Scholar]
- 38.Stavenga DG. Visual acuity of fly photoreceptors in natural conditions - dependence on UV sensitizing pigment and light-controlling pupil. J Exp Biol. 2004;207:1703–1713. doi: 10.1242/jeb.00949. [DOI] [PubMed] [Google Scholar]
- 39.Kirschfeld K, Franceschini N. Photostable pigments within the membrane of photoreceptors and their possible role. Biophysics of structure and mechanism. 1977;3:191–194. doi: 10.1007/BF00535818. [DOI] [PubMed] [Google Scholar]
- 40.Enright JM, Toomey MB, Sato S-y, Temple SE, Allen JR, Fujiwara R, Kramlinger VM, Nagy LD, Johnson KM, Xiao Y. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A 1 into A 2. Curr Biol. 2015;25:3048–3057. doi: 10.1016/j.cub.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stavenga DG, Meglic A, Pirih P, Koshitaka H, Arikawa K, Wehling MF, Belusic G. Photoreceptor spectral tuning by colorful, multilayered facet lenses in long-legged fly eyes (Dolichopodidae) J Comp Physiol A. 2017;203:23–33. doi: 10.1007/s00359-016-1131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernard GD, Miller WH. Interference filters in the corneas of Diptera. Invest Ophthalmol. 1968;7:416–434. [PubMed] [Google Scholar]
- 43.Stavenga DG. Colour in the eyes of insects. Journal of Comparative Physiology A Sensory Neural and Behavioral Physiology. 2002;188:337–348. doi: 10.1007/s00359-002-0307-9. [DOI] [PubMed] [Google Scholar]
- 44.Lunau K, Knuttel H. Vision through Colored Eyes. Naturwissenschaften. 1995;82:432–434. doi: 10.1007/BF01133678. [DOI] [PubMed] [Google Scholar]
- 45.Arikawa K, Mizuno S, Scholten DGW, Kinoshita M, Seki T, Kitamoto J, Stavenga DG. An ultraviolet absorbing pigment causes a narrow-band violet receptor and a single-peaked green receptor in the eye of the butterfly Papilio. Vision Res. 1999;39:1–8. doi: 10.1016/s0042-6989(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 46.Hardie RC. Functional organization of the fly retina. Progress in Sensory Physiology. 1985;5:1–79. [Google Scholar]
- 47.Kirschfeld K, Franceschini N, Minke B. Evidence for a sensitising pigment in fly photoreceptors. Nature. 1977;269:386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- 48.Toomey MB, Collins AM, Frederiksen R, Cornwall MC, Timlin JA, Corbo JC. A complex carotenoid palette tunes avian colour vision. J R Soc Interface. 2015;12:20150563. doi: 10.1098/rsif.2015.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.McCulloch KJ, Osorio D, Briscoe AD. Sexual dimorphism in the compound eye of Heliconius erato: a nymphalid butterfly with at least five spectral classes of photoreceptor. J Exp Biol. 2016;219:2377–2387. doi: 10.1242/jeb.136523. Female and male nymphalid H.erato butterfly eyes differ in Opsin number and spectral tuning: only females enhance their color vision by an additional UV opsin in the shortwavelengths and spectral tuning of an LW opsin in disctinct photoreceptors. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa Y, Kinoshita M, Stavenga DG, Arikawa K. Sex-specific retinal pigmentation results in sexually dimorphic long-wavelength-sensitive photoreceptors in the eastern pale clouded yellow butterfly, Colias erate. J Exp Biol. 2013;216:1916–1923. doi: 10.1242/jeb.083485. [DOI] [PubMed] [Google Scholar]
- 51.Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 52.Niven JE, Anderson JC, Laughlin SB. Fly photoreceptors demonstrate energy-information tradeoffs in neural coding. PLoS Biol. 2007;5:e116. doi: 10.1371/journal.pbio.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran D, Softley R, Warrant EJ. Eyeless Mexican Cavefish Save Energy by Eliminating the Circadian Rhythm in Metabolism. PLoS ONE. 2014;9:e107877. doi: 10.1371/journal.pone.0107877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moran D, Softley R, Warrant EJ. The energetic cost of vision and the evolution of eyeless Mexican cavefish. Sci Adv. 2015;1:e1500363. doi: 10.1126/sciadv.1500363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedrich M. Biological Clocks and Visual Systems in Cave-Adapted Animals at the Dawn of Speleogenomics. Integr Comp Biol. 2013;53:50–67. doi: 10.1093/icb/ict058. [DOI] [PubMed] [Google Scholar]
- 56.Friedrich M, Chen R, Daines B, Bao RY, Caravas J, Rai PK, Zagmajster M, Peck SB. Phototransduction and clock gene expression in the troglobiont beetle Ptomaphagus hirtus of Mammoth cave. J Exp Biol. 2011;214:3532–3541. doi: 10.1242/jeb.060368. [DOI] [PubMed] [Google Scholar]
- 57.Packard AS. The Cave Fauna of North America: With Remarks on the Anatomy of the Brain and Origin of the Blind Species. National Academy of Sciences; 1888. [Google Scholar]
- 58.Cronin TW, Bok MJ. Photoreception and vision in the ultraviolet. J Exp Biol. 2016;219:2790–2801. doi: 10.1242/jeb.128769. [DOI] [PubMed] [Google Scholar]
- 59.Manwaring KF, Whiting MF, Wilcox E, Bybee SM. A study of common scorpionfly (Mecoptera: Panorpidae) visual systems reveals the expression of a single opsin. Org Divers Evol. 2016;16:201–209. [Google Scholar]
- 60.Longden KD. Central Brain Circuitry for Color-Vision-Modulated Behaviors. Curr Biol. 2016;26:R981–R988. doi: 10.1016/j.cub.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 61.Kelber A. Colour in the eye of the beholder: receptor sensitivities and neural circuits underlying colour opponency and colour perception. Curr Opin Neurobiol. 2016;41:106–112. doi: 10.1016/j.conb.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Kawasaki M, Kinoshita M, Weckstrom M, Arikawa K. Difference in dynamic properties of photoreceptors in a butterfly, Papilio xuthus: possible segregation of motion and color processing. Journal of Comparative Physiology A Sensory Neural and Behavioral Physiology. 2015;201:1115–1123. doi: 10.1007/s00359-015-1039-y. [DOI] [PubMed] [Google Scholar]
- 63.Yang EC, Osorio D. Spectral sensitivities of photoreceptors and lamina monopolar cells in the dragonfly, Hemicordulia tau. J Comp Physiol A. 1991;169:663–669. [Google Scholar]
- 64.Yang EC, Osorio D. Spectral responses and chromatic processing in the dragonfly lamina. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1996;178:543–550. doi: 10.1007/BF00188161. [DOI] [PubMed] [Google Scholar]
- 65.Chen PJ, Arikawa K, Yang EC. Diversity of the photoreceptors and spectral opponency in the compound eye of the Golden Birdwing, Troides aeacus formosanus. PLoS One. 2013;8:e62240. doi: 10.1371/journal.pone.0062240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buschbeck EK, Ehmer B, Hoy RR. The unusual visual system of the Strepsiptera: external eye and neuropils. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2003;189:617–630. doi: 10.1007/s00359-003-0443-x. [DOI] [PubMed] [Google Scholar]
- 67.James M, Nandamuri P, Stahl A, Buschbeck EK. The unusual eyes of Xenos peckii (Strepsiptera: Xenidae) have green- and UV-sensitive photoreceptors. J Exp Biol. 2016;219:3866–3874. doi: 10.1242/jeb.148361. [DOI] [PubMed] [Google Scholar]
- 68.Chin AL, Lin CY, Fu TF, Dickson BJ, Chiang AS. Diversity and Wiring Variability of Visual Local Neurons in the Drosophila Medulla M6 Stratum. J Comp Neurol. 2014;522:3795–3816. doi: 10.1002/cne.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burkhardt D, DlM I. Electrophysiological studies on the eyes of Diptera, Mecoptera and Hymenoptera. In: Wehner R, editor. Information Processing in the Visual Systems of Arthropods. Springer-Verlag; 1972. pp. 147–153. [Google Scholar]
- 70.Meinertzhagen IA, Menzel R, Kahle G. The identification of spectral receptor types in the retina and lamina of the dragonfly Sympetrum rubicundulum. J Comp Physiol. 1983;151:295–310. [Google Scholar]
- 71**.Frentiu FD, Yuan F, Savage WK, Bernard GD, Mullen SP, Briscoe AD. Opsin Clines in Butterflies Suggest Novel Roles for Insect Photopigments. Mol Biol Evol. 2015;32:368–379. doi: 10.1093/molbev/msu304. Polymorphisms within the blue Opsin of 10 butterfly populations do not cause changes in spectral sensitivity, however are actively maintained in the genome. [DOI] [PubMed] [Google Scholar]
- 72.Thoen HH, How MJ, Chiou T-H, Marshall J. A Different Form of Color Vision in Mantis Shrimp. Science. 2014;343:411–413. doi: 10.1126/science.1245824. [DOI] [PubMed] [Google Scholar]
- 73.Land MF, Marshall JN, Brownless D, Cronin TW. The eye-movements of the mantis shrimp Odontodactylus scyllarus (Crustacea: Stomatopoda) J Comp Physiol A. 1990;167:155–166. [Google Scholar]
- 74**.Chen P-J, Awata H, Matsushita A, Yang E-C, Arikawa K. Extreme spectral richness in the eye of the Common Bluebottle butterfly, Graphium Sarpedon. Front Ecol Evol. 2016;4:18. Multimodal approach using electrophysiological, anatomical and molecular studies reveal fifteen different photoreceptor types - a record number for a single insect eye so far. Different strategies of spectral tuning enhance color vision based on solely 5 Opsins. [Google Scholar]
- 75.Hauser FE, van Hazel I, Chang BSW. Spectral Tuning in Vertebrate Short Wavelength-Sensitive 1 (SWS1) Visual Pigments: Can Wavelength Sensitivity be Inferred From Sequence Data? J Exp Zool Part B. 2014;322:529–539. doi: 10.1002/jez.b.22576. [DOI] [PubMed] [Google Scholar]
- 76.Perry MW, Desplan C. Love spots. Curr Biol. 2016;26:R484–R485. doi: 10.1016/j.cub.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Land MF, Eckert H. Maps of the acute zones of fly eyes. J Comp Physiol A. 1985;156:525–538. [Google Scholar]
- 78.Hardie RC. Projection and connectivity of sex-specific photoreceptors in the compound eye of the male housefly (Musca domestica) Cell & Tissue Research. 1983;233:1–21. doi: 10.1007/BF00222228. [DOI] [PubMed] [Google Scholar]
- 79.Hardie RC, Franceschini N, Ribi W, Kirschfeld K. Distribution and properties of sex-specific photoreceptors in the fly Musca domestica. Journal of Comparative Physiology. 1981;145:139–152. [Google Scholar]
- 80**.Perry M, Kinoshita M, Saldi G, Huo L, Arikawa K, Desplan C. Molecular logic behind the three-way stochastic choices that expand butterfly colour vision. Nature. 2016;535:280–284. doi: 10.1038/nature18616. Independent stochastic choices in two butterfly color-sensitive photoreceptors enhance color vision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Finkbeiner SD, Fishman DA, Osorio D, Briscoe AD. Ultraviolet and yellow reflectance but not fluorescence is important for visual discrimination of conspecifics by Heliconius erato. J Exp Biol. 2017;220:1267–1276. doi: 10.1242/jeb.153593. [DOI] [PubMed] [Google Scholar]
- 82.Ogawa Y, Awata H, Wakakuwa M, Kinoshita M, Stavenga DG, Arikawa K. Coexpression of three middle wavelength-absorbing visual pigments in sexually dimorphic photoreceptors of the butterfly Colias erate. J Comp Physiol A. 2012;198:857–867. doi: 10.1007/s00359-012-0756-8. [DOI] [PubMed] [Google Scholar]
- 83.Awata H, Wakakuwa M, Arikawa K. Evolution of color vision in pierid butterflies: blue opsin duplication, ommatidial heterogeneity and eye regionalization in Colias erate. Journal of Comparative Physiology A Sensory Neural and Behavioral Physiology. 2009;195:401–408. doi: 10.1007/s00359-009-0418-7. [DOI] [PubMed] [Google Scholar]
- 84*.Everett A, Tong X, Briscoe AD, Monteiro A. Phenotypic plasticity in opsin expression in a butterfly compound eye complements sex role reversal. BMC Evol Biol. 2012;12:232. doi: 10.1186/1471-2148-12-232. The authors describe a phenotypic plasticity in morphology and behavior indicating distinct adaptation to fluctuating seasonal environments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macias-Munoz A, Smith G, Monteiro A, Briscoe AD. Transcriptome-Wide Differential Gene Expression in Bicyclus anynana Butterflies: Female Vision-Related Genes Are More Plastic. Mol Biol Evol. 2016;33:79–92. doi: 10.1093/molbev/msv197. [DOI] [PubMed] [Google Scholar]
- 86.Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arikawa K, Stavenga DG. Random array of colour filters in the eyes of butterflies. J Exp Biol. 1997;200:2501–2506. doi: 10.1242/jeb.200.19.2501. [DOI] [PubMed] [Google Scholar]
- 88.Telles FJ, Rodriguez-Girones MA. Insect vision models under scrutiny: what bumblebees (Bombus terrestris terrestris L.) can still tell us. Science of Nature. 2015;102:4. doi: 10.1007/s00114-014-1256-1. [DOI] [PubMed] [Google Scholar]
- 89.Salcedo E, Zheng L, Phistry M, Bagg EE, Britt SG. Molecular basis for ultraviolet vision in invertebrates. J Neurosci. 2003;23:10873–10878. doi: 10.1523/JNEUROSCI.23-34-10873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


