Synopsis
Kaposi sarcoma herpes virus (KSHV)-associated multicentric Castleman disease (MCD) is a rare, polyclonal lymphoproliferative disorder characterized by flares of inflammatory symptoms, edema, cytopenias, lymphadenopathy and splenomegaly. Diagnosis is established with a lymph node biopsy. Pathogenesis is related to dysregulated inflammatory cytokines, including human and viral interleukin (IL)-6. Rituximab alone or in combination with chemotherapy, such as liposomal doxorubicin, has led to long-term overall survival of over 90%. Monoclonal antibodies targeting human interleukin-6 activity remain experimental as they do not target viral interleukin-6. KSHV-MCD patients are at high risk for Kaposi sarcoma and non-Hodgkin lymphomas, which may be concurrent.
Keywords: Kaposi sarcoma herpesvirus, human herpesvirus-8, multicentric Castleman disease, human interleukin-6, viral interleukin-6, rituximab, liposomal doxorubicin
Introduction
Castleman disease is a term used to describe a variety of pathologic entities ranging from indolent localized angiofollicular hyperplasia (unicentric Castleman disease), as first described by Benjamin Castleman in the 1950s, to multicentric lymphprolferations associated with inflammatory symptoms (multicentric Castleman disease, MCD). One epidemiogically distinct plasmablastic form of MCD described in patients with HIV and associated with high mortality1 was found to be caused by a newly discovered virus, called Kaposi sarcoma-associated herpesvirus (KSHV) or human herpesvirus-8 (HHV-8). KSHV was first identified as the etiologic agent for Kaposi sarcoma (KS) and is now recognized as the cause of almost all MCD in HIV-positive patients and rare cases of MCD in HIV-negative patients.2
KSHV-MCD is clinically characterized by intermittent inflammatory symptoms, cytopenias, edema lymphadenopathy and splenomegaly which often wax and wane. The diagnosis is confirmed pathologically, generally through a lymph node biopsy. Disease manifestations are associated with elevated levels of cytokines, especially interleukin (IL)-6 and IL-10.3–5 Untreated, KSHV-MCD is generally lethal within 2 years.5 Its rarity, intermittent manifestations, association with HIV, and non-specific symptoms make diagnosing KSHV-MCD a challenge. However, the past decade has seen the development of several effective therapies and substantial improvement in overall survival; therefore increased recognition and timely diagnosis is important.
Epidemiology
KSHV-MCD incidence is unknown and the disease is almost certainly underdiagnosed. Powles et. al. estimated the incidence of KSHV-MCD in HIV positive individuals to be 4.3 cases per 10,000 person-years and noted increasing incidence despite availability of effective antiretroviral therapy (ART) for HIV.6 KSHV-MCD often occurs in the setting of suppressed HIV, relatively preserved CD4+ T-cell counts and evidence of KSHV-specific CD8+ T-cell response.7,8 An improved understanding of the timing of KSHV-MCD diagnosis in relation to initiation of ART is required. It is possible that like KS and lymphoma, incidence is highest in the first year after ART initiation.9
KSHV-MCD is especially likely to be underdiagnosed in areas of sub-Saharan Africa with a high seroprevalence of both KSHV and HIV.10–12 Unlike developed countries where KSHV prevalence in the general population is 2–5%, KSHV is endemic in large parts of sub-Saharan African, with 40 to >80% of adults seropositive in much of the region.10,11 The lack of reported KSHV-MCD cases almost certainly represents underdiagnosis, as KSHV-MCD has been described among African immigrants.13,14 Due to lack of pathology services in many parts of sub-Saharan Africa, KS is sometimes treated empirically, and without evaluation for concurrent KSHV-MCD in suspected cases. Additionally, fevers and lymphadenopathy, when present, are often empirically treated as tuberculosis.13,15 Increased diagnostic capacity for KSHV-associated dieases, including KSHV-MCD, is needed in this setting.
Pathogenesis
KSHV is a gammaherpesvirus, most closely related to Epstein Barr virus, with latent and lytic phases characteristic of all herpesviruses. In addition to KSHV-MCD, it is the etiologic agent of KS, primary effusion lymphoma (PEL), and KSHV-associated diffuse large B cell lymphoma. Also, it is the cause of a newly identified condition called KSHV inflammatory cytokine syndrome (KICS), in which patients have severe inflammatory symptoms that mimic KSHV-MCD but lack the requisite pathologic findings of KSHV-MCD.16,17
KSHV encodes several proteins that allow for immune evasion via downregulation of surface proteins required for immune surveillance.18,19 The development of KSHV-MCD in HIV positive patients may be related to reduction or functional impairment of invariant natural killer T (iNKT) cells.20 iNKT cells play a major role in innate immunity and control of EBV infected B-cells through activation of glycolipid antigens presented by the major histocompatibiity complex class 1-related molecule, CD1d, as well as stimulating the expansion and maturation of other immune cells.21 In vitro studies of human tonsillar B cells suggest KSHV-MCD pathogenesis begins with KSHV infection via oral transmission of tonsillar IgM λ-expressing B cells that proliferate into plasmablasts characteristic of KSHV-MCD.22
Expression of latent and lytic genes varies among KSHV-associated disorders.23 In KS and PEL, the majority of genes expressed are latent genes with lytic proteins expressed in only a minority of cells, although in PEL, a KSHV-encoded viral interleukin 6 (vIL-6) is sometimes expressed in the absence of other lytic genes. In KSHV-MCD, however, a substantial proportion of the KSHV-infected plasmablasts in affected lymph nodes express lytic proteins. In some cases the full lytic repertoire is expressed, and in other cases only vIL-6 is expressed.23–25 Excess human cytokines, namely IL-6 (hIL-6), IL-10, tumor necrosis factor-α (TNFα), and IL-1 are also important in the pathogenesis of KSHV-MCD.5,26,27 vIL-6 shares 25% homology with its human counterpart. Unlike hIL-6, it binds directly to and signals through glycoprotein (gp)130, allowing it to affect a broad range of cells.28–30 By contrast, hIL-6 signaling requires binding of both the classical IL-6 receptor, gp80, as well its coreceptor, gp130, which is ubiquitously expressed. Similar to hIL-6, serum vIL-6 levels correlate with the symptoms and laboratory abnormalities associated with active disease.26,31 Although v-IL6 is often considered a lytic gene, it may be specifically upregulated in KSHV-MCD by X-box binding protein 1 (XBP-1).32 There is also evidence that vIL-6 itself activates hIL-6, further driving KSHV-MCD pathogenesis.33 Additional protein products of latently expressed genes also play a role in the pathogenesis of KSHV-MCD, particularly viral FLICE-inhibitory protein (vFLIP) which has been shown to induce significant disturbances in serum cytokines and expansion of suppressed myeloid cells allowing for host immune evasion, angiogenesis and tumor progression in mouse models.34
Diagnosis
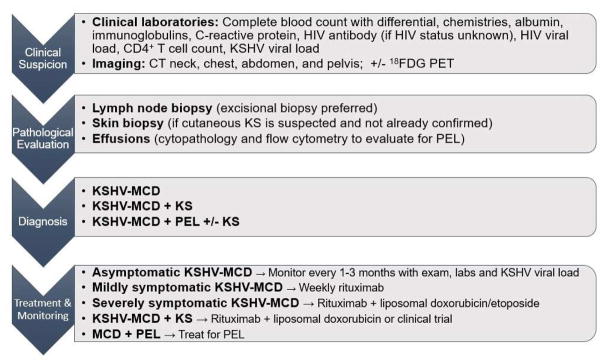
KSHV-MCD should be suspected in patients with an appropriate combination of risk factors and constellation of clinical and laboratory findings (Figure 1). Histopathologic confirmation of the diagnosis by lymph node biopsy is required. Populations at highest risk include men who have sex with men and sub-Saharan Africans. Diagnosis requires a high level of suspicion on the part of the clinician as the features of KSHV-MCD overlap significantly with those seen in uncontrolled infections and lymphoid malignancies. Clinical features of KSHV-MCD include fatigue, fevers, night sweats, weight loss, volume overload (including ascites and pulmonary effusions), rashes, and nonspecific neurologic, sinus, respiratory and gastrointestinal symptoms. The course may include relapsing and remitting symptoms. Many patients have concurrent KS and the clinician’s suspicion for KSHV-MCD should be raised in patients with KS and the aforementioned symptoms and laboratory findings. Computerized tomography classically shows diffuse lymphadenopathy and splenomegaly (Figure 2A).35 KSHV-MCD patients with active disease essentially always have an elevated C-reactive protein (CRP) and KSHV viral load.
Figure 1.
Schema for the Diagnosis and Management of KSHV-associated Multicentric Castleman Disease.
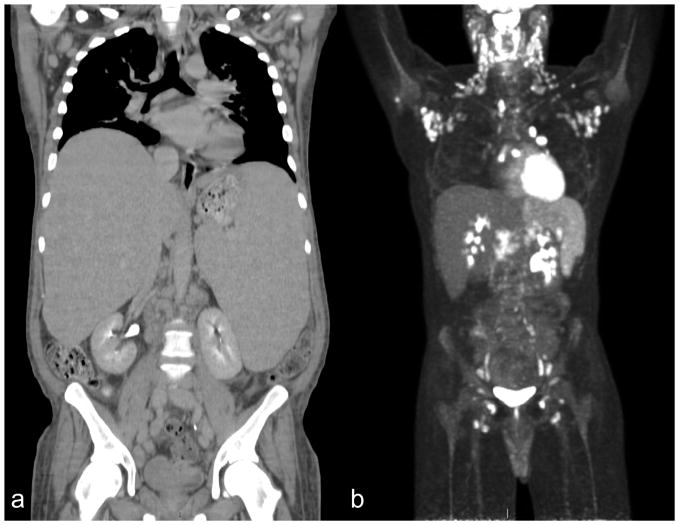
Figure 2.
Computerized Tomography (CT) and 18Fluorodeoxyglucose positron emission tomography (18FDG PET) in KSHV-associated Multicentric Castleman Disease (KSHV-MCD). (A) Computerized tomography scans of an HIV-positive patient with KSHV-MCD prior to treatment showing hepatosplenomegaly and axillary and retroperitoneal adenopathy. (B) 18FDG PET of an HIV-positive patient with KSHV-MCD showing characteristic 18FDG-avid cervical and axillary adenopathy, as well as splenomegaly with diffuse increased18FDG uptake.
Plasma or peripheral blood mononuclear cell (PBMC) measurements of KSHV viral load should be performed and monitored throughout the disease course as this correlates with active disease and response to treatment.5 Patients may exhibit several other laboratory abnormalities during active disease including cytopenias, hypoalbuminemia, hyponatremia and elevated γ-globulin.1,5,7 Biopsy confirmation of KS should be pursued if indicated, as concurrent KS has implications for the choice of KSHV-MCD treatment. All patients with KSHV-MCD should be tested for HIV. While plasma levels of hIL-6 and IL-10 correlate with disease activity and response to treatment, these cytokines are not routinely followed outside of the scope of research activities.5,27,36 Patients may become critically ill due to sepsis-like manifestations in the absence of infection driven by cytokine excess or exhibit pathologic and laboratory findings consistent with hemophagocytic syndrome.37
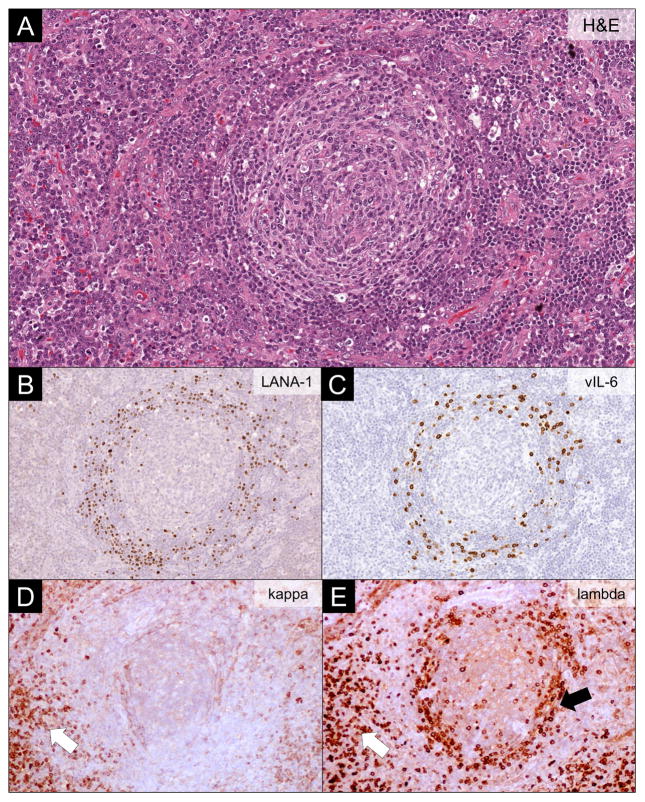
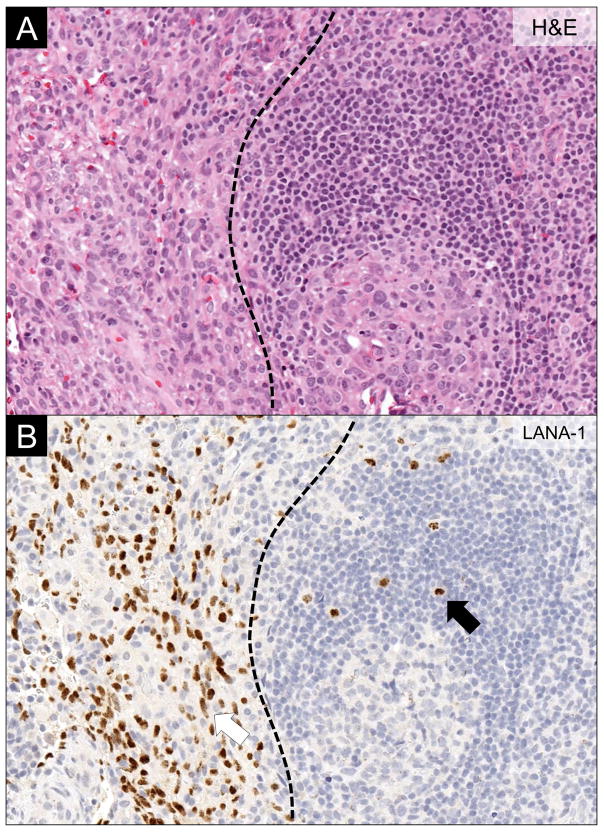
Histologically, KSHV-MCD-involved lymph nodes have expansion of KSHV-infected plasmablasts in the mantle zone of B cell follicles. They also have reactive KSHV-uninfected plasmablasts. KSHV-infected plasmablasts may form microscopic collections, sometimes referred to as microlymphomas, although these are generally polyclonal.38 KSHV infected plasmablasts stain positive for KSHV-associated latent nuclear antigen-1 (LANA-1), and a proportion also express vlL-6. Unlike KSHV-associated germinotropic lymphoproliferative disorder and some cases of PEL, KSHV-MCD plasmablasts are negative for Epstein-Barr virus.39,40 KSHV-infected plasmablasts express cytoplasmic IgM that is λ light-chain restricted but with a polyclonal pattern of immunoglobulin gene rearrangement (Figure 3).41 KS may be noted in the same lymph node (Figure 4). A bone marrow biopsy is sometimes performed to evaluate cytopenias, but is not required for diagnosis. In general, plasmacytosis is the predominant feature in the bone marrow with lymphoid aggregates and scattered KSHV-infected mononuclear cells less frequently seen.42
Figure 3.
Lymph Node Findings in KSHV-associated Multicentric Castleman Disease. (A) Hematoxylin and eosin (H&E) stain showing typical features of KSHV-associated multicentric Castleman disease. The involved lymph nodes have a regressed germinal center surrounded by layered mantle cells, vascular proliferation and hyalinization, and plasmacytosis in the interfollicular regions. (B) LANA-1 immunohistochemistry highlighting KSHV-infected plasmablasts residing in the mantle cell layers. (C) vIL-6 immunohistochemistry showing vIL-6 in a proportion of KSHV-infected plasmablasts. (D-E) Kappa and lambda light chain immunohistochemistry showing restricted lambda expression in the KSHV-infected plasmablasts (black arrow), while the interfollicular plasma cells are polytypic (white arrow).
Figure 4.
Concominant Lymph Node Kaposi Sarcoma and KSHV-associated Multicentric Castleman Disease. (A) H&E stain demonstrating concomitant Kaposi sarcoma (left of the dotted line) and multicentric Castleman disease (MCD) (right of the dotted line). (B) LANA-1 immunohistochemistry highlighting the KSHV-infected cells. Note the different cytomorphology of Kaposi sarcoma cells (red arrow) and the plasmablasts in MCD (black arrow).
It is possible to diagnose KSHV-MCD from a lymph node core needle biopsy but an excisional lymph node biopsy is often required. In general, the most easily accessible enlarged lymph node should be chosen for pathologic evaluation. However, if an excisional lymph node biopsy does not show KSHV-MCD and the diagnosis is strongly suspected, 18-fluorodeoxyglucose positron emission tomography (18FDG-PET) scan should be performed. In KSHV-MCD, the most common findings are hypermetabolic symmetric 18FDG-avid adenopathy with increased uptake notable in the spleen and bone marrow (Figure 2B).35 Suspicious lymph nodes, which are often the largest and/or most FDG avid, should be biopsied. Any effusions require cytopathologic examination. Effusions in KSHV-MCD may have elevated KSHV viral loads and show proliferation of polyclonal λ-restricted B cells; however, this finding has not been established as a method to definitively diagnose KSHV-MCD. More importantly, effusions should be examined to rule out PEL via cytopathology, flow cytometry and B-cell clonality. In addition to PEL, patients with KSHV-MCD are at high risk of developing other non-Hodgkin lymphomas.38,43,44
Treatment
Although ART is generally insufficient for the treatment of KSHV-MCD, it is indicated for all patients with HIV. ART should be used concurrently along with KSHV-MCD-specific treatment in HIV-positive patients.6,45 Patients with HIV-associated KSHV-MCD may obtain long remissions on ART with appropriate KSHV-MCD therapy. Although the effect of ART in preventing relapses is not proven, ART decreases mortality related to HIV.46,47
There is currently no Food and Drug Administration (FDA)-approved therapy for KSHV-MCD. Several cytotoxic chemotherapies with clinical activity in B-cell lymphomas have been used to treat KSHV-MCD, including etoposide, vincristine, vinblastine, cyclophosphamide and doxorubicin.1 However, chemotherapy alone is relatively ineffective. In one analysis between 1985 and 2006, the reported survival with these chemotherapies either alone or in combination was poor, with a median overall survival of 12 months. However, major improvement in treatment responses and survival came with the development of rituximab, a humanized monoclonal antibody against CD20 antigen on B-cells.
Three prospective treatment studies and several cohort studies all show a substantial treatment effect of rituximab in KSHV-MCD. It is interesting that rituximab is effective despite the fact that proliferating KSHV-infected B cells in KSHV-MCD are usually CD20 negative. This may be due to the fact that KSHV uninfected CD20 positive B cells within the microenvironment secrete inflammatory cytokines and also serve as a major potential reservoir of KSHV infection and replication.41 Two prospective phase 2 studies published in 2007 established the efficacy of rituximab in KSHV-MCD.48,49 In the CastlemaB Trial, 24 patients with chemotherapy-dependent HIV-associated KSHV-MCD were administered 4 weekly infusions of rituximab (375 mg/m2) after the discontinuation of chemotherapy. Overall survival was 92% and 71% remained in remission at 1 year. A common problem was exacerbation of KS, noted in 8 out of 12 patients with a previous diagnosis of KS despite the use of ART.49 Bower et. al. reported similar results in 21 previously untreated patients with symptomatic HIV-associated KSHV-MCD treated with 4 weekly doses of rituximab 375 mg/m2. At 2 years, overall survival was 95% and disease-free survival was 79%. However, more than one third of the patients had progression of KS.48
To address the issue of KS exacerbation with the use of rituximab, a prospective trial evaluated the treatment of HIV-associated KSHV-MCD with a combination of rituximab 375 mg/m2 and liposomal doxorubicin 20 mg/m2, an FDA-approved KS therapy, every 3 weeks for a median of 4 cycles. Another potential advantage of concurrent use of liposomal doxubicin is that it can potentially target KSHV-MCD plasmablasts. Twelve of the 17 patients treated had concurrent KS. Exacerbation of KS was seen in only 1 patient, and KS regressed in most patients. The 3-year overall survival with this combination regimen was 81% and the event-free survival was 69%.14 Rituximab also appears to be an effective therapy for KSHV-MCD in HIV-uninfected patients.50 Infusion reactions that include rigors and fevers are common during the first administration of rituximab and may be due to a cytokine release syndrome. Premedication with steroids and benadyl, as well as slow infusion rates during the first cycle are warranted. When infusion reactions do occur, they are generally short-lived and can be managed with meperidine. Infusions can be resumed at slower rates after resolution of symptoms. Although linked to exacerbation of KS, treatment with rituximab is associated with a substantially lower risk of non-Hodgkin’s lymphoma in KSHV-MCD patients.44
Anti-herpesvirus therapy has also been evaluated in KSHV-MCD Remissions have been described with the use of ganciclovir in one small retrospective study.51 Two KSHV lytic genes, ORF36 and ORF21, encode enzymes that can phosphorylate ganciclovir and AZT, respectively, leading to accumulation of triphosphate moieties which are toxic to KSHV-infected cells. Because of the role of viral enzymes in drug activation, non-KSHV-infected cells are spared.52 Based upon the principle of “virus activated cytotoxic therapy,” a pilot study showed activity of high-dose zidovudine (AZT) (600 mg orally every 6 hours) and valganciclovir (900 mg orally every 12 hours) in HIV positive KSHV-MCD patients. Twelve of 14 patients achieved major clinical benefit with an 86% overall survival at 4 years. However, the median progression-free survival was only 6 months. While rituximab-based therapy is generally the treatment of choice, high-dose AZT and valganciclovir may be a useful alternative for patients with mild disease or who cannot tolerate rituximab.53
We follow a risk-stratified approach to treatment of KSHV-MCD, which takes into account the presence of concurrent KS or severe symptoms (Figure 1 and Table 1). In patients with mild symptoms and laboratory abnormalities, 4 weekly doses of rituximab are generally sufficient to induce clinical remission in the vast majority of patients. Several case series have reported failure of rituximab in patients with aggressive disease, poor performance status, and organ failure.54,55 Therefore, in patients with severe symptoms or a concurrent diagnosis of KS, we usually treat patients with rituximab combined with liposomal doxorubicin every 3 weeks.14 More frequent initial rituximab dosing, i.e weekly during the first cycle, in combination with liposomal doxorubicin may also be appropriate. Excellent results of weekly rituximab combined with etoposide 100 mg/m2 for KSHV-MCD patients with severe symptoms have also been described, although etoposide is a less effective drug for treating KS.56 In the rituximab era, combination chemotherapy does not have a role in treating KSHV-MCD except in the setting of concurrent lymphoma.
Table 1.
Treatments for KSHV-MCD
| Therapy | Dose | Mechanism of action | When to use |
|---|---|---|---|
| Rituximab | 375 mg/m2 weekly x4 weeks | Depletes IL-6 secreting CD20+ B cells | Mild symptomatic disease |
| Rituximab + | Rituximab 375 mg/m2 | Addition of cytotoxic | Aggressive |
| liposomal doxorubicin | + liposomal doxorubicin 20 mg/m2 every 3 weeks until response plateau | chemotherapy to treat CD20 negative MCD plasmablasts and KS spindle cells | disease and/or concurrent KS |
| Rituximab + etoposide | Rituximab 375 mg/m2 + etoposide 100 mg/m2 IV weekly x4 weeks | Addition of cytotoxic chemotherapy to treat CD20 negative MCD plasmablasts | Aggressive disease |
| Zidovidine (AZT) + valganciclovir | Zidovidine 600 mg PO every 6 hours + valganciclovir 900 mg PO every 12 hours days 1–7 of 21-day cycle | Virus activated cytotoxic therapy | Mild disease with concurrent KS and/or patients allergic to rituximab |
Treatment of KSHV-MCD should be individualized. The initiation of KSHV-MCD therapy should not be based on abnormal radiographic findings alone, but instead upon symptoms and abnormal laboratory findings. Duration of therapy should also be symptom based. There are no consensus guidelines for treatment response in KSHV-MCD. The Castlemab Trial defined criteria for an MCD attack warranting treatment as the presence of fever, elevated CRP and presence of 3 clinical symptoms. The National Cancer Institute (NCI) Criteria for Treatment includes at least one clinical symptom; the presence of anemia, thrombocytopenia, or hypoalbuminemia; and an elevated CRP.53 We generally treat until resolution of clinical symptoms and significant improvement in these marker laboratory abnormalities. Like KSHV-MCD treatment, there is no standardized approach for patient follow-up. We generally see patients in clinic and measure their KSHV viral load every 3 months for the first year after therapy and less frequently thereafter. Patients with a persistently elevated KSHV viral load, or residual adenopathy or splenomegaly are at higher risk of relapse, and may require more frequent monitoring, although we generally do not treat patients with these findings in the absence of symptoms and laboratory abnormalities.57 Although consolidation or maintenance therapy have been used in the past, there is no clear role for these approaches. A recent study of HIV-positive KSHV-MCD patients treated with rituximab-based therapy found that the 5-year relapse-free survival was 82% without maintenance rituximab or anti-herpes drugs. In addition, all patients with disease relapse were successfully retreated with rituximab-based therapy.56 Therefore, there is little potential benefit to maintenance therapy as relapse rates are low and remission can be reinduced in the majority of patients. Importantly, KSHV-MCD patients should be monitored for the development of other KSHV-associated diseases.43
While rituximab has revolutionized KSHV-MCD treatment, there remains a need for rituximab-sparing approaches, especially for patients with concurrent advanced KS, HIV patients with CD4+ counts <100 cells/uL, for patients allergic to rituximab, and in patients in resource-limited settings. Monoclonal antibodies targeting hIL-6 activity have been developed for KSHV-negative (idiopathic) MCD and several rheumatologic conditions and are promising for KSHV-MCD given the role hIL-6 plays in the pathogenesis of symptomatic disease. Siltuximab, a monoclonal antibody that binds directly to hIL-6, is FDA-approved for the treatment of idiopathic MCD but has not been evaluated in KSHV-MCD58,59. Tocilizumab, a humanized monoclonal antibody against the IL-6 receptor is FDA-approved for rheumatoid arthritis, and has shown activity in other forms of MCD and also 2 KSHV-associated cases.60 Targeting hIL-6 is not expected to directly influence vIL-6 activity, which plays an essential role in pathogenesis of symptomatic KSHV-MCD. Therefore anti-IL6 agents may require combination with other approaches, such as virus-activated cytotoxic therapy.33 We are currently studying tocilizumab in KSHV-MCD in a clinical trial at the NCI (NCT01441063). Pomalidomide has been shown to be effective against KS, and in the laboratory it can prevent the KSHV-induced downregulation of MHC-1.61,62 Based on these findings,our group is exploring the combination of pomalidomide and liposomal doxorubicin in patients with concurrent KS and KSHV-MCD (NCT02659930). We hypothesize that combination chemoimmunotherapy will debulk high KS tumor burden while decreasing KSHV driven immune evasion and counteracting paracrine growth signals within the microenvironment.62
Conclusion
KSHV-MCD is a rare and potentially deadly B cell lymphoproliferative disorder with waxing and waning symptoms that make diagnosis a challenge. Physicians should have a high index of suspicion for KSHV-MCD, especially in high risk patients. Symptoms and laboratory abnormalities are associated with KSHV lytic activation and elevated inflammatory cytokines. Rituximab-based therapies have led to a drastic improvement with most recent studies reporting greater than 90% overall 5-year survival.56 As KSHV-MCD patients continue to live longer with established and experimental treatment approaches, clinicians must remain vigilant for KSHV-MCD relapse and development of other KSHV-associated diseases.
Key Points.
KSHV-MCD is a B-cell lymphoproliferative disorder caused by KSHV that is characterized by waxing and waning inflammatory symptoms, laboratory abnormalities, edema, adenopathy, and splenomegaly. It is most common in patients with HIV.
Four weekly doses of rituximab 375 mg/m2 leads to remission in the majority of mildly symptomatic patients but may lead to exacerbation of concurrent KS.
Rituximab 375 mg/m2 plus liposomal doxorubicin 20 mg/m2 administered every 3 weeks effectively treats patients with aggressive disease or concurrent KS.
Rituximab-based treatment has increased 5-year overall survival to over 90%.
Current studies are evaluating targeted rituximab-sparing approaches that may decrease toxicity and/or be appropriate for patients with concurrent Kaposi sarcoma.
Acknowledgments
We thank Hao-Wei Wang for pathology images.
Footnotes
Disclosures
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute. Research of the authors is supported in part by a CRADA between the National Cancer Institute and Celgene Corp. TSU and RY are co-inventors on a patent application related to the treatment of KSHV-associated diseases with pomalidomide, and the spouse of RY is a co-inventor on a patent related to the measurement of KSHV vIL-6. These inventions were all made as part of their duties as employees of the US Government, and the patents are or will be assigned to U.S. Department of Health and Human Services. The government may convey a portion of the royalties it receives from licensure of its patents to its employee inventors. Finally, RY and TSU have recently conducted clinical research using drugs supplied to the NCI by Merck and Co., Hoffman LaRoche, and Bayer Healthcare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oksenhendler E, Duarte M, Soulier J, et al. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10(1):61–67. [PubMed] [Google Scholar]
- 2.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- 3.Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74(4):1360–1367. [PubMed] [Google Scholar]
- 4.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990;86(2):592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood. 2000;96(6):2069–2073. [PubMed] [Google Scholar]
- 6.Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman's disease. Ann Oncol. 2009;20(4):775–779. doi: 10.1093/annonc/mdn697. [DOI] [PubMed] [Google Scholar]
- 7.Bower M, Newsom-Davis T, Naresh K, et al. Clinical Features and Outcome in HIV-Associated Multicentric Castleman's Disease. J Clin Oncol. 2011;29(18):2481–2486. doi: 10.1200/JCO.2010.34.1909. [DOI] [PubMed] [Google Scholar]
- 8.Guihot A, Oksenhendler E, Galicier L, et al. Multicentric Castleman disease is associated with polyfunctional effector memory HHV-8-specific CD8+ T cells. Blood. 2008;111(3):1387–1395. doi: 10.1182/blood-2007-03-080648. [DOI] [PubMed] [Google Scholar]
- 9.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011;117(5):1089–1096. doi: 10.1002/cncr.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat Med. 1996;2(8):925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 11.Maskew M, Macphail AP, Whitby D, Egger M, Wallis CL, Fox MP. Prevalence and predictors of kaposi sarcoma herpes virus seropositivity: a cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect Agent Cancer. 2011;6:22. doi: 10.1186/1750-9378-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shebl FM, Dollard SC, Pfeiffer RM, et al. Human herpesvirus 8 seropositivity among sexually active adults in Uganda. PLoS One. 2011;6(6):e21286. doi: 10.1371/journal.pone.0021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopal S, Liomba NG, Montgomery ND, et al. Characteristics and survival for HIV-associated multicentric Castleman disease in Malawi. J Int AIDS Soc. 2015;18:20122. doi: 10.7448/IAS.18.1.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uldrick TS, Polizzotto MN, Aleman K, et al. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood. 2014;124(24):3544–3552. doi: 10.1182/blood-2014-07-586800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal S, Wood WA, Lee SJ, et al. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119(22):5078–5087. doi: 10.1182/blood-2012-02-387092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polizzotto MN, Uldrick TS, Wyvill KM, et al. Clinical Features and Outcomes of Patients With Symptomatic Kaposi Sarcoma Herpesvirus (KSHV)-associated Inflammation: Prospective Characterization of KSHV Inflammatory Cytokine Syndrome (KICS) Clin Infect Dis. 2016;62(6):730–738. doi: 10.1093/cid/civ996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uldrick TS, Wang V, O'Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis. 2010;51(3):350–358. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coscoy L, Ganem D. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci U S A. 2000;97(14):8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115(5):1369–1378. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sbihi Z, Dossier A, Boutboul D, et al. iNKT and memory B-cell alterations in HHV-8 multicentric Castleman disease. Blood. 2017;129(7):855–865. doi: 10.1182/blood-2016-06-719716. [DOI] [PubMed] [Google Scholar]
- 21.Chung BK, Tsai K, Allan LL, et al. Innate immune control of EBV-infected B cells by invariant natural killer T cells. Blood. 2013;122(15):2600–2608. doi: 10.1182/blood-2013-01-480665. [DOI] [PubMed] [Google Scholar]
- 22.Hassman LM, Ellison TJ, Kedes DH. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J Clin Invest. 2011;121(2):752–768. doi: 10.1172/JCI44185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parravicini C, Chandran B, Corbellino M, et al. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am J Pathol. 2000;156(3):743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staskus KA, Sun R, Miller G, et al. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J Virol. 1999;73(5):4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katano H, Sato Y, Kurata T, Mori S, Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology. 2000;269(2):335–344. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 26.Polizzotto MN, Uldrick TS, Wang V, et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood. 2013;122(26):4189–4198. doi: 10.1182/blood-2013-08-519959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bower M, Veraitch O, Szydlo R, et al. Cytokine changes during rituximab therapy in HIV-associated multicentric Castleman disease. Blood. 2009;113(19):4521–4524. doi: 10.1182/blood-2008-12-197053. [DOI] [PubMed] [Google Scholar]
- 28.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum Immunol. 1999;60(10):921–927. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 29.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274(5293):1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 30.Aoki Y, Jones KD, Tosato G. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. J Hematother Stem Cell Res. 2000;9(2):137–145. doi: 10.1089/152581600319351. [DOI] [PubMed] [Google Scholar]
- 31.Aoki Y, Tosato G, Fonville TW, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood. 2001;97(8):2526–2527. doi: 10.1182/blood.v97.8.2526. [DOI] [PubMed] [Google Scholar]
- 32.Hu D, Wang V, Yang M, et al. Induction of Kaposi's Sarcoma-Associated Herpesvirus-Encoded Viral Interleukin-6 by X-Box Binding Protein 1. J Virol. 2015;90(1):368–378. doi: 10.1128/JVI.01192-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suthaus J, Stuhlmann-Laeisz C, Tompkins VS, et al. HHV-8-encoded viral IL-6 collaborates with mouse IL-6 in the development of multicentric Castleman disease in mice. Blood. 2012;119(22):5173–5181. doi: 10.1182/blood-2011-09-377705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballon G, Akar G, Cesarman E. Systemic expression of Kaposi sarcoma herpesvirus (KSHV) Vflip in endothelial cells leads to a profound proinflammatory phenotype and myeloid lineage remodeling in vivo. PLoS Pathog. 2015;11(1):e1004581. doi: 10.1371/journal.ppat.1004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polizzotto MN, Millo C, Uldrick TS, et al. 18F-fluorodeoxyglucose Positron Emission Tomography in Kaposi Sarcoma Herpesvirus-Associated Multicentric Castleman Disease: Correlation With Activity, Severity, Inflammatory and Virologic Parameters. J Infect Dis. 2015;212(8):1250–1260. doi: 10.1093/infdis/jiv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newsom-Davis T, Bower M, Wildfire A, et al. Resolution of AIDS-related Castleman's disease with anti-CD20 monoclonal antibodies is associated with declining IL-6 and TNF-alpha levels. Leuk Lymphoma. 2004;45(9):1939–1941. doi: 10.1080/10428190410001693533. [DOI] [PubMed] [Google Scholar]
- 37.Fardet L, Blum L, Kerob D, et al. Human herpesvirus 8-associated hemophagocytic lymphohistiocytosis in human immunodeficiency virus-infected patients. Clin Infect Dis. 2003;37(2):285–291. doi: 10.1086/375224. [DOI] [PubMed] [Google Scholar]
- 38.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406–1412. [PubMed] [Google Scholar]
- 39.Chadburn A, Hyjek EM, Tam W, et al. Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD) Histopathology. 2008;53(5):513–524. doi: 10.1111/j.1365-2559.2008.03144.x. [DOI] [PubMed] [Google Scholar]
- 40.Du MQ, Diss TC, Liu H, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100(9):3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 41.Wang HW, Pittaluga S, Jaffe ES. Multicentric Castleman disease: Where are we now? Semin Diagn Pathol. 2016;33(5):294–306. doi: 10.1053/j.semdp.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkataraman G, Uldrick TS, Aleman K, et al. Bone marrow findings in HIV-positive patients with Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Am J Clin Pathol. 2013;139(5):651–661. doi: 10.1309/AJCPKGF7U8AWQBVG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331–2336. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 44.Gerard L, Michot JM, Burcheri S, et al. Rituximab decreases the risk of lymphoma in patients with HIV-associated multicentric Castleman disease. Blood. 2012;119(10):2228–2233. doi: 10.1182/blood-2011-08-376012. [DOI] [PubMed] [Google Scholar]
- 45.Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116(22):4415–4421. doi: 10.1182/blood-2010-07-290213. [DOI] [PubMed] [Google Scholar]
- 46.Aaron L, Lidove O, Yousry C, Roudiere L, Dupont B, Viard JP. Human herpesvirus 8-positive Castleman disease in human immunodeficiency virus-infected patients: the impact of highly active antiretroviral therapy. Clin Infect Dis. 2002;35(7):880–882. doi: 10.1086/342696. [DOI] [PubMed] [Google Scholar]
- 47.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147(12):836–839. doi: 10.7326/0003-4819-147-12-200712180-00003. [DOI] [PubMed] [Google Scholar]
- 49.Gerard L, Berezne A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25(22):3350–3356. doi: 10.1200/JCO.2007.10.6732. [DOI] [PubMed] [Google Scholar]
- 50.Dossier A, Meignin V, Fieschi C, Boutboul D, Oksenhendler E, Galicier L. Human herpesvirus 8-related Castleman disease in the absence of HIV infection. Clin Infect Dis. 2013;56(6):833–842. doi: 10.1093/cid/cis1009. [DOI] [PubMed] [Google Scholar]
- 51.Casper C, Nichols WG, Huang M-L, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004;103(5):1632–1634. doi: 10.1182/blood-2003-05-1721. [DOI] [PubMed] [Google Scholar]
- 52.Davis DA, Singer KE, Reynolds IP, Haque M, Yarchoan R. Hypoxia enhances the phosphorylation and cytotoxicity of ganciclovir and zidovudine in Kaposi's sarcoma-associated herpesvirus infected cells. Cancer Res. 2007;67(14):7003–7010. doi: 10.1158/0008-5472.CAN-07-0939. [DOI] [PubMed] [Google Scholar]
- 53.Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117(26):6977–6986. doi: 10.1182/blood-2010-11-317610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuville S, Agbalika F, Rabian C, Briere J, Molina JM. Failure of rituximab in human immunodeficiency virus-associated multicentric Castleman disease. Am J Hematol. 2005;79(4):337–339. doi: 10.1002/ajh.20418. [DOI] [PubMed] [Google Scholar]
- 55.Buchler T, Dubash S, Lee V, et al. Rituximab failure in fulminant multicentric HIV/human herpesvirus 8-associated Castleman's disease with multiorgan failure: report of two cases. AIDS. 2008;22(13):1685–1687. doi: 10.1097/QAD.0b013e3282f7371f. [DOI] [PubMed] [Google Scholar]
- 56.Pria AD, Pinato D, Roe J, Naresh K, Nelson M, Bower M. Relapse of HHV8-positive multicentric Castleman disease following rituximab-based therapy in HIV-positive patients. Blood. 2017;129(15):2143–2147. doi: 10.1182/blood-2016-10-747477. [DOI] [PubMed] [Google Scholar]
- 57.Stebbing J, Adams C, Sanitt A, et al. Plasma HHV8 DNA predicts relapse in individuals with HIV-associated multicentric Castleman disease. Blood. 2011;118(2):271–275. doi: 10.1182/blood-2011-02-335620. [DOI] [PubMed] [Google Scholar]
- 58.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9(13):4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 59.van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman's disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15(9):966–974. doi: 10.1016/S1470-2045(14)70319-5. [DOI] [PubMed] [Google Scholar]
- 60.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 61.Polizzotto MN, Uldrick TS, Wyvill KM, et al. Pomalidomide for Symptomatic Kaposi's Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J Clin Oncol. 2016;34(34):4125–4131. doi: 10.1200/JCO.2016.69.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis DA, Mishra S, Anagho HA, et al. Restoration of immune surface molecules in Kaposi sarcoma-associated herpesvirus infected cells by lenalidomide and pomalidomide. Oncotarget. 2017 doi: 10.18632/oncotarget.17960. [DOI] [PMC free article] [PubMed] [Google Scholar]