Abstract
The majority of older patients who develop heart failure (HF), particularly older women, have a preserved left ventricular ejection fraction (HFpEF). The prevalence of this syndrome is increasing and prognosis is not improving in comparision to HF with reduced ejection fraction (HFrEF). Patients with HFpEF have severe symptoms of effort intolerance, poor quality-of-life, frequent hospitalizations, and increased mortality. Despite the importance of HFpEF, there are numerous major gaps in our understanding of its pathophysiology and management. Originally viewed as a disorder due solely to abnormalities in left ventricular diastolic function, our understanding has evolved such that HFpEF is now understood as a systemic syndrome, involving multiple organ systems, likely triggered by inflammation and other as yet unidentified circulating factors, and with important contributions of aging and multiple-comorbidities, features generally typical of other geriatric syndromes. Here we present an update on the pathophysiology, diagnosis, management, and future directions in this important disorder among older persons.
Keywords: heart failure, preserved ejection fraction, aging, elderly, comorbidities
Introduction
Clinical significance
Heart failure (HF) with preserved ejection fraction (HFpEF) is the most common form of HF in patients older than 65 years;1 among older women, >80% of new cases of HF are HFpEF.2 Among nonagenarians, nearly all patients with HF have preserved EF.3 In contrast to HF with reduced ejection fraction (HFrEF), the prevalence of HFpEF is increasing and its prognosis is not improving, which may be due to the combination of aging of the population and increased rates of obesity.4 The health and economic impact of HFpEF is at least as great as that of HFrEF.4;5 The combined mortality and readmission rates 90 day post-discharge are comparable to HFrEF (35%).6 One-year mortality for HFpEF ranges up to 29%,4;7 and increases with increased burden of comorbidities.8 While cardiovascular (CV) events are the most common cause of death, non-cardiac causes of death are very common, and account for a significant proportion of deaths in HFpEF.9 Patients with HFpEF have high rehospitalization rates,.6 and the majority of rehospitalizations are for non-cardiac causes.5 In addition, HFpEF patients have poor quality-of-life, similar in severity to patients with HFrEF.10
Clinical Manifestations of HFpEF
Clinical manifestations of HFpEF are generally similar to those of HFrEF. In the chronic, stable state, even when relatively euvolemic and well-compensated, HFpEF patients have severe exercise intolerance, characterized by exertional fatigue and dyspnea which is associated with poor quality-of-life. However, HFpEF patients also have intermittent acute exacerbations, with severe dyspnea, volume overload, body edema, and pulmonary edema. These acute exacerbations are ofte associated with dietary indiscretion, medication non-compliance, markedly elevated systolic blood pressure (BP), atrial fibrillation (AF), myocardial ischemia, renal dysfunction, and pulmonary infections, but can also occur in their absence.11
Diagnosis of HFpEF
Evaluation of new onset HF in an older patient should include an imaging test, such as an echocardiogram. Not only will an echocardiogram assess systolic function, but may also discover unexpected but important diagnoses, such as valvular abnormalities, large pericardial effusion, hypertrophic obstructive cardiomyopathy, and cardiac amyloidosis. While echocardiography is an important initial test, HFpEF is not necessarily an echocardiographic diagnosis; rather the echocardiogram can provide helpful supportive findings in addition to identifying other causes of HF symptoms. The 2013 American College of Cardiology / American Heart Association (ACC/AHA) Consensus Guidelines defined HFpEF largely as a diagnosis of exclusion: typical symptoms and signs of HF, preserved EF on an imaging study, and no other obvious cause to explain the patient’s symptoms, such as marked anemia or thyroid dysfunction.12 As suggested in the 2017 ACC/AHA Focused Update on HF, measurement of natriuretic peptide biomarkers [B-type natriuretic peptide (BNP) or N-terminal pro b-type natriuretic peptide (NT-proBNP)] can be helpful in the diagnosis of HF.12 However, multiple studies have reported that: natriuretic peptides are significantly lower in HFpEF patients compared with HFrEF;13 and natriuretic peptide levels are inversely related to body mass index, highly relevant since obesity is very common in HFpEF.14 Natriuretic peptide levels are paradoxically inversely related to treatment benefit,15 and their change does not correlate well with symptom improvement.16 In addition, BNP levels increase with age in normal populations free of LV dysfunction,17 and female gender is an independent predictor of BNP levels in the older adult population, even without cardiac dysfunction.18 Thus age and gender can affect BNP and NT-proBNP levels, further reducing their diagnostic value in older persons.17:18 Therefore, we believe that HFpEF remains a clinical diagnosis, and that the ACC/AHA guidelines above are appropriate for clinical practice.
Evolution in Our Understanding of the Pathophysiology of HFpEF
The earliest description of HFpEF was by Robert Luchi in the 1982 Journal.19 Dr. Luchi noted that in his patients aged ≥75 years admitted with acute congestive HF, nuclear imaging studies, a relatively new development at the time, frequently showed a relatively normal LVEF, rather than a severely reduced LVEF, which was universally thought to be a requisite for HF.19 Although this syndrome was first recognized in 1982, it was not until 2001 when it was mentioned in the ACC/AHA HF management guidelines, where it was termed “HF due to diastolic dysfunction”. In the 2005 guidelines, the syndrome was referred to as the more generic and less pathophysiologically presumptious term “HF with preserved EF”.20 Since then, HFpEF has become accepted as a true and distinct clinical syndrome.21 Thirty-five years after its initial description, the fundamental pathophysiological mechanisms of HFpEF remain incompletely defined.
One of the most commonly cited mechanisms of HFpEF has been LV diastolic dysfunction, consisting of abnormal LV active relaxation and /or increased LV passive stiffness.22 While diastolic dysfunction is a pivotal factor in many cases of HFpEF, many patients do not have echo-Doppler indices of severe diastolic dysfunction, at least at rest, or that differ greatly from that expected based on age and comorbidities.23;24 However, most HFpEF studies have only measured diastolic function at rest rather than during exercise where symptoms become manifest. 25 Significant LV hypertrophy was previously thought to be a uniform characteristic of HFpEF. However, many HFpEF patients have concentric remodeling without hypertrophy or even normal LV geometry and mass.26;27 In addition, a recent trial of well characterized HFpEF patients showed that only 8% of patients had LV hypertrophy at baseline, and 50% had significant or severe diastolic dysfunction at rest. In addition, while cardiac fibrosis is commonly present in HFpEF, the degree appears modest.28
These data have led to reconsideration of the initial hypothesis of HTN-induced LV hypertrophy, diastolic dysfunction, and cardiac fibrosis as the sole or fundamental underlying pathophysiological mechanisms of HFpEF. Additonally, this process was driven by the discovery of several novel contributing factors to HFpEF, including: mild LV systolic dysfunction;26;29 impaired systolic reserve in both LV and right ventricle (RV);30 impaired heart rate (HR) recovery and chronotropic incompetence (CI);31–33 abnormal ventricular vascular coupling;34 reduced vasodilator reserve;32;33 cardiac aging;35 neuroendocrine dysfunction;13 left atrial (LA) dysfunction;36;37 impaired resting pulmonary arterial (PA) and RV function; abnormal PA vasodilatory reserve as well as abnormal RV-PA coupling;30;38–40 altered pulmonary function and gas exchange including low diffusion capacity;41 impaired oxygen carrying capacity, peripheral oxygen extraction, and contributions from skeletal muscle dysfunction, including adipose infiltration, reduced muscle mass, capillarity, and mitochondrial density (Figure 1).42–45 As a result of this evolution in our patholophysiological understanding, this disorder underwent a name change approximately 10 years ago, from the presumptive term ‘Diastolic HF’, to the broader, more phenomenological term, HFpEF.21
Figure 1.
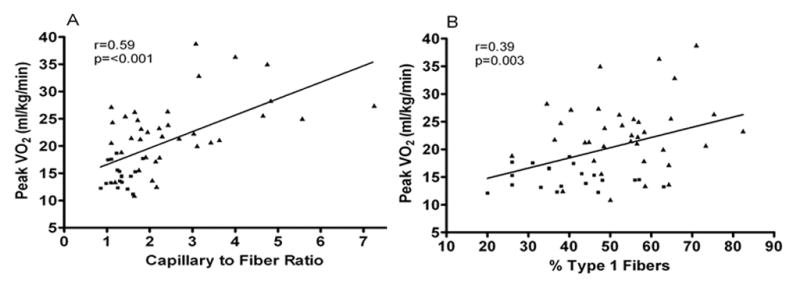
Relationship of capillary-to-fiber ratio (A) and percentage of type I muscle fibers (B) with peak O2 uptake (VO2) in older patients with heart failure with preserved ejection fraction (■) and age-matched healthy control subjects (▲).
Role of multimorbidity
Older patients hospitalized with a primary diagnosis of HF often have multiple non cardiac comorbidities (5.5 on average).46 Those with preserved EFs have more non cardiac comorbidities (4 on average) than those with reduced EF.47;48 The most important non-cardiac comorbidities are obesity, HTN, diabetes (DM), chronic obstructive lung disease (COPD), anemia, chronic kidney disease (CKD), fraility, cognitive dysfunction, dementia, cerebrovascular disease, peripheral arterial disease, liver disease and rheumatological disorders.47
Cardiac comorbidities
Coronary artery disease is present in 25% to 68% of HFpEF patients and is associated with greater deterioration in LV function and increased mortality, including CV death.49;50 An autopsy study recently showed that epicardial CAD was frequent and extensive in HFpEF.24 An important observation in this study was decreased coronary microvascular density present in HFpEF patients. Atrial fibrillation prevalence has been increasing due to aging of the general population and increased longevity. AF in HFpEF is associated with impaired LV systolic and diastolic functional reserves, larger LA with poor LA function, more severe neurohumoral activation, and impaired exercise tolerance.51:52 Hypertension is the most prevalent risk factor for HF, and precedes the diagnosis of HF in 75–85% of persons who develop HF. Along with afterload excess and LV hypertrophy, fibrosis and increased arterial stiffness, HTN also induces inflammation, oxidative stress and endothelial dysfunction in HFpEF.53
Non-Cardiac Comorbidities
In the older HFpEF population, non-cardiac comorbidities are very common and are related to the high rate of non-HF admissions and deaths.47 Metabolic risk factors: HFpEF patients have a high prevalence of obesity and DM. About 75% of normotensive well controlled DM patients without CAD show evidence of LV diastolic dysfunction with echocardiographic tissue Doppler imaging.54 Approximately 85% of older HFpEF patients are overweight or obese, a rate twice the general population, and the HFpEF epidemic has largely paralleled the obesity epidemic.55 Excess adipose tissue can produce impairments relevant to HFpEF by 2 general categories of mechanisms: local/mechanical and systemic/metabolic. Examples of local/mechanical mechanisms include enhanced pericardial restraint,56 decreased oxygen diffusion from capillaries to working skeletal muscle cells,57 accelerated coronary atherosclerosis associated with epicardial adipose, and accelerated renal dysfunction associated with perirenal fat. Excess adipose tissue can also produce a plethora of adverse systemic/metabolic effects, including promoting inflammation, HTN, insulin resistance, and dyslipidemia and impairing cardiac, arterial, skeletal muscle, and physical function,58–60 all of which are common in HFpEF and contribute to its pathophysiology.61
Up to one-third of HFpEF patients may have COPD,47;62 which contributes to increased symptoms and mortality.1;47;62 Moreover, HF patients with preserved EF are more likely than those with reduced EF to receive a COPD diagnosis.63 In addition, even in the absence of formal COPD diagnosis, patients with HFpEF have multiple pulmonary abnormalities that contribute to their worse symptoms and poor outcomes.64 Sleep-disordered breathing, pre and post capillary pulmonary HTN and restrictive lung disease are highly prevalent in patients with obesity and HTN and may contribute to clinical HF and dyspnea.65 Obstructive sleep apnea is common in patients with HFpEF with a prevalence of 69% to 81%, and is independently associated with a worse prognosis, even when HF therapy is optimal.66 In addition, the pulmonary vasculature responds to hypoxia with vasoconstriction leading to increases in both RV and LV afterload which in the chronic setting can lead to chronic pulmonary remodeling.67 Furthermore, RV dysfunction is common in HFpEF and predictive of clinical outcome.39 Anemia is more prevalent in HFpEF than in HFrEF patients and associated with increased risk of HF hospitalization and overall mortality.68 HFpEF and CKD commonly co-exist and have shared pathogenic mechanisms such as neurohumoral activation, inflammation and oxidative stress.69
Physical frailty and cognitive dysfunction
In older patients hospitalized primarily for HF, many factors outside the heart such as advanced age, globally reduced organ system reserve capacity, physical frailty, impaired cognition, and comorbidities (often numerous and severe) strongly influence outcomes.70 Frailty is associated with worse outcome in older patients with HF. It is a strong independent predictor of all-cause mortality and associated with 92% increased risk for emergency visits and 22% increased risk for hospitalization.71 Similarly, older patients with incident HF have significant functional and cognitive impairments.72 A recent study showed that there is also a high prevalence of subclinical cerebral infarction among patients with HFpEF, even in the absence of AF.73 In addition, the hospital environment—with immobilization, fasting, sleep deprivation, and disorientation—can dramatically worsen physical frailty with rapid, severe loss of muscle mass and function.70 Treatment of HFpEF is much more challenging in patients with cognitive limitations. Thus, when older HF patients are thought to be ready for discharge, their multiple comorbidities, globally reduced organ reserve, severe physical deconditioning, and cognitive dysfunction often remain unaddressed.70 The result is the “post-hospitalization syndrome,” with high rates of rehospitalization, mortality, and nursing home admission, prolonged physical disability, poor quality-of-life, and high health care costs.74 These multiple non-cardiac co-morbidities not only contribute to the pathophysiology of HFpEF, but are strong contributors to exercise intolerance in chronic HFpEF and to the high rate of clinical events, including hospitalizations and death. Indeed, in contrast to HFrEF, in HFpEF non-cardiac comorbidities account for the majority of all-cause hospitalizations and mortality.47
New Paradigm for the Pathophysiology of HFpEF
The involvement of this broad array of abnormalities in HFpEF, the recognition of the high frequency, severity, and impact of multiple non-cardiac comorbidities, systemic, multiorgan involvement, and its nearly exclusive existence in older persons, has led to recognition of HFpEF as a true geriatric syndrome. It has also led to a new paradigm of HFpEF, whereby aging along with multiple comorbidities in HFpEF may initiate and/or aggravate chronic systemic inflammation that may affect myocardial remodeling and dysfunction in HFpEF through a signaling cascade, which may begin with coronary microvascular endothelial dysfunction (Figure 2).8;75 This reduces myocardial nitric oxide (NO) bioavailability and leads to reduced protein kinase G (PKG) activity in cardiomyocytes, which become stiff and hypertrophied.8 These alterations also promote microvascular rarefaction and dysfunction in cardiac 24 and skeletal muscle.57;76
Figure 2.
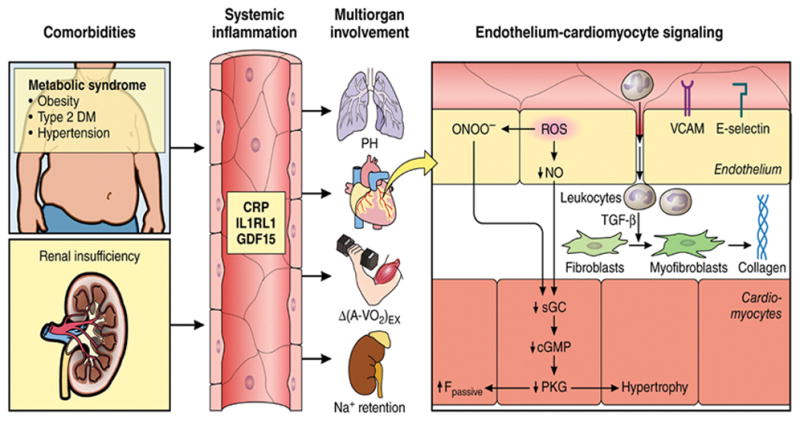
Systemic and myocardial signaling in HFpEF. Comorbidities induce systemic inflammation, evident from elevated plasma levels of inflammatory biomarkers such as soluble interleukin 1 receptor-like 1 (IL1RL1), C-reactive protein (CRP), and growth differentiation factor 15 (GDF15). Chronic inflammation affects the lungs, myocardium, skeletal muscle, and kidneys leading to diverse HFpEF phenotypes with variable involvement of pulmonary hypertension (PH), myocardial remodeling, deficient skeletal muscle oxygen extraction (ΔA-Vo2) during exercise (Ex), and renal Na+ retention. Myocardial remodeling and dysfunction begins with coronary endothelial microvascular inflammation manifest from endothelial expression of adhesion molecules such as vascular cell adhesion molecule (VCAM) and E-Selectin. Expression of adhesion molecules attracts infiltrating leukocytes secreting transforming growth factor β (TGF-β), which converts fibroblasts to myofibroblasts with enhanced interstitial collagen deposition. Endothelial inflammation also results in the presence of reactive oxygen species (ROS), reduced nitric oxide (NO) bioavailability, and production of peroxynitrite (ONOO– ). This reduces soluble guanylate cyclase (sGC) activity, cyclic guanosine monophosphate (cGMP) content, and the favorable effects of protein kinase G (PKG) on cardiomyocyte stiffness and hypertrophy. HFpEF indicates heart failure with preserved ejection fraction. Reproduced from Circulation with permission. Circulation. 2016;134:73–90.
Management of HFpEF
Because HFpEF is a relatively recently acepted disorder, and because most of the moderate number of clinical trials to date have generally been neutral on their primary outcomes, management remains largely empiric. Below we review general approaches, non-pharmacological interventions, the clinical medication trials reported to date, device interventions, and published consensus guideline recommendations (See Table 1-Clinical Synopsis).
Table 1.
Clinical Synopsis
|
HFpEF=heart failure with preserved ejection fraction; HF=heart failure; HTN=hypertension
General approach
Management goals in elders with HFpEF include relief of symptoms, improvement in functional capacity and quality-of-life, prevention of acute exacerbations and related hospital admissions, and prolongation of survival. A systematic approach should comprise several of the following elements: diagnosis and staging of disease, search for reversible etiology, judicious use of medications, patient education, enhancement of self-management skills, coordination of care across disciplines, and effective follow-up. HF patients should have a scale, weigh regularly, and know what steps to take if weight increases beyond pre-specified ranges. Diuretic adjustments can be performed as needed by nurses over the telephone, and in some cases by the patients themselves. There must be easy access to health care providers so that problems can be addressed early in order to avoid decompensation and hospitalizations. Examples include : periodic telephone calls, frequent follow-up appointments, and monitoring programs utilizing telephone and the internet.
Non-pharmacological Approaches
Exercise training
In a single-blind trial evaluating the effects of 16 weeks of endurance exercise training (ET) in older patients with HFpEF, ET was associated with increased peak oxygen consumption (VO2), ventilatory anaerobic threshold, 6 minute walk distance, and improved physical quality-of-life scores with ET.77 These results were confirmed in a subsequent multicenter, randomized trial of 3 months of combined ET and strength training in HFpEF patients.78 In a second, separate, randomized, attention-controlled, single-blind trial of 4 months of upper and lower extremity endurance ET, a significant increase in peak VO2 was demonstrated without alterations in carotid arterial stiffness or brachial artery flow mediated dilation.79 A subsequent multicenter trial confirmed that ET improves exercise capacity and symptoms.80 In a recent pilot study, 4 weeks of high-intensity interval training significantly improved peak VO2 and LV diastolic dysfunction in HFpEF patients.81 Further, a recent meta-analysis demonstrated ET in HFpEF patients was associated with improvements in cardiorespiratory fitness and quality-of-life without significant changes in LV systolic or diastolic function, and another study showed that endurance ET increased peak VO2 more in HFpEF than HFrEF.82;83
Since ET is one of the only proven interventions for improving symptoms and quality-of-life in HFpEF patients, what are the mechanisms for these improvements? ET may improve exercise capacity either by increasing exercise cardiac output (CO) (via increased HR or stroke volume), or by increasing arterio-venous oxygen difference (A-VO2 diff) by improvement in peripheral vascular function leading to increased diffusive oxygen transport or increased oxygen utilization by the skeletal muscle. Studies to date indicate that an ET-induced increase in A-VO2diff is the primary contributor to ET related improvement in peak VO2.84 Indeed, skeletal muscle oxidative metabolism is impaired at baseline in older patients with HFpEF, but it can be favorably shifted by ET to a more efficient muscle O2 utilization.42 In addition, there does not appear to be any ET-related beneficial effect on LV diastolic function in older HFpEF patients, even after 1 year of exercise.85 Although the above studies support mechanisms for the beneficial effects of ET that are independent of LV systolic or diastolic function, some studies have attributed ET related improvements to exercise-induced favorable changes in LV function and CO, atrial reverse remodeling and improved LV diastolic function.78;81
Dietary Modifications
As previosly stated, ≥80% of older HFpEF patients are overweight or obese. Because increased body adiposity promotes inflammation and impairs cardiac, arterial, renal, and skeletal muscle function, weight loss could be beneficial in the large proportion of patients with the obese HFpEF phenotype. Recently it has been shown that in older obese patients with clinically stable HFpEF, weight loss via caloric restriction diet was feasible and appeared safe and significantly improved exercise capacity and quality-of-life, and the effect was additive to ET (Figure 3, Online supplemetal Table 2).86 Caloric restriction improved quality-of-life much more than ET. The improvements from caloric restriction were associated with reduced total body and skeletal muscle adipose and reduced inflammation. In a recent trial in symptomatic patients with chronic HF (25% of whom had HFpEF), dietary sodium restriction was associated with increased risk of adverse outcomes, particularly HF hospitalization.87
Figure 3.
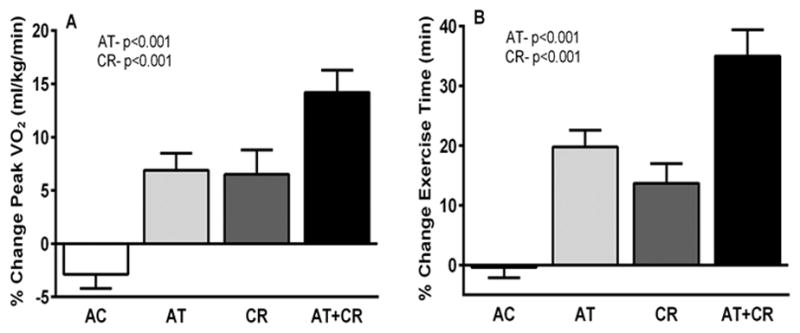
Effects of a 20-week caloric restriction diet on exercise capacity and quality of life in HFpEF. The graph displays percent changes ± standard errors at the 20-week follow-up relative to baseline by randomized group for peak VO2 (mL·kg–1·min–1, A) and exercise time (min), P values represent effects for AT and CR. AT indicates aerobic exercise training; and CR, caloric restriction diet.
Pharmacological Therapies
Online supplemetal Table 1 summarizes the pharmacological therapies tested to date in HFpEF.
Targeting Renin-Angiotensin-Aldosterone System (RAAS) Signaling
For a variety of reasons, the RAAS was the most obvious target for potential HFpEF therapies because RAAS antagonists are highly beneficial for HFrEF and HTN. However, of the 3 large randomized trials of angiotensin-converting enzyme inhibiotrs/angiotensin receptor blockers (ACEI/ARB) performed to date in HFpEF, only the CHARM-Preserved study found nominal benefit for candesartan in reducing HF hospitalizations over three years of follow-up.88–90 The aldosterone antagonist trials also showed no definite benefit.10;91;92 However, a post-hoc geographical analysis from TOPCAT indicated that the cohort from the Americas most closely matched characteristics observed in other randomized trials and also appeared most responsive to spironolactone.93 Despite these mixed results, given the lack of other alternatives and the relatively low cost and moderate rate of side effects of low dose spironolactone, some have suggested this agent for the purpose of reducing hospitalizations.
Targeting beta-Adrenergic Signaling and HR
Slowing the HR should result in an increase in the diastolic filling period in an abnormally stiff LV, thus potentially allowing greater end diastolic volume and stroke volume. However, none of the beta blocker trials had positive effects on their primary outcomes in HFpEF patients (Online supplemetal Table 1 ). The findings from beta blocker trials are consistent with multiple exercise physiology studies that have shown that HFpEF patients have a high rate of intrinsic chronotropic incompetence and that this is a significant contributor to their reduced exercise capacity.31–33;94
Targeting Defective cGMP Signaling
As discussed previously in the pathophysiology sectin, according to the new HFpEF paradigm, endothelial inflammation results in reduced NO bioavailability and production of peroxynitrite. This reduces soluble guanylate cyclase (sGC) activity, cGMP content and its effector kinase PKG activity. Thus, targeting defective cGMP-PKG signaling could represent a novel therapy for HFpEF treatment. Sildenafil is an inhibitor of phosphodiesterase-5 that increases cGMP levels, thus augmenting PKG activity in multiple organs relevant to HF. In the RELAX trial, sildenafil did not improve 6 minute walk distance or quality-of-life, and was associated with modest worsening of renal function and increases in neurohormone levels.95 Other pharmacological trials targeting cGMP–PKG signaling are summarized in Online supplemetal Table 1.
Targeting natriuretic peptide-cGMP signaling
Neprilysin is a zinc-dependent metalloprotease that degrades biologically active natriuretic peptides and does not affect the biologically inactive NTproBNP.96 LCZ696 is a new combination drug of the angiotensin II type-1 receptor blocker valsartan and the neprilysin inhibitor pro-drug AHU377. It has been shown to improve mortality in HFrEF. In PARAMOUNT, LCZ696 significantly lowered NT-pro BNP levels and, at 36 weeks, decreased LA size and showed a trend toward improved functional class.96 This phase-2 study led to an ongoing large, multi-center trial, PARAGON, in patients with HFpEF with the primary composite outcome of CV death or first hospitalization for HF (ClinicalTrials.gov NCT01920711).
Device Therapy
The CARDIOMEMS device is a wireless, implanted PA pressure monitor implanted in the distal PA during a right heart catheterization procedure. Patients transmit hemodynamic data daily using a wireless transmitter. The CHAMPION trial showed a significant reduction in HF hospitalizations.97 Device therapy trials in HFpEF are summarized in Online supplemetal Table 2.
Prevention of HFpEF
Since most therapies tested to date have been relatively ineffective for established HFpEF, additional emphasis should be placed on prevention. Many reports suggest that the incidence of new HFpEF cases can be reduced by the following factors: systolic HTN management, regular physical activity, prevention and treatment of obesity, and management of other aforementioned comorbidities, including DM, CAD and AF.48;82;86;98
Published Guidelines for Treatment of HFpEF
Given the lack of positive clinical outcome trials, there is little evidence to base strong guideline recommendations. Therefore, the ACC/AHA consensus guideline recommendations are relatively sparse regarding management of HFpEF. In patients with symptomatic HFpEF, diuretics are recommended to relieve symptoms due to volume overload (Class I with level of evidence B); however, the optimal diuretic dosing regimens have not been clarified.12 Control of HTN is suggested as an important treatment strategy for HFpEF (Class I with level of evidence B).12 However, this is empiric and based on data from studies in non-HF populations. The BP goals in the ACC/AHA HF guideline are similar to those in the general population, with the exception that the 2017 ACC/AHA HF guideline update became one of the first in the U.S. to recommend the lower systolic BP target of 130mmHg, based on the potent results of the SPRINT trial.12;99 In SPRINT, both HFpEF and HFrEF incident cases were significantly reduced, including specifically in patients >75 years old.100 ARBs and aldosterone antagonists receive a relatively weak recommendation (class IIb, level of evidence B) as reasonable to consider for decreasing hospitalizations in HFpEF.
Other ACC/AHA guidelines recommendations include treatment of common comorbidities, including overt myocardial ischemia, restoration and maintenance of sinus rhythm, control of HR in patients with permanent AF, treatment of anemia and formal sleep assessment in HF patients with suspicion of sleep disordered breathing or excessive daytime sleepiness. To restore sinus rhythm, cardioversion is recommended because catheter ablation of AF has had limited long-term success in HFpEF.101 If cardioversion is unsuccessful, rate control and permanent anticoagulation become mandatory.
Although it is proven that exercise is the only strategy (other than potentially diet) known to improve symptoms and exercise capacity (and probably quality-of-life), the Centers for Medicare and Medicaid Services (CMS) excluded HFpEF in their 2014 decision to reimburse for cardiac rehabilitation for HF patients. Unfortunately, it is currently unknown whether ET that begins outside of a monitored supervised setting is safe for older HFpEF patients. However, current ACC/AHA guidelines recommend moderate, regular physical activity for all HF patients, which seems reasonable.
HFpEF as a true geriatric syndrome
Given all the above, it is clear that HFpEF has emerged as a true geriatric syndrome, fulfilling all the following formal criteria.102 1) It is uncommon in younger persons, but highly prevalent in older adults, particularly women over age 80 years, in whom it comprises nearly 100% of new HF cases. 2) It is a chronic debilitating condition with multifactorial pathophysiology. 3) It is very heterogeneous clinically with underlying age-related changes, frequent multiple chronic co-morbidities and multi-organ involvement that render persons vulnerable to situational challenges. 4) It shares common risk factors—including older age, cognitive impairment, functional impairment and impaired mobility. 5) It is strongly associated with functional decline and poor clinical outcomes. 6) It is associated with frequent hospitalizations and high rate of readmissions due to non-cardiac causes. 7) Diagnostic strategies to identify the underlying causes can sometimes be ineffective, burdensome and costly. 8) Therapeutic management of the clinical manifestations can be helpful even in the absence of a firm diagnosis or clarification of the underlying causes. Thus, due to inherent complexity in caring for older adults with cardiovascular disease, it will be fruitful for geriatricians and cardiovascular specialists to collaborate and develop a new model in treating HFpEF patients employing geriatric principles.
Conclusions and Future Directions
HFpEF is currently the most common form of HF, is nearly unique to the older population, and is a true geriatric sydrome. Non-pharmacological therapies, including disease management, ET, and caloric restriction weight loss in obese patients, have been shown to be effective. However, pharmacological trials have generally been neutral on their primary outcomes, including both clinical endpoints and exercise function. An evolving paradigm suggests that, like other geriatric syndromes, HFpEF is complex and multi-factorial, likely systemic, with multi-factorial pathophysiology, underlying age-related changes, frequent multiple chronic co-morbidities, multi-organ involvement, and clinical heterogeneity. If so, novel approaches that have systemic effects and influence inflammation and multiple organ systems may be fruitful.98 In addition, future studies should account for emerging data indicating the presence of multiple, potentially distinct HFpEF phenotypes.98 Continued progress is imperative, since HFpEF is the most common cardiovascular disorder for which there are no therapies definitively shown to alter prognosis.
Supplementary Material
Acknowledgments
Supported in part by NIH grants R01AG18915, R01AG045551, and P30AG12232, and by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine
Footnotes
Author Contributions: Upadhya: drafting and critical review of manuscript. Pisani: critical review of final manuscript. Kitzman: concept and design, interpretation of data, critical review of, intellectual contributions to approve of final manuscript.
Potential Financial Conflicts of Interest:
Dr. Kitzman declares the following relationships: Consultant for: Abbvie; Bayer; Merck; Medtronic; St. Luke’s Medical Center, Kansas City, MO; GSK; Relypsa; Corvia Medical; Boehringer-Ingelheim; and Actavis; research grant funding from Novartis; and stock ownership in Gilead Sciences.
Dr. Upadhya has received research funding from Novarits and Corvia.
References
- 1.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 2.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin JM, Kitzman DW. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The Cardiovacular Health Study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 3.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 5.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure: A Report From the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 7.Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in olmsted county, minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulus W, Tschope C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Patel RB, Michel A, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69:556–569. doi: 10.1016/j.jacc.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Pfeffer M, Assmann S, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 11.Klapholz M, Maurer M, Lowe AM, et al. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: Results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–1438. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 14.Stavrakis S, Pakala A, Thomas J, Chaudhry MA, Thadani U. Obesity, Brain Natriuretic Peptide Levels and Mortality in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Systolic Function. Am J Med Sci. 2013;345:211–217. doi: 10.1097/MAJ.0b013e318271c012. [DOI] [PubMed] [Google Scholar]
- 15.Kociol RD, McNulty SE, Hernandez AF, et al. Markers of Decongestion, Dyspnea Relief, and Clinical Outcomes Among Patients Hospitalized With Acute Heart Failure. Circ Heart Fail. 2013;6:240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand IS, Claggett B, Liu J, et al. Interaction between Spironolactone and Natriuretic Peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT Trial. JACC Heart Fail. 2017;5:241–252. doi: 10.1016/j.jchf.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Sayama H, Nakamura Y, Saito N, Kinoshita M. Why is the concentration of plasma brain natriuretic peptide in elderly inpatients greater than normal? Coron Artery Dis. 1999;10:537–540. doi: 10.1097/00019501-199910000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Maisel AS, Clopton P, Krishnaswamy P, et al. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. Am Heart J. 2004;147:1078–1084. doi: 10.1016/j.ahj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Luchi RJ, Snow E, Luche JM. Left ventricular function in hospitalized geriatric patients. J Am Geriatr Soc. 1982;30:700–705. doi: 10.1111/j.1532-5415.1982.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 20.WRITING COMMITTEE. Hunt SA, Abraham WT, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Kitzman DW. Heart Failure: A rose by another name? Congest Heart Fail. 2006;12:166–168. doi: 10.1111/j.1527-5299.2006.05520.x. [DOI] [PubMed] [Google Scholar]
- 22.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman DW, Upadhya B, Vasu S. What the dead can teach the living: the systemic nature of heart failure with preserved ejection fraction. Circulation. 2015;131:522–524. doi: 10.1161/CIRCULATIONAHA.114.014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA. Exercise haemodynamics and outcome in patients with dyspnoea. Eur Heart J. 2014;35:3085–3087. doi: 10.1093/eurheartj/ehu350. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and Ventricular Systolic Stiffening in Hypertensive Heart Disease: Insights Into the Pathogenesis of Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zile MR, Gottdiener JS, Hetzel SJ, et al. Prevalence and Significance of Alterations in Cardiac Structure and Function in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 28.Su MY, Lin LY, Tseng YH, et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. doi: 10.1016/j.jcmg.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:446–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 33.Phan T, Shivu G, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 34.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer R, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 35.Kitzman DW, Taffet G. Effects of aging on cardiovascular structure and function. In: Halter JB, Ouslander JG, Tinetti ME, Studenski SA, High KP, Asthana S, editors. Hazzard’s Geriatric Medicine and Gerontology. 6. New York: McGraw Hill; 2009. pp. 883–895. [Google Scholar]
- 36.Freed BH, Daruwalla V, Cheng JY, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016:9. doi: 10.1161/CIRCIMAGING.115.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left Atrial Volume, Geometry, and Function in Systolic and Diastolic Heart Failure of Persons >=65 Years of Age (The Cardiovascular Health Study) Am J Cardiol. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 38.Andersen MJ, Hwang SJ, Kane GC, et al. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–550. doi: 10.1161/CIRCHEARTFAILURE.114.002114. [DOI] [PubMed] [Google Scholar]
- 39.Melenovsky V, Hwang S, Lin G, Redfield M, Borlaug B. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammed SF, Hussain I, Abou Ezzeddine OF, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson TP, Johnson BD, Borlaug BA. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:490–498. doi: 10.1016/j.jchf.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky SB, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina AJ, Bharadwaj MS, Van Horn C, et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016;4:636–645. doi: 10.1016/j.jchf.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 47.Ather S, Chan W, Bozkurt B, et al. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population With Heart Failure and Preserved Versus Reduced Ejection Fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 49.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 50.Rusinaru D, Houpe D, Szymanski C, Levy F, Marechaux S, Tribouilloy C. Coronary artery disease and 10-year outcome after hospital admission for heart failure with preserved and with reduced ejection fraction. Eur J Heart Fail. 2014;16:967–976. doi: 10.1002/ejhf.142. [DOI] [PubMed] [Google Scholar]
- 51.Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:123–130. doi: 10.1161/CIRCHEARTFAILURE.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lam CS, Rienstra M, Tay WT, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. 2017;5:92–98. doi: 10.1016/j.jchf.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Cohen RA, Tong X. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. J Cardiovasc Pharmacol. 2010;55:308–316. doi: 10.1097/fjc.0b013e3181d89670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyer JK, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–875. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 55.Ndumele CE, Coresh J, Lazo M, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–607. doi: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haykowsky MJ, Tomczak CR, Scott JM, Patterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 2015;119:739–744. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beavers K, Beavers D, Houston D, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Normandin E, Houston DK, Nicklas BJ. Caloric restriction for treatment of geriatric obesity: Do the benefits outweigh the risks? Curr Opin Cardiol. 2015;4:143–155. doi: 10.1007/s13668-015-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma K, Kass D. Heart Failure With Preserved Ejection Fraction: Mechanisms, Clinical Features, and Therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caruana L, Petrie MC, Davie AP, McMurray JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart faiure” or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitzman DW, Guazzi M. Impaired Alveolar Capillary Membrane Diffusion: A Recently Recognized Contributor to Exertional Dyspnea in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:499–501. doi: 10.1016/j.jchf.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Guazzi M, Borlaug B. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 66.Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11:602–608. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 67.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–127. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 68.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008;121:726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colombo P, Ganda A, Lin J, et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17:177–190. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 71.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and Healthcare Utilization Among Patients With Heart Failure in the Community. JACC Heart Fail. 2013;1:135–141. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murad K, Goff DC, Jr, Morgan TM, et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: The Cardiovascular Health Study. JACC Heart Fail. 2015;3:542–550. doi: 10.1016/j.jchf.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cogswell RJ, Norby FL, Gottesman RF, et al. High prevalence of subclinical cerebral infarction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.812. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krumholz HM. Post-Hospital Syndrome: An Acquired, Transient Condition of Generalized Risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franssen C, Chen S, Unger A, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2015;4:312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart hailure with preserved ejection fraction: the role of abnormal peripheral pxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edelmann F, Gelbrich G, Dungen H, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 79.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edelmann F, Gelbrich G, Dungen H, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 81.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: A pilot study. J Appl Physiol. 2014;95:15–27. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 82.Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pandey A, Kitzman DW, Brubaker P, et al. Response to endurance exercise training in older heart failure patients with preserved versus reduced ejection fraction. J Am Geriatr Soc. 2017;65:1698–1704. doi: 10.1111/jgs.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomised clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doukky R, Avery E, Mangla A, et al. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail. 2016;4:24–35. doi: 10.1016/j.jchf.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 89.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 90.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 91.Edelmann F Aldo-DHF investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The aldo-dhf randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 92.Deswal A, Richardson P, Bozkurt B, Mann D. Results of the Randomized Aldosterone Antagonism in Heart Failure With Preserved Ejection Fraction Trial (RAAM-PEF) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an Aldosterone Antagonist (TOPCAT) Trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 94.Abudiab MM, Redfield MM, Melenovsky V, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Redfield M, Chen H, Borlaug B, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Solomon S, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. The Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 97.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 98.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wright JT, Jr, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:1857–1865. doi: 10.1016/j.jacc.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 102.Flacker JM. What is a geriatric syndrome anyway? J Am Geriatr Soc. 2003;51:574–576. doi: 10.1046/j.1532-5415.2003.51174.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


