Abstract
Five patients with painful vascular malformations of the extremities that were refractory to standard treatment and that were confirmed as low flow using dynamic contrast-enhanced MRI were treated with magnetic resonance imaging guided high intensity focused ultrasound (MRgFUS). Daily pain scores improved (max, pre = 8.4±1.5; post = 1.6±2.2, P = 0.004) at median follow up of 9 months (range 4 – 36 months). The size of vascular malformations decreased on follow-up MR imaging (enhancing volume, 8.2 mL [0.7–10.1 mL] before treatment; 0 mL [0–2.3 mL] after treatment; P = .018) at a median follow-up of 5 months (range, 3–36 mo). No complications occurred.
Vascular malformations are common, with a prevalence of 4.5% (1). Venous malformations represent the most common type of vascular malformation, with an incidence of 1 in 10,000 persons and a prevalence of 1% (2). Injection of a sclerosant agent is a widely accepted first-line treatment of low-flow venous malformations (3). Technical success requires a safe route of access and the ability to visualize the vascular malformation continuously throughout the procedure. Most vascular malformations are evaluated by magnetic resonance (MR) imaging before treatment to determine their extent, depth, and involvement of adjacent structures, as well as to establish a working diagnosis (4). The procedure is usually performed with use of a combination of physical examination, ultrasound (US), and fluoroscopy. When the vascular malformation is difficult to visualize with US or is not amenable to percutaneous access because of proximity to critical structures such as bone, skin, or neurovascular structures, other options for treatment must be considered (3,5).
There is recent evidence that treatment of selected low-flow vascular malformations with radiofrequency ablation or cryoablation can be safe and effective (6–9). MR imaging–guided high-intensity focused ultrasound (US) is another option for ablative therapy that offers several advantages. It is noninvasive, allows for real-time MR imaging for lesion localization and thermal monitoring, and provides an immediate posttreatment assessment of effectiveness (10). The purpose of this preliminary study was to evaluate the safety and efficacy of MRgFUS for treating low-flow vascular malformations of the extremities.
MATERIALS AND METHODS
Study Population
After institutional review board approval had been obtained, a retrospective review was conducted of patients who had small low-flow vascular malformations in the lower extremities that were treated with MR imaging–guided high-intensity focused US at 2 institutions between 2011 and 2015. In this period, 6–8 patients per year with low-flow vascular malformations were treated with US-guided sclerotherapy, and 5 patients (3 male, 2 female), whose median age was 36 years (range, 18–54 y), were treated with MR-guided high-intensity focused US. Four vascular malformations were in the thigh and 1 was in the calf; and all were intramuscular.
The decision to proceed with MR–guided high-intensity focused US was made in a collaborative fashion by our institutions’ multidisciplinary vascular anomalies clinical team, which includes dermatologists, surgeons, radiologist, and pathologists. Patients with painful vascular malformations that had not shown a response to conservative measures and that were poorly visualized by US were considered for MR–guided high-intensity focused US. Dynamic contrast-enhanced MR imaging was used to confirm that vascular malformations were low-flow malformations and had acoustic windows suitable for MR–guided high-intensity focused US, such that the US beam from the transducer to the target did not traverse bone or reach within 1 cm of a major nerve or the skin, as determined by the operating physician. All vascular malformations were ≤ 5 cm in maximal diameter and contained less than 25% fat content, as estimated on pretreatment MR images by contouring the total volume of the lesion (as described in Outcomes) and comparing this versus the contoured volume of the fat-containing portion of the lesion derived from fat-only images (LAVA-Flex; described in Preprocedure and Postprocedure Imaging). This restriction resulted from the inability of proton resonance frequency shift thermometry to reliably measure temperatures during sonication in predominantly fat-containing tissues (11). Patients with contraindications to MR imaging or anesthesia were excluded.
Preprocedure and Postprocedure Imaging
MR imaging of the involved extremity was performed with the acquisition of multiplanar fast spin-echo T1-weighted (repetition time [TR] ~ 675 ms, echo time [TE] ~ 20 ms, field of view [FOV] = 20–30 cm, ST = 4 mm, matrix = 384 × 224) and T2-weighted images with and without fat saturation (TR ~ 4,500 ms, TE ~ 50 ms, FOV = 20–30 cm, ST = 4 mm, matrix = 320 × 224), followed by axial LAVA-Flex dynamic postcontrast images (TR ~ 5 ms, TE ~ 2 ms, FOV = 20–30 cm, ST = 3 mm, matrix = 256 × 224, 4 postcontrast phases acquired), obtained before and after injection of gadobutrol contrast agent (Bayer, Leverkusen, Germany). Delayed postcontrast T1-weighted gradient-echo fat-saturated high-resolution images (TR ~ 5 ms, TE ~ 1.3 ms, FOV = 22 cm, ST = 1 mm, matrix = 512 × 512) were also acquired approximately 10 minutes after injection of contrast agent. Images were acquired on a Discovery MR750 3-T magnet (GE Healthcare, Chicago, Illinois).
MR Imaging–Guided High-Intensity Focused US Procedure
MR imaging–guided high-intensity focused US was performed by using the ExAblate 2100 system (InSightec, Tirat Carmel, Israel) under general anesthesia by 2 operators with 6 and 7 years of experience with MR-guided high-intensity focused US. The procedure followed established methods (12). Briefly, the patient was positioned on the table to optimize the acoustic window such that the vascular malformation was aligned with the in-table US transducer, with acoustic coupling through a wet gel pad. Multiplanar T1- and T2-weighted MR imaging, as detailed earlier, was used to manually segment the contour of the vascular malformation and the skin surface. If necessary, adjacent nerves were demarcated so that the system would plan treatment without energy passing through these areas. Sonication typically began with 3 or 4 sonications in 2 or 3 different planes to align the beam with the target; low energies used for this calibration step were sufficient to heat but not ablate the targeted tissue. Generally, calibration required adjustment of the beam by 0–4 mm in the anterior–posterior, medial–lateral, and/or superior–inferior directions.
Typical sonication spot sizes used for these treatments were 5 × 15 mm (diameter × length), which was a length sufficient to cover from the superficial to the deep margin of the vascular malformation. The initial treatment plan produced by the ExAblate software was manually adjusted before and during treatment with modification of the spot density and US parameters (energy delivered and sonication duration) as needed to achieve a maximum temperature of at least 60 °C and thermal dose of 240 CEM within the targeted tissue. The order of sonication spots was chosen such that treatment began with a sonication spot that extended through the center of the vascular malformation, with subsequent spots surrounding the center and extending outward. Sonication was monitored by using MR multislice echo-planar imaging thermometry as previously described (10). The number, energy, and duration of sonications were recorded. All patients were managed as outpatients, with monitoring for 0.5–3 hours after treatment per hospital policy, including evaluation for injury to the skin and nerves.
Outcomes
Clinical pain scores and lesion size on imaging were assessed preoperatively and at least 3 months after focused US by 2 operators with 10 and 13 years of experience with MR imaging interpretation. Reduction in maximum and average daily pain as measured on a 10-point numeric rating scale was the primary clinical outcome, and decrease in enhancing volume and largest measured dimension of the vascular malformation on postcontrast axial T1-weighted MR imaging were the imaging outcomes. The immediate posttreatment nonperfused volume was also determined. Volume was measured by manually contouring the vascular malformation (Osirix; Pixmeo, Bernex, Switzerland). Complications were also recorded and classified according to Common Terminology Criteria for Adverse Events, version 4 (13). Technical parameters were recorded, including treatment time, which was defined as the time from start of the first sonication through the end of the last sonication.
Statistical Analysis
Statistical analysis was performed by using Stata software (version 10.0, StataCorp, College Station, Texas). Comparison of outcome variables between pretreatment and posttreatment levels was performed by using paired t tests.
RESULTS
MR–guided high-intensity focused US was performed on 5 symptomatic low-flow venous malformations of the lower extremity. Examples of treatments are shown in Figures 1 and 2. The sonication parameters are detailed in Table 1. Median treatment duration was 119 minutes (range, 50–202 min). Mean nonperfused volume after treatment was 6.7 mL (range, 5.1–8.4 mL); the mean ratio of nonperfused to initial tumor volume was 4.9 (range, 0.5–12). In the 3 cases in which the nonperfused volume exceeded the tumor volume, there was no residual viable tumor on subsequent imaging.
Figure 1.
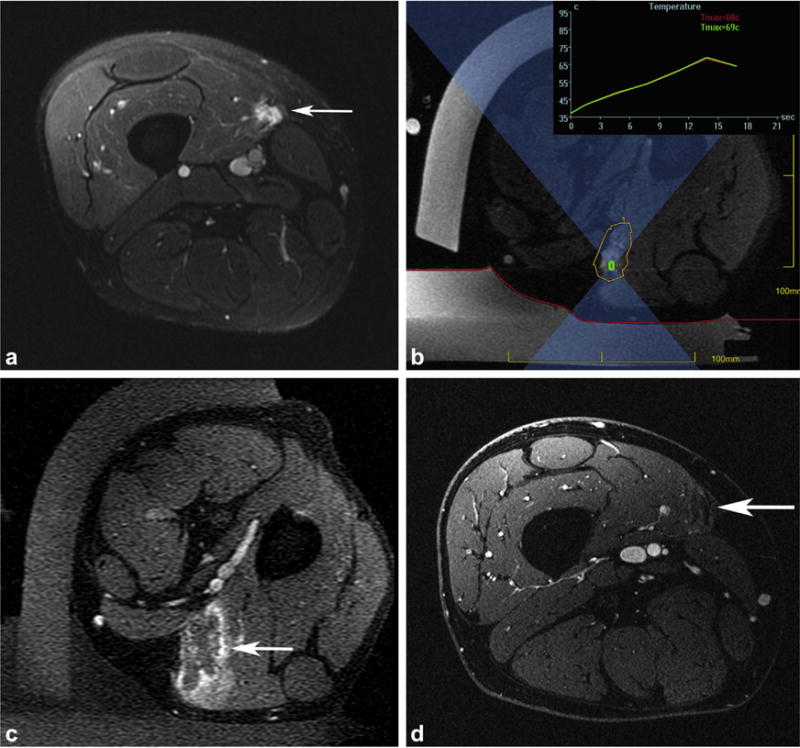
MR–guided high-intensity focused US treatment of a slow-flow vascular malformation in the medial thigh causing constant right thigh pain, rated as high as 10 of 10 in severity, refractory to medical management and US–guided anesthetic/steroid injection. (a) Axial postcontrast T1-weighted three-dimensional (3D) spoiled gradient recalled (SPGR) fat-suppressed image obtained 3 months before treatment shows an enhancing 1.3 × 1.2 × 5.2-cm mass (arrow) located in the vastus medialis muscle. (b) Axial MR thermometry magnitude image acquired during sonication with the patient in a left lateral decubitus position shows the region of treatment (yellow) around the vascular malformation, with a representative sonication (green rectangle) and beam path (blue hourglass). Temperature is displayed in real time during treatment (in the inset, the red curve represents the single hottest pixel and the green curve represents an average temperature in a 3 × 3-pixel area around the sonication target). The skin interface with the gel pad and water bath in the near field is demarcated (red line). (c) Axial postcontrast T1-weighted 3D SPGR fat-suppressed image acquired immediately after treatment reveals the ablated area of the vascular malformation (arrow), with surrounding inflammatory enhancement. (d) Axial postcontrast T1-weighted 3D SPGR fat-suppressed image obtained 9 months after treatment shows absence of residual vascular malformation at the treatment site (arrow). Pain improved to a score of 4 of 10 in maximum severity.
Figure 2.
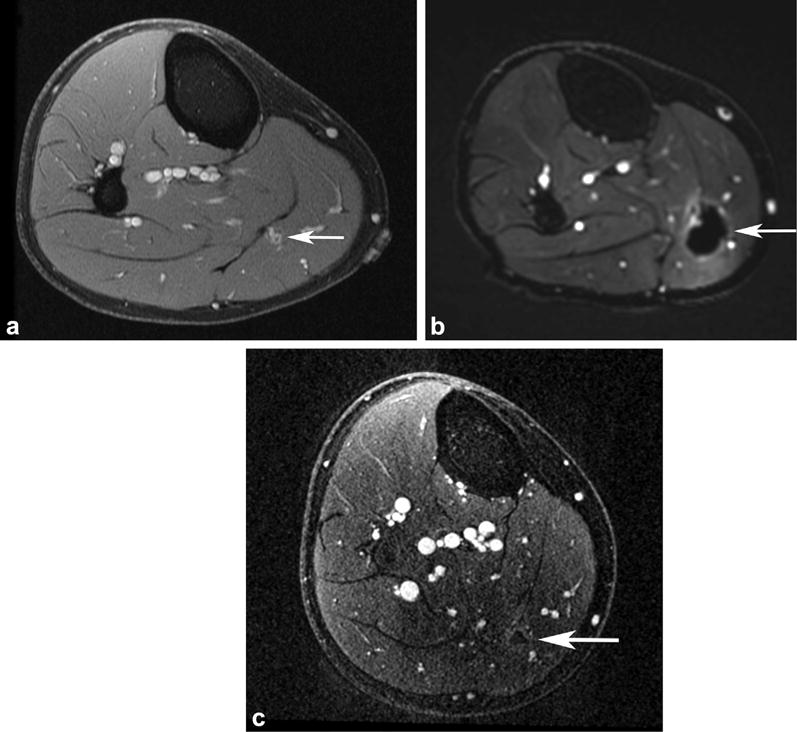
MR–guided high-intensity focused US treatment of a slow-flow vascular malformation in the medial calf causing intermittent right calf pain, rated as high as 10 of 10 in severity, refractory to medical management and US–guided anesthetic/steroid injection. (a) Axial postcontrast T1-weighted 3D SPGR fat-suppressed image obtained 5 months before treatment shows an enhancing 0.9 × 0.7 × 1.5-cm mass (arrow) located in the medial gastrocnemius muscle. (b) Axial postcontrast T1-weighted 3D SPGR fat-suppressed image acquired immediately after treatment reveals the ablated area of the vascular malformation (arrow), with surrounding inflammatory enhancement. (c) Axial postcontrast T1-weighted 3D SPGR fat-suppressed image obtained 5 months after treatment shows absence of residual vascular malformation at the treatment site (arrow). Pain resolved after treatment.
Table 1.
Technical Treatment Parameters
| Parameter | Value |
|---|---|
| Sonication | |
| Treatment time (min) | 123.8 (50–202) |
| Sonications per treatment | 36 (11–56) |
| Sonication duration (s) | 13.4 (7.6–20) |
| Energy per sonication (J) | 1967 (658–3,753) |
| Temperature at target (°C) | |
| Average | 55.3 (47.3–78.0) |
| Maximum | 62.4 (50.1–96) |
Note–Values presented as mean (range). Average is the average temperature in a 3 × 3-pixel area around the maximum temperature achieved during sonication. Transducer operating frequency was 1 MHz.
The median follow-up period for pain was 9 months (range, 4–36 mo). No complications occurred during this period. There were reductions in mean maximum pain and average daily pain on the 10-point numeric rating scale, with reductions in mean maximum pain of 81% (pretreatment, 8.4 ± 1.5; posttreatment, 1.6 ± 2.2; P = .004) and in average pain of 75% (pretreatment, 4.8 ± 1.3; posttreatment, 1.2 ± 1.8; P = .003). On follow-up MR imaging obtained a median of 5 months after treatment (range, 3–36 mo), the enhancing volume of the vascular malformation decreased by an average of 93% (range, 77%–100%), from a median of 8.2 mL (range, 0.7–10.1 mL) to 0 mL (range, 0–2.3 mL; P = .018), and maximum diameter of any residual enhancing portion of the vascular malformation decreased by 60% (range, 48%–100%), from 2.7 cm ± 2.0 to 0.9 cm ± 1.3 (P = .007; Table 2).
Table 2.
Response to MR–Guided Focused US Treatment
| Outcome | Before Treatment | After Treatment | P Value* |
|---|---|---|---|
| Daily pain per NRS (0–10) | |||
| Maximum | 8.4 ± 1.5 | 1.6 ± 2.2 | .004* |
| Range | 7–10 | 0–4 | |
| Average | 4.8 ± 1.3 | 1.2 ± 1.8 | .003* |
| Range | 3–6 | 0–4 | |
| Maximum lesion dimension (cm) | 2.7 ± 2.0 | 0.9 ± 1.3 | .007* |
| Lesion enhancing volume (mL)† | 8.2 (0.7–10.1) | 0.0 (0–2.3) | .018* |
Note–Vales presented as mean ± standard deviation. Maximal lesion dimension refers to the largest enhancing dimension measured on multiplanar contrast-enhanced MR imaging. Pain scores were assessed at a median of 9 months after treatment (range, 4–36 mo). Dimensions of any residual enhancing lesion were measured on MR images obtained a median of 5 months after treatment (range, 3–36 mo).
P values were determined by paired t tests.
Values presented as median (range).
Of the 4 patients who reported resolution of average daily pain, 3 had complete resolution of the vascular malformation on MR. Another patient had occasional episodes of mild to moderate pain; 2 treatments were planned in this case to allow repositioning to optimize access to a portion of vascular malformation that was deemed too near the sciatic nerve in the initial treatment position. The patient’s pain was greatly relieved after the first procedure, and she decided to defer further treatment.
DISCUSSION
The present study demonstrates statistically significant improvement in pain and reduction in lesion size in a small series of small, slow-flow lower-extremity malformations without any complications. The results suggest that MR imaging–guided high-intensity focused US can be a safe and efficacious noninvasive treatment for painful low-flow vascular malformations. For a patient who may be difficult to treat with conventional percutaneous injections because the vascular malformation is difficult to visualize sonographically, or in whom the vascular anatomy is unfavorable for injection of a sclerosant agent because of the likelihood of failure or risk of complications, MR–guided high-intensity focused US may offer an alternative.
The present results are consistent with those of a recently published case report (14) describing successful treatment of a painful small venous malformation of the medial left calf with the use of MR–guided high-intensity focused US. The authors reported pain relief and a 30% reduction in vascular malformation volume. The present series adds to that early experience, and these results were sustained for as long as 36 months of follow-up.
Conventional treatment options for symptomatic venous malformations include surgery and percutaneous sclerotherapy. Successful treatment after radical excision of these vascular malformations has been reported in as many as 81% of patients at 1 year after treatment, with complications of arteriovenous fistula, foot drop, skin necrosis, and wound dehiscence occurring in 19% in 1 study of 48 patients (15). Outcomes after sclerotherapy range from 75% to 95% initial success, with durable relief reported at 24 months in 82% of patients in a study of 87 patients and at 12 months in 49% in another study of 66 patients (16). Between 2 and 3 treatments are typically required (16,17). Complication rates range from 8% to 33%, with increased pain, infection, skin necrosis, and nerve damage reported in 1%–11% of cases (16,17).
MR–guided high-intensity focused US may confer several advantages over conventional treatments, not only in terms of conformal lesion targeting through MR imaging guidance, but also the real-time monitoring of the therapeutic effect with MR thermometry. For example, complete ablation of the vascular malformation may result in more durable symptom relief; however, this and other potential advantages of this noninvasive approach, such as a reduced risk of infection and faster recovery, remain to be proven in subsequent larger studies. The procedures do take several hours to complete, limiting the lesion volume that can be treated in a single session, but this may change as newer systems are developed that improve the efficiency of sonication by actively cooling the skin while delivering heating at the focal spot. Treatment parameters must also be modified to overcome perfusion of the vascular malformation, which might otherwise dissipate the heat delivered during sonication; specifically, sonication energies were delivered in these treatments over shorter durations (13.4 s) than is typical for soft-tissue tumor treatments (20–40 s). In addition, treatment of vascular malformations with large fat content is made challenging by the unreliability of standard proton resonant frequency shift thermometry in fat; incorporation of MR thermometry methods that allow for temperature measurement in fat (18) would broaden the application of this method to allow treatment of a greater variety of vascular malformations.
Although the present results are promising, our series is limited by the small sample size as well as the temporal heterogeneity of clinical and imaging follow-up among study subjects. The present feasibility study is promising, but future studies are necessary, including rigorous clinical and imaging follow-up, to determine whether there are advantages to MR–guided high-intensity focused US compared with current ablative treatments in terms of safety, efficacy, and durability.
Acknowledgments
This work was partially supported by National Institutes of Health Grant P01 CA159992-03.
K.B.P. receives research support from General Electric (Boston, Massachusetts) and InSightec (Tirat Carmel, Israel).
ABBREVIATIONS
- FOV
field of view
- SPGR
spoiled gradient recalled [imaging]
- TE
echo time
- 3D
three-dimensional
- TR
repetition time
Footnotes
From the SIR 2017 Annual Meeting.
None of the other authors have identified a conflict of interest.
References
- 1.Verajankorva E, Rautio R, Giordano S, Koskivuo I, Savolainen O. Clinical study: the efficiency of sclerotherapy in the treatment of vascular malformations: a retrospective study of 63 patients. Plast Surg Int. 2016;2016:2809152. doi: 10.1155/2016/2809152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behravesh S, Yakes W, Gupta N, et al. Venous malformations: clinical diagnosis and treatment. Cardiovasc Diagn Ther. 2016;6:557–569. doi: 10.21037/cdt.2016.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee BB, Baumgartner I, Berlien P, et al. Diagnosis and treatment of venous malformations. Consensus Document of the International Union of Phlebology (IUP): updated 2013. Int Angiol. 2015;34:97–149. [PubMed] [Google Scholar]
- 4.Legiehn GM, Heran MKS. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am. 2008;46:545–597. doi: 10.1016/j.rcl.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.van der Vleuten CJM, Kater A, Wijnen MHWA, Schultze Kool LJ, Rovers MM. Effectiveness of sclerotherapy, surgery, and laser therapy in patients with venous malformations: a systematic review. Cardiovasc Intervent Radiol. 2014;37:977–989. doi: 10.1007/s00270-013-0764-2. [DOI] [PubMed] [Google Scholar]
- 6.Childs DD, Emory CL. Successful treatment of intramuscular venous malformation with image-guided radiofrequency ablation. J Vasc Interv Radiol. 2012;23:1391–1393. doi: 10.1016/j.jvir.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis F, Havez M, Labreze C, et al. Percutaneous cryoablation of symptomatic localized venous malformations: preliminary short-term results. J Vasc Interv Radiol. 2013;24:823–827. doi: 10.1016/j.jvir.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Wang X, Suo W. Management of venous malformations with percutaneous radiofrequency thermal ablation. Br J Dermatol. 2012;167:637–642. doi: 10.1111/j.1365-2133.2012.10963.x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson SM, Callstrom MR, McKusick MA, Woodrum DA. Initial results of image-guided percutaneous ablation as second-line treatment for symptomatic vascular anomalies. Cardiovasc Intervent Radiol. 2015;38:1171–1178. doi: 10.1007/s00270-015-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging. 2008;27:391–399. doi: 10.1002/jmri.21261. [DOI] [PubMed] [Google Scholar]
- 11.Rieke V, Butts Pauly K. Echo combination to reduce proton resonance frequency (PRF) thermometry errors from fat. J Magn Reson Imaging. 2008;27:673–677. doi: 10.1002/jmri.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghanouni P, Dobrotwir A, Bazzocchi A, et al. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: a retrospective multicenter study. Eur Radiol. 2017;27:732–740. doi: 10.1007/s00330-016-4376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Bethesda, MD: National Institutes of Health; 2009. NIH Publication 09-7473. Available at, http://evs.nci.nih.gov/ftp1/CTCAE/About.html. [Google Scholar]
- 14.van Breugel JMM, Nijenhuis RJ, Ries MG, et al. Non-invasive magnetic resonance-guided high intensity focused ultrasound ablation of a vascular malformation in the lower extremity:a case report. J Ther Ultras. 2015;23:1–7. doi: 10.1186/s40349-015-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh YN, Do YS, Park KB, et al. The results of surgical treatment for patients with venous malformations. Ann Vasc Surg. 2012;26:665–673. doi: 10.1016/j.avsg.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Bowman J, Johnson J, McKusick M, Gloviczki P, Driscoll D. Outcomes of sclerotherapy and embolization for arteriovenous and venous malformations. Semin Vasc Surg. 2013;26:48–54. doi: 10.1053/j.semvascsurg.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Ali S, Weiss CR, Sinha A, Eng J, Mitchell SE. The treatment of venous malformations with percutaneous sclerotherapy at a single academic medical center. Phlebology. 2016;31:603–609. doi: 10.1177/0268355516633380. [DOI] [PubMed] [Google Scholar]
- 18.Ozhinsky E, Kohi MP, Ghanouni P, Rieke V. T2-based temperature monitoring in abdominal fat during MR-guided focused ultrasound treatment of patients with uterine fibroids. J Ther Ultras. 2016;4:26. doi: 10.1186/s40349-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


