Abstract
The dorsomedial striatum (DMS) is an important sensorimotor region mediating the acquisition of goal-directed instrumental reward learning and behavioral flexibility. However, whether the DMS also regulates Pavlovian cue-food learning is less clear. The current study used excitotoxic lesions to determine whether the DMS is critical in Pavlovian appetitive learning and behavior, using discriminative conditioning and reversal paradigms. The results showed that DMS lesions transiently retarded cue-food learning and subsequent reversal of this learning. Rats with DMS lesions selectively attenuated responding to a food cue but not a control cue, early in training, suggesting the DMS is involved when initial associations are formed. Similarly, initial reversal learning was attenuated in rats with DMS lesions, which suggests impaired flexibility to adjust behavior when the cue meaning is reversed. We also examined the effect of DMS lesions on food intake during tests with access to a highly palatable food along with standard chow diet. Rats with DMS lesions showed an altered pattern of intake, with an initial reduction in HFD followed by an increase in chow consumption. These results demonstrate that the DMS has a role in mediating cue-food learning and its subsequent reversal, as well as changes in food intake when a choice is provided. Together, these results demonstrate the DMS is involved in reward associative learning and reward consumption, when behavioral flexibility is needed to adjust responding or consumption to match the current value.
Keywords: dorsomedial striatum, appetitive learning, feeding, reversal learning, lesion
The dorsal striatum was originally viewed as a sensorimotor processing area critical for regulation of locomotion, but more recent anatomical evidence suggested a broader function in the control of behavioral and cognitive outputs (Alexander et al., 1990). Recent structural and functional evidence support its function in reward-related learning and behavior (Balleine & O’Doherty, 2010; Haber & Knutson 2010). In particular, the dorsomedial striatum (DMS) is necessary for goal-directed appetitive learning. For example, antagonism of NMDA receptors within the DMS impairs acquisition of lever-pressing for sucrose pellets (McKee et al., 2010). The acquisition of instrumental appetitive learning has been shown to induce plasticity in the DMS (Shan et al., 2014), while lesion or inactivation of the DMS impairs goal-directed instrumental learning (Yin et al., 2005; Shiflett et al., 2010). Furthermore, the DMS is necessary for behavioral flexibility when reward outcomes change, such as in reversal learning (e.g., Ragozzino, 2007; Castañé et al., 2010).
In contrast, little is known regarding the role of the DMS in Pavlovian appetitive learning. We recently demonstrated increased neuronal activation in the DMS (Fos induction) during cue-food pairings in well-trained rats (Cole et al., 2015), however whether the DMS is critical for this learning is unknown. Therefore, here we used excitotoxic lesions to determine whether the DMS is necessary for Pavlovian appetitive learning and behavior, using discriminative conditioning and reversal learning.
In addition to mediating goal-directed instrumental learning and behavior, the DMS has also been implicated in palatable food consumption and obesity. A recent study from DiFeliceantonio and colleagues (2012) found that consumption of palatable food (chocolate) corresponded with increased enkephalin release in the DMS, and mu-opioid receptor stimulation markedly increased consumption. In humans, body mass index and activity in the dorsal striatum in response to palatable food are correlated (e.g., Stice et al., 2008). Therefore, in addition to determining DMS function in reward associative learning, we examined the effect of DMS lesions on consumption of a palatable food.
Materials and Methods
Subjects and Surgery
Twenty experimentally naïve, male Long-Evans rats (250–275 g) were obtained from Taconic Biosciences and housed and maintained as described previously (Cole et al., 2013). All procedures were approved by the Boston College Institutional Animal Care and Use Committee and were in accordance with the NIH Guidelines on the Care and Use of Laboratory Animals.
All surgeries were performed using a mixture (1 ml/kg body weight) of ketamine (50 mg/ml) and xylazine (10 mg/ml) to achieve anesthesia before placing the subject in a stereotaxic frame (Kopf Instruments). Rats received four 0.25 μl injections (two in each hemisphere) in the DMS using a 1 μl 32 gauge Hamilton ‘Neuros’ syringe, at a rate of 0.1 μl/min, driven by a Quintessential Stereotaxic Injector (Stoelting). Injections consisted of either 0.15 M N-methyl-D-aspartate (NMDA; Sigma-Aldrich) in phosphate buffered saline (PBS) to produce lesions, or PBS alone. The flat-skull coordinates of the injections from bregma were as follows: anteroposterior, +1.20 mm; mediolateral, +1.8 mm; dorsoventral, −4.50 mm and anteroposterior, −0.10 mm; mediolateral, +1.8 mm; dorsoventral −4.50 mm. After each infusion the needle was left in place for 2 minutes to allow for diffusion of the injectate.
Apparatus
Training was performed in a set of behavioral chambers described previously (Cole et al., 2013). The conditioned stimuli (CSs) were a 10 s 75 dB, 2 kHz tone and a 10 s 75 dB white noise. The unconditioned stimulus (US) consisted of two food pellets (formula 5TUL, 45 mg: Test Diets) delivered to the food-cup. The stimuli were controlled by GraphicState 3.0 software system (Coulbourn Instruments). For consumption testing both standard lab chow (3.1 kcal/g), and a high fat diet (HFD; 8.7 kcal/g) were given. The HFD consisted of a 9:1 mix of hydrogenated vegetable shortening (Crisco® All-Vegetable shortening, J. M. Smucker Co) and sugar (cane granulated sugar, Domino Foods Inc.).
Behavioral Testing Procedure
Rats were gradually reduced to 90% of their ad libitum weight, and remained food-restricted throughout training (acquisition and reversal). All animals initially received 2 days of habituation to the behavioral chambers (32 min exposures with no additional stimuli). Following habituation all animals received 1 g of the US food pellets in their home cage to familiarize them with the taste.
Acquisition
To assess the effects of DMS lesions on Pavlovian learning, all rats received 10 days of training in a discriminative conditioning paradigm. In each daily 30 min session, rats received twelve presentations of two different auditory cues (six presentations of each; order intermixed). One 10 s cue (e.g., tone) was immediately followed by delivery of the food US (CS+), while the other 10 s cue (e.g., noise) was presented alone (CS−). The two auditory cues used as the CS+ and CS− were counterbalanced within groups. The inter-trial intervals (ITI) between the CSs were random (range of 60 s to 219 s) and varied across days of training, as did the order of CSs. Rats were weighed and given 1 hr of access to chow for maintenance of bodyweight 30–60 min following each daily training session.
Reversal
After Acquisition training all rats remained in their home cages for 5 days prior to the start of Reversal training. Reversal consisted of ten daily sessions identical to Acquisition, except that the cues that served as the CS+ and CS− were reversed, now referred to as rCS+ and rCS−. Following each session rats were weighed and given daily chow access for maintenance of bodyweight as during Acquisition.
Restricted high fat diet access
Following Reversal, rats remained in their home cages for 5 days with ad libitum access to food (laboratory chow). All rats then received six sessions (over 8 days) with access to HFD for 6 hours. To examine any potential effects on initial and/or sustained eating, consumption was measured at both 1 and 6 hours. Rats were placed into a clean, bedding-free cage with a glass dish of HFD (10 g). Water and a pre-weighed quantity of chow (20 g) were also available. After 1 hour, the chow and HFD were removed for weighing and replaced with additional chow (40 g) and HFD (20 g). After 5 hours (6 hours from start), the chow and HFD were removed for weighing, and the rats were returned to their home cage. The daily chow consumed following each HFD session (18 hours later) was also recorded.
Immunohistochemistry
In order to verify lesion placements, after completion of behavioral testing, brain tissue was collected and processed with immunohistochemistry for NeuN detection. Rats were anaesthetized with tribromoethanol (1.25 mL/100 g body weight, i.p.) and transcardially perfused with 0.9% saline followed by ice cold 4% paraformaldehyde in 0.1 M borate buffer. The brains were stored for 20–24 hours at 4°C in the fixative with 12% sucrose and then rapidly frozen in hexanes cooled with dry ice and stored at −80°C. Frozen brains were cut into 40 μm coronal sections using a sliding microtome (Leica Biosystems) and collected into three serially adjacent series.
Immediately following slicing, sections from one series were incubated for 1 hour in a blocking solution (potassium phosphate-buffered saline [KPBS] containing normal horse serum [NHS], Triton X-100, and milk), and then incubated with mouse antiserum against NeuN (1:1000, MAB377; Millipore) in the blocking solution for 72 hours at 4°C. Sections were subsequently rinsed with KPBS, NHS, and milk, incubated with biotinylated secondary antibody against mouse (1:500, BA-2001; Vector Laboratories) in the blocking solution for 45 min, rinsed in KPBS, incubated in avidin biotin complex (ABC, PK-6100; Vector Laboratories) for 45 min, and rinsed again in KPBS.
Nuclei labeled for NeuN were visualized as grey with nickel-intensified 3, 3′-diaminobenzidine (SK-4100; Vector Laboratories). Sections were rinsed, mounted on SuperFrost slides (Fisher Scientific), dried at 40°C, dehydrated through graded alcohols, cleared in xylenes, and coverslipped with DPX Mountant (Electron Microscopy Services). The second series of sections were mounted from KPBS onto gelatin-coated slides and stained with thionin for identification of nuclear borders as defined in Swanson (2004), and secondary verification of lesion extent.
Data Analysis
Scoring of behavior was made from recorded training sessions by a trained experimenter, unaware of experimental condition. There were two measures of conditioning during Acquisition and Reversal. The primary measure was the percentage of time rats expressed food-cup behavior during the CS. Food-cup behavior was defined as nose pokes into the recessed-food cup, or standing immediately in front of and directly facing the food-cup. Behavior was scored every 1.25 s during the 10 s CS, with the number of food-cup observations summed and converted to a percentage. Rats’ behavior during the 10 s immediately preceding each CS presentation (pre-CS) was also scored in the same manner. The second measure of conditioning was latency to initiate food-cup behavior. Latency was the time elapsed in seconds from the onset of the CS until the rat showed food-cup behavior as defined above. Latency included the 10 s CS period, as well as the 10 s immediately following the CS. After this time any food–cup behavior was considered unspecific to the cue presentation, and so a maximum latency of 20 s was assigned to any trial during which food-cup behavior was made later or did not occur.
For the consumption phase of the experiment, the amount of chow and HFD consumed (in grams) by the rats in the initial (0–1 hours) and sustained (1–6 hours) consumption periods during tests were weighed separately. Consumption in each of these periods were averaged and analyzed separately.
Most data were analyzed using two-way analysis of variance (ANOVA) with group (Sham, Lesion) as between-subjects factor, and either CS/rCS presentation or day of training (acquisition or reversal), or food consumption testing as within-subjects factors. For Consumption testing a three-way ANOVA was used with factors of group, testing day, and food type. The statistical package SPSS (v.21) was used and type I error was controlled at 0.05 unless otherwise noted.
Results
Histology
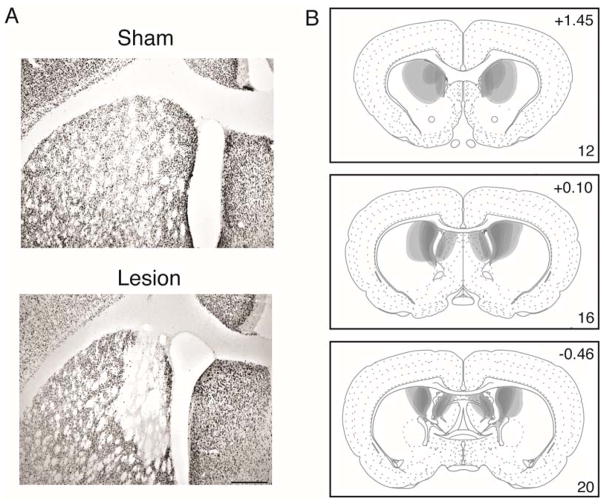
Histological analysis was performed to determine the accuracy and extent of the lesions. Example images of NeuN stained tissue from Sham and Lesion brains are shown in Figure 1A. Representations of lesion placements included in the final analyses are shown in Figure 1B. The majority of lesions targeted the posterior DMS (pDMS: here considered as posterior to Level 15 (+0.45 from Bregma) in Swanson [2004]), although some lesions extended rostrally to encompass some of the anterior DMS. Six subjects were excluded due to insufficient cell damage (less than 50% of structure damaged in each hemisphere) resulting in a total of 14 rats included in the analyses (Sham, n=8 and Lesion, n=6).
Figure 1.
(A) NeuN-stained sections showing typical sham (top) and NMDA excitotoxic lesions (bottom) of the DMS. Scale bar = 200μm (B) Lesion extent of all included subjects, drawn with 50% opacity in Adobe Illustrator CS4. Numbers in the top right refer to distance in mm from Bregma, and those in the bottom right refer to the corresponding plate number in Swanson (2004), adapted from Swanson, L.W. (2004) Brain Maps: Structure of the Rat Brain, 3rd edition.
Acquisition
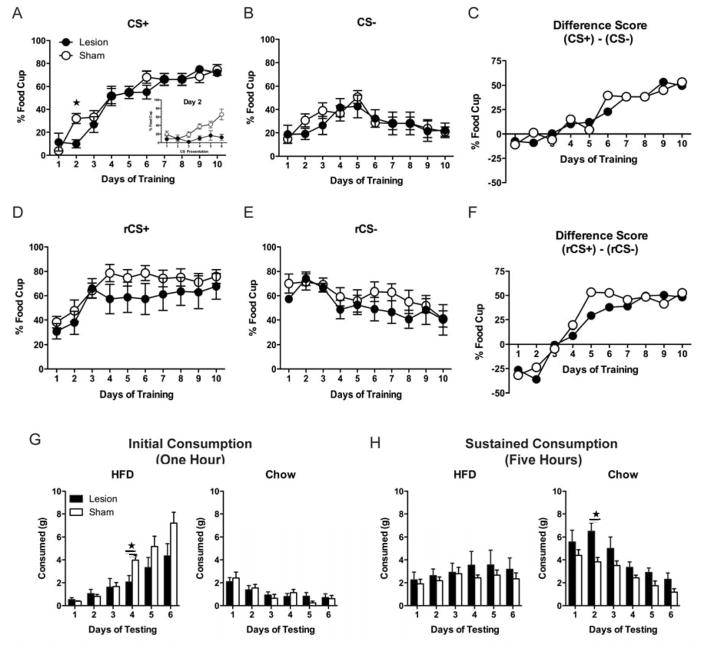
There were no differences between groups in pre-CS responding prior to CS+ (F(1,12) = 0.027, p > 0.05) or CS− presentations (F(1,12) = 0.015, p > 0.05). All rats increased food-cup responding during CS+ presentations across Acquisition (Figure 2A). A group (Sham, Lesion) by training day repeated measures ANOVA on CS+ responding showed a main effect of day (F(1,9) = 66.712, p < 0.01), confirming that food-cup responding increased across sessions. There was no significant main effect of group (F(1,12) = 0.396, p > 0.05) although there was a significant group by training day interaction (F(1,9) = 2.476, p < 0.05). Follow-up analyses of CS+ training focused on Day 2, during which the Lesion group exhibited attenuated food-cup responding compared to Sham animals (Figure 2A, inset). There was a significant main effect of group (F(1,12) = 15.514, p < 0.01), confirming that the Lesion group displayed significantly less food-cup responding than Shams. There was also a significant main effect of CS+ presentation (F(1,5) = 5.876, p < 0.01) and a significant group by CS+ interaction (F(1,5) = 3.584, p < 0.01), confirming that only Sham animals increased CS+ responding across the session.
Figure 2.
Percentage of time (mean ± SEM) rats expressed food-cup behavior during CS+ presentations across training days (Day 2 inset) (A), during CS− presentations across days (B), and expressed as a difference score (CS+ − CS−) (C). Percentage of time (mean ± SEM) rats expressed food-cup behavior during Reversal to rCS+ (previously CS−) (D), to rCS− (previously CS+) (E), and expressed as a difference score (rCS+ − rCS−) (F). Consumption (mean + SEM) of chow and HFD across days of testing. Consumption was measured after 1 hour (G) and then after an additional 5 hours (H). ★ denotes p < 0.05.
Similarly, all rats showed a decrease in latency to approach the food-cup in response to CS+ presentations across Acquisition (Supplementary Figure 1). A group (Sham, Lesion) by training day repeated measure ANOVA revealed a main effect of day (F(1,9) = 68.138, p < 0.01). There was no significant main effect of group (F(1,12) = 0.246, p > 0.05), however there was a significant group by training day interaction (F(1,9) = 3.528, p < 0.01). On Day 2, Lesion animals displayed a greater latency to approach the food-cup in response to CS+ presentation. There was a significant main effect of group (F(1,12) = 8.847, p < 0.05), confirming that Lesion animals had a higher latency compared to Sham animals. There was no significant main effect of CS+ presentation (F(1,5) = 1.501, p > 0.05) and no significant interaction (F(1,5) = 1.187, p > 0.05) (Supplementary Figure 1).
All rats showed a change in food-cup responding during CS− presentations across Acquisition (Figure 2B). A group (Sham, Lesion) by training day repeated measures ANOVA on responding during CS− showed a main effect of day (F(1,9) = 4.685, p < 0.01), confirming that food-cup responding changed across training days. This change was a significant quadratic trend (F(1,12) = 14.231, p < 0.01), because responding to the CS− at first increased, and then decreased across training (Figure 2B). There was no significant main effect of group (F(1,12) = 0.262, p > 0.05) and no significant group by training day interaction (F(1,9) = 0.633, p > 0.05).
Similarly, while latency to approach the food-cup during CS− presentations changed across training days (F(1,9) = 6.101, p < 0.01), there was no significant main effect of group (F(1,12) = 0.848, p > 0.05) and no significant group by training day interaction (F(1,9) = 0.718, p > 0.05). (Supplementary Figure 1).
To further examine learning during Acquisition, we examined the difference in responding within-subjects to the training cues, by subtracting the mean food-cup responding to CS− from the mean responding during CS+ presentations (CS+ − CS−). All rats showed increased discrimination between the cues across training (Figure 2C). A group (Sham, Lesion) by training day repeated measures ANOVA on the difference score revealed a main effect of day (F(1,9) = 12.027, p < 0.01), confirming that the difference in food-cup responding between the two cues increased across Acquisition. There was no effect of group (F(1,12) = 0.001, p > 0.05), and no significant interaction between training day and group (F(1,9) = 0.666, p > 0.05), indicating that there was no difference between groups overall in discrimination across Acquisition.
Reversal
There was no difference between groups in pre-CS responding prior to rCS+ (F(1,12) = 0.011, p > 0.05), or rCS− presentations (F(1,12) = 0.208, p > 0.05). All rats acquired reversal learning, with increased food-cup behavior during rCS+ presentations across days (Figure 2D). A group (Sham, Lesion) by reversal day repeated measures ANOVA on responding during rCS+ showed a significant main effect of day (F(1,9) = 13.941, p < 0.01), confirming reversal learning with an increase in responding to the rCS+ (previously CS−). There was no significant main effect of group (F(1,12) = 1.480, p > 0.05) and no significant group by reversal day interaction (F(1,9) = 1.055, p > 0.05).
Similarly, animals in both groups showed learning during rCS− presentations as evidenced by decreased food-cup responding across training days (Figure 2E). A group (Sham, Lesion) by reversal day repeated measures ANOVA of responding during rCS− showed a significant main effect of day (F(1,9) = 7.989, p < 0.01), confirming that decreased food-cup responding occurred across days to the rCS− (previously CS+). There was no significant main effect of group (F(1,12) = 0.828, p > 0.05) and no significant main effect of group by reversal day interaction (F(1,9) = 1.003, p > 0.05).
To further examine reversal learning, we also examined the difference in responding within-subjects to the reversed cues (rCS+ − rCS−). All rats showed increased discrimination between the reversed cues across training (Figure 2F). A group (Sham, Lesion) by training day repeated measures ANOVA on the difference score revealed a main effect of day (F(1,9) = 52.738, p < 0.01), confirming that the difference in food-cup responding between the two cues increased across Reversal. There was no effect of group (F(1,12) = 0.345, p > 0.05), however there was a significant quadratic interaction between training day and group (F(1,9) = 7.293, p < 0.05) with the rate of discrimination across Reversal initially attenuated in the Lesion animals. Analyses of simple effects with alpha adjusted to 0.025 confirmed that there was a quadratic trend of discrimination in Sham animals (F(1,7) = 27.099, p < 0.01), but not in Lesion animals (F(1,5) = 6.788, p > 0.025).
Consumption
During the initial consumption phase (1 hour) (Figure 2G) all rats increased HFD and decreased chow consumption across days but Lesion rats consumed less HFD than Sham rats. A group (Sham, Lesion) by food type (Chow, HFD) by day repeated measures ANOVA revealed a significant effect of both day (F(1,5) = 33.789, p < 0.01) and food type (F(1,5) = 16.985, p < 0.01), indicating that overall consumption increased across days, and overall more HFD was consumed than chow. This difference between chow and HFD consumption increased across testing days as evidenced by a significant day by food type interaction (F(1,5) = 8.705, p < 0.01). There was a significant interaction between group and day (F(1,5) = 3.111, p < 0.05), as well as group and food type (F(1,1) = 7.141, p < 0.05), revealing that the difference in consumption between groups increased across testing, and was greater for HFD than chow. Follow-up testing revealed that Lesion animals ate less HFD than Sham animals during Day 4 (t(12) = 2.863, p < 0.05). There was no significant effect of group overall (F(1,12) = 2.148, p > 0.05), or group by food type by day interaction (F(1,5) = 1.648, p > 0.05).
During the sustained consumption phase (5 hours) (Figure 2H) both groups ate similar amounts of HFD, which did not change across days, but Lesion rats ate more chow than Sham rats as confirmed by a significant effect of group (F(1,12) = 9.104, p < 0.05). There was also a significant effect of day (F(1,5) = 5.702, p < 0.01) indicating overall consumption decreased across testing, and a significant day × food type interaction (F(1,5) = 14.811, p < 0.01). Follow-up testing revealed that Lesion animals ate more chow than Sham animals during Day 2 (t(12) = −3.694, p < 0.01). No other effects or interactions reached significance (Fs < 3.959, ps > 0.05).
In the 18 hours immediately following HFD access (overnight), all rats consumed less chow across test days. A group (Sham, Lesion) by day repeated measures ANOVA of overnight consumption showed a significant main effect of day (F(1,5) = 3.192, p < 0.05), confirming that overnight chow consumption decreased across days. There was no significant effect of group (F(1,12) = 0.987, p > 0.05) and no significant group by day interaction (F(1,5) = 0.478, p > 0.05) (Supplementary Figure 2A).
There was a significant change in body weight gain across days of HFD access (F(1,5) = 426.826, p < 0.01), but no significant effect of group (F(1,12) = 0.516, p > 0.05) and no significant group by day interaction (F(1,5) = 0.011, p > 0.05).
Discussion
Here, we examined the effect of DMS excitotoxic lesions on Pavlovian discriminative appetitive conditioning, reversal learning, and food intake. We found that DMS lesions transiently attenuated responding to a food cue early in discriminative training, and also retarded initial reversal of this learning. In addition, during consumption tests with access to standard lab chow and a highly palatable food, rats with these lesions showed an altered pattern of food intake. Interestingly, this change consisted of an initial reduction in HFD intake, followed by a sustained increase in chow consumption.
The initial lower responding during acquisition training demonstrates that the DMS regulates early Pavlovian cue food learning. It is important to note that this reduction in responding in Lesion rats is not likely due to a general motor deficit, because overall across days of training there were no significant differences in food cup activity or latency to approach the food cup between Lesion and Sham rats, and no differences in activity during the CS−.
These results demonstrate that the DMS not only regulates learning of goal-directed actions, but also cue-food associations. Anatomically, the DMS is well-positioned to act as an integrative site mediating such learning. It receives innervation from the prelimbic area of the medial prefrontal cortex (PL), and the more ventral infralimbic region (ILA) (Berendse et al., 1992). The PL and ILA show increased activity (Fos induction) during cue-food pairings in well-trained rats (Cole, et al., 2015), and critically mediate feeding behaviors that rely on such cue-food associations (e.g., Petrovich et al., 2007; Homayoun and Moghaddam, 2009). The DMS also receives inputs from the anterior part of the basolateral nucleus of the amygdala (BLAa) (Kita and Kitai, 1990 and Corbit et al., 2013), which, similarly to the PL and ILA, is recruited by cue-food learning and critically mediates a range of cue-driven appetitive behaviors (Hatfield et al. 1996; Holland et al. 2002; Setlow et al. 2002; Pickens et al. 2003; Corbit and Balleine 2005; Prévost et al. 2012; Cole et al., 2013). Importantly, the BLAa has been proposed to modulate the PL/ILA and DMS because it is selectively recruited during early learning. Thus, the DMS likely regulates appetitive cue-food learning, and subsequent cue driven behaviors, including reversal learning via a network with the BLAa, and PL/ILA.
The current study adds to a previous investigation of the role of the DMS in cue-food learning. Corbit and Janak (2010) demonstrated that temporarily inactivating the pDMS during training of a Pavlovian appetitive task had no effect during this learning, but instead led to a subsequent failure to observe outcome-specific devaluation. This would seem to contradict the current finding, however there are a number of significant differences between the two studies that need to be considered. First, here we examined discrimination learning, where one cue signaled food, while a second cue had no associated outcome. In contrast, the prior study employed a task where each cue signaled a distinct food outcome (both cues were followed by food delivery). Second, in the current study the reduction in acquisition responding was both transient, and early in training, while in the Corbit and Janak study, all rats received six sessions of training where both cues were followed by delivery of the same food prior to any DMS manipulation. Given these differences, it is difficult to compare the two studies in a meaningful way regarding the pDMS function.
We also found that reversal learning, when the outcome paired with the cues (food or no food) was switched, was initially retarded in Lesion animals. This is consistent with the established role for the DMS in behavioral flexibility. For example, DMS inactivation interferes with reversal learning of turn discrimination (Pisa and Cyr, 1990), instrumental spatial discrimination (Castañé et al., 2010), and place reversal learning (Ragozzino et al., 2009), as well as a visual set-shifting task (Ragozzino et al., 2002). The current findings add to these studies, by revealing for the first time that DMS function also mediates reversal of discriminative cue-food Pavlovian learning.
Interestingly, we found that DMS lesions significantly shifted the pattern of intake during the HFD access time, initially causing a reduction in HFD consumption followed by a more sustained increase in chow intake. When the total daily consumption is expressed in kcal, it is evident that overall more HFD than chow was consumed by both Sham and Lesion rats (Supplementary Figure 2B). The changes in consumption observed in Lesion rats may be related to impairments in palatability (a decrease in HFD or an increase in chow palatability) in agreement with prior work (e.g., DiFeliceantonio et al., 2012). Alternatively, the changes in consumption may be due to behavioral inflexibility to adjust intake when two options are available. Further studies are needed to examine these possibilities.
In conclusion, we found that the DMS mediates initial reward learning and consumption, particularly when behavioral flexibility is needed to adjust responding or intake to match the current value.
Supplementary Material
Acknowledgments
We thank Heather Mayer for technical assistance.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health grant R01DK085721 to GDP.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, ‘prefrontal’ and ‘limbic’ functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;5:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. The Journal of Comparative Neurology. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Castañé A, Theobald DEH, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behavioural Brain Research. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Powell DJ, Petrovich GD. Differential recruitment of distinct amygdalar nuclei across appetitive associative learning. Learning & Memory. 2013;20:294–299. doi: 10.1101/lm.031070.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Hobin MP, Petrovich GD. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. The Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. European Journal of Neuroscience. 2010;31:1312–1321. doi: 10.1111/j.1460-9568.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Leung BK, Balleine BW. The role of the amygdala-striatal pathway in the acquisition and performance of goal-directed instrumental actions. The Journal of Neuroscience. 2013;33:17682–17690. doi: 10.1523/JNEUROSCI.3271-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFeliceantonio A, Mabrouk O, Kennedy R, Berridge K. Enkephalin surges in dorsal neostriatum as a signal to eat. Current Biology. 2012;22:1918–1924. doi: 10.1016/j.cub.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. The Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Differential representation of Pavlovian-instrumental transfer by prefrontal cortex subregions and striatum. The European Journal of Neuroscience. 2009;29:1461–1476. doi: 10.1111/j.1460-9568.2009.06679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. The Journal of Comparative Neurology. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- McKee BL, Kelley AE, Moser HR, Andrzejewski ME. Operant learning requires NMDA-receptor activation in the anterior cingulate cortex and dorsomedial striatum, but not in the orbitofrontal cortex. Behavioral Neuroscience. 2010;124:500–509. doi: 10.1037/a0020270. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. The Journal of Neuroscience. 2007;27:6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. The Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa M, Cyr J. Regionally selective roles of the rat’s striatum in modality-specific discrimination learning and forelimb reaching. Behavioural Brain Research. 1990;37:281–292. doi: 10.1016/0166-4328(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Prévost C, Liljeholm M, Tyszka JM, O’Doherty JP. Neural correlates of specific and general Pavlovian-to-Instrumental Transfer within human amygdalar subregions: a high-resolution fMRI study. The Journal of Neuroscience. 2012;32:8383–8390. doi: 10.1523/JNEUROSCI.6237-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. The Annals of the New York Academy of Science. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiology of Learning and Memory. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behavioral Neuroscience. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Shan Q, Ge M, Christie MJ, Balleine BW. The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. The Journal of Neuroscience. 2014;34:9196–9201. doi: 10.1523/JNEUROSCI.0313-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Brown RA, Balleine BW. Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. The Journal of Neuroscience. 2010;30:2951–2959. doi: 10.1523/JNEUROSCI.1778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by taqia a1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; 2004. [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. The European Journal of Neuroscience. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.