Abstract
Introduction
Massive transfusion (MT) is frequently required during liver transplantation. Risk stratification of transplant patients at risk for MT is an appealing concept, but remains poorly developed. Thrombelastography (TEG) has recently been shown to reduce mortality when employed for trauma resuscitation. We hypothesize that preoperative TEG can be used to risk stratify patients for massive transfusion.
Material and Methods
Liver transplant patients had blood drawn prior to surgical incision and assayed via TEG. Pre-operative TEG measurements were collected in addition to standard laboratory coagulation tests. TEG variables including R-time (reaction time), angle, MA (maximum amplitude), and LY30 (clot lysis 30 minutes after MA) were correlated to red blood cell (RBC) units, plasma (FFP), cryoprecipitate (Cryo), and platelets (Plt) during the first 24 hours following surgery, and tested for their performance using a receiver operating characteristic (ROC) curve.
Results
28 patients were included in the analysis with a median MELD (Model for End-Stage Liver Disease) score of 17; 36% received a massive transfusion. The TEG variables associated with MT (defined as ≥10 RBC units/24hr) were a low MA (p<0.001) and low angle (p=0.014). A high INR (International Normalized Ratio of prothrombin time) (p=0.003) and low platelet count (p=0.007) were also associated with massive transfusion. MA had the highest area under the curve (0.861) followed by INR (0.803). A MA of less than 47mm has a sensitivity of 90% and specificity of 72% to predict a massive transfusion. MA was the only coagulation variable that correlated strongly to all blood products transfused.
Conclusion
TEG MA has a high predictability of massive transfusion during liver transplantation. The use of TEG pre-operatively may help guide more cost effective blood bank preparation for this procedure as only a third of patients required a massive transfusion.
Introduction
Early experience in liver transplant surgery was associated large volumes of blood products during the perioperative period. Starzl’s first hundred transplants averaged 26 units of red blood cells (RBC) during the operation(1). A more contemporary analysis of liver transplantation has demonstrated a marked reduction in blood product usage, averaging 14–17 units of RBC during the perioperative period(2, 3). In trauma, early identification of patients at risk of massive transfusion (MT) and implementation of a protocol to prepare transfusions, have been associated with improved survival(4). Conversely, in liver transplant it is anticipated that the majority of patients will undergo large blood product resuscitation; however, many patients may not require massive transfusion because liver transplantation in certain scenarios has become a virtually bloodless procedure(5). Therefore, risk stratification for bleeding in these patients can lead to more optimal utilization of the blood bank, and aid in patient education for anticipated postoperative outcomes.
Risk of blood product use in transplant has been associated with an elevated international normalized ratio of prothrombin time (INR) and low platelet count(6), but these have limitations for predicting bleeding risk in patients with liver disease(7). The shortcoming of using these traditional coagulation assays is partitioning the coagulation system into plasma and cellular components. The cellular based model of hemostasis emphasizes the importance of retaining the cellular components of blood when assessing coagulation(8). Thrombelastography (TEG) is a whole blood assay, which provides comparable coagulation information to five conventional laboratory assays(9). TEG-guided transfusion for procedures in cirrhotic patients reduces unnecessary transfusions compared to traditional assays(10). TEG-guided resuscitation in critically injured trauma patients also reduces blood product usage and reduces mortality by up to 50%(11). The same benefit of fewer transfusions while using TEG- based resuscitation exists for liver transplantation(12).
There is growing evidence supporting the use of TEG as a technique to guide transfusion strategies in patients with severe bleeding that are at risk for massive transfusion(13, 14). Currently, there are no consistent guidelines on predicting the risk of massive transfusion for patients undergoing liver transplant surgery. With refined technique, we can use devices to identify patients at risk for bleeding, guide and lessen the usage of blood products, and reduce massive transfusion. The objective of our study is assess if preoperative TEG in patients undergoing liver transplant surgery can predict massive transfusion. We hypothesize that preoperative TEG can be used to risk stratify patients for massive transfusion.
Material and Methods
Subjects
Liver transplant recipients were enrolled pre-operatively in a Colorado Multi-Institutional Review Board study to prospectively collect blood samples for the first 24 hours following surgery. All patients were transplanted at the University of Colorado Hospital; which averages ~100 liver transplants a year. Enrollment criteria were adult (>18 years) and cadaveric liver donor recipient. Patient demographics were recorded; including age, sex, co-morbidities, and model for end-stage liver disease (MELD) calculated the day of surgery. TEG assays were not performed on these patients as part of their routine preoperative coagulation assessment, which is currently limited to International Normalized Ratio of prothrombin time (INR) and platelet count.
Thrombelastography (TEG)
Pre-operative blood samples were drawn in the operating room after intubation and before incision. Blood was collected in citrated tubes and assayed at room temperature between 20 minutes and 2 hours after blood draw, per manufacturer’s guidelines. The citrated samples were re-calcified, and assayed using the TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics, Niles IL) per manufacturer instructions. Clot formation, strength, and fibrinolysis were measured using standard TEG measurements including reaction time (R-time), angle, maximum amplitude (MA), and clot lysis 30 minutes after MA (LY30). All TEG tracings were used for research purposes and not available to the surgeons or anesthesiologists.
Outcomes
Total blood product use was calculated at 24 hours after the operation. Massive transfusion was defined as ≥10 units of red blood cells transfused within 24 hours of surgery(15), and intra- operative mortality was also recorded as an outcome.
Statistical Analysis
Statistical analysis was performed using SPSS 22 software (Microsoft, Armonk, NY). Normally distributed data were described as mean and standard deviation and non-normally distributed data were described as the median value within the 25th to 75th percentile values. Clinical variables and outcomes were contracted between patients that underwent a massive transfusion using a Mann Whitney U test. Correlation between coagulation parameters and blood products were assessed with Spearman’s Rho test, and a high correlation was considered a value > 0.5. Receiver operating characteristic (ROC) curves were generated with all coagulation tests that were significant between patients that underwent massive transfusion (MT), and patients that did not undergo massive transfusion (no-MT). Alpha was set to 0.05 for significance. Youden index was used to determine a threshold for predicting MT.
Results
There were 28 patients included in the analysis, 61% were male, and the median age was 56 years (53–61). Median values for standard lab data were: MELD = 17 (10–27), INR = 1.9 (1.3–12.7), Platelet Count = 81 (56–110). Median values for total units transfused within 24 hours of starting surgery were: 5 units of RBC, 4 units of plasma, and 1 unit of platelets. Only 25% of patients received cryoprecipitate. The intraoperative mortality rate was 7%, and 36% underwent a massive transfusion. Extended mortality at both 30 days and 90 days was 0%.
Differences in MT patients versus no-MT patients are listed in table 1. Notable differences were that MT patients presented lower hemoglobin (Hgb) (p=0.006), higher INR (p=0.003), lower platelet count (p=0.007), lower angle (p=0.014), and lower MA (p<0.001) than no-MT patients. There were no differences in R-time (p=0.763) or LY30 (p=0.945). Mortality rate was 20% in MT versus 0% in no-MT patients (p=0.119).
Table 1.
Comparison of MT versus No MT patients.
| MT (n=10) | No-MT (n=18) | P value | |
|---|---|---|---|
| Age (years) | 53 (52–59) | 60 (54–63) | 0.126 |
| Percent Male | 45% | 74% | 0.238 |
| MELD | 31 (25–39) | 12 (8–19) | <0.001 |
| Hgb | 9.9 (9.2–11.0) | 13.6 (10.4–15.5) | 0.006 |
| INR | 2.5 (1.9–3.3) | 1.4 (1.1–2.0) | 0.003 |
| Platelet Count | 64 (31–80) | 100 (58–123) | 0.007 |
| R-Time (minutes) | 11 (7–13) | 10 (8–12) | 0.763 |
| Angle (degrees) | 36 (3–43) | 52 (40–57) | 0.014 |
| MA (mm) | 37 (14–41) | 56 (44–64) | 0.001 |
| LY30 (%) | 0.0 (0.0–2.1) | 0.1 (0.0–0.9) | 0.945 |
MT= massive transfusion; MELD= model for end stage liver disease score; Hgb = hemoglobin; INR = international normalized ratio of prothrombin time; Platelet count = 1,000 per microliter of blood; R-time = reaction time; MA= maximum amplitude; LY30 = lysis at 30 minutes after clot reaches MA
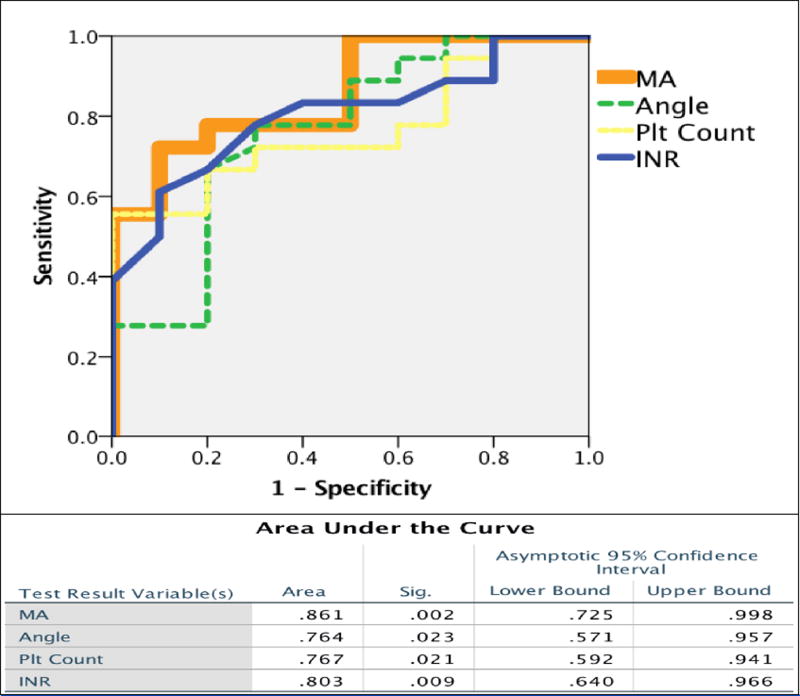
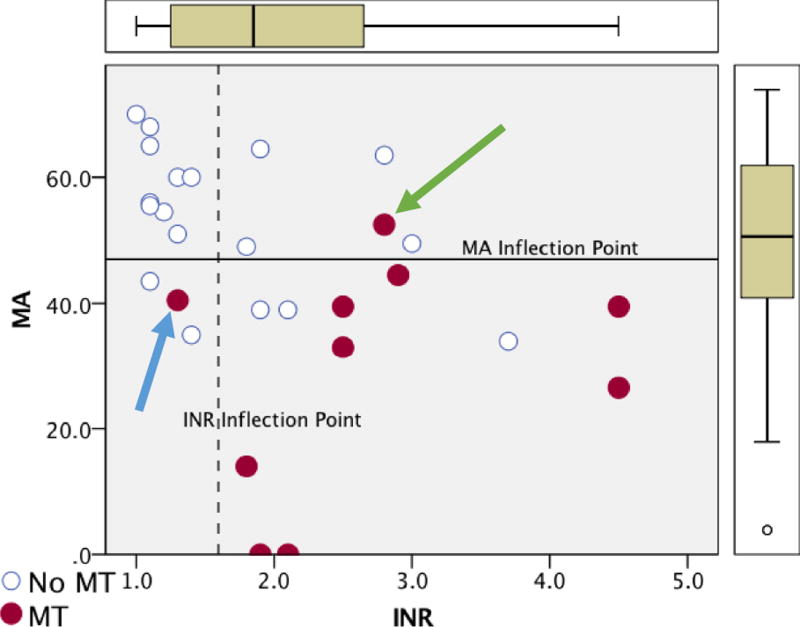
Area under the curve (AUC) of ROC curves was highest for MA 0.861, followed by INR 0.803, platelet count 0.767, and angle 0.764 (Figure 1). Inflection points determined by the Youden index of each of the coagulation assays are listed in table 2. The MA and INR had a high sensitivity that retained high specificity, while platelet count and angle also had high sensitivity but lacked specificity. In transplant recipients with an MA < 47mm, 67% underwent a massive transfusion, and MA could exclude those who would not require a massive transfusion in 93% of patients. MA and INR had a significant correlation (Spearman’s Rho -0.525, p=0.004), and using the optimal inflection points to identify patients at risk of massive transfusions each marker only missed one patient that required a massive transfusion; however, both assays missed a different individual (Figure 2).
Figure 1. Receiver Operating Characteristic (ROC) Curves of Coagulation Variables Associated with Massive Transfusion.
Figure 1 represents the ROC curves of coagulation variables that were significantly associated with massive transfusion. Their specific area under the curve metrics are listed in the table below with associated confidence intervals.
Table 2.
Receiver Operating Characteristic (ROC) Curve Results
| Youden Index | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| MA (mm) | <47 | 90% | 72% | 64% | 93% |
| INR | >1.5 | 90% | 61% | 57% | 92% |
| Plt Count | <96 | 100% | 56% | 56% | 100% |
| Angle (degrees) | <41 | 81% | 59% | 72% | 70% |
INR = international normalized ratio of prothrombin time; Platelet count = 1,000 per microliter of blood; MA= maximum amplitude
Figure 2. Scatter Plot of MA vs INR and Patients that Underwent a Massive Transfusion.
MT = massive transfusion; MA = maximum amplitude (mm); INR = international normalized ratio of prothrombin time
The y-axis represents the patient’s MA and the x-axis represents INR. Patients in red required a massive transfusion. All dots above the solid line on the X axis represent patients that had a MA above the threshold to predict massive transfusion. All dots to the left of the dotted line represent patients that had an INR below the threshold to predict massive transfusion. The blue arrow identifies a patient that was missed by using INR as a predictor for MT, while the green arrow represents a patient missed using MA as a predictor for MT.
MA had a strong correlation with all four blood products used during surgery (Table 3), while INR and Hgb were limited to RBC and plasma transfusions. The platelet count was strongly associated with cryoprecipitate and platelet transfusions. Angle had a strong correlation to cryoprecipitate transfusions.
Table 3.
Spearman’s Rho correlations between blood products and lab assays
| RBC units | FFP units | Plt units | Cryo units | |
|---|---|---|---|---|
| INR (correlation) | 0.534* | 0.755* | 0.357 | 0.211 |
| P value | 0.003 | 0.000 | 0.062 | 0.281 |
| Plt Count (correlation) | −0.424 | −0.489 | −0.611* | −0.696* |
| P value | 0.025 | 0.008 | 0.001 | 0.000 |
| Hgb (correlation) | −0.614* | −0.514* | −0.236 | −0.338 |
| P value | 0.001 | 0.005 | 0.227 | 0.078 |
| R-time (correlation) | 0.144 | 0.162 | 0.378 | 0.082 |
| P values | 0.465 | 0.411 | 0.047 | 0.678 |
| Angle (correlation) | −0.441 | −0.440 | −0.483 | −0.553* |
| P value | 0.019 | 0.019 | 0.009 | 0.002 |
| MA( correlation) | −0.620* | −0.711* | −0.639* | −0.622* |
| P Value | <0.001 | <0.001 | <0.001 | <0.001 |
| LY30 (correlation) | −0.012 | −0.023 | 0.069 | −0.121 |
| P value | 0.954 | 0.906 | 0.729 | 0.385 |
RBC = red blood cells; FFP = fresh frozen plasma; Plt = platelet; Cryo = cryoprecipitate;
Strong Spearman’s Rho correlation (> 0.500);
INR = international normalized ratio of prothrombin time; Plt count = 1,000 per microliter of blood; R-time = reaction time; MA= maximum amplitude; LY30 = lysis at 30 minutes after clot reaches MA
Discussion
Multiple coagulation variables assessed in this study identified liver transplant recipients at risk of massive transfusion, but MA had the greatest area under the curve when analyzing each variables respective predictability of massive transfusion. While most lab measurements were sensitive for predicting who will require a massive transfusion, MA and INR retained the highest specificity. Only MA strongly correlated with all blood products transfused in the first 24 hours from surgery, while INR, Hgb, platelet count, and TEG angle only correlated with one or two of the blood products transfused. The clinical relevance of identifying these patients at risk of massive transfusions is twofold: first, patients can be more accurately warned preoperatively of a higher mortality risk, and second, the blood bank can selectively prepare for large volume blood product resuscitation, rather than anticipating that all patients need full blood bank mobilization.
Thrombelastography has been used in liver transplant surgery since the 1960’s to guide intraoperative blood transfusions and, in fact was the first clinical arenas to rely on TEG to manage intraoperative coagulopathy (16). This assay has been prospectively validated to reduce total blood product use(17) and can reduce intraoperative plasma transfused by nearly 50%(12). However, TEG has been primarily advocated for use during surgery as a reactive approach to correcting a patients coagulation abnormalities(13), rather than predicting their risk for blood product utilization. Our study demonstrates that a preoperative TEG may also provide predictive value. TEG has previously been demonstrated to predict massive transfusion in trauma (9, 18). However, conventional coagulation assays to predict massive transfusion during liver transplantation have not been as effective to predict high blood product requirements. Findlay et al. in 2000(19) analyzed over 500 liver transplant patients and failed to identify a predictive score for blood product use based on patient demographics and laboratory values, while utilizing log transformation in an attempt to normalize blood product distribution and fit a regression model. Ritter et al. in 1989(20) measured specific coagulation factor levels and did not find an association with blood loss during surgery in 66 patients.
Our study differs from these previous studies, as we focused on a binary output of massive transfusion rather than the actual number of blood products transfused. We chose the cut off of ≥10 RBC units within 24 hours from surgery, as this threshold has been associated with increased morbidity, poorer graft function and increased mortality(21). Other investigators have also assessed the risk for massive transfusion in liver transplant. Mor et al. in 1993(21) found a significant association with low platelet counts and other conventional assays to predict a massive transfusion. Using an alternative definition of 6 or more RBC units in 24 hours following surgery, McCluskey et al.(22) were able to identify 7 preoperative variables—including INR and platelet count—to reliably predict a massive transfusion. However, other investigators using higher cut offs of 20+ or 30+ RBC units found an association with an elevated INR and low platelet count when predicting massive blood product utilization, but these variables lacked significance after adjusting for confounders(6). While these studies have large numbers of patients included in their analysis, they employed databanks of patients over decades in which the surgical techniques were diverse. In addition, they did not perform whole blood assays such as TEG to measure coagulation. TEG measurement of coagulation in patients with end stage liver disease has proven to be a more reliable tool than INR when ruling out the need for preemptive blood products for invasive procedures(10).
This study is limited to a relatively small patient population, but has the benefit of a cohort of transplant patients undergoing the same surgical technique without the use of venous bypass. Due to our small number we could not conclude superiority of a MA over INR in prediction of massive transfusion, as this would require a larger population and multicenter evaluation. In addition, the causality for TEG abnormalities predicting massive transfusion warrants continued investigation. To our surprise the R-time in TEG, which is historically attributed to the enzymatic phase of coagulation(23), had no predictability of blood product utilization during surgery and did not correlate with plasma transfusions or INR. The etiology of a prolonged INR in pathologic clinical settings remains unclear as it cannot be completely attributed to coagulation factor deficiencies(24). Compensatory mechanisms exist during liver failure to make these patients paradoxically hypercoagulable at times(25). Furthermore, the risk of over correcting these patient pre-operative coagulation abnormalities may be real, as a recent study identified that a elevated preoperative TEG MA as an associated risk factor for hepatic artery thrombosis(26).
In conclusion, TEG appears to be a preoperative tool to risk stratify patients for massive transfusion. A low MA was correlated to all blood products transfused in the first 24 hours of surgery, and had the highest area under the curve retaining high specificity and sensitivity to predict those patients which went on to a massive transfusion. In the modern era of liver transplantation, in which only a third of patients underwent a massive transfusion, it is becoming increasingly clinically valuable and cost effective to identify these patients for optimal resource utilization, rather than assuming all patients are at risk of massive transfusion. TEG may provide a valuable role in the preoperative assessment of liver transplant in reducing blood product administration and reducing the mobilization of large quantities of unused blood during surgery.
Acknowledgments
This study was supported in part by National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, in addition to the National Heart Lung and Blood Institute UM1-HL120877. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional research support was provided by Haemonetics with shared intellectual property.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Academic Surgical Congress Las Vegas NV, February 2017
References
- 1.Lewis JH, Bontempo FA, Awad SA, Kang YG, Kiss JE, et al. Liver transplantation: intraoperative changes in coagulation factors in 100 first transplants. Hepatology. 1989;9:710–714. doi: 10.1002/hep.1840090509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rana A, Petrowsky H, Hong JC, Agopian VG, Kaldas FM, et al. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. Journal of the American College of Surgeons. 2013;216:902–907. doi: 10.1016/j.jamcollsurg.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Reichert B, Kaltenborn A, Becker T, Schiffer M, Klempnauer J, et al. Massive blood transfusion after the first cut in liver transplantation predicts renal outcome and survival. Langenbecks Arch Surg. 2014;399:429–440. doi: 10.1007/s00423-014-1181-y. [DOI] [PubMed] [Google Scholar]
- 4.Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. The Journal of trauma. 2009;66:41–48. doi: 10.1097/TA.0b013e31819313bb. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 5.Jabbour N, Gagandeep S, Mateo R, Sher L, Strum E, et al. Live donor liver transplantation without blood products: strategies developed for Jehovah's Witnesses offer broad application. Annals of surgery. 2004;240:350–357. doi: 10.1097/01.sla.0000133352.25163.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cywinski JB, Alster JM, Miller C, Vogt DP, Parker BM. Prediction of intraoperative transfusion requirements during orthotopic liver transplantation and the influence on postoperative patient survival. Anesthesia and analgesia. 2014;118:428–437. doi: 10.1213/ANE.0b013e3182a76f19. [DOI] [PubMed] [Google Scholar]
- 7.Mallett SV. Clinical Utility of Viscoelastic Tests of Coagulation (TEG/ROTEM) in Patients with Liver Disease and during Liver Transplantation. Seminars in thrombosis and hemostasis. 2015;41:527–537. doi: 10.1055/s-0035-1550434. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thrombosis and haemostasis. 2001;85:958–965. [PubMed] [Google Scholar]
- 9.Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. The Journal of trauma. 2011;71:407–414. doi: 10.1097/TA.0b013e31821e1bf0. discussion 414-407. [DOI] [PubMed] [Google Scholar]
- 10.De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63:566–573. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Annals of surgery. 2016;263:1051–1059. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplantation proceedings. 2010;42:2590–2593. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 13.Wikkelsoe AJ, Afshari A, Wetterslev J, Brok J, Moeller AM. Monitoring patients at risk of massive transfusion with Thrombelastography or Thromboelastometry: a systematic review. Acta anaesthesiologica Scandinavica. 2011;55:1174–1189. doi: 10.1111/j.1399-6576.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 14.Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. The Cochrane database of systematic reviews. 2016:Cd007871. doi: 10.1002/14651858.CD007871.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleland S, Corredor C, Ye JJ, Srinivas C, McCluskey SA. Massive haemorrhage in liver transplantation: Consequences, prediction and management. World journal of transplantation. 2016;6:291–305. doi: 10.5500/wjt.v6.i2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation. Archives of surgery. 1966;92:71–79. doi: 10.1001/archsurg.1966.01320190073016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesthesia and analgesia. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 18.Moore HB, Moore EE, Chin TL, Gonzalez E, Chapman MP, et al. Activated clotting time of thrombelastography (T-ACT) predicts early postinjury blood component transfusion beyond plasma. Surgery. 2014;156:564–569. doi: 10.1016/j.surg.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Findlay JY, Rettke SR. Poor prediction of blood transfusion requirements in adult liver transplantations from preoperative variables. J Clin Anesth. 2000;12:319–323. doi: 10.1016/s0952-8180(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 20.Ritter DM, Owen CA, Jr, Bowie EJ, Rettke SR, Cole TL, et al. Evaluation of preoperative hematology-coagulation screening in liver transplantation. Mayo Clinic proceedings. 1989;64:216–223. doi: 10.1016/s0025-6196(12)65676-6. [DOI] [PubMed] [Google Scholar]
- 21.Mor E, Jennings L, Gonwa TA, Holman MJ, Gibbs J, et al. The impact of operative bleeding on outcome in transplantation of the liver. Surgery, gynecology & obstetrics. 1993;176:219–227. [PubMed] [Google Scholar]
- 22.McCluskey SA, Karkouti K, Wijeysundera DN, Kakizawa K, Ghannam M, et al. Derivation of a risk index for the prediction of massive blood transfusion in liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12:1584–1593. doi: 10.1002/lt.20868. [DOI] [PubMed] [Google Scholar]
- 23.Salooja N, Perry DJ. Thrombelastography. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2001;12:327–337. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Davenport RA, Guerreiro M, Frith D, Rourke C, Platton S, et al. Activated Protein C Drives the Hyperfibrinolysis of Acute Traumatic Coagulopathy. Anesthesiology. 2017;126:115–127. doi: 10.1097/ALN.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baccouche H, Labidi A, Fekih M, Mahjoub S, Kaabi H, et al. Haemostatic balance in cirrhosis. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2017;28:139–144. doi: 10.1097/MBC.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 26.Zahr Eldeen F, Roll GR, Derosas C, Rao R, Khan MS, et al. Preoperative Thromboelastography as a Sensitive Tool Predicting Those at Risk of Developing Early Hepatic Artery Thrombosis After Adult Liver Transplantation. Transplantation. 2016;100:2382–2390. doi: 10.1097/TP.0000000000001395. [DOI] [PubMed] [Google Scholar]