Abstract
Background
Acute lung injury and respiratory distress syndrome is characterized by uncontrolled inflammation of the lungs after a severe inflammatory stimulus. We have previously demonstrated an ameliorated syndrome and improved survival in mice with early administration of valproic acid (VPA), a broad-spectrum histone deacetylase inhibitor, while studies in humans have shown no benefit when anti-inflammatories are administered late. The current study tested the hypothesis that early treatment would improve outcomes in our gram-negative pneumonia-induced acute lung injury.
Materials and methods
Mice (C57BL/6) had 50 × 106 Escherichia coli (strain 19,138) instilled endotracheally and VPA (250 mg/kg) administered intraperitoneally 3, 4, 6, and 9 h (n = 12/group) later. Six hours after VPA administration, the animals were killed, and bronchoalveolar lavage (BAL) fluid interleukin-6 (IL-6), tumor necrosis factor, neutrophils and macrophages as well as theE coli colony-forming units were quantified. Plasma IL-6 was also measured. A separate group of mice (n = 12/group) were followed prospectively for 7 days to assess survival.
Results
BAL IL-6 and tumor necrosis factor as well as plasma IL-6 were significantly lower in the animals administered VPA within 3 h (P < 0.05) but not when administered later (4, 6, 9 h). There was no difference in the BAL E coli colony-forming units, macrophage, or neutrophil numbers at any time point. Survival improved only when VPA was administered within 3 h.
Conclusions
A narrow therapeutic window exists in this murine model of gram-negative pneumonia-induced acute lung injury and likely explains the lack of response in studies with late administration of anti-inflammatory therapies in clinical studies.
Keywords: Acute lung injury, Acute respiratory distress syndrome, Histone deacetylase inhibitors, Valproic acid, Timing of administration, Therapeutic window
Background
Acute lung injury and respiratory distress syndrome (ARDS) describes a spectrum of pulmonary condition that manifests clinically with severe respiratory failure.1 The syndrome develops as the lungs mount an exaggerated immune response to a severe inflammatory stimulus resulting from local or systemic insults. Neutrophils recruited to the lungs damage the pulmonary vascular endothelium and alveolar epithelium, leading to increased permeability across and between cells, intrapulmonary hemorrhage, and pulmonary edema due to disruption of the blood-alveolar barrier.2 These pathologic events taking place at a microscopic level eventually compromise gas exchange and lead to severe hypoxia and respiratory failure, typically necessitating ventilator support.3
This inflammatory condition affects more than 200,000 patients annually in the United States alone3,4 and is associated with over 3.6 million hospital days3 and excessive long-term functional disability and health care utilization.5 The mortality of the syndrome is also very high, exceeding 45%—approaching 60% in the most severe cases6—making ARDS more lethal than embolic stroke and acute coronary syndromes.7,8 Despite recent advances in critical care, treatment for ARDS remains largely supportive,9–11 and targeted anti-inflammatory therapies have failed to improve clinically relevant outcomes in humans. However, these have been typically administered past the exudative stage of the clinical syndrome or around its zenith,12–14 leaving little room for meaningful improvement. At the same time, subjects on anti-inflammatory therapies at baseline for different, non–ARDS-related indications appear to develop an ameliorated clinical syndrome, if at all, and have improved outcomes, when compared to counterparts not on long-term anti-inflammatory regimens.15,16 This observation has raised the question: Does timing of therapy play as an important role in the treatment of ARDS, or perhaps even more so, than the therapy itself? Does a therapeutic time window exist, during which, if targeted anti-inflammatory therapies are administered, the maximum clinical benefit can be derived (Fig. 1)? We have previously demonstrated an ameliorated inflammatory phenotype in a well validated and commonly used murine model of gram-negative pneumonia-induced acute lung injury17–20 utilizing a broad-spectrum histone deacetylase inhibitor (HDACI), valproic acid (VPA), administered early.21 HDACI is thought to exert at least part of their potent anti-inflammatory effects by interacting with the toll-like receptor, myeloid differentiation factor 88 (MyD88), interleukin-1 receptor kinase (IRAK1), and NF-kB pathway. Pathogen-associated molecular patterns interface with macrophage plasma membrane toll-like receptors and signal through MyD88 and IRAK1 to stimulate nuclear translocation of NF-kB, subsequently turning on the transcription of proinflammatory cytokines.22,23 HDACIs are known to decrease MyD88 and IRAK1 protein expression, likely by enhancing their degradation due to heat-shock protein 90 hyperacetylation, thereby preventing NF-kB nuclear translocation and cytokine expression in vivo.24 This represents at least one possible mechanism by which the kB-regulated proteins become underexpressed with HDACIs.
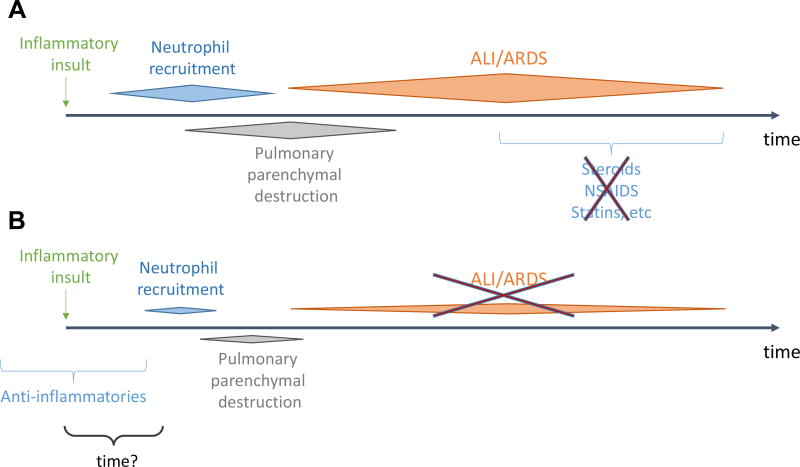
Fig. 1.
Proposed mechanism of effect of timing of administration of anti-inflammatory agents. (A) Anti-inflammatory therapies are of no clinical benefit when administered at the peak of the ARDS syndrome. (B) Patients on chronic anti-inflammatory therapies for nonpulmonary indications appear to develop a much more benign, if at all, clinical syndrome. (Color version of figure is available online.)
With the current project, we explore whether timing of administration of a previously proven to be effective therapy for ARDS21 has the same efficacy at improving the animal inflammatory profile and overall survival when administered at varied time intervals.
Methods
Mice
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) 6–8 wk old (n = 12/group) were allowed to acclimate for a minimum of 3 d and maintained under pathogen-free conditions in a full-barrier facility until the time of experimentation. All mice were provided food and water ab libitum, and all procedures were conducted after approval by Boston University’s Institutional Animal Care and Use Committee and in accordance with local and international guidelines for ethical animal treatment.
Escherichia coli
E coli (serotype 06:K3:H1, strain 19,138, American Type Culture Collection, Manassas, VA) were cultured in Luria broth to the desired concentration and subsequently quantified by optical density and verified by serial dilutions on Luria broth plates.
Endotracheal instillations and VPA administration
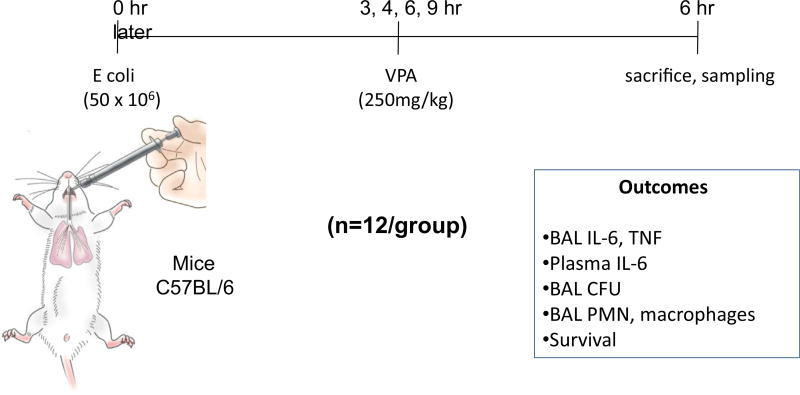
After anesthesia with inhaled isoflurane, mice underwent direct endotracheal instillation. They were suspended by their front incisors on an angled surgical board and their tongue retracted outward with DeBakey forceps to allow access to their hypopharynx. Fifty million of E coli diluted in 50 µL of saline was instilled endotracheally by pipette. Mice were placed back into their cages upon return to consciousness. Mice were administered intraperitoneally 250 mg/kg of VPA (Selleck Chemicals, Boston, MA) 3, 4, 6, and 9 h after E coli instillation (n = 12/group) (Fig. 2). Another set of animals followed the same timeline, without VPA or vehicle administration (n = 12/group).
Fig. 2.
Experimental paradigm of varied timing of administration of valproic acid (VPA) after endotracheal Escherichia coli instillation leading to a severe multilobar gram-negative pneumonia, progressing to ARDS. CFU = colony-forming unit. (Color version of figure is available online.)
Animals were killed 6 h after VPA administration (or E coli instillation without VPA) and underwent thoracotomy. Cardiac puncture was performed, and systemic blood samples were obtained. The left hilum was ligated with 3-0 silks, while the trachea was surgically exposed, and a tracheostomy with a 22G angiocath was obtained. Bronchoalveolar lavage (BAL) of the right lung was performed with HBSS EDTA in a stepwise fashion until 1 mL of BAL fluid (BALF) was obtained. Cell counts and differentials were obtained in the BALF by Cytospin (ThermoScientific, Waltham, MA).
Cytokine expression
The proinflammatory cytokines tumor necrosis factor-α (TNF) and interleukin-6 (IL-6) were quantified in BALF samples by ELISA as per the manufacturers’ instructions [TNF and IL-6: eBioscience (San Diego, CA)]. IL-6 was also quantified in the systemic circulation. These cytokines were selected for comparison, as they are implicated in the pathophysiology of acute lung injury and ARDS in both human and animals and have been previously demonstrated to correlate with pulmonary and systemic inflammation, as well as overall survival (plasma IL-6) in our murine model of ARDS, as previously published.21
Bacterial burden
Viable bacteria were colony quantified,25,26 after serial dilutions of BALF were plated on blood agar, and colony-forming units/mL were enumerated after incubation at 37°C for 48 h.
Survival
A separate set of animals was instilled endotracheally with 50 × 106 of E coli diluted in 50 µL of saline and again underwent intraperitoneal injections of 250 mg/kg of VPA (Selleck Chemicals, Boston, MA) at 3, 4, 6 and 9 h after E coli instillation (n = 12/group), this time without killing. Instead, these mice were followed prospectively for 7 days to assess survival.
Statistics
Continuous variables were compared with Student’s t-test and survival with the log-rank test and Kaplan–Meier analysis. Statistical significance was declared with a two-sided P-value of P < 0.05. Statistical analyses were performed with Prism 6.0 (GraphPad Software, La Jolla, CA) or Stata 13.1 (StataCorp, College Station, TX).
Results
Cytokine expression as a marker of pulmonary and systemic inflammatory response
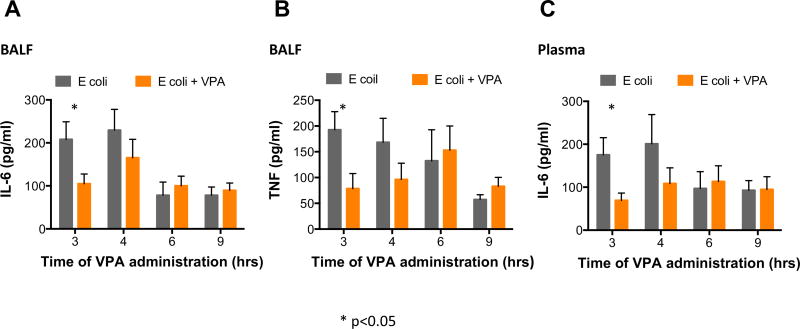
The BALF proinflammatory cytokines TNF and IL-6 were noted to be significantly decreased in animals injected with VPA at 3 h, compared to animals that did not receive VPA, but not in those that received VPA at later intervals. Similarly, systemic IL-6, a marker previously identified to correlate closely with survival in murine models of pneumonia,21,27 was also noted to improve with the 3-h administration of VPA but not when administered later (Fig. 3).
Fig. 3.
Early histone deacetylase inhibition (≤3 h) improves pulmonary [IL-6 (A) and TNF (B)] and systemic [IL-6 (C)] proinflammatory cytokines. (Each value represents mean ± SEM, * = P < 0.05 comparing Escherichia coli to E coli — VPA). SEM = standard error of mean. (Color version of figure is available online.)
Pulmonary inflammatory cell colonies with varied VPA timing of administration
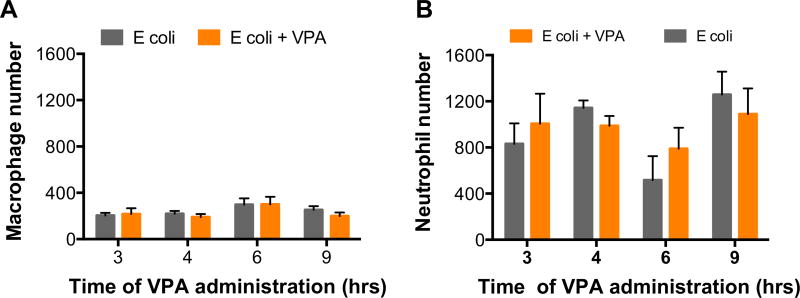
BAL cytology demonstrated few pulmonary macrophages in both E coli only and VPA-injected mice, regardless of administration or timing of HDACI. There were many more neutrophils recruited to the pulmonary parenchyma of the E coli–instilled mice. There was no difference in the number of neutrophils quantified between the E coli only and VPA-injected mice, regardless of administration or timing of HDACI (Fig. 4).
Fig. 4.
Histone deacetylase inhibition does not affect pulmonary macrophage or neutrophil colonies at any of the studied timing intervals. (Each value represents mean ± SEM). SEM = standard error of mean. (Color version of figure is available online.)
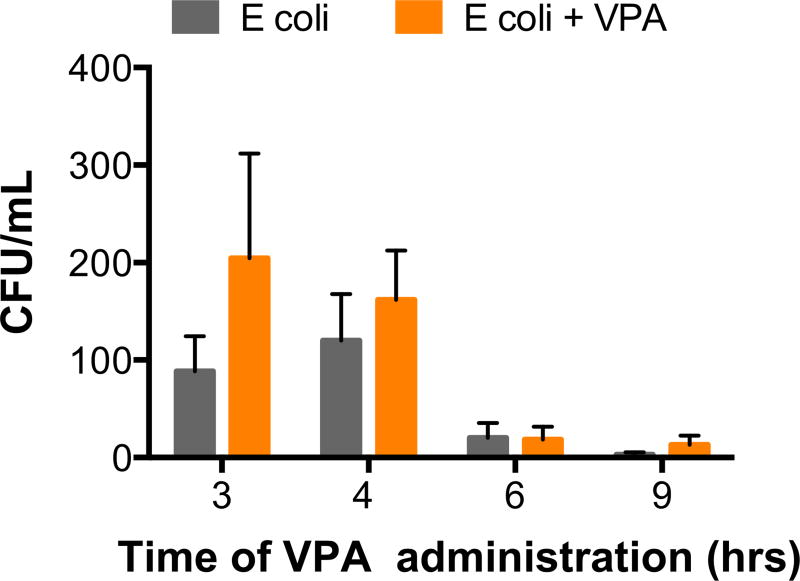
Pulmonary bacterial burden
Bacterial infections in immunocompetent hosts routinely trigger an inflammatory response that in turn attracts immune cells and activates bactericidal pathways, in an attempt to fight the bacterial infection. By mitigating this immune response early and aggressively, we have previously demonstrated that bacterial clearance may be hindered.21 Although an increase in bacterial burden was seen with the 3-h administration of VPA and to a lesser extent at 4 h, neither achieved statistical significance. At later time points (6 h and 9 h) of HDACI, only few bacteria could be isolated from the BALF, as by this time, the innate bactericidal pathways likely have nearly eliminated the infectious process (Fig. 5).
Fig. 5.
Histone deacetylase inhibition does not significantly worsen pulmonary bacterial burden at any of the studied time points of administration. (Each value represents mean ± SEM). CFU = colony-forming unit; SEM = standard error of mean. (Color version of figure is available online.)
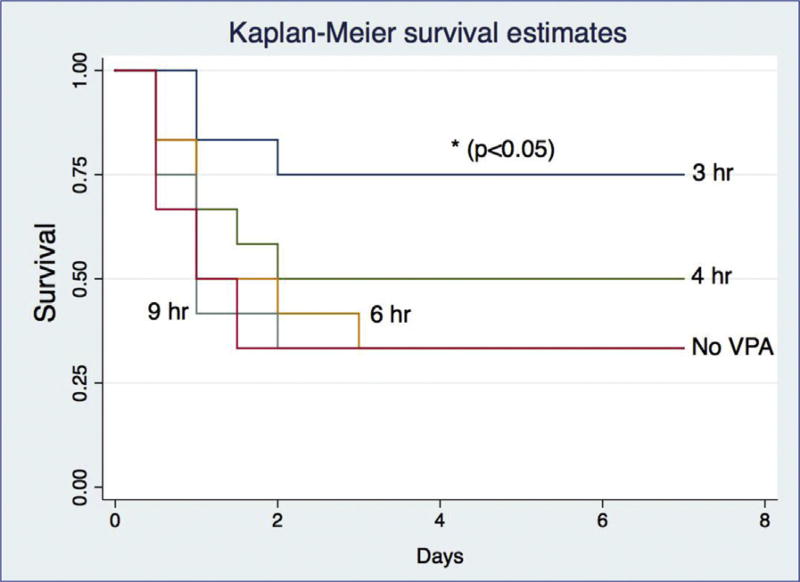
Survival
Survival only improved with the early administration (3 h) of HDACI, compared to the survival curve seen in untreated with VPA animals. When VPA was administered at later time points (4, 6, 9 h), there was no discernible survival improvement (Fig. 6). Our findings are summarized on Table.
Fig. 6.
Early histone deacetylase inhibition (≤3 h) improves survival in a gram-negative pneumonia-induced model of murine ARDS. (Color version of figure is available online.)
Table.
Summary of the effects of the timing of valproic acid administration, a broad-spectrum HDACI, including time points studied at earlier experiments (30 min).
| Timing of VPA administration |
BAL IL-6 | BAL TNF-α | BAL CFU | BAL macrophages | BAL neutrophils | Plasma IL-6 | Survival |
|---|---|---|---|---|---|---|---|
| 3 h | ↓ | ↓ | No change | No change | No change | ↓ | ↑ |
| 4 h | No change | No change | No change | No change | No change | No change | No change |
| 6 h | No change | No change | No change | No change | No change | No change | No change |
| 9 h | No change | No change | No change | No change | No change | No change | No change |
CFU = colony-forming unit.
Discussion
Bacterial infections in immunocompetent hosts routinely trigger a robust inflammatory cascade that attracts neutrophils to the primary infectious site and activates a variety of bactericidal pathways, in an attempt to control microbial proliferation. This process, specifically when exaggerated, may lead to host parenchymal destruction and end up being perhaps more detrimental than the original infectious process per se.28 ARDS represents an example of such a disease process, in which the exaggerated pulmonary immune response may be more detrimental than the infectious process triggering it, and leads to potentially lethal complications.
Previous clinical trials with aggressive anti-inflammatory therapies in human ARDS subjects have failed to show clinically relevant benefits, including survival,13,14,29 likely because they were administered in the proliferative stage of the disease, after irreversible pulmonary parenchymal damage has reached its zenith and the systemic proinflammatory cascade has surpassed the point of clinically meaningful reversal with medical management. More recently, however, new hope has been found with two studies that demonstrated an ameliorated ARDS clinical syndrome and improved clinical outcomes in patients on anti-inflammatory therapies at baseline for various indications, who were hospitalized with ARDS. In the former, Harr et al. secondarily analyzed severely injured blunt trauma patients, who were enrolled in the Glue Grant database.16 Subjects at risk for developing multiorgan failure (MOF) (age >45 y, base deficit >6mEq/L or systolic blood pressure <90 mm Hg, who received a blood transfusion) and who were on antiplatelet therapy (aspirin, clopidogrel and/or ticlopidine) at baseline for nonpulmonary indications had smaller risk for developing ARDS (58% versus 64%, P = 0.011) and MOF (17% versus 21%, P = 0.029 respectively) compared to their nonantiplatelet counterparts, after adjusting for confounders.16 Mortality was also slightly lower in the antiplatelet-receiving cohort, although the difference did not reach statistical significance (P = 0.06). They concluded that not only platelets are likely to be involved in the pathogenesis of ARDS (a topic we are currently investigating in a separate project), a finding endorsed by other investigators too,30 but that early combined antiplatelet and anti-inflammatory management may be beneficial in trauma patients at risk for ARDS and MOF. Similarly, Chen et al.15 demonstrated in a secondary analysis of the Validating Acute Lung Injury Markers for Diagnosis study that patients on prehospital aspirin had a significantly lower prevalence of ARDS (27% versus 34%, P = 0.034). Even after adjusting for age, gender, race, presence of sepsis, and degree of physiologic derangement (APACHE II), the odds ratio for developing ARDS was favorable (0.60, 95% confidence interval: 0.41–0.90, P < 0.05).
The above observations naturally raised the question on whether aspirin could be utilized as therapy for ARDS31,32 and shifted the focus of ARDS management from treatment to primary prevention.33–35 Despite the initial enthusiasm and encouraging results with aspirin as potential prevention or treatment for ARDS,36 a prospective randomized trial of aspirin in at-risk patients presenting to the emergency department (The LIPS-A Randomized Clinical Trial) failed to reduce risk of ARDS compared to placebo, and the participating authors concluded that the results of their phase 2b trial did not support continuation to a larger phase 3 trial.37
These findings suggest that for an intervention to be successful at minimizing prevalence, morbidity and mortality from ARDS, broader inflammatory pathways should be targeted and that the timing of administration of these measures may play as a big role as the intervention itself. We have previously demonstrated that mitigating this immune response with VPA, a far more broad-spectrum anti-inflammatory agent at high doses, decelerates neutrophil migration to the pulmonary parenchyma and reduces resultant oxidative tissue destruction.21 In addition to the local effects, systemic inflammation is also muted, lessening the likelihood of MOF, a key cause of mortality in ARDS victims.6,38 With the current project, we aim to identify if a narrow therapeutic window exists within which, if VPA is administered in our murine model of gram-negative pneumonia-induced ARDS, local and systemic inflammation as well as survival can be improved. We demonstrate that VPA administered systemically within up to 3 h from the primary inflammatory stimulus, both pulmonary and the systemic immune response can be muted to the extent that overall survival can be improved. Unlike previous experience with very early HDACI,21 VPA administration at this time point does not affect neutrophil migration to the lungs nor local bacterial clearance. These findings are consistent with and may help explain why clinical trials with anti-inflammatories late in the stage of the ARDS syndrome failed to produce any significant clinical gains.
There are several limitations to our model, including lack of assessment of pulmonary function and long-term outcomes, optimal dosing of HDACI, and the discrepancies of our model’s kinetics to that of the human clinical syndrome; however, we aim to tackle these research questions one at a time.
Acknowledgments
This project was funded by the NIH NIGMS grant R21 AI112887-01.
Footnotes
Authors’ contributions: G.K., M.D.G., and D.R. contributed for project design. N.D., P.O., M.D.G., and G.K. conducted experiments. P.O., N.D., M.D.G., and G.K. helped in data management and analysis. G.K. drafted the manuscript. G.K., J.P.M., and D.R. participated in manuscript review and overall project supervision.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 6.Sharif N, Irfan M, Hussain J, Khan J. Factors associated within 28 days in-hospital mortality of patients with acute respiratory distress syndrome. Biomed Res Int. 2013;2013:564547. doi: 10.1155/2013/564547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 8.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 9.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 11.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 12.Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 14.McAuley DF, Laffey JG, O’Kane CM, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Janz DR, Bastarache JA, et al. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: a propensity-adjusted analysis. Crit Care Med. 2015;43:801–807. doi: 10.1097/CCM.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harr JN, Moore EE, Johnson J, et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit Care Med. 2013;41:399–404. doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaney J, Horie S, Masterson C, et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70:625–635. doi: 10.1136/thoraxjnl-2015-206813. [DOI] [PubMed] [Google Scholar]
- 19.Wan T, Yuan G, Ren Y, et al. Diet-induced obese mice exhibit altered immune responses to acute lung injury induced by Escherichia coli. Obesity (Silver Spring) 2016;24:2101–2110. doi: 10.1002/oby.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Files DC, Ilaiwy A, Parry TL, et al. Lung injury-induced skeletal muscle wasting in aged mice is linked to alterations in long Q4 chain fatty acid metabolism. Metabolomics. 2016;12 doi: 10.1007/s11306-016-1079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasotakis G, Galvan M, King E, et al. Valproic acid mitigates the inflammatory response and prevents acute respiratory distress syndrome in a murine model of Escherichia coli pneumonia at the expense of bacterial clearance. J Trauma Acute Care Surg. 2017;82:758–765. doi: 10.1097/TA.0000000000001389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Jin S, Wang C, Jiang R, Wan J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010;34:1676–1683. doi: 10.1007/s00268-010-0493-5. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Alam HB. Modulation of acetylation: creating a prosurvival and anti-inflammatory phenotype in lethal hemorrhagic and septic shock. J Biomed Biotechnol. 2011;2011:523481. doi: 10.1155/2011/523481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong W, Li Y, Liu B, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid attenuates Toll-like receptor 4 signaling in lipopolysaccharide-stimulated mouse macrophages. J Surg Res. 2012;178:851–859. doi: 10.1016/j.jss.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizgerd JP, Lupa MM, Kogan MS, Warren HB, Kobzik L, Topulos GP. Nuclear factor-kappaB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am J Respir Crit Care Med. 2003;168:810–817. doi: 10.1164/rccm.200303-412OC. [DOI] [PubMed] [Google Scholar]
- 26.Mizgerd JP, Peschon JJ, Doerschuk CM. Roles of tumor necrosis factor receptor signaling during murine Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2000;22:85–91. doi: 10.1165/ajrcmb.22.1.3733. [DOI] [PubMed] [Google Scholar]
- 27.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Lee KY. Pneumonia, acute respiratory distress syndrome, and early immune-Modulator therapy. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troncy E, Collet JP, Shapiro S, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998;157:1483–1488. doi: 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 30.Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L915–L923. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care. 2015;19:374. doi: 10.1186/s13054-015-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle AJ, Di Gangi S, Hamid UI, et al. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Crit Care. 2015;19:109. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mezidi M, Guerin C. Aspirin for prevention of acute respiratory distress syndrome (ARDS): let’s not throw the baby with the water! Ann Transl Med. 2016;4:376. doi: 10.21037/atm.2016.07.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reilly JP, Christie JD. Is it possible to prevent ARDS? JAMA. 2016;315:2403–2405. doi: 10.1001/jama.2016.5988. [DOI] [PubMed] [Google Scholar]
- 35.Rubenfeld GD. Who cares about preventing acute respiratory distress syndrome? Am J Respir Crit Care Med. 2015;191:255–260. doi: 10.1164/rccm.201408-1574CP. [DOI] [PubMed] [Google Scholar]
- 36.Panka BA, de Grooth HJ, Spoelstra-de Man AM, Looney MR, Tuinman PR. Prevention or treatment of Ards with aspirin: a review of Preclinical models and Meta-analysis of clinical studies. Shock. 2017;47:13–21. doi: 10.1097/SHK.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kor DJ, Carter RE, Park PK, et al. Effect of aspirin on Development of ARDS in at-risk patients presenting to the emergency department: the LIPS-a randomized clinical trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eachempati SR, Hydo LJ, Shou J, Barie PS. Outcomes of acute respiratory distress syndrome (ARDS) in elderly patients. J Trauma. 2007;63:344–350. doi: 10.1097/TA.0b013e3180eea5a1. [DOI] [PubMed] [Google Scholar]