Abstract
Background
This study identifies the overall survival status of lung cancer patients with bone metastasis and metastasis patterns. Poor prognostic factors were identified to develop a scoring system for estimating survival period after bone metastasis.
Methods
A retrospective cohort analysis was performed at Chiang Mai University for the period January 1, 2006 and December 31, 2013. Time-to-event analysis was performed to estimate survival rate. The primary end point was death related to lung cancer. Univariate and multivariate analysis of the prognostic variables was done using the Cox's regression model. The score was derived from the corresponding estimated regression coefficients of significantly poor prognostic factors.
Results
A total of 505 lung cancer with bone metastasis patients were analyzed. Four hundred two cases (79.6%) were concurrent diagnosis and 103 (20.4%) were subsequent diagnosis. The median survival time of lung cancer after bone metastasis 148 days. Male gender and ECOG 3–4 were significant poor prognostic factors for lung cancer after bone metastasis, with hazard ratios of 1.42 (95% CI 1.17–1.73), and 1.30 (95% CI 1.06–1.60), respectively. Prognosis score was determined using the binary term present/not-present for those factors. The curve from prognostic score summations of 2, 1 and 0 presented a good discrimination of survival expectancy, showing an expected median survival time of approximately 109, 146, and 225 days, respectively.
Conclusions
Prognostic score is a clinically simple and easy method for estimating life expectancy and for guiding interventions in bone metastasis of lung cancer.
Abbreviations: CMU-PAC, Chiang Mai University-Picture Archive Communication system; CT, Computed tomography (CT); ECOG, Eastern Cooperative Oncology Group; EGFR, Epidermal growth factor receptor; IARC, International Agency for Research on Cancer (IARC); ICD-O, International Classification of Disease for Oncology; ICD-9, International Classification of Disease-9; ICD-10, International Classification of Disease-10; HR, Hazard ratio; NSCLC, Non-small cell lung cancer; NCCN, National Comprehensive Cancer Network; SREs, Skeletal-related events; SMI, Suandok Medical Informatics
Keywords: Bone metastasis, Clinical prediction rule, Lung cancer, Prognostic factors, Skeletal-related events, Survival rate
Highlights
-
•
Median survival time of lung cancer after bone metastasis is 148 days.
-
•
Male, and ECOG 3–4 are poor prognosis factors.
-
•
Prognostic score is specific to bone metastasis from lung cancer.
-
•
Prognostic score can help guide optimizing intervention in specific group.
1. Introduction
Lung cancer is the most prevalent type of cancer and the leading cause of cancer-related death worldwide [1], [2], and is the most common cancer in the Thai population [3]. The skeletal system is one of the common sites for lung cancer metastasis. The presentation of skeletal related events (SREs) during the course of treatment impairs the quality of life and decreases performance status which indirectly affects overall survival [4]. Bone metastasis is usually under-diagnosed in lung cancer patients because the sensitive investigation often is only recommended after the appearance of clinical signs [5]. The frequency of SREs is likely to increase as survival rates improve with new treatment modalities [6], [7]. The increasing number of surgical interventions [8] and with the introduction of other medical agents are helping prevent complications from bone metastasis [9]. Therefore, optimal treatment becomes a major concern which involves weighing in the balance the risks from treatment and quality of life over the remaining survival period.
Survival estimation is one of the critical steps which determine the direction of management for SREs. Under and over estimation can lead to under treatment or overly aggressive intervention, respectively. Stable fixation of limbs and decompressive spine surgery can help improve ambulatory ability and overall performance status and can increase the opportunity for further treatment either with chemotherapy or with other targeted therapies. The question is whether post-operative recovery and/or complications from intervention will on balance be a benefit or not for the remaining survival time. There are several survival estimating system introduced for optimizing between invasive surgical interventions and remaining survival time in bone metastases. Those scores derived from several types of cancer which have different patterns of metastasis, the prognostic ability might not be able to work properly in bone metastases of lung cancer patients who have a very short expected survival time.
The biology of the disease and accessibility of treatment plays an important role in determining the metastasis of cancers and the survival patterns of patients. There is limited data on survival rates and bone metastasis patterns in lung cancer among Thai people. This study identified the overall survival of lung cancer after bone metastasis, as well as other important characteristics and bone metastasis patterns. Poor prognostic factors were identified and a scoring system was developed to estimate the survival period after bone metastasis. That scoring system can assist survival estimates which affect skeletal management decisions and efforts to maintain quality of life in a palliative care program.
2. Material and methods
2.1. Data sources
Retrospective analysis was performed on 505 patients with lung cancer and bone metastasis treated at Chiang Mai University Hospital between 1 January 2006 and 31 Dec 2013. This study was approved by Ethical committee of Faculty of Medicine, Chiang Mai University (EC-ORT-10AVG010294). Data source was reviewed from “Chiang Mai Cancer Registry” which is the categories B qualification following International Agency for Research on Cancer (IARC) classification system [10]. Related clinical courses including skeletal-related events and ECOG scores were obtained from the Digicard system at Chiang Mai University Hospital. Laboratory results and radiography details were obtained from Suandok Medical Informatics (SMI) and the Chiang Mai University-Picture Archive Communication (CMU-PAC) system.
All patients with lung cancer were treated by multi-disciplinary teams including oncologists, pulmonologists, radiologists, pathologists, orthopedic surgeons, and thoracic surgeons. Computed tomography (CT) of the chest was performed in all cases to evaluate lung mass, mediastinal lymph nodes and metastatic lesions. CT brain and bone scans were performed in symptomatic patients, e.g., bone pain, headache or neurological deficits, cases of suspected brain metastasis, and those first presenting with advanced disease (at least stage IIIA). Surgical treatment included anatomical resection (sublobar resection, lobectomy or pneumonectomy) with systematic mediastinal lymph node dissection or sampling in stage I, II, some stage IIIa, and palliative resection in advanced disease cases. ECOG score was used to evaluate the status of all patients. Cisplatinum-based doublet chemotherapy was used in cases of neo-adjuvant, adjuvant, and first-line metastatic disease. Single agents were used for some second and third-line metastasis. The regimens and doses of chemotherapy followed National Comprehensive Cancer Network (NCCN) guidelines.
2.2. Study flow and definition of parameters
Lung cancer patient records with International Classification of Disease for Oncology (ICD-O) code C34 (lung cancer) and lung cancer with bone metastasis were retrieved from the Cancer Registry of Chiang Mai University. Patient code C34 were sourced with Skeletal-related events (SREs) by ICD-10 code: secondary malignant neoplasm of bone and bone marrow (C79.5), pathological fracture (M84.XX), spinal cord compression (G95.2), hypercalcemia (E83.5), palliative care (Z51.5), and ICD-9 code: operation on bone (78.XX), reduction of fracture and dislocation (79.XX), decompressive laminectormy (03.09), radiation procedure (92.24) and (92.29). The date of lung cancer diagnosis was defined as the date of tissue diagnosis. The primary end point was death from disease with a cutoff of 31 December 2015. Date of bone metastasis was defined as either 1) the date of lung cancer diagnosis in patients who initially presented with SREs or who had radiographic evidence of bone metastasis (concurrent diagnosis) or 2) the date of radiographic evidence with or without clinical presentation after lung cancer had been diagnosed (subsequent diagnosis). The primary end point of bone metastasis was death from disease with a cutoff of 31 December 2015. Laboratory parameters and performance score were recorded within 14 days before or after the date of diagnosis of bone metastasis.
2.3. Statistical analysis
Time-to-event analysis was performed using the Kaplan-Meier curve to estimate the median survival time and overall survival rate of patients with lung cancer after bone metastasis. Differences in survival rates from the Kaplan-Meier curve for each variable were analyzed using the log-rank test. Univariate and multivariate analysis was performed using the Cox's regression model and STATA version 10.1 to identify poor prognostic factors. Variables with a p-value less than 0.1 were selected for further multivariate analysis. Scores were derived from the corresponding estimated regression coefficients of the poor prognostic factors. Scores were then rounded off to the nearest integer to develop a prognostic score [11]. The prognostic score was calculated by adding scores for individual factors and estimated survival using the Kaplan-Meier method.
3. Results
3.1. Demographic data and survival rates
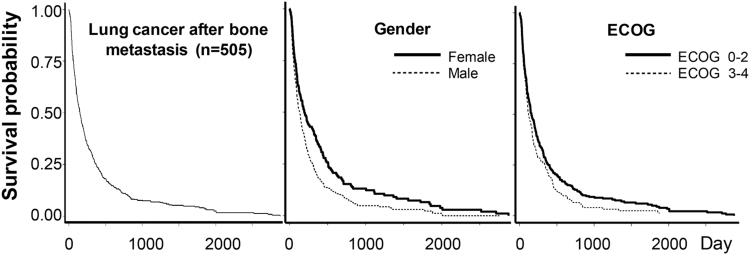
There were 505 cases of diagnosed bone metastasis of which 402 (79.6%) were concurrent and 103 (20.4%) were subsequent diagnoses (cut off time was 30 days after lung cancer had been diagnosis). In subsequent diagnosis group, the median time of SREs presented on 129 days after lung cancer was diagnosed, and interquartile range was 57–280 days. Predominate group was male gender 328 (64.9%). The median survival time of lung cancer patients after bone metastases was 148 days, and the 1-, 3-, and 5-year overall survival rates was 25.3%, 7.5%, and 4.1% respectively (Fig. 1). Adenocarcinoma was the common pathology found in lung cancer with bone metastasis 310 (61.4%), squamous cell, small cell, large cell, and others was 131 (25.9%), 30 (5.9%), and 3 (0.6%), respectively.
Fig. 1.
Kaplan-Meier curve showing survival pattern of lung cancer after bone metastasis, and the significant poor prognostic variables (gender and ECOG).
3.2. Prognostic factors of cancer related-deaths in lung cancer patients with bone metastasis
Potential prognostic factors of outcomes were analyzed for 505 cases of bone metastasis using the log-rank test. General health condition and associated underlying conditions were weighed as potential prognostic factors for survival rate. However, the study failed to identify any statistically significant association between those factors and survival rate (Table 1). Three factors were identified as borderline and significantly prognostic factors for poor outcomes based on univariate analysis (p<0.1): gender, ECOG score of 3–4, non-small cell histology subtype. Multivariate analysis identified only two significant poor prognostic factors: male gender, ECOG score of 3–4 (Table 2). Kaplan-Meier curves of the significant prognostic factors are shown in Fig. 1; the hazard ratio of each factor is presented in Table 2.
Table 1.
The differences of survival patterns for each variable analyzed by log-rank test.
| Variable | Number (%) | 3-M survival (%) | 6-M survival (%) | 1-Y Survival (%) | p-value |
|---|---|---|---|---|---|
| Total | 505 (100) | 64.5 | 44.7 | 25.3 | – |
| Gender | |||||
| Female | 177 (35.1) | 69.9 | 52.7 | 35.9 | |
| Male | 328 (64.9) | 61.7 | 40.3 | 19.6 | 0.008* |
| Age of bone metastasis | |||||
| < 60 year | 264 (52.3) | 64.5 | 44.2 | 25.6 | |
| ≥ 60 year | 241 (47.7) | 64.5 | 45.1 | 25.0 | 0.892 |
| ECOG score | |||||
| ECOG 0–2 | 364 (72.1) | 66.4 | 47.4 | 26.3 | |
| ECOG 3–4 | 141 (27.9) | 59.7 | 37.6 | 22.8 | 0.026* |
| Histology diagnosis | |||||
| Small cell | 30 (5.9) | 62.3 | 32.0 | 10.7 | |
| Non-small cell | 475 (94.1) | 64.7 | 45.4 | 26.0 | 0.098* |
| Type of SREs | |||||
| Other symptoms | 427 (84.5) | 64.6 | 46.0 | 25.4 | |
| Parapariasis | 78 (15.5) | 63.9 | 36.8 | 25.0 | 0.407 |
| Site of bone metastasis | |||||
| Single | 150 (29.7) | 64.7 | 48.6 | 26.0 | |
| Multiple | 355 (70.3) | 64.5 | 43.0 | 25.0 | 0.692 |
| Extraosseous metastasis | |||||
| No | 354 (70.1) | 67.9 | 46.6 | 25.4 | |
| Yes | 151 (29.9) | 56.7 | 40.1 | 25.1 | 0.286 |
| Creatinine level | |||||
| < 1.4 mg/mL | 333 (87.9) | 66.6 | 47.9 | 28.5 | |
| ≥ 1.4 mg/mL | 46 (12.1) | 58.4 | 40.4 | 17.9 | 0.311 |
| Hematocrit level | |||||
| < 34% | 175 (45.7) | 69.0 | 48.5 | 29.9 | |
| ≥ 34% | 208 (54.3) | 64.4 | 46.2 | 24.5 | 0.243 |
| Albumin level | |||||
| ≥ 3.4 mg/mL | 155 (30.7) | 60.9 | 44.3 | 28.7 | |
| < 3.4 mg/mL | 193 (38.2) | 72.4 | 51.7 | 26.2 | 0.399 |
| AST level | |||||
| < 60 mg/mL | 315 (62.4) | 75.8 | 48.3 | 28.7 | |
| ≥ 60 mg/mL | 34 (6.7) | 76.5 | 41.2 | 20.6 | 0.231 |
| ALT level | |||||
| < 31 mg/mL | 263 (52.1) | 67.7 | 49.0 | 28.9 | |
| ≥ 31 mg/mL | 87 (17.2) | 61.8 | 42.9 | 24.2 | 0.455 |
| Serum calcium level | |||||
| < 9.2 mg/mL | 161 (31.9) | 60.5 | 47.7 | 29.6 | |
| ≥ 9.2 mg/mL | 141 (27.9) | 65.2 | 46.9 | 24.0 | 0.268 |
Table 2.
Multivariate analysis of prognostic factors and predictive scores.
| Variables | Regression coefficient | Standard error | Hazard Ratio | 95% CI | p-value | Score |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | Reference | 0 | ||||
| Male | 0.351 | 0.101 | 1.42 | 1.166–1.729 | > 0.001 | 1 |
| ECOG | ||||||
| 0–2 | Reference | 0 | ||||
| 3–4 | 0.260 | 0.104 | 1.30 | 1.056–1.591 | 0.013 | 1 |
| Histological subtype | ||||||
| Small cell | Reference | |||||
| Non-small cell | 0.035 | 0.207 | 1.42 | 0.945–2.131 | 0.091 | – |
3.3. Prognostic score development
The estimated regression coefficients (natural logarithm of the hazard ratio) of significant poor prognostic factors were multiplied by four and rounded off to the nearest integer. Values for male gender, and ECOG 3–4 were 0.351, and 0.260 which were transformed into 1.35, and 1.0 then rounded off to 1, and 1, respectively (Table 2). Each score was coded as a binary term, either present or not present, for the factors. The possible summation scores for an individual case were thus 0, 1, and 2.
3.4. The range of survival rate of prognostic scores
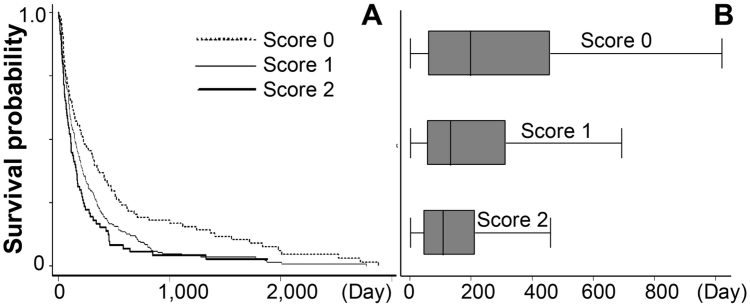
Prognostic scores were calculated by summing individual poor prognostic factor scores in the same cohort. The estimated median survival curve presented good discrimination when the prognostic scores were categorized into three groups (Table 3 and Fig. 2A-B). Scores of 2 represented a significantly different expected time compared to scores of 1 and 0. However, there was no significant difference in life expectancy between prognostic scores of 1 and scores of 0 (Table 3).
Table 3.
Discriminatory ability of predictive scores and range of survival periods.
| Summation of scores | Number (%) | Median time survival: Q1-Q3 (Days) | 95% CI | p-value | 95% CI | p-value |
|---|---|---|---|---|---|---|
| 0 | 120 (23.8) | 225: 66–566 | Reference | – | – | – |
| 1 | 301 (59.6) | 146: 60–346 | 1.153–1.830 | 0.002 | Reference | – |
| 2 | 84 (16.6) | 109: 45–223 | 1.357–2.468 | >0.001 | 0.975–1.616 | 0.078 |
Fig. 2.
Kaplan-Meier curve showing survival curve of each score categories (A), and expected time distribution of each score categories (B).
4. Discussion
Lung cancer is considered to be a fast progressing disease with a short latency period after clinical diagnosis. Cancer cells are capable of infiltrating and colonizing remote organs including bone, contralateral lungs and the brain simultaneously [12]. Most of the bone metastases in this series (79.6%) were found concurrently at the time of diagnosis which is the main pattern of bone metastasis in lung cancer. In our population, male gender, and ECOG score 3 or 4, were significant poor prognostic factors after bone metastasis. On the other hand, previous studies have found that performance status 0 or 1, single bone metastasis and presentation with EGFR mutation were good prognosis for lung cancer after bone metastasis [13], [14]. Current results agree with previous study that the performance status of patient plays the most important role. Performance status represented physical fitness of individual to perform selfcare, daily and physical activity [15] and it becomes the strong prognostic factor than any others particularly in very short survival expectancy as lung cancer. However, an evaluation has been performed subjectively and less reliable therefore the significant result shown in the group of obviously good (ECOG 0–1) for good prognostic factor or poor (ECOG 3–4) for poor prognostic factor.
Male gender plays important role in both an increased risk for SREs from previous studies [16], [17] and poor prognosis after bone metastasis in this study. Female gender has been studied as the independent good prognosis for a better survival in non-small cell lung cancer [18]. Recent reviews of gender indicate that males have a higher susceptibility to cigarette smoking-attributable lung cancer than females [19]. The exact pathophysiology is still not clear, although there are several hypotheses, e.g., gender differences in baseline exposure to environmental pollution [20], the tendency of males to inhale more deeply than females, gender differences in consuming cigarettes with lower tar and nicotine yields [21], or differences in genetic susceptibility to the metabolites of tobacco carcinogens between males and females [22], [23].
In this study, we could not find any significant different of survival expectation between single and multiple bone metastasis. Misclassification between two groups due to the reliability of evaluation might be the main problem of this parameter since sensitive investigation have not been recommended routinely for initial staging process [24]. As accessibility of targeted therapy in the Thai population has been low during the recent period, therefore this parameter was not included in the analysis.
Several survival estimated systems have been used for optimizing invasive procedures during palliative care in metastasis patients. Tokuhashi et al. introduced a system of survival estimation for guiding decisions regarding surgical intervention for spine metastases [25]. The Tokuhashi score is computed based on grouping primary cancers by behavior, number of metastasis sites, association with extraosseous metastasis, and neurological level [25]. Although the system is suggestive of actual survival in a good prognosis group, it is less accurate for patients with an estimated survival of less than 12 months [26]. Tomita et al. introduced a scoring system which relies on only three factors (type of cancer, site of metastasis, and association with other organ metastases) which has a predictive ability similar to the Tokuhashi score [27], [28]. Katagiri et al. developed a predictive score for general bone metastasis which relies on the type of primary cancer, whether the metastasis is visceral or cerebral, performance status, previous chemotherapy, and multiple skeletal metastases [11]. Those scores were derived based on many types of primary cancer which presented wide spectrum. Since this study focused only lung cancer which have a similar metastasis pattern, the result found that other prognostic factor including visceral of cerebral metastasis, number of skeletal metastasis did not play role for prognosis in very short survival of lung cancer.
This study included only lung cancer patients with bone metastasis in deriving a prognostic score. The prognostic ability was good, particularly in a very short survival group presenting a high cumulative score (score=2: average 3.5 month). The discriminatory ability of the scores is clearly seen in the survival curve over a narrow survival period range. With those patients, any surgical intervention should be avoided, however non-invasive ablation for pain control and temporary splinting would be recommended. Surgical intervention for the intermediate survival expectancy (score=1: averaged 5 months) and long survival expectancy (score=0: averaging 7.5 month) would normally be aimed at maintaining their performance status, and preventing secondary SREs which had been reported to occur around 26.9% of bone metastasis in lung cancer [29]. The limitation of this study is that the group of EGFR mutations and treatment were not analyzed. The scoring system appeared to work well for estimating survival period under similar patterns of survival rate and health care. This scoring system, however, still requires further validation.
5. Conclusions
Bone metastasis from lung cancer comprises the major group of palliative care patients because lung cancer is the leading form cancer in Thailand. Bone metastases are frequently found concurrent with a diagnosis of advanced stage, which indicates a short survival time. Male gender and performance 3–4 status plays role in poor prognostic factors after bone metastasis. Prognostic scores deriving based on only lung cancer is clinically simple and easy to use to estimate life expectancy.
Acknowledgements
This research received funding the Faculty of Medicine, Chiang Mai University. Additional support was provided by the Excellence Center in Osteology Research and Training Center (ORTC), Chiang Mai University, and the Thailand National Research University (NRU) Fund. The authors would also like to express their sincere thanks to Dr. G. Lamar Robert, Ph.D., for editing the English manuscript.
Acknowledgments
Declarations
Ethics approval and consent to participant
This study does not contain any individual personal data therefore patients and/or family members do not ask to provided consent. Retrospective analysis was approved by Ethical committee of Faculty of Medicine, Chiang Mai University (EC-ORT-10AVG010294).
Authors’ contributions
AP, and AT carried out the statistical analysis. UA, JK, PC and PT participated in the data acquisition, DP and JS conceived of the study, and helped to draft manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Dumnoensun Pruksakorn, Email: dumnoensun.p@cmu.ac.th.
Areerak Phanphaisarn, Email: aphanphaisarn@hotmail.com.
Jongkolnee Settakorn, Email: jsettakorn@gmail.com.
Urarat Arpornchayanon, Email: bee_183@yahoo.com.
Apichat Tantraworasin, Email: ohm_med@hotmail.com.
Parunya Chaiyawat, Email: p_chaiyawat@hotmail.com.
Jeerawan klangjorhor, Email: jeerawan.klangjorhor@gmail.com.
Pimpisa Teeyakasem, Email: pimpis.mk@gmail.com.
References
- 1.Houston K.A., Henley S.J., Li J., White M.C., Richards T.B. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer. 2014;86(1):22–28. doi: 10.1016/j.lungcan.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lortet-Tieulent J., Soerjomataram I., Ferlay J., Rutherford M., Weiderpass E., Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84(1):13–22. doi: 10.1016/j.lungcan.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Cancer in thailand, New Thammada Press (Thailand) Co., Ltd., Bangkok, Thailand, 2015.
- 4.Sugiura H., Yamada K., Sugiura T., Hida T., Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008;466(3):729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Addario G., Früh M., Reck M., Baumann P., Klepetko W., Felip E., Group E.G.W. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;21(Suppl 5):v116–v119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A., Gray R., Perry M.C., Brahmer J., Schiller J.H., Dowlati A., Lilenbaum R., Johnson D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Rosen G., Nirenberg A., Caparros B., Juergens H., Kosloff C., Mehta B.M., Marcove R.C., Huvos A.G. Osteogenic sarcoma: eight-percent, three-year, disease-free survival with combination chemotherapy (T-7) Natl. Cancer Inst. Monogr. 1981;56:213–220. [PubMed] [Google Scholar]
- 8.Harrington K.D. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614–1627. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1614::aid-cncr12>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Langer C., Hirsh V. Skeletal morbidity in lung cancer patients with bone metastases: demonstrating the need for early diagnosis and treatment with bisphosphonates. Lung Cancer. 2010;67(1):4–11. doi: 10.1016/j.lungcan.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Incidence in Five Continents Volume X, The International Agency for Research on Cancer (IARC), Lyon, France, 2014.
- 11.Katagiri H., Takahashi M., Wakai K., Sugiura H., Kataoka T., Nakanishi K. Prognostic factors and a scoring system for patients with skeletal metastasis. J. Bone Jt. Surg. Br. 2005;87(5):698–703. doi: 10.1302/0301-620X.87B5.15185. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen D.X., Bos P.D., Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 13.Bae H.M., Lee S.H., Kim T.M., Kim D.W., Yang S.C., Wu H.G., Kim Y.W., Heo D.S. Prognostic factors for non-small cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer. 2012;77(3):572–577. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 14.Ulas A., Bilici A., Durnali A., Tokluoglu S., Akinci S., Silay K., Oksuzoglu B., Alkis N. Risk factors for skeletal-related events (SREs) and factors affecting SRE-free survival for nonsmall cell lung cancer patients with bone metastases. Tumour Biol. 2016;37(1):1131–1140. doi: 10.1007/s13277-015-3907-z. [DOI] [PubMed] [Google Scholar]
- 15.Oken M.M., Creech R.H., Tormey D.C., Horton J., Davis T.E., McFadden E.T., Carbone P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 16.Sekine I., Nokihara H., Yamamoto N., Kunitoh H., Ohe Y., Tamura T. Risk factors for skeletal-related events in patients with non-small cell lung cancer treated by chemotherapy. Lung Cancer. 2009;65(2):219–222. doi: 10.1016/j.lungcan.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Ulas A., Bilici A., Durnali A., Tokluoglu S., Akinci S., Silay K., Oksuzoglu B., Alkis N. Risk factors for skeletal-related events (SREs) and factors affecting SRE-free survival for nonsmall cell lung cancer patients with bone metastases. Tumour Biol. 2015 doi: 10.1007/s13277-015-3907-z. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H., Ando K., Shinmyo T., Morita K., Mochizuki A., Kurimoto N., Tatsunami S. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann. Thorac. Cardiovasc Surg. 2011;17(5):469–480. doi: 10.5761/atcs.oa.10.01637. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y., Liu H., Zheng S., Ding Z., Chen Z., Jin W., Wang L., Wang Z., Fei Y., Zhang S., Ying K., Zhang R. Gender susceptibility for cigarette smoking-attributable lung cancer: a systematic review and meta-analysis. Lung Cancer. 2014;85(3):351–360. doi: 10.1016/j.lungcan.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Kiyohara C., Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend. Med. 2010;7(5):381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 21.De Matteis S., Consonni D., Pesatori A.C., Bergen A.W., Bertazzi P.A., Caporaso N.E., Lubin J.H., Wacholder S., Landi M.T. Are women who smoke at higher risk for lung cancer than men who smoke? Am. J. Epidemiol. 2013;177(7):601–612. doi: 10.1093/aje/kws445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung R.J., McKay J.D., Gaborieau V., Boffetta P., Hashibe M., Zaridze D., Mukeria A., Szeszenia-Dabrowska N., Lissowska J., Rudnai P., Fabianova E., Mates D., Bencko V., Foretova L., Janout V., Chen C., Goodman G., Field J.K., Liloglou T., Xinarianos G., Cassidy A., McLaughlin J., Liu G., Narod S., Krokan H.E., Skorpen F., Elvestad M.B., Hveem K., Vatten L., Linseisen J., Clavel-Chapelon F., Vineis P., Bueno-de-Mesquita H.B., Lund E., Martinez C., Bingham S., Rasmuson T., Hainaut P., Riboli E., Ahrens W., Benhamou S., Lagiou P., Trichopoulos D., Holcátová I., Merletti F., Kjaerheim K., Agudo A., Macfarlane G., Talamini R., Simonato L., Lowry R., Conway D.I., Znaor A., Healy C., Zelenika D., Boland A., Delepine M., Foglio M., Lechner D., Matsuda F., Blanche H., Gut I., Heath S., Lathrop M., Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 23.Amos C.I., Wu X., Broderick P., Gorlov I.P., Gu J., Eisen T., Dong Q., Zhang Q., Gu X., Vijayakrishnan J., Sullivan K., Matakidou A., Wang Y., Mills G., Doheny K., Tsai Y.Y., Chen W.V., Shete S., Spitz M.R., Houlston R.S. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat. Genet. 2008;40(5):616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vansteenkiste J., Crinò L., Dooms C., Douillard J.Y., Faivre-Finn C., Lim E., Rocco G., Senan S., Van Schil P., Veronesi G., Stahel R., Peters S., Felip E., Members P. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann. Oncol. 2014;25(8):1462–1474. doi: 10.1093/annonc/mdu089. [DOI] [PubMed] [Google Scholar]
- 25.Tokuhashi Y., Matsuzaki H., Toriyama S., Kawano H., Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila. Pa 1976) 1990;15(11):1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zoccali C., Skoch J., Patel A.S., Walter C.M., Avila M.J., Martirosyan N.L., Demetri S., Baaj A.A. World Neurosurg; 2015. The Surgical Anatomy of the Lumbo-Sacro-Iliac Triangle: A Cadaveric Study. [DOI] [PubMed] [Google Scholar]
- 27.Tomita K., Kawahara N., Kobayashi T., Yoshida A., Murakami H., Akamaru T. Surgical strategy for spinal metastases. Spine (Phila. Pa 1976) 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 28.Aoude A., Amiot L.P. A comparison of the modified Tokuhashi and Tomita scores in determining prognosis for patients afflicted with spinal metastasis. Can. J. Surg. 2014;57(3):188–193. doi: 10.1503/cjs.012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J.-M., Ahn J.S., Lee S., Kim J.A., Lee J., Park Y.H., Park H.C., Ahn M.-J., Ahn Y.C., Park K. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer. 2011;71(1):89–93. doi: 10.1016/j.lungcan.2010.04.003. [DOI] [PubMed] [Google Scholar]