Abstract
Background
Brucellosis is a zoonotic infection transmitted to humans through direct contact with infected animals, their products, or excreta such as urine or dung. Brucellosis is associated with significant morbidity in Southwestern Uganda, where cattle and goat rearing are a major economic industry. As in many settings in sub-Saharan Africa, diagnosis and management of brucellosis remain a challenge due to the presence of comorbidities and limitations in resources for diagnostic testing and therapy.
Methods
A chart review was conducted to characterize the clinical manifestations, diagnosis, comorbidities, and management of 101 patients treated for brucellosis at the Kabale Regional Referral Hospital from September 2002 to May 2010.
Results
Patients presented with substantial comorbidities. The most common manifestation of illness was osteoarticular, but disease manifestations were quite varied. A high rate of focal illness in this cohort (77%) was observed.
Conclusions
Clinicians in this setting should be cognizant of the varied presentations, comorbidities, and treatment options for this disease.
Keywords: brucellosis/complications, developing countries, global health, Uganda, zoonoses
Brucellosis, caused by intracellular Gram-negative bacteria of the genus Brucella, is one of the most common zoonoses worldwide [1]. Transmission to humans most commonly occurs via consumption of insufficiently cooked meat or unpasteurized milk products. Transmission may also occur via direct contact with animals or animal secretions with breaks in the skin or via inhalation of aerosolized particles, which may occur in abbatoirs or laboratories.
The annual worldwide incidence exceeds 500000 cases per year, although it is likely underdiagnosed and underreported [2]. Brucellosis is most commonly identified in Mediterranean countries, India, the Balkans, the Middle East, Central and South America, and the Asian republics of the former Soviet Union. Disease due to Brucella spp is also encountered in sub-Saharan Africa, where clinical disease may coexist with or mimic other endemic illnesses such as malaria, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), and tuberculosis. One such country, Uganda, has an incidence reported as 0.9 per 100000 population, although the true incidence may be up to 20 times the reported figure [3]. Contributing to the incidence in Uganda is endemic brucellosis in herd animals, with a prevalence as high as 55% in cattle herds [4] and 13%–43% in goat herds [5]. The potential for transmission to humans is compounded by informal milk processing, resulting in 92% of milk products passing to market unpasteurized [6]. As a consequence, Brucella has been identified in 12.6% to 29% of informally marketed milk in Uganda [7, 8]. The lack of herd vaccination and an informal system of milk distribution provides a ready source for Brucella exposure in Uganda.
Brucellosis may involve 1 or more organ systems, including musculoskeletal, hematopoetic, nervous, genitourinary, gastrointestinal, cardiovascular, hepatobiliary, pulmonary, ocular, or cutaneous, or it may manifest with nonspecific systemic symptoms or pyrexia [9]. Data on the manifestations of brucellosis is limited in sub-Saharan Africa, with one recent systemic review failing to reveal a single high-quality study from the region [10]. The purpose of the present study was to identify and report the epidemiologic and clinical features of human brucellosis identified at a regional referral hospital in rural southwestern Uganda.
MATERIALS AND METHODS
A single-center retrospective chart review was conducted at Kabale Regional Referral Hospital in Uganda. The study was approved by the Kabale Regional Referral Hospital and the Institutional Review Board at the University of Connecticut Health Center. The Kabale Regional Referral Hospital is located in the Kabale District of southwestern Uganda and is a 226-bed facility that frequently has a patient census in excess of bed capacity, with more than 12000 inpatient admissions and more than 82000 outpatient visits per year [11]. It is located approximately 400 kilometers from the capital, Kampala, and is 1 of the 14 Regional Referral Hospitals in Uganda. The hospital serves a population of approximately 2 million people in its catchment area. It serves as the regional clinical referral hub for the Districts of Kanungu, Kisoro, and Rukungiri, some parts of Ntungamo, as well as serving people from parts of the neighboring countries of Rwanda and the Democratic Republic of Congo. Typical of many hospitals in rural sub-Saharan Africa, the site has limited diagnostic testing and therapeutic options.
A review of available hospital records from the general adult medical ward from September 28, 2002 through May 17, 2010 was performed. All records with a diagnosis indicating brucellosis on the discharge face sheet were selected for detailed chart audit. A standard case definition for brucellosis was established. This included a recorded discharge diagnosis of brucellosis, laboratory data demonstrating a Brucella agglutinin titer of ≥1:160, and implementation of a treatment plan to complete an antibiotic treatment course specific to Brucella spp. Brucella antibody testing was performed using somatic antigens to Brucella aboruis (Cypress Diagnostics, Lonadorp, Belgium).
Features of presenting complaints were obtained from available admission medical records. Manifestations of disease spectrum were then classified based upon the organ system involved including osteoarticular, neurologic, cardiovascular, hepatobiliary, gastrointestinal, respiratory, genitourinary, cutaneous, ocular, or nonfocal involvement, as noted by the treating physician. Charts were reviewed for additional comorbidities. Results of all available testing including radiology and serology were reviewed, including hepatitis B surface antigen, rapid plasma reagin (RPR), Treponema pallidum hemagglutination assay (TPHA), HIV antibody rapid test, HIV p24 antigen, Widal test, urinalyses, and cerebrospinal fluid analyses. Patient demographics, occupation, documented exposure to cattle or goats, urban or rural residence, and consumption of unboiled milk were identified, when available, through chart review. Furthermore, clinical presentations were compared in those with and without laboratory-confirmed coinfection with HIV. Human immunodeficiency virus infection was identified by either (1) a reported past medical history or (2) laboratory testing confirming a positive HIV antibody or p24 antigen.
Data were entered onsite into Microsoft Excel (2013) and imported into IBM SPSS Statistics for Windows, version 22.0. (IBM Corp, Armonk, NY). For measures of a continuous nature, sample sizes, means, medians, and standard deviations were produced. For nominal measures, sample sizes and percentages were produced. Relationships between variables were evaluated using χ2 or Student’s t test as appropriate.
RESULTS
Patient Demographics
Two hundred forty-six chart audits were performed. One hundred twenty-eight (52%) did not meet the required laboratory definition, and 17 (7%) were not prescribed antimicrobial therapy directed at Brucella at time of hospital discharge. Of these, 6 had inadequate documentation, and 11 had alternative diagnoses established in lieu of brucellosis including hepatitis (1), pelvic inflammatory disease (1), tuberculosis (1), urinary tract infection (1), and syphilis ([7] 4 of which were neurosyphilis). One hundred one patients were identified who met the strict case definition of brucellosis.
Of the 101 patients with who met the strict case definition, the mean age was 44.7 years (range, 13–90) and 55 (54%) were male. There were no age differences between male and females. Occupational history was obtained in 65 (64%), 58 (90%) of whom were noted to be peasants or peasant farmers. Thirty-three patients (33%) were identified as having direct contact with cattle, and 6 patients (6%) were identified as having direct contact with goats. Four patients (4%) reported direct exposure to both goats and cattle. Four patients (4%) reported unpasteurized milk consumption.
Comorbidities were identified in 45 patients (45%). Fourteen patients (14%) had multiple comorbidities (Table 1). Human immunodeficiency virus coinfection with Brucella occurred in 16 patients (16%). Syphilis coinfection occurred in 12 patients (12%), and tuberculosis occurred in 3 patients (3%). Other comorbidities were identified in smaller numbers.
Table 1.
Comorbidities Among the Study Cohorta
| Comorbidities | Patients (%) |
|---|---|
| Any comorbidity | 45 (45) |
| ≥2 comorbidities | 14 (14) |
| Syphilis | 12 (12) |
| HIV/AIDS | 16 (16) |
| Alcohol abuse | 6 (6) |
| Cryptosporidium | 4 (4) |
| Diabetes | 3 (3) |
| Hypertension | 3 (3) |
| Tuberculosis | 3 (3) |
| Congestive cardiac failure | 2 (2) |
| Epilepsy | 2 (2) |
| Hepatitis B | 2 (2) |
| Reactive arthritis | 2 (2) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
aOne each (1%) of bipolar disorder, chlamydia, cryptococcal meningitis, coronary artery disease, malignancy, orthopedic injury, rheumatic heart disease, and urinary tract infection.
Patient Presentations
Patients presented with a wide variety of initial symptoms (Table 2). The most common complaints were osteoarticular pain (n = 46, 46%), fever (n = 36, 36%), abdominal pain (n = 31, 31%), cough (n = 28, 28%), anorexia (n = 26, 26%), and headache (n = 23, 23%). Of those with osteoarticular complaints, 23 (50%) noted pain in the lower back, 14 (30%) noted pain in the sacroiliac region, 19 (41%) noted pain in other specific locations including hip, knee, shoulder, and 20 (44%) noted pain in more than 1 location.
Table 2.
Presenting Symptoms
| Symptom | Number (%) |
|---|---|
| Osteoarticular pain | 46 (46) |
| Lower back | 23 (50) |
| Sacroiliac | 14 (30) |
| Knees/legs | 3 (7) |
| Shoulders | 1 (2) |
| Hip | 1 (2) |
| Multiple locations | 20 (44) |
| Fever | 36 (36) |
| Abdominal pain | 31 (31) |
| Cough | 28 (28) |
| Anorexia | 26 (26) |
| Headache | 23 (23) |
| Palpitations | 16 (16) |
| Emesis | 14 (14) |
| Cognitive difficulty | 8 (8) |
| Constipation | 8 (8) |
| Diarrhea | 8 (8) |
| Skin changes | 7 (7) |
Among the classified clinical syndromes, skeletal manifestations were most commonly identified, occurring in 35 (35%) of all patients, followed by nonfocal febrile illness (23%), central nervous system (19%), hepatobiliary (8%), respiratory (6%), cardiovascular (5%), gastrointestinal (2%), and genitourinary (2%) manifestations. There were no statistically significant differences in presentation between HIV-seropositive patients and HIV-seronegative/sero-unknown patients (Table 3).
Table 3.
Clinical Syndromes
| Clinical Syndrome | HIV Negative or Unknown N (%) |
HIV Positivea N (%) |
|---|---|---|
| Skeletal | 35 (35) | 4 (25) |
| Nonfocal/FUO | 23 (23) | 3 (19) |
| Central Nervous System | 19 (19) | 5 (31) |
| Hepatobiliary | 8 (8) | 2 (13) |
| Respiratory | 6 (7) | 2 (13) |
| Cardiovascular | 5 (5) | 0 |
| Gastrointestinal | 2 (2) | 0 |
| Genitourinary | 2 (2) | 0 |
| Cutaneous | 1 (1) | 1 (6) |
| Ocular | 0 | 0 |
Abbreviations: FUO, fever of unknown origin; HIV, human immunodeficiency virus; NS, not significant.
a P = NS for all values between HIV positives and HIV negative/unknowns using Bonferroni correction.
Laboratory Findings
Brucella agglutination testing was available for all cases of confirmed Brucella. The mean titer was 1:234 ± 142, range 1:160–1:1280, with 64% of cases presenting with a titer of 1:160. Bacteriologic cultures were not available due to inherent laboratory biohazard risks and resource constraints.
Other laboratory data were obtained in 65 patients (80%) (Table 4). A leukocyte count was obtained on 31 patients (31%) identifying neutropenia in 23% of patients and leukocytosis in 13% of patients. The mean total leukocyte count was 6.3 × 103 cells/mm3 (range, 3.2–16.2). Erythrocyte sedimentation rate was obtained in 31 patients (38%) with a mean of 73.8 mm/hour (range, 14–126 mm/hour).
Table 4.
Laboratory Findings
| Laboratory Finding | N (%) | Mean [Range]/N (%) Positive |
|---|---|---|
| WBC | 31 (31) | 6.3 [3.2–16.2] |
| <4000 cells/mcL | 7 (23) | |
| 4000–11000 cells/mcL | 20 (65) | |
| >11000 cells/mcL | 4 (13) | |
| ESR | 24 (24) | 73.8 [14–126] |
| >30 mm/hour | 18 (75) | |
| Hemoglobin | 16 (16) | 10.1 [5.2–16.2] |
| <12 mg/dL | 11 (69) | |
| CD4 count | 4 (25) | 315 [28–504] |
| Syphilis serology | 58 (57) | 12 positive (20) |
| Blood smear (malaria) | 18 (18) | 1 positive (6) |
| Brucella agglutinin titer | 101 (100) | 1:234 [1:160–1:1280] |
Abbreviations: ESR, erythrocyte sedimentation rate; WBC, white blood cells.
Pre-existing HIV positivity was noted in 16 patients (16%), with 11 patients (11%) aware of serostatus at presentation, and an additional 5 patients were diagnosed during the course of hospitalization. Of the newly diagnosed HIV+ patients, antibody seropositivity was present in 2 patients, and 3 had a positive p24 antigen consistent with acute HIV infection. CD4 count was obtained in 4 HIV-infected patients (25%), with a mean of 315 cells/mm3 (range, 28–504). Fifty-eight patients (64%) were tested for syphilis, typically with a screening RPR and confirmatory TPHA, 12 (20%) of whom tested positive.
Treatment and Outcomes
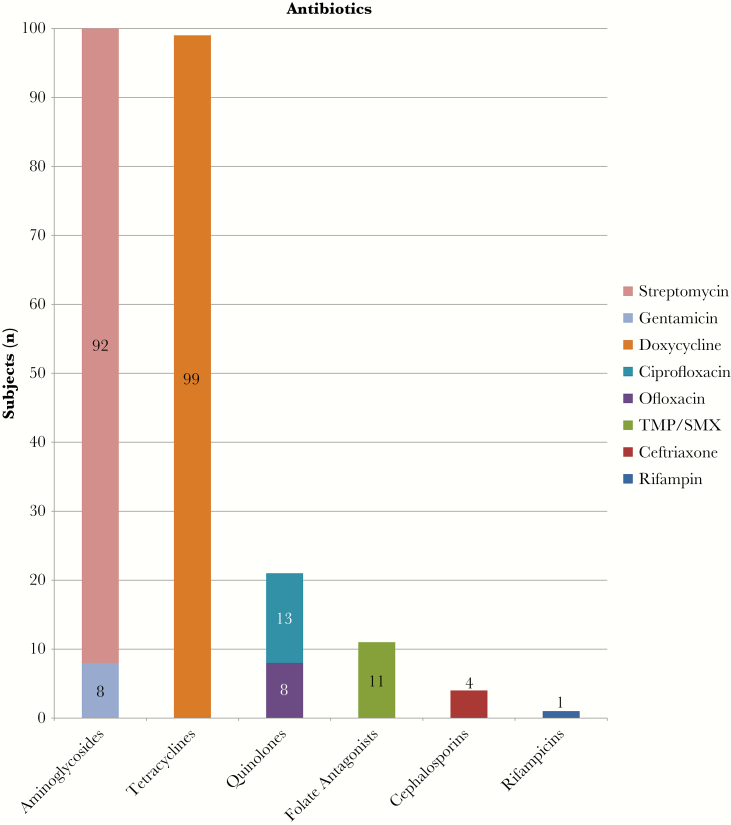
Prescribed treatment courses were characterized by specific agents administered and by number of antibiotics with anti-Brucella activity prescribed. Ninety-seven patients (96%) were treated with both doxycycline and an aminoglycoside, with gentamicin used in 8 (8%) and streptomycin in 92 (91%). Trimethoprim/sulfamethoxazole (TMP/SMX) was used in 11 cases (11%), quinolones were used in 21 cases (21%), ceftriaxone was used in 4 cases (4%), and rifampin was used in 1 case (1%) (Figure 1). Twenty-eight patients (28%) received 3 antibiotic regimens, and 3 patients (3%) received 4 antibiotic regimens. All patients had treatment courses planned for 6 weeks, except 1 who was treated for 20 weeks [12].
Figure 1.
Antibiotic regimens. TMP/SMX, trimethoprim/sulfamethoxazole.
Six deaths (6%) were recorded during patients’ hospitalizations (3 with neurologic, 2 with hepatobiliary, one with gastrointestinal disease), 2 patients (2%) were transferred to other facilities, and 93 patients (92%) were discharged from the hospital with plans to follow up in community health units or outpatient clinics. Follow-up and completion of the antibiotic course was unable to be determined in this cohort. Improvement in clinical status was noted in 73 patients (72%) at the time of discharge, and the clinical status of 21 patients (21%) was recorded as not changed or unknown.
DISCUSSION
Brucellosis remains an important zoonotic illness in developing countries, and it continues to have a significant impact on patient morbidity in the Kabale District (Kigezi region) of Uganda. Recognition and clinical management of disease due to Brucella spp may be challenging in rural African settings due to other concurrent comorbidities and limitations of diagnostic and therapeutic options. Brucella abortus has traditionally been considered the primary agent of human brucellosis in Uganda [13]. However, Brucella melitensis is also known to be prevalent in goats in Uganda, with 10% of goats and 43% of goat herds affected [5], thus also posing a potential risk to humans.
Limitations in medical infrastructure and social challenges common to sub-Saharan Africa have been shown to lead to delays in diagnosis of brucellosis in other African cohorts [14]. Symptoms are classically protean in presentation, consisting most classically of joint pain, fever, weakness, diaphoresis, and lower back pain [15]. Symptoms may overlap with other highly endemic illnesses such as malaria, HIV/AIDS, and tuberculosis, which can make the clinical diagnosis challenging. Infectious comorbidities including syphilis, HIV/AIDS, malaria, and tuberculosis were commonly identified in this cohort of patients in rural Uganda.
Physical examination may be nonspecific and may reveal hepatomegaly, splenomegaly, and lymphadenopathy [16]. Brucella can affect nearly every organ system [10], although it involves the osteoarticular system most commonly [17]. Consistent with prior studies, the most common organ system involved in our overall patient population was osteoarticular (35%). In our cohort, 45% had concomitant comorbidities, likely increasing the difficulty in arriving at a diagnosis. Unlike other studies that report the prevalence of focal findings ranging between 27.7% and 43.2% [15, 18], the presence of focal findings was much higher in our study at 77%. It is likely that additional cases of brucellosis infection resulting in nonfocal, systemic, or febrile illness are present in the region but are either not recognized or are misclassified as being due to other endemic infectious illnesses.
Despite Brucella spp having a predilection for the reticuloendothelial system, suggesting that immunodeficiency secondary to HIV might lead to altered clinical manifestations, prior studies of HIV-positive patients with brucellosis revealed no significant differences in the clinical presentation of the disease compared with HIV-seronegative patients [19]. The present evaluation is consistent with prior studies and did not identify any significant differences in the clinical presentation of brucellosis in HIV-seropositive patients compared with seronegative patients.
Diagnostic testing may be limited to serum antibody testing in the rural sub-Saharan African setting. The present study identified Brucella infection on the basis of serological tests, but these are incapable of distinguishing infection with B abortus from that of B melitensis. Bacteriologic culture, polymerase chain reaction, the Rose Bengal plate agglutination test, and enzyme-linked immunosorbent assay were not available and are often limited in the rural African setting. Serum antibody testing using the serum agglutination testing is most effective when using a definition of a 4-fold or greater rise in Brucella agglutination titer between acute- and convalescent-phase serum specimens obtained ≥2 weeks apart [20]; however, this approach is not practical in many settings in sub-Saharan Africa due to difficulties in obtaining laboratory studies and limitations in patient follow up. Antibodies at a single time point may be affected by a high rate of background exposure in endemic settings and has been noted to be positive in 11% of residents of another area of western Uganda [8]. False-positive Brucella antibody test results can also be caused by cross-reacting antibodies to Escherichia coli O157, Francisella tularensis, Moraxella phenylpyruvica, Yersinia enterocolitica, certain Salmonella serotypes, and from persons vaccinated against Vibrio cholera [21]. Serial dilutions evaluating for a prozone phenomenon are not routinely performed, and thus testing may fail to identify symptomatic patients with very high titers of antibody. A high baseline prevalence of antibody requires a titer of at least 1:160 in endemic areas to rule out low-level background positivity in symptomatic patients [22, 23].
Given the resource limitation of resources available at Kabale Regional Referral Hospital, supporting laboratory data with brucellosis was incomplete. All 101 patients with confirmed brucellosis had a study-defined titer of at least 1:160 (median, 1:160; range, 1:160–1:1280) and a clinical presentation consistent with brucellosis. Of the patients with further serological studies, the most common abnormalities were an elevated erythrocyte sedimentation rate and anemia, which is consistent with prior literature [15].
Management of brucellosis is complicated by limited health infrastructure, limited antibiotic availability, requirement of parenteral administration of aminoglycosides, and the concern for development of resistance to streptomycin and/or rifampin in cases where infection with Mycobacterium tuberculosis may be present. Brucellosis typically requires prolonged therapy with at least 2 agents to which it is susceptible. Antibiotics active against Brucella spp include tetracycline, gentamicin, streptomycin, ceftriaxone, ciprofloxacin, levofloxacin, ofloxacin, and rifampin [24]. Treatment typically consists of a tetracycline plus rifampin or tetracycline plus aminoglycoside for at least 6 weeks [25]. The Uganda clinical guidelines [26] recommend that initial therapy consist of a course doxycycline for 6 weeks combined with an aminoglycoside or ciprofloxacin for 2 weeks, or treatment with TMP/SMX for 6 weeks combined with gentamicin for 2 weeks. Rifampin, ceftriaxone, and ofloxacin may be effective [16]. The addition of a third agent is recommended in some combinations and in neurologic disease [12, 16]. In this cohort, 97 (96%) met the national treatment standard, typically doxycycline combined with an aminoglycoside. Use of additional agents including TMP/SMX, ceftriaxone, rifampin, and fluoroquinolones (ciprofloxacin, ofloxacin) were frequently added to this core regimen. Thirty-one patients (31%) had 3 or more antibiotics prescribed. Duration of antimicrobial therapy was typically 6 weeks, but it was extended if there was an incomplete clinical response. The longest duration of treatment noted at this hospital was 20 weeks [12].
Contact with animals and animal products are common in this cohort. Rural patients are likely to be either raising livestock or to come in contact with livestock given the pastoral grazing practice in this region of Uganda. Seroprevalence for brucellosis in local cattle herds ranging between 55% and 100% provides a ready zoonotic reservoir for disease transmission [4, 27]. Given a high prevalence (92%) of unpasteurized milk reaching the consumer in this region, it is likely that unboiled milk consumption may also play a significant mode of transmission, and this is likely underreported in these rural patients [6]. A systematic practice of cattle herd vaccination and milk pasteurization could potentially have a large impact on the public health burden caused by this illness.
There are several limitations to this study to be noted. The study was conducted at a single clinical site and thus may not be reflective of other geographic locales. Medical documentation was sparse in this cohort, and thus many subjects had limited data documented in the available record. This may lead to potential skewing of data towards those with higher acuity levels who required more aggressive medical and laboratory evaluation that is recorded and thus available for analysis. The antibody testing criteria for study inclusion, a value of 1:160 or greater at a single time point, is imperfect. Titers may lead to false positives in a region with high background endemicity for brucellosis and coexisting comorbid illnesses. False negatives may be seen in patients who may have acute brucellosis but have not yet mounted a significant antibody titer at the time of phlebotomy, those with chronic brucellosis, immunologic impairment, or those with the prozone phenomenon [12, 16].
CONCLUSIONS
In conclusion, brucellosis remains an important cause of morbidity and mortality in southwestern Uganda. Clinicians in this setting should be aware of brucellosis as a cause of disease in this setting that may occur in isolation or in conjunction with other comorbidities. Recognition of clinical disease due to Brucella spp remains challenging given a high background rate of exposure and low-level antibody positivity, limited testing modalities, and the protean nature of the illness. Clinical management may also present challenges due to a high frequency of confounding comorbidities and limited therapeutic options in sub-Saharan Africa.
Acknowledgments
We acknowledge Drs. Vishal Joshi, Ankita Kadakia, and Benjamin Busman, who contributed to project operations, chart review, and data management.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ariza J, Bosilkovski M, Cascio A et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med 2007; 4:e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis 2007; 7:775–86. [DOI] [PubMed] [Google Scholar]
- 3. Pappas G, Papadimitriou P, Akritidis N et al. The new global map of human brucellosis. Lancet Infect Dis 2006; 6:91–9. [DOI] [PubMed] [Google Scholar]
- 4. Bernard F, Vincent C, Matthieu L et al. Tuberculosis and brucellosis prevalence survey on dairy cattle in Mbarara milk basin (Uganda). Prev Vet Med 2005; 67:267–81. [DOI] [PubMed] [Google Scholar]
- 5. Kabagambe EK, Elzer PH, Geaghan JP et al. Risk factors for Brucella seropositivity in goat herds in eastern and western Uganda. Prev Vet Med 2001; 52:91–108. [DOI] [PubMed] [Google Scholar]
- 6. Staal SJ, Kaguaongo WN . The Ugandan Dairy Subsector Targeting Development Opportunities . Nairobi, Kenya: International Livestock Research Institute (ILRI); 2003. [Google Scholar]
- 7. Makita K, Fevre EM, Waiswa C et al. How human brucellosis incidence in urban Kampala can be reduced most efficiently? A stochastic risk assessment of informally-marketed milk. PLoS One 2010; 5:e14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller R, Nakavuma JL, Ssajjakambwe P et al. The prevalence of brucellosis in cattle, goats and humans in rural Uganda: a comparative study. Transbound Emerg Dis 2016; 63:e197–210. [DOI] [PubMed] [Google Scholar]
- 9. Young EJ. An overview of human brucellosis. Clin Infect Dis 1995; 21:283–9. [DOI] [PubMed] [Google Scholar]
- 10. Dean AS, Crump L, Greter H et al. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis 2012; 6:e1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. USAID/SUSTAIN. Kabale Regional Referral Hospital Profile . November, 2015. Available at: http://www.sustainuganda.org/content/kabale-regional-referral-hospital-profile. Accessed 27 May 2017. [Google Scholar]
- 12. Kyebambe P, Kasyaba R, Nkakyekorera S. Cervical spondylitic myeloradiculopathy due to chronic brucellosis in a Ugandan teenager. Afr Health Sci 2012; 12:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Makita K, Fèvre EM, Waiswa C et al. Spatial epidemiology of hospital-diagnosed brucellosis in Kampala, Uganda. Int J Health Geogr 2011; 10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kunda J, Fitzpatrick J, Kazwala R et al. Health-seeking behaviour of human brucellosis cases in rural Tanzania. BMC Public Health 2007; 7:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buzgan T, Karahocagil MK, Irmak H et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis 2010; 14:e469–78. [DOI] [PubMed] [Google Scholar]
- 16. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med 2005; 352:2325–36. [DOI] [PubMed] [Google Scholar]
- 17. Colmenero JD, Reguera JM, Martos F et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore) 1996; 75:195–211. [DOI] [PubMed] [Google Scholar]
- 18. Kursun E, Turunc T, Demiroglu Y, Arslan H. Evaluation of four hundred and forty seven brucellosis cases. Intern Med 2013; 52:745–50. [DOI] [PubMed] [Google Scholar]
- 19. Moreno S, Ariza J, Espinosa FJ et al. Brucellosis in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis 1998; 17:319–26. [DOI] [PubMed] [Google Scholar]
- 20. Godfroid J, Cloeckaert A, Liautard JP et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res 2005; 36:313–26. [DOI] [PubMed] [Google Scholar]
- 21. Brucellosis Reference Guide: Exposures, Testing, and Prevention . Centers for Disease Control and Prevention; 2017. Available at: https://www.cdc.gov/brucellosis/pdf/brucellosi-reference-guide.pdf. Accessed 29 September 2017. [Google Scholar]
- 22. Araj GF. Update on laboratory diagnosis of human brucellosis. Int J Antimicrob Agents 2010; 36(Suppl 1):S12–7. [DOI] [PubMed] [Google Scholar]
- 23. Galińska EM, Zagórski J. Brucellosis in humans–etiology, diagnostics, clinical forms. Ann Agric Environ Med 2013; 20:233–8. [PubMed] [Google Scholar]
- 24. Tanyel E, Coban AY, Koruk ST et al. Actual antibiotic resistance pattern of Brucella melitensis in Central Anatolia. An update from an endemic region. Saudi Med J 2007; 28:1239–42. [PubMed] [Google Scholar]
- 25. Skalsky K, Yahav D, Bishara J et al. Treatment of human brucellosis: systematic review and meta-analysis of randomised controlled trials. BMJ 2008; 336:701–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ugandan Clinical Guidelines: National Guidelines for Management of Common Conditions . Kampala; 2012. Available at: health.go.ug/docs/UCG_2012.pdf. Accessed 27 May 2017. [Google Scholar]
- 27. Magona JW, Walubengo J, Galiwango T, Etoori A. Seroprevalence and potential risk of bovine brucellosis in zerograzing and pastoral dairy systems in Uganda. Trop Anim Health Prod 2009; 41:1765–71. [DOI] [PubMed] [Google Scholar]